Abstract
The family of multidrug resistance-associated proteins (MRPs) belongs to the superfamily of adenosine triphosphate-binding-cassette (ABC) transporters, which have the ability to function as outward pumps for chemotherapeutic drugs and therefore might be involved in drug resistance. In this study the expression of the MRP2, MRP3, MRP4, MRP5, and SMRP genes was measured using TaqMan real-time polymerase chain reaction (PCR) in 103 children with previously untreated acute lymphoblastic leukemia (ALL) (precursor B-cell ALL [B-ALL], n = 71; T-cell ALL [T-ALL], n = 32). All 5 genes were expressed with a great variability. Only MRP3 expression was associated with a significantly worse prognosis (P = .008). The median expression of MRP3 was 10-fold higher in T-ALL than in precursor B-ALL (P < .001) and 4-fold higher in male patients than in female patients (P < .001). The prognostic impact of MRP3 was independent of immunophenotype or sex. Higher levels of MRP3 were found in patients with a poor in vivo response to prednisone, but this could not be confirmed in an independent case-control study (40 patients) for prednisone response. In healthy donors, the median expression of MRP4 was 4-fold higher in bone marrow and 8-fold higher in CD34+ stem cells compared with peripheral blood (P = .002). Our results suggest that MRP3 is involved in drug resistance in childhood ALL. It therefore represents an interesting target to overcome multidrug resistance. High levels of MRP3 could possibly be the reason for the poorer prognosis of male patients or patients who have T-ALL. Similar to other members of the family of ABC transporters, MRP4 seems to be a marker for immature stem cells. (Blood. 2003;102:4493-4498)
Introduction
Acute lymphoblastic leukemia (ALL) is the most frequent malignancy in childhood. Major improvements in the treatment of ALL have been achieved over the last 30 years, and more than 70% of children with ALL can be cured with current therapeutic regimens.1-3 However, some patients still fail to respond to therapy and others relapse with resistant disease.
Several mechanisms of drug resistance have been identified. One of these is the overexpression of adenosine triphosphate (ATP)-dependent membrane proteins that function as drug efflux pumps.4 The best-characterized drug efflux pump is the permeability glycoprotein (P-gp), which is encoded by the multidrug resistance gene 1 (MDR1). Expression of P-gp/MDR1 has been identified as an independent adverse prognostic factor for complete remission and survival in patients with acute myeloid leukemia (AML).5-7 Recent studies suggested that the results in the treatment of AML can be improved by combining chemotherapy with drugs that inhibit the function of P-gp.8,9 However, the clinical relevance of P-gp seems to be much smaller in childhood ALL than in AML. Some studies found a prognostic impact of P-gp expression in childhood ALL,10,11 but others failed to show such an association.12,13
Our group could recently demonstrate that the expression of the breast cancer resistance protein (BCRP), which is a close relative of MDR1, was also associated with a poor response to chemotherapy in AML patients,14 but not in ALL patients.15
Like MDR1 and BCRP, the family of multidrug resistance-associated proteins (MRPs) belongs to the superfamily of ATP-binding-cassette (ABC) transporters. Their structure, function, and substrate specificity have been studied intensively.16,17 In transfection studies, all MRPs were shown to confer resistance to drugs that are used in the treatment of ALL (MRP1, MRP2, and MRP3: doxorubicin, vincristine, methotrexate; MRP4: methotrexate, thioguanine, 6-mercaptopurin; MRP5 and SMRP: thioguanine, 6-mercaptopurine18 ). So far, little is known about the clinical relevance of MRPs in hematologic malignancies. Only the expression of MRP1 has been studied in larger groups of ALL patients. No association with response to chemotherapy was found.13
The aims of our study were to find out whether the more recently discovered members of the MRP family (MRP2, MRP3, MRP4, MRP5, and SMRP) are expressed in childhood ALL and whether they are associated with a poor response to chemotherapy.
Patients and methods
Patients, diagnosis, and therapy
All 103 patients were diagnosed with previously untreated ALL. The main patient characteristics are summarized in Table 1. The initial diagnosis of ALL was determined by Pappenheim-stained bone marrow smears and cytochemistry reactions (periodic acid-Schiff reaction, acid phosphatase, alphanaphthyl acetate esterase, and myeloperoxidase reaction). Immunophenotype and chromosomal rearrangements were determined by standard methods.1,19 Written consent was given for the use of all patient samples for this study.
Initial patient data
. | No. . |
|---|---|
| Number of patients | 103 |
| Median age, y (range) | 6.8 (0.2-17.1) |
| Sex, male/female | 54/49 |
| Median WBC, 109/L (range) | 48 (2-688) |
| Median percentage of leukemic cells, WBC (range) | 83 (1-100)* |
| BCR/ABL, yes/no/NA | 1/44/58 |
| MLL/AF4, yes/no/NA | 0/45/58 |
| TEL/AML 1, yes/no/NA | 4/41/58 |
| Liver, more than 3 cm below costal margin, yes/no/NA | 52/48/3 |
| Spleen, more than 3 cm below costal margin, yes/no/NA | 51/49/3 |
| Response to prednisone, good† poor‡ NA | 44/9/50 |
| Immunophenotype | |
| Precursor B lineage, male/female | 71 (34/37) |
| T lineage, male/female | 32 (20/12) |
. | No. . |
|---|---|
| Number of patients | 103 |
| Median age, y (range) | 6.8 (0.2-17.1) |
| Sex, male/female | 54/49 |
| Median WBC, 109/L (range) | 48 (2-688) |
| Median percentage of leukemic cells, WBC (range) | 83 (1-100)* |
| BCR/ABL, yes/no/NA | 1/44/58 |
| MLL/AF4, yes/no/NA | 0/45/58 |
| TEL/AML 1, yes/no/NA | 4/41/58 |
| Liver, more than 3 cm below costal margin, yes/no/NA | 52/48/3 |
| Spleen, more than 3 cm below costal margin, yes/no/NA | 51/49/3 |
| Response to prednisone, good† poor‡ NA | 44/9/50 |
| Immunophenotype | |
| Precursor B lineage, male/female | 71 (34/37) |
| T lineage, male/female | 32 (20/12) |
NA indicates not available.
Peripheral blood was used only to analyze MRP expression in patients with more than 80% of leukemic cells before and more than 90% after Ficoll-Hypaque density gradient centrifugation.
Less than 109 leukemic cells/L on day 8.
More than 109 leukemic cells/L on day 8.
All patients were treated according to multicenter studies in Germany: ALL-VI/80 (7 patients), ALL-VII/81 (41 patients), ALL-VIII/87 (10 patients),2,20,21 ALL-Berlin-Frankfurt-Munster (BFM)-90 (16 patients),1 ALL-BFM-95 (19 patients),22 and ALL-BFM-2000 (10 patients). The main drugs that were used in all studies were steroids, methotrexate, cytosine-arabinoside, anthracycline, asparaginase, and vincristine. The event-free survival was 41% in ALL-VI/80, 58% in ALL-VII/81, 74% in ALL-VIII/87,2,20,21 78% in ALL-BFM-90,1 and 79% in ALL-BFM-95.22 The improvement of the treatment results from 1980 to 1990 was mainly achieved by an intensification of chemotherapy. The therapy regimens of the studies ALL-BFM-90/95 and 2000 are similar.
To assess the association of prednisone response and MRP3 expression, an independent study was performed. This study was designed as a case-control study for prednisone response. Cases were patients with poor prednisone response (n = 20); controls were patients with good prednisone response (n = 20).
According to ALL-BFM criteria,1 prednisone good-response was defined as the reduction of leukemic blasts in the peripheral blood to lower than 1000 per μL on day 8 after 7 days of monotherapy with prednisone and a single intrathecal application of methotrexate on treatment-day 1. Prednisone poor-response is defined as the presence of 1000 per μL or more peripheral blood blasts on treatment-day 8.
All patients of the case-control study have been treated according to trial ALL-BFM 2000. Matching criteria were initial white blood cell (WBC) count, age at diagnosis, sex, and immunophenotype (Table 2). All samples were negative for BCR/ABL, TEL/AML1, and MLL/AF4 rearrangements.
Initial data of patients included in the independent casecontrol study for the analysis of the association of MRP3 expression and prednisone response
. | Poor response to prednisone . | Good response to prednisone . |
|---|---|---|
| Number of patients | 20 | 20 |
| Sex | ||
| Female | 10 | 10 |
| Male | 10 | 10 |
| Age, y | ||
| Younger than 1 | 0 | 0 |
| Older than 1; less than 10 | 12 | 12 |
| Older than 10 | 8 | 8 |
| Initial WBC count, 109/L | ||
| Less than 10 | 4 | 4 |
| More than 10; less than 50 | 6 | 6 |
| More than 50; less than 100 | 5 | 5 |
| More than 100 | 5 | 5 |
| Immunophenotype | ||
| T-ALL | 10 | 10 |
| Common ALL | 10 | 10 |
| MRP3, median (range) | 0.00022 (0-0.0033) | 0.00023 (0-0.0052) |
. | Poor response to prednisone . | Good response to prednisone . |
|---|---|---|
| Number of patients | 20 | 20 |
| Sex | ||
| Female | 10 | 10 |
| Male | 10 | 10 |
| Age, y | ||
| Younger than 1 | 0 | 0 |
| Older than 1; less than 10 | 12 | 12 |
| Older than 10 | 8 | 8 |
| Initial WBC count, 109/L | ||
| Less than 10 | 4 | 4 |
| More than 10; less than 50 | 6 | 6 |
| More than 50; less than 100 | 5 | 5 |
| More than 100 | 5 | 5 |
| Immunophenotype | ||
| T-ALL | 10 | 10 |
| Common ALL | 10 | 10 |
| MRP3, median (range) | 0.00022 (0-0.0033) | 0.00023 (0-0.0052) |
Cases and controls were matched for age, sex, immunophenotype, and initial WBC count. All samples were negative for BCR/ABL, TEL/AML 1, and MLL/AF4 rearrangements.
Healthy donors
There were 5 samples of peripheral blood CD34+ stem cells (3 male, 2 female; aged 22 to 38 years) and 6 samples of bone marrow (3 male, 3 female; aged 30 to 45 years) obtained from healthy adults who donated for stem cell transplantation or bone marrow transplantation. Written consent was given for the use of these samples for this study.
The laboratory staff donated 14 samples of peripheral blood; 8 of these samples (5 male, 3 female; aged 25 to 48 years) were used to analyze all mononuclear cells. The other 6 samples (3 male, 3 female; aged 23 to 46 years) were used for a separate analysis of T lymphocytes and B lymphocytes.
Sample collection and processing
Leukemic cells and mononuclear cells of healthy donors were isolated from bone marrow or peripheral blood by Ficoll-Hypaque density gradient centrifugation. After this procedure, the percentage of leukemic cells was more than 90% in all patient samples as determined by May-Gruenwald-Giemsa-stained cytospins. All patient samples were collected prior to the beginning of chemotherapy. All samples were cryopreserved in liquid nitrogen.
CD34+ stem cells of healthy donors were isolated using the CliniMACS system; T lymphocytes (CD3+) and B lymphocytes (CD19+) of healthy donors were isolated using the magnetic-activated cell sorter (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the cell populations was more than 90% as determined by flow cytometry.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using RNeasy Mini Kit including DNase digestion (Qiagen, Hilden, Germany). The amount of RNA was measured by photometry, and a stock solution of 2 μg RNA in 40 μL was prepared. RNA was transcribed into cDNA using Omniscript (Qiagen).
Quantitative PCR was performed using the ABI Prism 7700 Sequence Detector (Applied Biosystems, Weiterstadt, Germany). Primers and TaqMan probes for MDR1,23 MRP2, MRP5, and SMRP24 were used as described previously. Primers and TaqMan probes for MRP3 and MRP4 were as follows: MRP3: forward 5′-GCACCATTGTCGTGGCTACA-3′, reverse 5′-GCAGGACACCCAGGACCAT-3′, and TaqMan probe 5′-CATCCTCTCCCACCTGTCCAAGCTCA-3′; MRP4: forward 5′-TGGATTCTGTGGCTTTGAACAC-3′, reverse 5′-AGCCAAAATGAGCGTGCAA-3′, and TaqMan probe 5′-CGTACGCCTATGCCACGGTGCTG-3′.
Final concentration of the primers was 900 nM (300 nM for MRP3 and SMRP); final concentration of the TaqMan probes was 300 nM (200 nM for MRP3 and SMRP). All TaqMan probes were labeled with 6-carboxy fluorescein (FAM) and 6-carboxytetramethyl rhodamine (TAMRA).
The expression of the resistance genes was standardized for expression of beta-2-microglobulin, which was measured using Pre-Developed Assay Reagents (Applied Biosystems).
The final volume for each PCR was 30 μL including 1.5 μL of the investigated sample. Universal PCR Master Mix (Applied Biosystems) was used according to the manufacturer's instructions.
Serial dilutions of cDNA of reference cell lines were used to generate standard curves. The reference cell lines were as follows: MCF7/CH1000
(MRP2 and MRP3), K562 (MRP4, MRP5, and SMRP), and CEM-ADR5000 (MDR1). The expression of each gene in each sample was analyzed in duplicate. The regression coefficients of the standard curves ranged between 0.994 and 0.999. The variation of the duplicate measurements was extremely small compared with the variation between different samples. In the few cases where there was a substantial difference between the 2 values, the sample was reanalyzed.
In 20 samples the measurement of the expression of MRP3 was repeated using the probe and primers supplied by Assays-on-Demand from Applied Biosystems. The Spearman correlation coefficient of the 2 measurements was 0.985 with P < .001.
Statistical methods
Cox regression analysis was used to estimate the prognostic relevance of each MRP gene. In order to prevent a bias due to the different outcomes in the 6 studies, all Cox regression analyses were calculated with the number of the study as a stratification variable. Kaplan-Meier statistics and log-rank tests were calculated to estimate the significance of differences between survival curves. Since the levels of MRP expression did not follow a normal distribution, expression of MRP in different groups of patients was compared using the Mann-Whitney test for 2 groups and the Kruskal-Wallis test for more than 2 groups. The correlation between MRP expression and other pretherapeutic findings was investigated by means of Spearman correlation coefficient, and the MRP expression at presentation and at relapse was compared using the Wilcoxon test. All P values are given for 2-sided tests. All calculations were performed using the SPSS 11.0 program (SPSS, Chicago, IL).
Results
Expression of MRPs in healthy controls
Measurable amounts of all 5 MRP genes were found in all samples from healthy controls (Figure 1). For MRP2, MRP5, and SMRP we found no significant differences between the different types of controls that were analyzed (peripheral blood mononuclear cells, bone marrow mononuclear cells, peripheral blood T lymphocytes [CD3+], peripheral blood B lymphocytes [CD19+], and peripheral blood stem cells [CD34+]).
Expression of MRP2 and MRP3 relative to cell line MCF7/CH1000 and expression of MRP4, MRP5, and SMRP relative to cell line K562 in 71 samples of precursor B-ALL, 32 samples of T-ALL, 8 samples of healthy peripheral blood mononuclear cells (PB), 6 samples of healthy bone marrow mononuclear cells (BM), 5 samples of healthy peripheral blood CD34+ stem cells (SC), 6 samples of healthy peripheral blood B lymphocytes (BL), and 6 samples of healthy peripheral blood T lymphocytes (TL). Mann-Whitney test for MRP3 in T-ALL versus precursor B-ALL: P < .001. Kruskal-Wallis test for MRP4 in PB versus BM versus SC: P = .002.
Expression of MRP2 and MRP3 relative to cell line MCF7/CH1000 and expression of MRP4, MRP5, and SMRP relative to cell line K562 in 71 samples of precursor B-ALL, 32 samples of T-ALL, 8 samples of healthy peripheral blood mononuclear cells (PB), 6 samples of healthy bone marrow mononuclear cells (BM), 5 samples of healthy peripheral blood CD34+ stem cells (SC), 6 samples of healthy peripheral blood B lymphocytes (BL), and 6 samples of healthy peripheral blood T lymphocytes (TL). Mann-Whitney test for MRP3 in T-ALL versus precursor B-ALL: P < .001. Kruskal-Wallis test for MRP4 in PB versus BM versus SC: P = .002.
The median expression of MRP4 was 4-fold higher in bone marrow and 8-fold higher in CD34+ stem cells compared with peripheral blood (P = .002; Figure 1).
The median expression of MRP3 was 10-fold higher in samples that contained all mononuclear cells from bone marrow or peripheral blood compared with samples that contained only isolated T lymphocytes or B lymphocytes (P values, < .01; Figure 1). This result suggests that the expression of MRP3 in blood and bone marrow is mainly attributed to high levels in monocytes or myeloid precursor cells.
None of the 5 MRP genes differentiated significantly between male and female individuals in any type of control samples. None of the 5 MRP genes was associated with the age of the donor.
Expression of MRPs in childhood ALL
All 5 genes were expressed with a great variability (Figure 1). Measurable amounts of MRP2, MRP4, MRP5, and SMRP were found in all patients. MRP3 was not detectable in 29 patients. The variation from the 10th percentile to the 90th percentile was 8-fold for MRP2, 15-fold for MRP4, 9-fold for MRP5, and 18-fold for SMRP. The variation from the lowest measurable value for MRP3 to the 90th percentile was 30-fold. The median levels of all 5 MRP genes were higher in ALL samples than in healthy B or T lymphocytes (P values, < .05; Figure 1).
Association of MRPs with other diagnostic features of ALL
All genes were investigated for their association with sex, age, immunophenotype, initial WBC count, and the percentage of leukemic cells in peripheral blood. The median expression of MRP2, MRP5, and SMRP was about 2 times higher in patients with T-cell ALL (T-ALL) compared with precursor B-cell ALL (B-ALL) (P values, < .01). Median expression of MRP3 was even 10 times higher in T-ALL (P < .001; Figure 1).
Higher levels for MRP2, MRP3, MRP5, and SMRP were associated with higher initial WBC counts and higher percentages of leukemic cells. However, the Spearman correlation coefficients were relatively small (range, 0.24-0.40; P values, < .05).
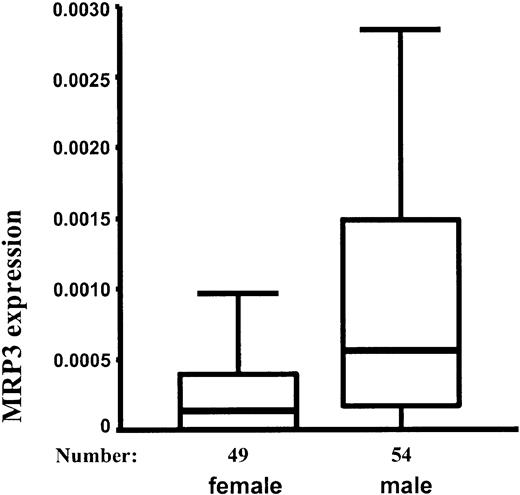
Surprisingly, the median expression of MRP3 was 4-fold higher in male patients than in female patients (P < .001; Figure 2). There was a higher proportion of T-ALLs in male patients (Table 1), but this did not explain the difference between MRP3 expression in boys and girls. When analyzing precursor B-ALL and T-ALL separately, the expression of MRP3 was still significantly higher in male patients (only precursor B-ALL, P = .035; only T-ALL, P = .003). None of the other MRP genes differentiated between male and female patients. None of the MRP genes was associated with the age of the patients.
Expression of MRP3 (10th, 25th, 50th, 75th, and 90th percentile) relative to cell line MCF7/CH1000 in 49 female and 54 male patients. Mann-Whitney test: P < .001.
Expression of MRP3 (10th, 25th, 50th, 75th, and 90th percentile) relative to cell line MCF7/CH1000 in 49 female and 54 male patients. Mann-Whitney test: P < .001.
In a previous study we measured the expression of the MDR1 gene in 38 patients who were also included in the present study. None of the MRP genes was significantly associated with the expression of MDR1.
Only in the more recent studies, ALL-BFM-90/95 and 2000, were patients routinely investigated for the chromosomal rearrangements BCR/ABL and TEL/AML1.1 Of the 45 patients, 1 was found positive for BCR/ABL and 4 patients were found positive for TEL/AML1. As far as we can tell from these small numbers, there was no trend for a particularly high or low expression of the MRP genes in these patients.
Expression of MRPs and survival
A univariate Cox regression analysis was calculated to estimate the prognostic relevance of each MRP gene. Only the expression of MRP3 was associated with a significantly worse prognosis (P = .008). Table 3 provides the data for overall survival in patients with high levels of MRP3 and in patients with low levels of MRP3 as defined by different cutoffs for high and low expression. Survival curves using the 75th percentile as cutoff are given in Figure 3A. The results were similar when relapse-free survival was calculated instead of overall survival.
Prognostic impact of MRP3 expression using quartiles and thirds as cutoffs for high and low expression in all patients (n = 103)
. | Low expression of MRP3 . | . | High expression of MRP3 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Percentile used as cutoff . | Overall survival after 10 years, % . | Standard error, % . | Overall survival after 10 years, % . | Standard error, % . | Log-rank test, P . | ||
| 25 | 64 | 11 | 55 | 6 | .14 | ||
| 33 | 66 | 9 | 52 | 6 | .04 | ||
| 50 | 62 | 7 | 52 | 7 | .05 | ||
| 66 | 63 | 6 | 46 | 9 | .009 | ||
| 75 | 64 | 6 | 38 | 10 | .002 | ||
. | Low expression of MRP3 . | . | High expression of MRP3 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Percentile used as cutoff . | Overall survival after 10 years, % . | Standard error, % . | Overall survival after 10 years, % . | Standard error, % . | Log-rank test, P . | ||
| 25 | 64 | 11 | 55 | 6 | .14 | ||
| 33 | 66 | 9 | 52 | 6 | .04 | ||
| 50 | 62 | 7 | 52 | 7 | .05 | ||
| 66 | 63 | 6 | 46 | 9 | .009 | ||
| 75 | 64 | 6 | 38 | 10 | .002 | ||
(A) Overall survival in patients with high levels of MRP3 and in patients with low levels of MRP3. The 75th percentile was used as cutoff for high and low expression. N indicates number of patients; OS, overall survival; and SE, standard error. Log-rank test:P = .002. (B) Same analysis as in panel A, but restricted to patients who were treated in the more recent studies ALL-BFM 90/95 and 2000. Log-rank test: P = .071.
(A) Overall survival in patients with high levels of MRP3 and in patients with low levels of MRP3. The 75th percentile was used as cutoff for high and low expression. N indicates number of patients; OS, overall survival; and SE, standard error. Log-rank test:P = .002. (B) Same analysis as in panel A, but restricted to patients who were treated in the more recent studies ALL-BFM 90/95 and 2000. Log-rank test: P = .071.
There were 45 patients included in the main study who were treated according to the more recent studies ALL-BFM-90, ALL-BFM 95, and ALL-BFM 2000. In these studies very similar therapy regimens have been used, and the results were much better than in the preceding studies. So far, only 4 of those 45 patients succumbed to their disease. This number is too small for statistically significant analyses of risk factors. However, the data presented in Table 4 and Figure 3B suggest that the prognostic impact of MRP3 was not or only in part overcome by the more intensive therapy in recent studies.
Prognostic impact of MRP3 expression using quartiles and thirds as cutoffs for high and low expression in patients who were treated only in the studies ALL-BFM 90/95 or 2000 (n = 45)
. | Low expression of MRP3 . | . | High expression of MRP3 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Percentile used as cutoff . | Overall survival after 5 years, % . | Standard error, % . | Overall survival after 5 years, % . | Standard error, % . | Log-rank test, P . | ||
| 25 | 100 | * | 87 | 6 | .21 | ||
| 33 | 100 | * | 85 | 7 | .14 | ||
| 50 | 100 | * | 80 | 9 | .04 | ||
| 66 | 96 | 4 | 79 | 11 | .07 | ||
| 75 | 93 | 5 | 82 | 12 | .28 | ||
. | Low expression of MRP3 . | . | High expression of MRP3 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Percentile used as cutoff . | Overall survival after 5 years, % . | Standard error, % . | Overall survival after 5 years, % . | Standard error, % . | Log-rank test, P . | ||
| 25 | 100 | * | 87 | 6 | .21 | ||
| 33 | 100 | * | 85 | 7 | .14 | ||
| 50 | 100 | * | 80 | 9 | .04 | ||
| 66 | 96 | 4 | 79 | 11 | .07 | ||
| 75 | 93 | 5 | 82 | 12 | .28 | ||
Standard errors cannot be computed if survival is 100%.
MRP3 expression was strongly correlated with T-cell immunology and male sex, both of which are known to be indicators of a poor prognosis.1,25 However, when sex or immunophenotype was used as a stratification variable, Cox regression analysis still showed a significant association between MRP3 expression and poor outcome (P values, < .01). This illustrates that the prognostic impact of MRP3 is independent of immunophenotype or sex.
In a multivariate stepwise Cox regression analysis with the continuous variables (MRP3 expression, WBC count, enlargement of liver below coastal arch, and enlargement of spleen below coastal arch) and the dichotomous variables (sex, immunophenotype [T-ALL versus precursor B-ALL], and age [younger than 2 years versus older than 2 years]), only the WBC count and the expression of MRP3 sustained independent prognostic relevance. The inclusion of the other variables did not significantly improve the prognostication compared with those 2 variables alone.
None of the other 4 MRP genes was associated with a poor prognosis. Even when testing a large set of different cutoffs for high and low expression, we found no convincing trend for a prognostic implication of MRP2, MRP4, MRP5, or SMRP.
Expression of MRPs and response to prednisone
As the initial treatment response to prednisone is the strongest prognostic factor of outcome within the experience of the BFM study group, response to prednisone was determined in all patients in whom the therapy was started after January 1988 (n = 53). Of these patients, 9 showed a poor response and 44 were classified as prednisone good-responders.
No association with response to prednisone was found for MRP2, MRP4, MRP5, or SMRP. The median expression of MRP3 was 4 times higher in patients with a poor response to prednisone (P = .01), but the proportions of patients with male sex (67% versus 48%) and with T-ALL (44% versus 25%) were also higher in patients with a poor response to prednisone than in patients with a good response. In order to analyze whether the association of MRP3 with the response to prednisone was independent of these factors, an independent case-control study for prednisone response was performed.
We found no trend for an association of MRP3 gene expression with response to prednisone in this group of patients (Table 2). This finding suggests that the association of MRP3 with response to prednisone in the original study population was due mainly to the higher proportion of T-ALL and male patients in the group with a poor response.
Consistent with the original study population, we found that within the independent case-control study the median expression of MRP3 was 5 times higher in male patients than in female patients (P = .019) and 4 times higher in T-ALL than in precursor B-ALL (P = .045).
Expression of MRPs at the time of diagnosis and at relapse
In 11 patients the expression of the 5 MRP genes was measured at the time of diagnosis as well as in first relapse. The expression levels of all 5 genes were very similar at both time points. None of the 5 genes was significantly up-regulated at the time of relapse.
Discussion
To our knowledge, this study provides the first data on the association between the response to chemotherapy and the expression of MRP2, MRP3, MRP4, MRP5, and SMRP in ALL patients. Our results suggest that MRP3 is involved in drug resistance in ALL. This is consistent with our observations in childhood AML, where MRP3 showed a strong prognostic impact.26 The observation that there is no association of MRP3 expression and prednisone response does not contradict that conclusion, as MRP3 has not been described as being specifically associated with steroid resistance. MRP3 therefore represents an interesting marker for risk-adapted therapy and a possible target for the development of specific drugs to overcome multidrug resistance. Sirotnak et al27 found that the function of MRPs can be inhibited by probenecid and that the efficacy of chemotherapeutics in mice could be enhanced by the coadministration of probenecid.
One of the most interesting results of this study is that MRP3 strongly differentiates between male and female patients. Many large studies have indicated that boys who suffer from ALL do worse than girls.1,25 A reason for this difference could not yet be found. Our results suggest that higher levels of MRP3 expression might account for the poorer prognosis in male patients. Future studies should address the biologic mechanism for this differential expression. Likewise, MRP3 seems to be one reason for the worse prognosis of patients with T-ALL compared with precursor B-ALL.
It was recently described that the ABC transporters MDR1 and BCRP are indicators of immature stem cells and that these proteins probably play an important role in cell differentiation.28,29 Our results show that MRP4 is highly expressed in CD34+ stem cells with intermediate levels in bone marrow and low levels in peripheral blood (Figure 1). These findings suggest that MRP4 could be another member of the ABC-transporter family, which is an indicator of immature stem cells and is involved in cell differentiation.
Prospective studies, including specific functional assays and the analysis of protein expression, are necessary to confirm our results. In a study on lung cancer cell lines, Young et al30 could demonstrate a good correlation between mRNA and protein levels for MRP2 and MRP3.
Our results do not indicate a clinical relevance of MRP2, MRP4, MRP5, or SMRP in childhood ALL. Either the expression of these genes in the leukemic cells is too small to cause a significant efflux of chemotherapeutic drugs or the levels of mRNA do not strongly correlate with the amount of functional protein in the cell membrane.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2002-11-3461.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Douglas D. Ross from the University of Maryland Greenebaum Cancer Center for providing the cell line MCF7/CH1000, which was used to generate standard curves for MRP2 and MRP3.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal