Abstract
Bone marrow transplantation (BMT) is the only curative therapy for sickle cell disease (SCD). However, the morbidity and mortality related to pretransplantation myeloablative chemotherapy often outweighs the morbidity of SCD itself, thus severely limiting the number of patients eligible for transplantation. Although nonmyeloablative transplantation is expected to reduce the risk of BMT, it will likely result in mixed-chimerism rather than complete replacement with donor stem cells. Clinical application of nonmyeloablative transplantation thus requires knowledge of the effect of mixed chimerism on SCD pathophysiology. We have, therefore, created a panel of transplanted SCD mice that received transplants displaying an array of red blood cell (RBC) and white blood cell (WBC) chimerism. A significant enrichment of RBC over WBC chimerism occurred in these mice, because of the dramatic survival advantage of donor over sickle RBCs in the peripheral blood. Increasing levels of RBC chimerism provided progressive correction of hematologic and pathologic abnormalities. However, sickle bone marrow and splenic hematopoiesis was not corrected until peripheral blood sickle RBCs were fully replaced with donor RBCs. These results have important and unexpected implications for nonmyeloablative BMT for SCD. As the critical hematopoietic organs were not corrected without full RBC replacement, 100% peripheral blood RBC chimerism becomes the most important benchmark for cure after nonmyeloablative BMT. (Blood. 2003;102:4582-4593)
Introduction
Although bone marrow transplantation (BMT) remains the only cure for sickle cell disease (SCD), the morbidity and mortality associated with conventional myeloablative BMT has limited its use to those patients who suffer the most devastating disease complications.1 Furthermore, the limitations of posttransplantation immunosuppression make BMT available only to those patients with immunologically matched donors. Together, these 2 limitations severely curtail the number of patients offered BMT, with 1 study estimating that of all the potential transplant recipients, only approximately 1% met strict criteria for eligibility and also had an available HLA-matched donor.1 Given these serious limits to conventional BMT, there is growing interest in developing nonmyeloablative protocols designed to induce mixed hematopoietic chimerism rather than complete replacement with donor marrow.2 The vast clinical experience with transient mixed red blood cell (RBC) chimerism after simple or exchange transfusion,3 as well as a small number of patients who received conventional transplants who serendipitously developed mixed chimerism,2,4 and had improvement in SCD complications,2,4 suggests that this approach may be a clinically feasible method of treating many of the complications of SCD. A key question then becomes: What level of mixed RBC chimerism is sufficient to provide long-term correction of the complications of SCD?
We have previously shown that mixed white blood cell (WBC) chimerism and transplantation tolerance across allogeneic barriers can be achieved in mice when a nonmyeloablative conditioning dose of busulfan is used in conjunction with peritransplantation T-cell costimulation blockade of the B7 and CD40 costimulation pathways.5 That work demonstrated that, although chimerism across major histocompatibility complex (MHC) barriers failed to develop in the absence of busulfan pretreatment, in the presence of busulfan and costimulation blockade stable chimerism was titratable by increasing the dose of transplanted bone marrow. In a congenic transplantation model, low levels of chimerism developed even in the absence of busulfan pretreatment when large numbers of donor bone marrow cells were infused.5
We next showed that in a murine model of SCD, the combination of busulfan, bone marrow, and peritransplantation costimulation blockade resulted in mixed WBC chimerism, and full replacement of the peripheral RBC compartment in 8 of 11 mice that received transplants and that these mice demonstrated cure of SCD pathophysiology.6 Interestingly, in that study, a small proportion of mice that did not receive busulfan pretreatment developed significant peripheral blood RBC chimerism even in the setting of extremely low levels of WBC chimerism.6 In agreement with our and others' previous work showing that sickle RBCs are cleared extremely rapidly from peripheral blood,7,8 these results suggested significant enrichment of RBC versus WBC chimerism and further that the RBC chimerism may have a substantial ameliorative effect on SCD pathophysiology.6 However, that previous work did not allow us to determine the threshold of WBC and RBC chimerism necessary to cure SCD.
To begin to answer the question of the threshold of chimerism necessary to cure SCD, Iannone et al9 showed that when murine sickle bone marrow was transplanted into healthy recipients, a dose-dependent increase in sickle pathology occurred. However, because that study focused on “reverse chimeras,” in which healthy mice were used as transplant recipients, the effect of mixed chimerism on established sickle pathology could not be addressed. Although our previous work produced a small number of mice with mixed RBC chimerism, the vast majority showed full replacement of the peripheral RBC compartment with donor RBCs, thereby preventing assessment of mixed RBC chimerism on the sickle phenotype.6 Given the importance of determining the threshold of RBC chimerism for cure of SCD, we have now created a panel of transplanted sickle mice that received transplants with increasing levels of RBC chimerism. This study has allowed us to address mechanistic questions that are of paramount importance to nonmyeloablative transplantation for SCD. First, we determined the mechanism of enrichment of donor over sickle RBCs that occurred in sickle mice receiving transplants. Second, we analyzed the effect of increasing amounts of donor RBCs on hematologic, physiologic, and pathologic parameters to determine the minimal level of RBC chimerism that consistently gave a total-body cure. Our results provide the first chimerism targets for nonmyeloablative BMT for SCD and suggest that the most rigorous benchmark for cure of SCD is full replacement of the peripheral blood with donor RBCs.
Materials and methods
Animals
Sickle mice were originally supplied by Dr Paszty (then at the Lawrence Berkley National Laboratory10 ). They express exclusively human α- and sickle β-globin and were originally bred by selective mating and exist on a mixed genetic background (strains FVB/N, 129, DBA/2, C57BL/6, and Black Swiss10 ). Expression of exclusively sickle β-globin in sickle mice was confirmed by differential hemoglobin electrophoresis as described in “Analysis of RBC chimerism.” Enhanced green fluorescent protein (eGFP) mice (B6-TgN(β-act-EGFP)osbY01) were generously supplied by Dr Okabe.11 Both the eGFP and sickle strains are now maintained at Emory University according to recommendations of the institutional animal care and use committee (IACUC). Sickle transplant recipients were both male and female, aged 7 to 12 weeks. Transplant recipients and donors were always both age- and sex-matched prior to BMT. Although the donor eGFP mice and recipient sickle mice share homology at the MHC locus (both are H2-Kb), they originate from different strain backgrounds10,11 and are expected to be mismatched at a variety of minor histocompatibility loci.
BMT protocol
Bone marrow was obtained from donor eGFP mice by flushing femurs and tibiae with normal saline through a 23-guage needle. Nucleated cells were counted, and the bone marrow was suspended at the following concentrations: 20 × 107/mL, 15 × 107/mL, 10 × 107/mL, 5 × 107/mL, and 1 × 107/mL. A volume of 100 μL of each of these suspensions was injected intravenously (through the retroorbital venus plexus) into groups of 4 to 8 sickle mice so that the final dose of bone marrow transplanted was 20 × 106, 15 × 106, 10 × 106, 5 × 106, and 1 × 106 cells, respectively. In addition, given that donor and recipient animals were expected to have multiple minor histocompatibility mismatches, all mice that received transplants also received 500 μg each of hamster antimouse-CD40L monoclonal antibody (mAb; MR1; BioExpress, Lebanon, NH) and human CTLA4-immunoglobulin (Ig; Bristol-Myers Squibb, Princeton, NJ) (for costimulation blockade) intraperitoneally on days 0, 2, 4, and 7 relative to BMT. Control mice received preconditioning with busulfan (Busulfex; 20 mg/kg intraperitoneally; Orphan Medical, Minnetonka, MN) on day -1, transplant of 20 × 106 eGFP bone marrow cells on day 0, and 500μg each of anti-CD40L and CTLA4-Ig on days 0, 2, 4, and 7.
Analysis of RBC chimerism
RBC chimerism was determined by differential hemoglobin electrophoresis of donor murine “single” β-globin and recipient human sickle β-globin.6,10 Electrophoresis was performed with the Helena Titan III electrophoresis system (Helena Laboratories, Beaumont, TX). The percentage of donor or recipient hemoglobin was determined by densitometry with the use of Kodak 1-D Image Analysis software (Kodak, Rochester, NY).
Flow cytometry
Peripheral blood was analyzed by staining with fluorochrome-conjugated antibodies followed by RBC lysis and washing with a whole blood lysis kit (R&D Systems, Minneapolis, MN). At 6 to 10 months after transplantation, bone marrow was obtained by flushing the femurs and tibiae of mice that received transplants with Hanks balanced saline solution (HBSS; Sigma, St Louis, MO), and splenocytes were obtained by passing spleens through a sterile screen into HBSS. Cell suspensions were then counted with a hemocytometer and stained with fluorescent antibodies. Antibodies (Pharmingen, San Diego, CA) included anti-Ter-119, anti-pan-CD45, anti-CD45.1, anti-Sca-1, anti-c-kit, anti-B220, anti-CD3, anti-Mac-1, anti-GR-1, anti-H2-Kb, and Annexin-V. Dead cells were identified by staining with propidium iodide (PI; Sigma) just prior to flow cytometry, and PI+ cells were excluded from subsequent analysis. Both directly fluorochrome-conjugated and biotinylated antibodies were used. When biotinylated antibodies were used, a secondary incubation with Streptavidin conjugated to allophycocyanin (APC), Cychrome, or APC-Cy7 (Pharmingen) was used. Flow cytometry was performed on FACScan (for 3-color analysis), FACScalibur (for 4-color analysis), or FACSVantage (for 5-color analysis) flow cytometers (Becton Dickinson, Mountain View, CA) and analyzed by using WinList analysis software (Verity Software House, Topsham, ME).
Analysis of bone marrow and spleen stem cells
Stem cells were defined as Sca-1+/c-kit+/Ter-119-/B220-/CD3-/Mac-1-/GR-1- (Sca-1+/c-kit+/Lineage-)12 cells in the bone marrow and spleen. To identify these cells, bone marrow cells and splenocytes were stained with directly conjugated antibodies to Sca-1 and c-kit and with biotinylated antibodies to the lineage markers. After incubation with Strepdavidin-APC-Cy7 and staining with PI to exclude dead cells, flow cytometry was performed by using a FACSVantage flow cytometer. The percentage of total Sca-1+/c-kit+/Lineage- cells that were either donor (eGFP+) or recipient (eGFP-) was identified by using WinList analysis software.
Analysis of RBC ontogeny
The ontogeny of RBC progenitors was determined as previously described.13 Briefly, bone marrow cells and splenocytes were triple labeled with Ter-119, CD71, and PI (to exclude dead cells). Four populations were identified for further analysis: region I (proerythroblasts, Ter-119med/CD71high), region II (basophilic erythroblasts, Ter-119high/CD71high), region III (late basophilic and chromatophilic erythroblasts, Ter-119high/CD71med), and region IV (orthochromatophilic erythroblasts, Ter-119high/CD71low).13 The percentage of each population in relation to total Ter-119+/PI- cells was determined by using WinList flow cytometry analysis software.
Analysis of RBC progenitor engraftment
As others have previously shown, there is a subpopulation of early RBC progenitors in the bone marrow and spleen that express CD45 in addition to Ter-119 and CD71.14 These cells also express eGFP and thus could be easily distinguished as donor (Ter-119+/CD71+/pan-CD45+/eGFP+/CD45.1-) or recipient (Ter-119+/CD71+/pan-CD45+/eGFP-/CD45.1+). Bone marrow cells and splenocytes were thus labeled with Ter-119, CD71, CD45, and CD45.1 and analyzed flow cytometrically with the use of a FACSVantage flow cytometer. The percentage of total Ter-119+/CD71+/CD45+ cells that were either donor (eGFP+/CD45.1-) or recipient (eGFP-/CD45.1+) was determined with WinList analysis software to directly measure the percentage of engraftment of donor RBC progenitors.
Determining hematopoietic balance (lymphomyeloid/erythroid ratio) in the hematopoietic organs
Hematopoietic balance was determined as the percentage of total bone marrow cells or splenocytes that were either lymphomyeloid (LM; CD45+/PI-) or erythroid (E; Ter-119+/PI-).
Analysis of hematologic characteristics
Complete blood counts were performed on a Hemavet 1500 blood analyzer (CDC Technologies, Oxford, CT). Reticulocytes were analyzed flow cytometrically and were defined as the percentage of peripheral blood cells that were Ter-119+ and Thiazole-Orange+ (a nucleic acid staining dye; Sigma).
Urine osmolarity
Urine (10 μL) was collected from both mice that received transplants and control mice that did not receive transplants. For consistency, this collection was always performed in the early morning, although for mice (in contrast to humans), early morning urine does not represent the most concentrated sample. Urine osmolarity was then determined by using a Vapro osmometer (Wescor, Logan UT) according to manufacturer's instructions.
Soluble vascular cell adhesion molecule measurement
Soluble vascular cell adhesion molecule (sVCAM) concentration in serum was determined by enzyme-linked immunosorbent assay (ELISA) with the use of a commercially available kit (R&D Systems).
RBC half-life
RBC population half-life was determined by a pulsed biotinylation experiment.6,15 N-hydroxysuccidimide biotin (50 mg/kg; Calbiochem, San Diego, CA) was injected into animals that received transplants or control animals. The percentage of biotinylated peripheral RBCs was determined by staining for RBCs with Ter-119 and for biotin with fluorescent-labeled Streptavidin (Ter-119+/Strepdavidin-Cychrome+). The percentage of Ter-119+/Strepdavidin-Cychrome+ cells remaining over time was analyzed flow cytometrically to determine the half-life of the RBC population. Given the chimerism present in the mice that received transplants, there was a biphasic nature to the RBC half-life curves, indicating the presence of 2 separate RBC populations (short-lived sickle RBCs and long-lived donor RBCs) with dramatically different RBC life spans. Although these 2 populations clearly have separate RBC life spans, thus precluding an interpolation of a single RBC half-life in the chimeric mice, the time at which half the biotinylation signal had disappeared (referred to as the biotinylation50 time) could be directly interpolated from the biotinylation decay curves.
Analysis of pathology
Dissections, weights, and microscopic studies were performed by 1 pathologist (E.A.M.) to provide uniformity of diagnoses. Tissue was fixed in neutral-buffered formalin at room temperature for at least a week, dehydrated in graded alcohols, embedded in paraffin, cut at 5 to 7 μm, and stained with hematoxylin and eosin. Three levels of each block were cut. Special stains, including Masson trichrome and Gomori-modified iron stains, were accompanied by appropriate controls. Although sickling of the erythrocytes made blinding of the pathologist to presence or absence of sickle hemoglobin impossible, blinding to the percentage of correction was maintained throughout the microscopic studies. Tissues surveyed included brain, heart, lungs, thymus, liver, pancreas, kidneys, gonads, adrenals, bone marrow, eyes, skeletal muscle, adipose, mesentery, penis, intestines, lymph nodes, thyroid, spinal cord, and bone. Microscopic sections were studied for changes detectable by routine light microscopy, and the changes were subjectively graded on a scale of 0 (absence) to 4+ (abundant/severe). The findings that were present most consistently included cardiac vascular ectasia (dilatation of the medium-sized arteries), increased thickness of the media of the pulmonary arteries (measured by ocular micrometer), ectasia of medium-sized pulmonary arteries, hepatic fibrosis consistent with remote infarct (focal increase in fibrous tissue by trichrome stain), recent hepatic infarcts (coagulative necrosis with/without inflammatory infiltrates and pigmented histiocytes), hepatic iron deposition (increased staining on Gomori-modified iron stain in both hepatocytes and Kupffer cells), renal glomerular enlargement (increased diameter of appropriately oriented glomerular tufts measured by ocular micrometer), renal mesangial glomerular changes (focal increase in mesangial tissue), renal tubular iron deposition, splenic red pulp/white pulp architectural integrity, splenic hematopoiesis (increased amount of hematopoietic cells within the red pulp), and estimation of ongoing injury in heart, lungs liver, kidneys, or spleen (sickle-related changes of chronic ischemia).
Results
Titratable chimerism after nonmyeloablative transplantation in murine SCD
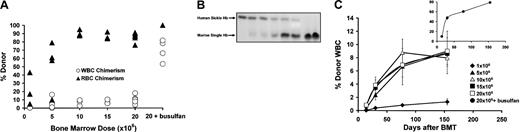
We used the transgenic eGFP strain of mice as bone marrow donors for transplantation into sickle mice and produced a panel of animals with a wide range of WBC and RBC chimerism (Figure 1A). WBC chimerism was easily detected by eGFP fluorescence without the need for antibody labeling. RBC chimerism was detected by the differential electrophoretic mobilities of donor and recipient hemoglobin isoforms (Figure 1B). The donor eGFP mice and the recipient sickle mice were MHC-matched at MHC class I, both being H-2Kb.10,11 Thus, as we have previously shown,5 we anticipated that acquisition of transplantation tolerance and donor chimerism with this transplant pair would occur readily, without the need for pretransplantation myelosuppression with busulfan. However, the eGFP donor mice and the sickle recipients were derived from different strain backgrounds,5,10,11 thus, despite being MHC-matched, these 2 strains were likely to have multiple minor histocompatibility mismatches. We, therefore, included our well-established method of inducing transplantation tolerance through T-cell costimulation blockade as part of the transplantation regimen.5,6 We transplanted sickle mice with either 1 × 106 (n = 5), 5 × 106 (n = 5), 10 × 106 (n = 5), 15 × 106 (n = 5), or 20 × 106 (n = 8) whole eGFP bone marrow cells on day 0 and gave costimulation blockade (with anti-CD40L and CTLA4-Ig) on days 0, 2, 4, and 7. As a positive control for engraftment an additional 4 mice were pretreated with busulfan (20 mg/kg) before transplantation of 20 × 106 whole bone marrow cells and treatment with anti-CD40L and CTLA4-Ig.6 Of the 32 mice that received transplants, 5 died during procedures (anesthesia- or phlebotomy-related deaths) prior to any analysis, and an additional 4 were analyzed partially (up to 3 months after transplantation) but died prior to terminal experiments. The remaining 23 mice were analyzed for 6 to 10 months prior to terminal experiments. Dose-dependent chimerism developed, with the highest levels occurring after pretreatment with busulfan (Figure 1A-C). Mice that received very low doses of bone marrow (1 × 106 or 5 × 106 cells) developed 0.1% to 16% WBC chimerism and 5% to 79% RBC chimerism (Figure 1A,C). In all but 1 mouse that received more than 10 × 106 bone marrow cells, RBC chimerism was more than 80% (84%-95%), whereas WBC chimerism was much lower (3%-18%). Multilineage WBC chimerism was present, including donor B cells, T cells, macrophages, and granulocytes (data not shown). Busulfan-pretreated mice developed significantly higher WBC chimerism (53%-81%) and complete replacement of the peripheral blood with donor RBCS (Figure 1A,C).
Durable, titratable WBC and RBC chimerism was produced in the sickle mice receiving transplants. (A) Increasing amounts of donor bone marrow yielded titratable WBC and RBC chimerism. Unmanipulated bone marrow cells from donor eGFP mice were transplanted into sickle recipients, and chimerism was determined at the terminal time point (6-10 months after transplantation). ▴ = percentage of donor WBCs, ▴ = percentage of donor Hb in the peripheral blood, respectively, for individual mice. (B) A wide range of RBC chimerism developed in the mice that received transplants. Shown is a representative hemoglobin electrophoresis with blood from animals that received transplants that demonstrates increasing amounts of donor Hb: From left to right, 0% (sickle control), 17% (no. 1582), 43% (no. 1550), 57.5% (no. 1636), 79% (no. 1630), 90% (no. 70), 100% (no. 62), and 100% (eGFP control). (C) Chimerism is stable over time. WBC chimerism was determined as the percentage of eGFP+/CD45+ cells in the peripheral blood. The average WBC chimerism over time for each of the 6 different treatment conditions is shown: ♦ = 1 × 106 donor bone marrow cells, ▴ = 5 × 106, ▵ = 10 × 106, ▪ = 15 × 106, □ = 20 × 106, • = 20 × 106 after pretreatment with 20 mg/kg busulfan (inset). For each bone marrow dose, n = 3-7. Error bars show SEM.
Durable, titratable WBC and RBC chimerism was produced in the sickle mice receiving transplants. (A) Increasing amounts of donor bone marrow yielded titratable WBC and RBC chimerism. Unmanipulated bone marrow cells from donor eGFP mice were transplanted into sickle recipients, and chimerism was determined at the terminal time point (6-10 months after transplantation). ▴ = percentage of donor WBCs, ▴ = percentage of donor Hb in the peripheral blood, respectively, for individual mice. (B) A wide range of RBC chimerism developed in the mice that received transplants. Shown is a representative hemoglobin electrophoresis with blood from animals that received transplants that demonstrates increasing amounts of donor Hb: From left to right, 0% (sickle control), 17% (no. 1582), 43% (no. 1550), 57.5% (no. 1636), 79% (no. 1630), 90% (no. 70), 100% (no. 62), and 100% (eGFP control). (C) Chimerism is stable over time. WBC chimerism was determined as the percentage of eGFP+/CD45+ cells in the peripheral blood. The average WBC chimerism over time for each of the 6 different treatment conditions is shown: ♦ = 1 × 106 donor bone marrow cells, ▴ = 5 × 106, ▵ = 10 × 106, ▪ = 15 × 106, □ = 20 × 106, • = 20 × 106 after pretreatment with 20 mg/kg busulfan (inset). For each bone marrow dose, n = 3-7. Error bars show SEM.
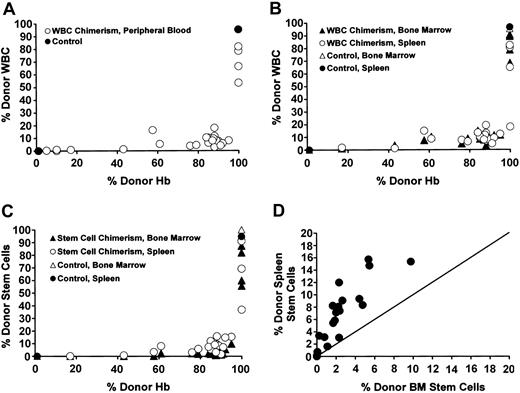
WBC chimerism (defined as the percentage of total CD45+/PI- cells that were also eGFP+) was similar in both the peripheral blood and the hematopoietic organs and was significantly lower than peripheral blood RBC chimerism (Figure 2A-B). Likewise, stem cell chimerism (defined as the percentage of all Sca-1+/c-kit+/PI-/lineage- cells in either the bone marrow or the spleen that were also eGFP+)12 showed a similar pattern to peripheral blood WBC chimerism (Figure 2C). Thus, donor stem cell chimerism in the bone marrow and spleen was significantly lower than donor RBC chimerism in the peripheral blood (Figure 2C). Although bone marrow and splenic stem cell chimerism were both significantly lower than peripheral blood RBC chimerism, the percentage of stem cells in the spleen that were donor in origin (ie, the percentage of total splenic c-kit+/Sca-1+/PI-/lineage- cells that were eGFP+) was significantly higher than the percentage of stem cells in the bone marrow that were donor in origin (Figure 2D). This difference was not attributable to different organ sizes, as each measurement was a percentage compared with total numbers of stem cells for that organ (either bone marrow or spleen). These higher levels of engraftment may reflect preferential homing or expansion of stem cells in the spleen over bone marrow in the setting of hyperactive splenic hematopoiesis7 in the sickle mice.
Enrichment of peripheral blood RBC chimerism compared with WBC chimerism in the blood, hematopoietic organs, and stem cells. (A) In the individual mice, a striking enrichment of peripheral blood RBC versus WBC chimerism occurred. The RBC chimerism (expressed as percentage of donor Hb, x-axis) versus WBC chimerism (y-axis) for each of the mice that received transplants is shown (▴). Control sickle (0% donor Hb) or eGFP (100% donor Hb) are shown (•). (B) Enrichment of peripheral RBC chimerism (x-axis) compared with WBC chimerism (y-axis) in the bone marrow (▴) and spleen (▴) occurred in mice that received transplants. Control sickle mice that did not receive transplants (0% donor Hb) or eGFP mice (100% donor Hb) are shown as ▵ (bone marrow) and • (spleen). (C) Enrichment of peripheral RBC chimerism (x-axis) compared with stem cell chimerism (y-axis) in the bone marrow (▴) and spleen (▴) also occurred in the mice that received transplants. The stem cell compartment was defined as those bone marrow cells or splenocytes that were Sca-1+/c-kit+/PI-/lineage-. The percentage of these stem cells that were either donor derived (eGFP+/CD45.1-) or recipient derived (eGFP-/CD45.1+) was then determined. Control sickle mice that did not receive transplants (0% donor Hb) or eGFP mice (100% donor Hb) are shown as ▵ (bone marrow) and • (spleen). (D) A higher percentage of splenic stem cells than bone marrow stem cells were donor derived in the chimeric mice. Stem cells were defined as described in panel C. The percentage of stem cells that were donor in the bone marrow (x-axis) versus spleen (y-axis) was plotted (•). The line depicts the theoretical 1:1 ratio of bone marrow to spleen stem cells.
Enrichment of peripheral blood RBC chimerism compared with WBC chimerism in the blood, hematopoietic organs, and stem cells. (A) In the individual mice, a striking enrichment of peripheral blood RBC versus WBC chimerism occurred. The RBC chimerism (expressed as percentage of donor Hb, x-axis) versus WBC chimerism (y-axis) for each of the mice that received transplants is shown (▴). Control sickle (0% donor Hb) or eGFP (100% donor Hb) are shown (•). (B) Enrichment of peripheral RBC chimerism (x-axis) compared with WBC chimerism (y-axis) in the bone marrow (▴) and spleen (▴) occurred in mice that received transplants. Control sickle mice that did not receive transplants (0% donor Hb) or eGFP mice (100% donor Hb) are shown as ▵ (bone marrow) and • (spleen). (C) Enrichment of peripheral RBC chimerism (x-axis) compared with stem cell chimerism (y-axis) in the bone marrow (▴) and spleen (▴) also occurred in the mice that received transplants. The stem cell compartment was defined as those bone marrow cells or splenocytes that were Sca-1+/c-kit+/PI-/lineage-. The percentage of these stem cells that were either donor derived (eGFP+/CD45.1-) or recipient derived (eGFP-/CD45.1+) was then determined. Control sickle mice that did not receive transplants (0% donor Hb) or eGFP mice (100% donor Hb) are shown as ▵ (bone marrow) and • (spleen). (D) A higher percentage of splenic stem cells than bone marrow stem cells were donor derived in the chimeric mice. Stem cells were defined as described in panel C. The percentage of stem cells that were donor in the bone marrow (x-axis) versus spleen (y-axis) was plotted (•). The line depicts the theoretical 1:1 ratio of bone marrow to spleen stem cells.
These data show that in murine SCD, even without any preconditioning with busulfan, significant WBC and RBC chimerism could be produced and was stable for more than 6 months after an MHC-matched BMT. Furthermore, we observed dramatic enrichment of peripheral blood RBC chimerism over WBC chimerism in the mice that received transplants.
Enrichment of RBC chimerism is caused by the survival advantage of donor RBCs in the peripheral blood
Two models can explain the enrichment in peripheral blood RBC chimerism compared with WBC chimerism in the animals that received transplants: (1) Enhanced survival of healthy RBCs in the peripheral blood compared with the rapidly cleared sickle RBC; (2) enrichment in the hematopoietic organs of donor erythroid progenitors, because of increased survival of healthy progenitors or, possibly, enhanced erythroid progenitor engraftment. Although we and others have previously shown that donor RBCs have enhanced survival compared with sickle RBCs in the periphery,7,8 no previous study has investigated the possibility of enrichment at the level of an erythroid progenitor. To differentiate these possibilities, we looked at the following 3 parameters:
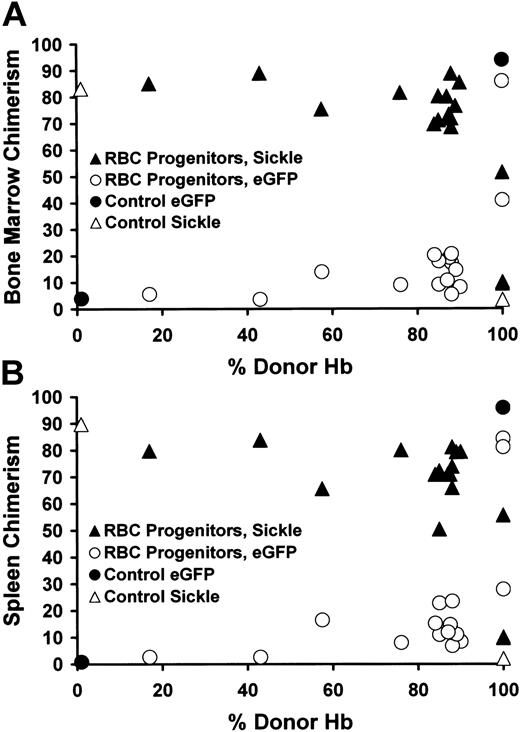
First, we directly determined the source of early RBC progenitors in the hematopoietic organs to investigate whether enhanced engraftment of RBCs compared with WBC progenitors had occurred. We studied the small population of early RBC progenitors in the bone marrow and spleen that express both Ter-119high and CD71high and that still express the pan-WBC marker CD45 on their cell surface,14 as these cells also express eGFP and thus can be easily identified as donor or recipient. Donor Ter-119high/CD71high/CD45+ cells are eGFP+ and CD45.1-, allowing identification of the Ter-119high/CD71high/pan-CD45+ cells as either donor (eGFP+/CD45.1-) or recipient (eGFP-/CD45.1+) (Figure 3A-B). This analysis showed that the percentage of donor RBC progenitors in mice with less than 100% peripheral RBC chimerism was extremely low and did not increase until full replacement of the peripheral RBC compartment with donor cells had occurred (Figure 3A-B). Thus, progressively increasing engraftment of donor RBC progenitors did not account for the enrichment in peripheral blood RBCs over WBC chimerism observed in the mice that received transplants. Furthermore, a comparison of Figure 2A-C (which shows the enrichment of peripheral blood RBC chimerism compared with WBC chimerism) with Figure 3A-B (which shows the enrichment of peripheral blood RBC chimerism compared with bone marrow [Figure 3A] or spleen [Figure 3B] early RBC progenitor chimerism) reveals strikingly similar patterns. This finding strengthens the argument that peripheral blood WBC chimerism, which can be accessed routinely, serves as a reliable surrogate for both WBC chimerism in the bone marrow and spleen and RBC progenitor chimerism in these same hematopoietic organs.
Enrichment of peripheral blood RBC chimerism compared with RBCprogenitor chimerism in the bone marrow and spleen. Early RBC progenitors (defined as Ter-119high/CD71high/pan-CD45+/PI-) were quantified by flow cytometry, and their origin (donor, eGFP+/CD45.1-) or host (eGFP-/CD45.1+) was determined. In both bone marrow (A) and spleen (B), sickle RBC progenitors predominated despite near complete replacement of the peripheral blood with donor RBCs. The x-axis shows the percentage of donor Hb in the peripheral blood. The y-axis depicts the percentage of eGFP+/CD45.1- donor (▴) or eGFP-/CD45.1+ sickle (▴) RBC progenitors in individual mice receiving transplants. In sickle (0% donor Hb) or eGFP (100% donor Hb) control animals the percentage of donor (•) or host (▵) RBC progenitors is also shown.
Enrichment of peripheral blood RBC chimerism compared with RBCprogenitor chimerism in the bone marrow and spleen. Early RBC progenitors (defined as Ter-119high/CD71high/pan-CD45+/PI-) were quantified by flow cytometry, and their origin (donor, eGFP+/CD45.1-) or host (eGFP-/CD45.1+) was determined. In both bone marrow (A) and spleen (B), sickle RBC progenitors predominated despite near complete replacement of the peripheral blood with donor RBCs. The x-axis shows the percentage of donor Hb in the peripheral blood. The y-axis depicts the percentage of eGFP+/CD45.1- donor (▴) or eGFP-/CD45.1+ sickle (▴) RBC progenitors in individual mice receiving transplants. In sickle (0% donor Hb) or eGFP (100% donor Hb) control animals the percentage of donor (•) or host (▵) RBC progenitors is also shown.
To further explore the relationship between peripheral blood RBC chimerism and the regulation of hematopoiesis, we examined the ontogeny of RBCs from proerythroblasts through orthochromatophilic erythroblasts in animals that received transplants with increasing levels of RBC chimerism. Socolovsky et al13 have previously shown that labeling with Ter-119 and CD71 allows flow cytometric differentiation of erythroid progenitors. After double-labeling, proerythroblasts (region I) are Ter119med/CD71high. Basophilic erythroblasts (region II) are Ter119high/CD71high. Late basophilic and chromatophilic erythroblasts (region III) are Ter119high/CD71med and orthochromatophilic erythroblasts (region IV) are Ter119high/CD71low13 (Figure 4). We noted a marked difference in the balance of erythroid progenitors in the hematopoietic organs when recipient sickle mice and donor eGFP mice were compared (Figure 4A-B). Thus, although eGFP mice have approximately equivalent numbers of early (regions I + II + III) and mature (region IV) erythrocytes in the bone marrow and predominantly mature (region IV) erythrocytes in the spleen (Figure 4A-D), sickle mice have a predominance of early erythroid progenitors (Figure 4A-D). In the chimeric mice, the balance of erythroid progenitors in the hematopoietic organs closely resembled that found in sickle mice that did not receive transplants and did not normalize until complete replacement of the peripheral blood with donor RBCs occurred (Figure 4B-D). For example, even when more than 80% of the peripheral RBCs were donor in origin, the hematopoietic organs still produced RBC progenitors in a balance resembling sickle mice that did not receive transplants, and this production did not change until fully 100% peripheral RBC chimerism was present. These data argued against enhanced survival of donor erythroid progenitors in the hematopoietic organs of chimeric mice, as a predicted shift of erythroid progenitor balance toward donor type did not occur until full replacement with donor RBC existed. Of importance, both the bone marrow and spleen were perfused with peripheral blood in the live animals prior to this analysis, and no manipulations were made to remove this blood from the hematopoietic organs prior to flow cytometry. Thus, it is possible that some contribution to the erythroid progenitor balance observed in these organs came from the peripheral blood. However, a comparison of the reticulocyte percentage in the peripheral blood (Figure 6C) and the progenitor balance in the bone marrow and spleen (Figure 4) shows this contribution to be minimal. Thus, in a representative animal with 90% donor chimerism, the peripheral blood reticulocyte count was only 4% (Figure 6C, closely resembling donor mice), but the percentage of early RBC progenitors (regions I + II + III) in the bone marrow and spleen were 84% and 44%, respectively, closely resembling sickle mice that did not receive transplants (Figure 4C-D). The balance of RBC progenitors in the bone marrow and spleen was, therefore, significantly different than the balance in the peripheral blood. This experiment thus indicates that enhanced survival of erythroid progenitors in the hematopoietic organs does not explain the enrichment of RBC versus WBC chimerism present in mice that received transplants.
The erythroid progenitor balance in mice receiving transplants closely resembled the sickle mice not receiving transplants until complete replacement of the peripheral blood with donor RBCs occurred. (A) Representative flow cytometric analysis of RBC ontogeny in sickle and eGFP spleens. Red cell maturation was mapped by determining the relative proportions of Termed/CD71high (region I), Terhigh/CD71high (region II), Terhigh/CD71med (region III), and Terhigh/CD71low (region IV) cells.13 Representative density-contour maps show the splenic RBC progenitor balance from eGFP and sickle mice. (B) Comparison of regions I to IV from spleens of control sickle (▪) and eGFP (▦) mice and representative mice with either 90% (▤) or 100% (□) peripheral RBC chimerism. This analysis gives a vivid illustration of the similarity of mice with 90% RBC engraftment to sickle mice that did not receive transplants and demonstrates that correction of RBC progenitor balance occurred only after 100% replacement with donor RBCs. (C-D) In both bone marrow (C) and the spleen (D), the RBC progenitor balance did not resemble donor eGFP mice until complete replacement of the peripheral blood with donor RBC occurred. For clarity, the percentage of early RBC progenitors (regions I, II, and III) were combined (▴,▵) and compared with the percentage of mature RBC progenitors (region IV; •,▴). For individual mice that received transplants, the percentage of RBC progenitors in the bone marrow (C) or spleen (D) that resided in region I + II + III (▴) or IV (▴) was determined (y-axis) and plotted against the percentage of donor Hb in the peripheral blood (x-axis). Early RBC progenitors (regions I + II + III) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as [▵]. Mature RBC progenitors (region IV) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as •.
The erythroid progenitor balance in mice receiving transplants closely resembled the sickle mice not receiving transplants until complete replacement of the peripheral blood with donor RBCs occurred. (A) Representative flow cytometric analysis of RBC ontogeny in sickle and eGFP spleens. Red cell maturation was mapped by determining the relative proportions of Termed/CD71high (region I), Terhigh/CD71high (region II), Terhigh/CD71med (region III), and Terhigh/CD71low (region IV) cells.13 Representative density-contour maps show the splenic RBC progenitor balance from eGFP and sickle mice. (B) Comparison of regions I to IV from spleens of control sickle (▪) and eGFP (▦) mice and representative mice with either 90% (▤) or 100% (□) peripheral RBC chimerism. This analysis gives a vivid illustration of the similarity of mice with 90% RBC engraftment to sickle mice that did not receive transplants and demonstrates that correction of RBC progenitor balance occurred only after 100% replacement with donor RBCs. (C-D) In both bone marrow (C) and the spleen (D), the RBC progenitor balance did not resemble donor eGFP mice until complete replacement of the peripheral blood with donor RBC occurred. For clarity, the percentage of early RBC progenitors (regions I, II, and III) were combined (▴,▵) and compared with the percentage of mature RBC progenitors (region IV; •,▴). For individual mice that received transplants, the percentage of RBC progenitors in the bone marrow (C) or spleen (D) that resided in region I + II + III (▴) or IV (▴) was determined (y-axis) and plotted against the percentage of donor Hb in the peripheral blood (x-axis). Early RBC progenitors (regions I + II + III) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as [▵]. Mature RBC progenitors (region IV) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as •.
Effect of increasing RBC chimerism on sickle physiology and hematopoiesis. The various physiologic parameters (y-axis) are plotted against peripheral blood percentage of donor Hb (x-axis). For panels A-G, sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as ▴. Mice that received transplants are shown as •. (A) Hematocrit versus percentage of donor Hb in the peripheral blood. (B) Peripheral WBC versus percentage of donor Hb in the peripheral blood. (C) Reticulocyte percentage versus percentage of donor Hb in the peripheral blood. (D) Annexin-V binding versus percentage of donor Hb in the peripheral blood. (E) Urine osmolarity versus percentage of donor Hb in the peripheral blood. (F) sVCAM concentration versus percentage of donor Hb in the peripheral blood. (G) Spleen weight (as percentage of total body weight) versus percentage of donor Hb in the peripheral blood. (H) Spleen apoptosis versus percentage of donor Hb in the peripheral blood. Apoptosis was determined either by Annexin-V binding (•,▴) or terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay (▴,▵). Sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as ▴ and ▴ for the Annexin-V binding and TUNEL assays, respectively. Animals that received transplants are shown as • and ▵ for the Annexin-V binding and TUNEL assays, respectively. (I) Normalization of lymphomyeloid-erythroid (LM/E) ratio occurred only in mice with 100% donor Hb in the peripheral blood. Splenic LM cells (•,▴) were defined as CD45+/PI-. Splenic E cells (▴,▵) were defined as Ter119+/PI-. The percentage of total splenocytes that were LM or E (y-axis) is plotted against peripheral blood percentage of donor Hb (x-axis). Sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as • (LM) and ▵ (E). Mice that received transplants are shown as ▴ (LM) and ▴ (E).
Effect of increasing RBC chimerism on sickle physiology and hematopoiesis. The various physiologic parameters (y-axis) are plotted against peripheral blood percentage of donor Hb (x-axis). For panels A-G, sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as ▴. Mice that received transplants are shown as •. (A) Hematocrit versus percentage of donor Hb in the peripheral blood. (B) Peripheral WBC versus percentage of donor Hb in the peripheral blood. (C) Reticulocyte percentage versus percentage of donor Hb in the peripheral blood. (D) Annexin-V binding versus percentage of donor Hb in the peripheral blood. (E) Urine osmolarity versus percentage of donor Hb in the peripheral blood. (F) sVCAM concentration versus percentage of donor Hb in the peripheral blood. (G) Spleen weight (as percentage of total body weight) versus percentage of donor Hb in the peripheral blood. (H) Spleen apoptosis versus percentage of donor Hb in the peripheral blood. Apoptosis was determined either by Annexin-V binding (•,▴) or terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay (▴,▵). Sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as ▴ and ▴ for the Annexin-V binding and TUNEL assays, respectively. Animals that received transplants are shown as • and ▵ for the Annexin-V binding and TUNEL assays, respectively. (I) Normalization of lymphomyeloid-erythroid (LM/E) ratio occurred only in mice with 100% donor Hb in the peripheral blood. Splenic LM cells (•,▴) were defined as CD45+/PI-. Splenic E cells (▴,▵) were defined as Ter119+/PI-. The percentage of total splenocytes that were LM or E (y-axis) is plotted against peripheral blood percentage of donor Hb (x-axis). Sickle (0% donor Hb) and eGFP (100% donor Hb) controls are shown as • (LM) and ▵ (E). Mice that received transplants are shown as ▴ (LM) and ▴ (E).
Having ruled out enhanced engraftment or survival of donor erythroid progenitors to explain the enrichment of RBC over WBC chimerism in mice that received transplants, we next studied the effect of differential survival of peripheral blood RBCs on RBC chimerism. We have previously shown that the half-life of RBCs in the peripheral blood of healthy animals is approximately 20-fold longer than for sickle RBCs.6,7 Furthermore, we and others have demonstrated that the mechanism of anemia in sickle mice is the rapid clearance of sickle RBCs and reticulocytes from the peripheral blood and not because of ineffective erythropoiesis in the hematopoietic organs.7,8 To confirm these previous observations, the peripheral blood of the animals that received transplants was biotinylated, and the amount of biotin that remained over time was measured.15 This experiment (Figure 5A-B) showed that, in keeping with the chimeric nature of the animals that received transplants, there is a biphasic shape to the biotinylation survival curves, having both a short component (from the sickle RBCs) and a long component (from the donor RBCs). Given this mixture of 2 separate RBC populations, a single RBC half-life could not be directly interpolated from the chimeric biotinylation curves. However, the measurement of the time required for half the original biotin signal to disappear (referred to here as the biotinylation50 time) did give important information about the correction of peripheral blood hemolytic anemia in the chimeric animals. This analysis showed that mice with low levels of donor hemoglobin (Hb) had composite biotinylation50 times resembling sickle mice that did not receive transplants, whereas a progressive increase in this measurement occurred with increasing levels of donor Hb (Figure 5A-B). This correlation between peripheral RBC chimerism and RBC biotinylation50 time gives direct support for the hypothesis that enhanced survival of healthy RBCs in the peripheral blood is the cause of the enrichment in peripheral RBC over WBC chimerism. Furthermore, most of the mice that received transplants had peripheral RBC/WBC chimerism ratios of approximately 20 or less (the ratio of the differential survival of healthy and sickle RBCs as shown in Figure 5 and in our previous work7 ), indicating that the differential peripheral blood survival of donor over sickle RBCs is sufficient to explain the enrichment in RBC versus WBC chimerism observed in the chimeric mice.
Effect of increasing RBC chimerism on the decay kinetics of biotinylated peripheral RBCs. (A) Biotinylation curves for representative mice that received transplants and control mice. y-axis = percentage of biotinylated RBCs remaining. x-axis = time (days) after biotinylation. Average survival curves for mice with 0% (sickle mice that did not receive transplants, •), 17% to 43% (▴), 61% to 76% (▴), 87% to 91% (▵), and 100% RBC chimerism (▪) are shown. (B) The relationship between the time at which 50% of the biotinylation signal had disappeared (biotinylation50 time, y-axis) and the percentage of donor Hb in the peripheral blood (x-axis) is shown for sickle (0% donor Hb) and eGFP (100% donor Hb) controls (▴) and for the mice that received transplants (•).
Effect of increasing RBC chimerism on the decay kinetics of biotinylated peripheral RBCs. (A) Biotinylation curves for representative mice that received transplants and control mice. y-axis = percentage of biotinylated RBCs remaining. x-axis = time (days) after biotinylation. Average survival curves for mice with 0% (sickle mice that did not receive transplants, •), 17% to 43% (▴), 61% to 76% (▴), 87% to 91% (▵), and 100% RBC chimerism (▪) are shown. (B) The relationship between the time at which 50% of the biotinylation signal had disappeared (biotinylation50 time, y-axis) and the percentage of donor Hb in the peripheral blood (x-axis) is shown for sickle (0% donor Hb) and eGFP (100% donor Hb) controls (▴) and for the mice that received transplants (•).
The survival advantage of donor versus sickle RBCs also explains the differential effect of busulfan on WBC versus RBC chimerism in the animals that received transplants. In mice not treated with busulfan, increasing bone marrow dose led to increasing WBC chimerism, ranging from 0.1% to 18% (Figure 1A). Given the survival advantage of healthy versus sickle RBCs, the concomitant RBC chimerism measured in the peripheral blood was much higher, ranging from 5% to 95%. When mice were pretreated with busulfan, chimerism further increased. Peripheral blood WBC chimerism increased from an average of 6.4% without busulfan to an average of 57% with busulfan pretreatment. RBC chimerism also increased, from an average of 86% when 20 × 106 bone marrow cells were transplanted without busulfan to 100% with busulfan pretreatment. Although there appears to be a more subtle effect of busulfan on RBC chimerism than WBC chimerism, this effect is due to reaching the ceiling of 100% chimerism that exists. Because one cannot measure more than 100% donor cells in the chimeric animals, the potential to measure an effect of busulfan on WBC chimerism (from an initial measurement of 6.4% chimerism) was much greater than the potential to measure an effect of busulfan on RBC chimerism (from an initial measurement of 86%). However, a comparison of peripheral blood, bone marrow, or spleen WBC chimerism after busulfan pretreatment (average = 57%, 81%, 75%, respectively; Figures 1A and 2A-B) with bone marrow and spleen RBC progenitor chimerism after busulfan pretreatment (average = 63% and 64%, respectively; Figure 3A-B) showed a similar effect of busulfan on both myelolymphoid and erythroid progenitor populations.
Chimeric mice demonstrate normalization of hematologic and physiologic parameters
We next determined the level of RBC chimerism necessary for correction of hematologic abnormalities and sickle pathophysiology. We examined a wide range of parameters in the chimeric mice, including hematocrit (Hct), WBC, reticulocyte percentage, RBC phosphatidylserine (PS) exposure, urine osmolarity, and sVCAM expression. The Hct and reticulocyte percentage in mice with low RBC chimerism resembled sickle mice that did not receive transplants but demonstrated progressive normalization, especially in animals with more than 70% to 80% donor Hb (Figure 6A,C). Likewise, the WBC count was reduced toward normal levels with increasing amounts of RBC chimerism, such that mice with more than 60% donor Hb had normal WBC counts (Figure 6B). We and others have shown that sickle RBCs display increased PS on their cell surface that correlates with clinical disease severity and with endothelial adhesion.7,16-20 In a similar manner to the Hct and reticulocyte count, mice with more than 70% to 80% normal levels of Hb showed minimal RBC PS exposure, whereas those with low RBC chimerism closely resemble sickle mice that did not receive transplants (Figure 6D). Sickle mice demonstrate kidney pathology6,10 and hyposthenuria. Chimeric mice progressively improved their urine-concentrating ability (Figure 6E). This finding provided a key physiologic correlation between correction of hematologic parameters and functional correction of end-organ damage in the animals that received transplants. Sickle mice and patients with sickle cell disease also demonstrate overexpression of endothelial adhesion molecules that is reflected in increased levels of sVCAM.21-23 Chimeric mice demonstrated progressive decrease in the level of sVCAM (Figure 6F). This normalization may have great importance, as many of the clinical complications in patients with sickle cell disease arise from abnormalities in adhesion and inflammatory pathways.
Analysis of pathology reveals progressive correction with increasing RBC chimerism
An exhaustive analysis of pathology in the mice that received transplants identified 12 areas of characteristic sickle pathology that improved after transplantation, including (1) cardiac vascular ectasia, (2) pulmonary artery medial thickness, (3) pulmonary vascular ectasia, (4) remote hepatic infarct, (5) recent hepatic infarct, (6) hepatocyte iron deposition, (7) hepatic Kupffer cell iron deposition, (8) renal glomerular hypertrophy, (9) renal mesangial hypercellularity, (10) renal tubular iron deposition (11) splenic loss of architectural integrity and vascular congestion, and (12) evidence of ongoing multiorgan injury. Evidence of ongoing multiorgan injury included primarily small-vessel changes (ectasia, perivascular fibrosis, and congestion), parenchymal chronic ischemic changes, pericentral vein sclerosis and hepatocytic ischemic changes in the liver, and evidence of ongoing hemolysis (especially in the liver and kidneys). A pathology score of 0 (no pathology) to 4 (sickle pathology) was given to each of these 12 characteristics (Table 1). Representative histopathology is shown (Figure 7A-T). The sum of the pathology scores for each mouse was also plotted against the percentage of donor Hb (Figure 8). This analysis showed a progressive correction of organ pathology with increasing amounts of donor peripheral RBCs.
Pathologic analysis of chimeric mice
Mouse ID . | Donor Hb, % . | Cardiac vascular ectasia . | Pulmonary artery medial thickness, μ . | Pulmonary vascular ectasia . | Remote hepatic infarct . | Recent hepatic infarct . | Hepatocyte iron . | Hepatic Kupffer cell iron . | Glomerular hypertrophy . | Renal mesangial hypercellularity . | Renal tubular iron . | Splenic architectural disruption . | Ongoing multiorgan injury . | Spleen cellularity . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFP1 | 100 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| eGFP2 | 100 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| eGFP3 | 100 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| eGFP4 | 100 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Sickle 1 | 0 | 4 | 1.1 | 4 | 3 | 4 | 2 | 3 | 2 | 3 | 3 | 4 | 4 | 4 |
| Sickle 2 | 0 | 4 | 2.7 | 4 | 4 | 4 | 2 | 4 | 2 | 3 | 4 | 4 | 4 | 4 |
| Sickle3 | 0 | 3 | 4 | 4 | 3 | 2 | 1 | 3 | 3 | 3 | 4 | 4 | 2 | 4 |
| Sickle4 | 0 | 4 | 2.7 | 4 | 3 | 3 | 2 | 4 | 2 | 3 | 4 | 4 | 4 | 4 |
| 62 | 100 | 2 | 1.6 | 1 | 0 | 0 | 0 | 0 | 2 | 3 | 2 | 0 | 2 | 0 |
| 63 | 100 | 0 | 0.8 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 3 |
| 65 | 100 | 0 | 2.7 | 3 | 0 | 0 | 0 | 1 | 4 | 2 | 2 | 0 | 0 | 0 |
| 66 | 100 | 1 | 2.1 | 2 | 0 | 2 | 1 | 1 | 2 | 3 | 2 | 1 | 3 | 2 |
| 68 | 76 | 3 | 2.4 | 0 | 1 | 1 | 1 | 2 | 3 | 3 | 2 | 2 | 2 | 3 |
| 70 | 90 | 3 | 2.7 | 3 | 0 | 0 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 4 |
| 72 | 85 | 0 | 1.3 | 0 | 4 | 2 | 0 | 1 | 1 | 3 | 1 | 1 | 3 | 4 |
| 1550 | 43 | 4 | 2.7 | 2 | 1 | 4 | 2 | 4 | 3 | 4 | 4 | 4 | 3 | 4 |
| 1582 | 17 | 4 | 4 | 2 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 1 | 3 | 4 |
| 1604 | 61 | 3 | 1.3 | 0 | 3 | 4 | 2 | 3 | 2 | 2 | 4 | 4 | 2 | 4 |
| 1630 | 79 | 2 | 1.3 | 0 | 3 | 3 | 1 | 2 | 0 | 1 | 4 | 2 | 2 | 4 |
| 1636 | 57.5 | 3 | 3.2 | 0 | 3 | 2 | 2 | 3 | 2 | 3 | 4 | 4 | 3 | 4 |
| 1661 | 88 | 2 | 2.7 | 3 | 0 | 2 | 1 | 2 | 1 | 1 | 3 | 1 | 3 | 4 |
| 1664 | 95 | 0 | 2.1 | 0 | 0 | 1 | 1 | 2 | 3 | 3 | 3 | 1 | 0 | 4 |
| 1710 | 89 | 0 | NA | NA | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 3 | 1 | 4 |
| 1718 | 88 | 1 | 2.7 | 4 | 0 | 1 | 1 | 2 | 2 | 2 | 3 | 1 | 0 | 4 |
| 1725 | 85 | 3 | 1.9 | 3 | 0 | 2 | 1 | 2 | 1 | 2 | 3 | 1 | 3 | 4 |
| 1748 | 92 | 0 | 2.7 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 3 | 1 | 0 | 4 |
| 1749 | 84 | 2 | 2.7 | 2 | 0 | 2 | 1 | 3 | 3 | 1 | 4 | 1 | 3 | 3 |
| 1780 | 91 | 0 | 1.6 | 0 | 2 | 3 | 2 | 2 | 1 | 1 | 3 | 2 | 2 | 3 |
| 1790 | 87 | 0 | 1.3 | 0 | 4 | 1 | 0 | 1 | 3 | 4 | 2 | 1 | 0 | 3 |
| 1798 | 88 | 3 | 1.9 | 0 | 4 | 1 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 |
Mouse ID . | Donor Hb, % . | Cardiac vascular ectasia . | Pulmonary artery medial thickness, μ . | Pulmonary vascular ectasia . | Remote hepatic infarct . | Recent hepatic infarct . | Hepatocyte iron . | Hepatic Kupffer cell iron . | Glomerular hypertrophy . | Renal mesangial hypercellularity . | Renal tubular iron . | Splenic architectural disruption . | Ongoing multiorgan injury . | Spleen cellularity . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFP1 | 100 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| eGFP2 | 100 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| eGFP3 | 100 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| eGFP4 | 100 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Sickle 1 | 0 | 4 | 1.1 | 4 | 3 | 4 | 2 | 3 | 2 | 3 | 3 | 4 | 4 | 4 |
| Sickle 2 | 0 | 4 | 2.7 | 4 | 4 | 4 | 2 | 4 | 2 | 3 | 4 | 4 | 4 | 4 |
| Sickle3 | 0 | 3 | 4 | 4 | 3 | 2 | 1 | 3 | 3 | 3 | 4 | 4 | 2 | 4 |
| Sickle4 | 0 | 4 | 2.7 | 4 | 3 | 3 | 2 | 4 | 2 | 3 | 4 | 4 | 4 | 4 |
| 62 | 100 | 2 | 1.6 | 1 | 0 | 0 | 0 | 0 | 2 | 3 | 2 | 0 | 2 | 0 |
| 63 | 100 | 0 | 0.8 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 3 |
| 65 | 100 | 0 | 2.7 | 3 | 0 | 0 | 0 | 1 | 4 | 2 | 2 | 0 | 0 | 0 |
| 66 | 100 | 1 | 2.1 | 2 | 0 | 2 | 1 | 1 | 2 | 3 | 2 | 1 | 3 | 2 |
| 68 | 76 | 3 | 2.4 | 0 | 1 | 1 | 1 | 2 | 3 | 3 | 2 | 2 | 2 | 3 |
| 70 | 90 | 3 | 2.7 | 3 | 0 | 0 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 4 |
| 72 | 85 | 0 | 1.3 | 0 | 4 | 2 | 0 | 1 | 1 | 3 | 1 | 1 | 3 | 4 |
| 1550 | 43 | 4 | 2.7 | 2 | 1 | 4 | 2 | 4 | 3 | 4 | 4 | 4 | 3 | 4 |
| 1582 | 17 | 4 | 4 | 2 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 1 | 3 | 4 |
| 1604 | 61 | 3 | 1.3 | 0 | 3 | 4 | 2 | 3 | 2 | 2 | 4 | 4 | 2 | 4 |
| 1630 | 79 | 2 | 1.3 | 0 | 3 | 3 | 1 | 2 | 0 | 1 | 4 | 2 | 2 | 4 |
| 1636 | 57.5 | 3 | 3.2 | 0 | 3 | 2 | 2 | 3 | 2 | 3 | 4 | 4 | 3 | 4 |
| 1661 | 88 | 2 | 2.7 | 3 | 0 | 2 | 1 | 2 | 1 | 1 | 3 | 1 | 3 | 4 |
| 1664 | 95 | 0 | 2.1 | 0 | 0 | 1 | 1 | 2 | 3 | 3 | 3 | 1 | 0 | 4 |
| 1710 | 89 | 0 | NA | NA | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 3 | 1 | 4 |
| 1718 | 88 | 1 | 2.7 | 4 | 0 | 1 | 1 | 2 | 2 | 2 | 3 | 1 | 0 | 4 |
| 1725 | 85 | 3 | 1.9 | 3 | 0 | 2 | 1 | 2 | 1 | 2 | 3 | 1 | 3 | 4 |
| 1748 | 92 | 0 | 2.7 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 3 | 1 | 0 | 4 |
| 1749 | 84 | 2 | 2.7 | 2 | 0 | 2 | 1 | 3 | 3 | 1 | 4 | 1 | 3 | 3 |
| 1780 | 91 | 0 | 1.6 | 0 | 2 | 3 | 2 | 2 | 1 | 1 | 3 | 2 | 2 | 3 |
| 1790 | 87 | 0 | 1.3 | 0 | 4 | 1 | 0 | 1 | 3 | 4 | 2 | 1 | 0 | 3 |
| 1798 | 88 | 3 | 1.9 | 0 | 4 | 1 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 |
Mice were immersion fixed after death, sectioned, stained, and analyzed in a blinded manner by a single pathologist. A pathologic score of 0 (no sickle pathology) to 4 (classic sickle pathology) was assigned to the indicated characteristics. Mouse ID = internal identifier. eGFP1-4 refer to control mice; Sickle 1-4, untransplanted sickle mice; and 62-1798, transplanted sickle mice. NA indicates not assessed.
Analysis of pathology revealed correction of characteristic sickle pathology in mice that received transplants. The 4 columns (from left to right) show microscopic findings in the sickle controls (panels A,E,I,M,Q), a representative mouse that received a transplant with less than 50% RBC chimerism (panels B,F,N,R [mouse no. 1582], J [mouse no. 1550]), a representative mouse that received a transplant with more than 70% to 80% RBC chimerism (panels C,G,K,O,S [mouse no. 70]) and a healthy eGFP control (panels D,H,L,P,T). The 5 rows (top to bottom) show sections of cardiac medium-sized arteries (panels A-D; hematoxylin and eosin [H&E] original magnification × 100), sections of a wall of a pulmonary artery (panels E-H: H&E stain; original magnification × 400), representative hepatic section (panels I-L: H&E stain; original magnification × 400) with iron stain inset (original magnification, × 400), representative renal section (panels M-P: trichrome stain; original magnification × 100) with iron stain inset (original magnification, × 400), and representative spleen section (panels Q-T: H&E stain; original magnification × 100). For the sickle controls and mice with less than 50% RBC chimerism, the figure illustrates ectasia of cardiac medium-sized arteries; increased thickness of the media of the pulmonary artery; hepatic infarcts; severe iron deposition in hepatocytes, hepatic Kupffer cells, and hepatic histiocytic cells; cortical renal infarcts with chronic inflammatory infiltrates; severe deposition of iron in renal tubular epithelium; and loss of splenic architecture with increased hematopoietic cells, ectasia of medium-sized arteries, and sinusoidal congestion. Mice that received transplants with more than 70% to 80% RBC chimerism showed changes intermediate between the healthy eGFP control and sickle control in the cardiac vascular ectasia, pulmonary artery wall thickness, and hepatic and renal tubular iron deposition. They showed no recent hepatic or renal infarcts, they demonstrated fibrosis consistent with remote infarcts, and they showed some return of the normal nodular architecture of the splenic white pulp.
Analysis of pathology revealed correction of characteristic sickle pathology in mice that received transplants. The 4 columns (from left to right) show microscopic findings in the sickle controls (panels A,E,I,M,Q), a representative mouse that received a transplant with less than 50% RBC chimerism (panels B,F,N,R [mouse no. 1582], J [mouse no. 1550]), a representative mouse that received a transplant with more than 70% to 80% RBC chimerism (panels C,G,K,O,S [mouse no. 70]) and a healthy eGFP control (panels D,H,L,P,T). The 5 rows (top to bottom) show sections of cardiac medium-sized arteries (panels A-D; hematoxylin and eosin [H&E] original magnification × 100), sections of a wall of a pulmonary artery (panels E-H: H&E stain; original magnification × 400), representative hepatic section (panels I-L: H&E stain; original magnification × 400) with iron stain inset (original magnification, × 400), representative renal section (panels M-P: trichrome stain; original magnification × 100) with iron stain inset (original magnification, × 400), and representative spleen section (panels Q-T: H&E stain; original magnification × 100). For the sickle controls and mice with less than 50% RBC chimerism, the figure illustrates ectasia of cardiac medium-sized arteries; increased thickness of the media of the pulmonary artery; hepatic infarcts; severe iron deposition in hepatocytes, hepatic Kupffer cells, and hepatic histiocytic cells; cortical renal infarcts with chronic inflammatory infiltrates; severe deposition of iron in renal tubular epithelium; and loss of splenic architecture with increased hematopoietic cells, ectasia of medium-sized arteries, and sinusoidal congestion. Mice that received transplants with more than 70% to 80% RBC chimerism showed changes intermediate between the healthy eGFP control and sickle control in the cardiac vascular ectasia, pulmonary artery wall thickness, and hepatic and renal tubular iron deposition. They showed no recent hepatic or renal infarcts, they demonstrated fibrosis consistent with remote infarcts, and they showed some return of the normal nodular architecture of the splenic white pulp.
Total pathology score showed progressive correction of sickle pathology. Total pathologic score (y-axis) versus percentage of normal Hb levels in the peripheral blood (x-axis). The total pathology score includes the following individual parameters: (1) cardiac vascular ectasia, (2) pulmonary artery medial thickness, (3) pulmonary vascular ectasia, (4) remote hepatic infarct, (5) recent hepatic infarct, (6) hepatocyte iron deposition, (7) hepatic Kupffer cell iron deposition, (8) renal glomerular hypertrophy, (9) renal mesangial hypercellularity, (10) renal tubular iron deposition, (11) splenic loss of architectural integrity and vascular congestion, and (12) evidence of ongoing multiorgan injury. This evidence includes primarily small-vessel changes (ectasia, perivascular fibrosis, and congestion), parenchymal chronic ischemic changes, pericentral vein sclerosis and hepatocytic ischemic changes in the liver, and evidence of ongoing hemolysis (especially in the liver and kidneys). Each of these parameters received a pathology score from 0 to 4 (Table 1). The summed scores were plotted against the percentage of donor Hb.
Total pathology score showed progressive correction of sickle pathology. Total pathologic score (y-axis) versus percentage of normal Hb levels in the peripheral blood (x-axis). The total pathology score includes the following individual parameters: (1) cardiac vascular ectasia, (2) pulmonary artery medial thickness, (3) pulmonary vascular ectasia, (4) remote hepatic infarct, (5) recent hepatic infarct, (6) hepatocyte iron deposition, (7) hepatic Kupffer cell iron deposition, (8) renal glomerular hypertrophy, (9) renal mesangial hypercellularity, (10) renal tubular iron deposition, (11) splenic loss of architectural integrity and vascular congestion, and (12) evidence of ongoing multiorgan injury. This evidence includes primarily small-vessel changes (ectasia, perivascular fibrosis, and congestion), parenchymal chronic ischemic changes, pericentral vein sclerosis and hepatocytic ischemic changes in the liver, and evidence of ongoing hemolysis (especially in the liver and kidneys). Each of these parameters received a pathology score from 0 to 4 (Table 1). The summed scores were plotted against the percentage of donor Hb.
Hematopoietic cure occurred only with 100% replacement with donor hemoglobin
Although progressive correction of multiple areas of sickle pathophysiology occurred in the chimeric mice, a notable exception was the lack of progressive correction of important hematopoietic abnormalities in the bone marrow and spleen. Given the importance of splenic hematopoiesis in the sickle mice, a detailed examination of many aspects of splenic function was undertaken. The spleen is greatly enlarged in murine SCD, with sickle spleens typically representing approximately 5% total body weight compared with less than 1% in healthy mice.6,7,10 Given that the spleen is a normal site of hematopoiesis in mice, this enlargement likely occurs because of the hemolysis-driven high erythropoietic rate in these animals in the setting of size-constrained limits on bone marrow hematopoiesis. Therefore, murine splenic hematopoiesis likely serves as a model of human bone marrow hematopoiesis.7,10 Although the spleen displayed progressive correction in selected physiologic and pathologic parameters, in others, full RBC replacement was required before correction occurred. Thus, although spleen architecture (Table 1; Figure 7), size (Figure 6G), and RBC Annexin-V binding and apoptosis (Figure 6H) all progressively normalized with increasing RBC chimerism, other critical indicators of hematopoietic regulation remained abnormal without 100% RBC chimerism. The spleens (and bone marrow) of the animals that received transplants displayed uncorrected RBC progenitor balance despite significant (> 80%-90%) RBC chimerism and did not show correction until 100% RBC chimerism was created (Figure 4A-D). Furthermore, splenic lymphomyeloid-erythroid (LM/E) ratio (highly skewed toward erythropoiesis in sickle mice7,10 ) resembled sickle mice that did not receive transplants even with more than 90% RBC chimerism (Figure 6I) and did not normalize without 100% RBC chimerism. Pathologic analysis of the spleen corroborated these results, in that even in animals with significant RBC chimerism, splenic hyperactive erythropoiesis remained (Figure 7; Table 1). In those animals with 100% RBC chimerism, both spleen size and hematopoiesis normalized, and it is expected that these mice reverted to the use of the bone marrow as the predominant hematopoietic organ. These data thus distinguish hematopoiesis from other sickle abnormalities and support a threshold of 100% peripheral blood RBC replacement for complete hematopoietic correction.
Discussion
The growing interest in nonmyeloablative BMT for hematologic disease is based on the assumption that partial chimerism may be sufficient to produce a clinical cure. Indeed, there are clinical data supporting this hypothesis. Chronic transfusion leads to fewer complications, although patients are not disease free.3 Further, the few patients with SCD that serendipitously developed mixed chimerism after conventional BMT experienced fewer complications in short-term follow-up.2 However, important clinical and mechanistic questions remain. Although hematologic indices may improve with increasing amounts of healthy peripheral RBCs, the effect of a persistent state of mixed RBC chimerism on total-body SCD pathophysiology had not been previously determined. Indeed, for SCD, with its many downstream inflammatory and thrombotic complications,21,22,24-26 the persistence of significant amounts of abnormal RBCs may have deleterious clinicopathologic effects. With ever-improving technologies for producing transplantation tolerance and mixed chimerism,5,6,27-30 it may be possible one day to titrate pretransplantation conditioning to produce a desired amount of chimerism while minimizing conditioning-related morbidity. Thus, it is crucial to determine the effects of mixed chimerism on hematologic and physiologic SCD abnormalities, to develop meaningful targets for transplantation.
We and others2,6,9 have consistently seen an enrichment of RBC over WBC chimerism when transplanting patients or murine models of SCD. This study provided the first evidence suggesting that this enrichment occurs entirely on the basis of the survival advantage of donor RBCs over the rapidly cleared sickle RBCs in the peripheral blood and not because of enhanced engraftment or survival of healthy erythroid progenitors (Figures 3, 4, 5). This peripheral enrichment of RBC chimerism compared with stem cell chimerism creates a scenario in which donor RBC and WBC progenitors can exist as a small percentage of total hematopoietic progenitors despite complete or near-complete replacement of the peripheral blood with donor RBCs. The implications are 2-fold: (1) Gentle transplantation regimens could produce significant levels of peripheral RBC chimerism, especially when combined with a tolerance-induction protocol. In this study, donors and recipients were MHC matched but, because of strain background differences, were expected to have multiple minor histocompatibility mismatches.10,11 We, therefore, used a transplantation regimen that included agents known to induce tolerance through blockade of T-cell costimulation pathways.5,6,30 Even in the absence of pretransplantation chemotherapy, mice that received transplants with this regimen developed stable WBC chimerism and significant RBC chimerism and, with the exception of their hematopoietic organs, significant improvement in SCD pathophysiology. This improvement may be very important for adults with SCD, who are exquisitely sensitive to transplantation preconditioning,1 and for whom conventional transplantation techniques are currently thought to be too risky. (2) The lack of enrichment of donor RBC progenitors in the hematopoietic organs also implies that at low levels of stem cell engraftment, transplantation burnout may become a problem clinically. Thus, significant peripheral RBC chimerism may develop in the setting of low levels of donor stem cells, and these transplantations, over the lifetime of a young patient, may not be stable. Our studies were carried out for 6 to 10 months (one quarter to one half the average murine life span) and indicate that WBC chimerism levels between 0.1% and 82% may be stable in the long term, but the longevity of low levels of stem cell chimerism (even in the face of high peripheral RBC chimerism) in patients cannot be predicted. The addition of robust tolerance-induction protocols to nonmyeloablative transplantations may be crucial to produce stable, mixed chimerism after BMT. Although these protocols are now well established in murine models,5,27-30 success has not yet been achieved in producing transplantation tolerance in either patients or their closest preclinical model, the nonhuman primates. The establishment of tolerance in these immunologically complex, out-bred populations represents the current vanguard of nonmyeloablative BMT research.
One of the critical issues in developing nonmyeloablative BMT for SCD is determining the targets for RBC chimerism. Although the data from patients with SCD who receive transplants is very limited, there is a wealth of data from patients undergoing chronic transfusion. In this cohort, less than 30% sickle RBCs in the peripheral blood leads to improvement in acute hematologic and end-organ complications.3 However, the efficacy of transfusion at improving long-term disease status remains unproven. Our cohort of engrafted mice allows the first direct examination of 3 areas of sickle pathology relevant to the determination of transplantation benchmarks: hematologic abnormalities, end-organ abnormalities, and hematopoietic abnormalities. We find that, although progressive improvement occurs in many hematologic and end-organ abnormalities in mice with more than 70% to 80% normal levels of Hb (Figures 6, 7, 8; Table 1), correction of bone marrow and splenic hematopoietic abnormalities does not occur despite high levels (> 90%) of healthy RBCs in the peripheral blood (Figures 4 and 6; Table 1). Thus, because the enrichment of RBC versus WBC chimerism occurred exclusively as a result of the peripheral survival advantage of healthy versus sickle RBCs, the hematopoietic organs in the mice that received transplants were still predominantly sickle, even in the face of significant (as high as 90%) peripheral RBC chimerism. Thus, sickle splenic and bone marrow hyperactive erythropoiesis7,8 continued to exist in these animals, despite high peripheral blood RBC chimerism. The high output of sickle RBCs into the peripheral blood was cleared with the same rapid kinetics observed in sickle mice that did not receive tranplants,7,8 leading to a final mixture of donor and sickle RBCs that greatly overestimated the amount of stem cell chimerism present in the hematopoietic organs of these animals. Two striking conclusions are drawn from this observation: (1) Sickle-mediated solid-organ pathology is determined by the peripheral blood chimerism. This determination is shown in Table 1 and Figures 6 and 7 and indicates that, for much of the organ pathology that plagues patients with SCD, significant improvement can be expected as progressive increases in peripheral blood RBC chimerism occur. (2) Sickle-mediated hematopoietic organ pathology reflects stem cell chimerism rather than peripheral blood RBC chimerism. Thus, given low levels of stem cell chimerism, most of the hematopoietic precursors in the chimeric mice are still recipient (sickle) rather than donor, and “cure” of these organs does not occur until stem cell chimerism reaches significant levels (> approximately 50% in this study, as shown in Figure 2C). This has important implications for nonmyeloablative transplantation in that it suggests that partial peripheral blood RBC chimerism, even if it is significant (> 70%), will likely arise from a highly abnormal hematopoietic compartment. Indeed, on the basis of our cohort of chimeric animals, a target of 50% or more stem cell chimerism (leading to 100% replacement of sickle with healthy RBCs in the peripheral blood) emerges as a benchmark at which all sickle-mediated abnormalities, including hematopoiesis, can be corrected. One important caveat to this target of more than 50% stem cell chimerism must be considered. This panel of mice did not include any with stem cell chimerism intermediary between 18% (which did not produce full RBC replacement) and 50% (which did produce full RBC replacement). There may be a level of WBC chimerism between these 2 limits that also produces full RBC replacement and cure of all SCD pathophysiology.
These results represent the first preclinical trial of nonmyeloablative transplantation for SCD designed to produce RBC as well as WBC mixed chimerism. They allowed us to investigate the mechanism of donor RBC enrichment as well as to determine that, for a complete reversal of all categories of sickle pathology, complete replacement of the peripheral blood with donor RBCs is required. WBC chimerism is, therefore, a reasonable goal for nonmyeloablative BMT for SCD, but anything less than 100% replacement of the diseased RBC compartment leaves important sickle pathology uncorrected.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-03-0712.
Supported in part by research grants DK/AI40519 (C.P.L.), CA74364-03 (C.P.L.), AI44644 (C.P.L.), R29HL60127 (D.R.A.), CURE Childhood Cancer (D.R.A.), The Jill Andrews Beat Leukemia Celebrity Classic (D.R.A.), the Carlos and Marguerite Mason Trust (C.P.L.), and a grant from the National Heart, Lung, and Blood Institute (N01-HB-07086) (E.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Diane Hollenbaugh, Robert Peach, and Alejandro Aruffo (Bristol-Myers Squibb) for providing CTLA4-Ig. We further thank Robert Karaffa for assistance with the Vantage flow cytometer.

![Figure 4. The erythroid progenitor balance in mice receiving transplants closely resembled the sickle mice not receiving transplants until complete replacement of the peripheral blood with donor RBCs occurred. (A) Representative flow cytometric analysis of RBC ontogeny in sickle and eGFP spleens. Red cell maturation was mapped by determining the relative proportions of Termed/CD71high (region I), Terhigh/CD71high (region II), Terhigh/CD71med (region III), and Terhigh/CD71low (region IV) cells.13 Representative density-contour maps show the splenic RBC progenitor balance from eGFP and sickle mice. (B) Comparison of regions I to IV from spleens of control sickle (▪) and eGFP (▦) mice and representative mice with either 90% (▤) or 100% (□) peripheral RBC chimerism. This analysis gives a vivid illustration of the similarity of mice with 90% RBC engraftment to sickle mice that did not receive transplants and demonstrates that correction of RBC progenitor balance occurred only after 100% replacement with donor RBCs. (C-D) In both bone marrow (C) and the spleen (D), the RBC progenitor balance did not resemble donor eGFP mice until complete replacement of the peripheral blood with donor RBC occurred. For clarity, the percentage of early RBC progenitors (regions I, II, and III) were combined (▴,▵) and compared with the percentage of mature RBC progenitors (region IV; •,▴). For individual mice that received transplants, the percentage of RBC progenitors in the bone marrow (C) or spleen (D) that resided in region I + II + III (▴) or IV (▴) was determined (y-axis) and plotted against the percentage of donor Hb in the peripheral blood (x-axis). Early RBC progenitors (regions I + II + III) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as [▵]. Mature RBC progenitors (region IV) in control sickle (0% donor Hb) or eGFP (100% donor Hb) mice are shown as •.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-03-0712/6/m_h82435383004.jpeg?Expires=1764986096&Signature=M6P4a1I5kd3~kfmb1qzuCp9I6ljHCIyI2rYJgu~juCs7hgU0svmMcjF-Y5kti-kWLZcKggIUNsY-q9h8cL9aS1mLguD6t8mkbrPyH7e8e7spR1eD0pHD~FUz3c27r2nG7Z27rp7UG3Rr0IAxWBqNCffrdIq9MGPSMzHprp-thzmYB7t4Qpcdh8QMmbslocH5mdFQmbQCpNkxaXS9YHfPtUPND~3UZfeUAf3K-PTrOIUpsTqcMQX3NmONmDJeYpkVx8nma93JLcIuhXjXq-XJ9bJCN2KTstZ7L1P-zHwOHbBJIdS6WaAruj1bMwRswxviQB6t2kdYhJg8OIQ~7n43ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
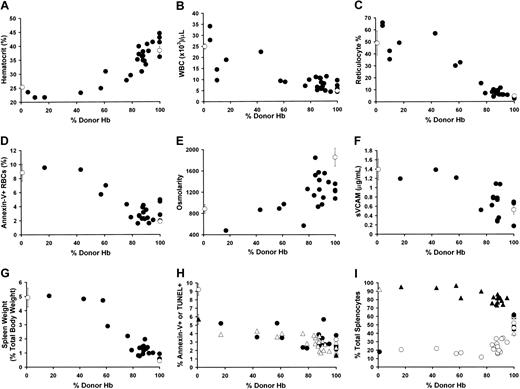
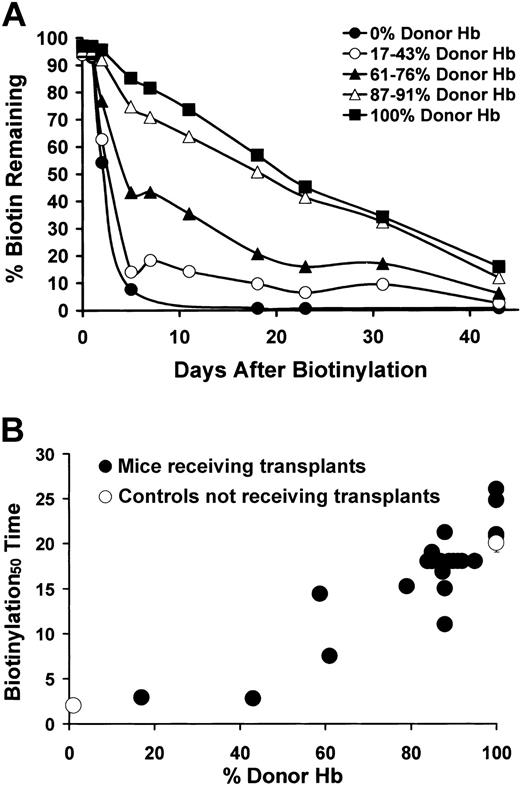
![Figure 7. Analysis of pathology revealed correction of characteristic sickle pathology in mice that received transplants. The 4 columns (from left to right) show microscopic findings in the sickle controls (panels A,E,I,M,Q), a representative mouse that received a transplant with less than 50% RBC chimerism (panels B,F,N,R [mouse no. 1582], J [mouse no. 1550]), a representative mouse that received a transplant with more than 70% to 80% RBC chimerism (panels C,G,K,O,S [mouse no. 70]) and a healthy eGFP control (panels D,H,L,P,T). The 5 rows (top to bottom) show sections of cardiac medium-sized arteries (panels A-D; hematoxylin and eosin [H&E] original magnification × 100), sections of a wall of a pulmonary artery (panels E-H: H&E stain; original magnification × 400), representative hepatic section (panels I-L: H&E stain; original magnification × 400) with iron stain inset (original magnification, × 400), representative renal section (panels M-P: trichrome stain; original magnification × 100) with iron stain inset (original magnification, × 400), and representative spleen section (panels Q-T: H&E stain; original magnification × 100). For the sickle controls and mice with less than 50% RBC chimerism, the figure illustrates ectasia of cardiac medium-sized arteries; increased thickness of the media of the pulmonary artery; hepatic infarcts; severe iron deposition in hepatocytes, hepatic Kupffer cells, and hepatic histiocytic cells; cortical renal infarcts with chronic inflammatory infiltrates; severe deposition of iron in renal tubular epithelium; and loss of splenic architecture with increased hematopoietic cells, ectasia of medium-sized arteries, and sinusoidal congestion. Mice that received transplants with more than 70% to 80% RBC chimerism showed changes intermediate between the healthy eGFP control and sickle control in the cardiac vascular ectasia, pulmonary artery wall thickness, and hepatic and renal tubular iron deposition. They showed no recent hepatic or renal infarcts, they demonstrated fibrosis consistent with remote infarcts, and they showed some return of the normal nodular architecture of the splenic white pulp.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-03-0712/6/m_h82435383007.jpeg?Expires=1764986096&Signature=PNvdY74FC6qBCGNcqbsLq58o4YbSRiSQ0nx5lAE80kdaAKFDQxOtObvQMc8NDnUPH7c~Xw~HJ2UvT7EwxrvsnnHh~Ou1IW0BLyRLR3FB1haARmeRIHFP3OSXeMEPHKCcp3qDDFpWeHyXF6IkpU1M8Z3U40M9kd8~U6~dB38DGGN81HkZlf~siBTV8ct8A3hsBfl8Kyf6TLlFuPXcDh6n96SA6pLNoukt9h2IPIAUv8UAFuX1r-rqNeImbJ-pP2OAJv1TMB14xSg7~V~G1s1sflaerjTqJX2YnKFkhmE5x0n4Is0bfXg1FIP71jBKEoL2y6AhVlr3C0AfgbkXB7AqvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
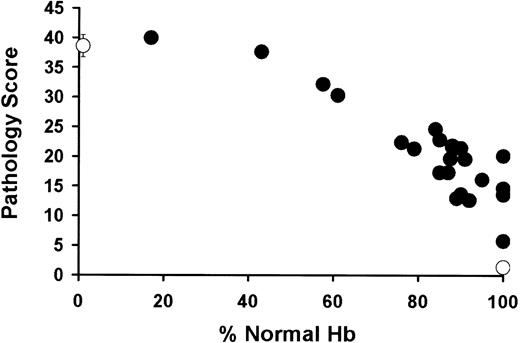
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal