Abstract
Elevated expression of multidrug efflux pumps such as P-glycoprotein (Pgp) have been associated with resistance to cytotoxic drugs used in the treatment of leukemias and other cancers. Imatinib mesylate (STI-571 or Gleevec) is a potent inhibitor of the BCR/ABL and c-KIT tyrosine kinases. It has displayed considerable efficacy in treatment of patients with Philadelphia-positive acute lymphoblastic leukemia and chronic myelogenous leukemia and those with gastrointestinal stromal tumors (GISTs). However, recently imatinib-resistant relapse has emerged as a significant problem. Although a major cause of resistance appears to be point mutation in the kinase domain of the target enzyme, the potential contribution of elevated multidrug efflux activity has not been systematically evaluated. The imatinib-sensitive human leukemic cell line K562, which is dependent on the activity of BCR/ABL for survival and growth, provides a convenient system for evaluating modulation of drug activity. By expressing Pgp at high levels in these cells, we have demonstrated that this pump provides minimal protection against cell growth inhibition and apoptosis induced by imatinib. In contrast, overexpression of Bcl-xL, which blocks apoptosis, resulted in partial protection against the drug. We conclude that Pgp up-regulation is not likely to be a significant contributor to imatinib resistance. (Blood. 2003;102:4499-4503)
Introduction
Imatinib mesylate, also known as STI571 (Novartis, Basel, Switzerland), is a potent inhibitor of the BCR/ABL and c-KIT protein tyrosine kinases1-3 and is being used for the treatment of chronic myelogenous leukemia (CML), Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL), and gastrointestinal stromal tumors (GISTs). Imatinib was found to be highly efficacious in phase 2 trials4,5 and has recently been approved by the Food and Drug Administration (FDA) for the treatment of Ph+ chronic-phase CML and Kit+ GISTs. The development of resistance to imatinib in some patients, especially those with CML in blast crisis or ALL, is a major limitation for therapy. In patients resistance has been correlated in a few cases with BCR/ABL gene amplification and, in many cases, with the presence of point mutations leading to amino acid substitutions within the kinase domain of BCR/ABL.6-11 Furthermore, some of the BCR/ABL mutants identified in patients were able to confer varying degrees of resistance to the growth inhibitory effects of imatinib when expressed in cell lines.11 However, not all leukemic samples from resistant patients displayed overexpression or mutation of BCR/ABL; therefore, alternative or additional mechanisms of drug resistance to imatinib remain to be determined.
Cell lines selected for imatinib resistance in vitro have been obtained by exposure to increasing doses of imatinib over a period of 3 to 6 months.12-14 Analysis of these cell lines has shown that, in contrast to data from patients, resistance was often associated with up-regulation of BCR/ABL but, where examined, point mutations in the kinase domain were not found. One report demonstrated that imatinib-resistant LAMA-84 cells overexpress P-glycoprotein (Pgp) in addition to BCR/ABL.14 Although the addition of the Pgp inhibitor, verapamil, did not affect growth of these cells in 1 μM imatinib (the dose used for selection), its addition did result in a reduction of viability in the presence of 2 μM imatinib. Also, verapamil enhanced the inhibition of BCR/ABL phosphorylation by imatinib. These data suggest that Pgp can confer some resistance to the drug. Furthermore, analysis of the effect of imatinib on the adenosine triphosphatase (ATPase) activity of Pgp and the other major drug efflux pump, multidrug resistance-associated protein (MRP), in a model system indicated that the drug can interact with Pgp but not MRP. Also imatinib could inhibit calcein extrusion from HL60 cells overexpressing Pgp, presumably by competing with the dye for binding to the pump.15
The question of whether Pgp can mediate resistance to imatinib is clinically important because expression of Pgp has been observed in blast crisis CML and in GISTs.16-18 To determine if Pgp up-regulation could confer resistance to imatinib, Pgp was overexpressed in K562 cells, which were derived from a patient with CML and depend on BCR/ABL activity for survival and growth. The imatinib sensitivity of K562 cells overexpressing Pgp was compared to parental K562 cells and to K562 cells overexpressing the antiapoptotic factor Bcl-xL. Imatinib has been shown to induce apoptosis in BCR/ABL+ cells by suppressing expression of the antiapoptotic factor Bcl-xL, which is induced by the oncogene in a signal transducer and activator of transcription 5 (Stat-5)-dependent manner.19,20
Materials and methods
Construction of expression plasmids
The 4.1-kilobase (kb) MDR1 complementary DNA (cDNA) (GenBank accession no. M14758) coding for Pgp was obtained from the American Type Culture Collection (Rockville, MD) as an EcoR1 fragment within the pGEM3Zf(-) vector. An XbaI fragment containing the MDR1 cDNA was excised and blunt-ended using DNAPolI Klenow fragment (Pharmacia, Buckinghamshire, United Kingdom) as per the manufacturer's instructions. The fragment was cloned into the HpaI site of the pRUFneo retroviral expression vector21 and checked for correct orientation.
The Bcl-xL cDNA was isolated from a cDNA library constructed from leukemic cell lines. The cDNA was amplified by high-fidelity polymerase chain reaction (PCR) using Elongase (Stratagene, La Jolla, CA) as per the manufacturer's instructions and cloned into the pRUFneo vector. The cDNA was sequenced to confirm the product cloned was identical to the Bcl-xL cDNA (GenBank accession no. Z23115).
The pRUFneo(MDR1) and pRUFneo(Bcl-xL) constructs were expanded in DH10β Escherichia coli and the plasmids purified using a midi-prep kit (Qiagen, Hilden, Germany).
Expression of Pgp and Bcl-xL in K562 cells
The retroviral expression vectors pRUFneo(MDR1) and pRUFneo(Bcl-xL) were transfected into amphotropic packaging cells BING22 using Fugene (Roche, Indianapolis, IN) according to the manufacturer's instructions. The culture medium (Iscove modified Dulbecco medium [IMDM]/10% fetal call serum [FCS]) was changed after 24 hours and the viral supernatant harvested after 48 hours. The viral supernatants were filtered (0.45 μm) and stored at -70°C. Viral supernatant (1 mL) was added directly to 3 × 105 K562 cells, incubated for 3 hours at 37°C, then diluted to 5 mL with RPMI/10% FCS and cultured for a further 48 hours. The infected K562 cells were selected in RPMI/10% FCS containing 1 mg/mL G418 for 1 week until uninfected K562 cells were killed. The K562(vector control), K562(Pgp), and K562(Bcl-xL) cell lines were expanded and cryopreserved.
Selection for high levels of expression of Pgp
The K562 cells infected with viruses carrying the MDR1 cDNA were analyzed for expression of Pgp on the cell surface by indirect immunofluorescence and flow cytometry using monoclonal antibody (Mab) U1C-2 (Coulter, Hialeah, FL) and negative isotype-matched Mab (1D4.5 anti-Salmonella) as described previously.23 The cells were sorted for high levels of Pgp expression using a fluorescence-activated cell sorting (FACS) Vantage cell sorter (Becton Dickinson, San Jose, CA) and expanded to give the K562(Pgp+) subline. The uniform high expression of surface Pgp on these cells was confirmed by flow cytometry.
Analysis of drug efflux
The K562(Pgp+) cells were analyzed in a drug efflux assay as described previously.24 The assay was conducted using daunorubicin as the drug and verapamil as the specific inhibitor of Pgp. Briefly, 1 × 106 cells/mL were exposed to 5 μg/mL daunorubicin for 30 minutes at 37°C/5%CO2. At various times following this incubation, aliquots of the cells were taken, verapamil was added to a final concentration of 6 μg/mL, and the samples were placed on ice. After all the time point samples were obtained, the cells were centrifuged, resuspended in RPMI 1640, and analyzed immediately for the level of daunorubicin fluorescence (FL1) using a FACScalibur flow cytometer (Becton Dickinson).
Analysis of growth inhibition by daunorubicin or imatinib
The parental K562 cell line K562(none), the K562 cell line expressing Bcl-xL K562(Bcl-xL) and the K562 cell line sorted for high levels of Pgp expression K562(Pgp+) were analyzed for the growth inhibitory effects of daunorubicin or imatinib using a colorimetric assay (Cell Titre96 Aqueous one solution MTS reagent; Promega, Madison, WI) that measures viable cell number as described previously.25 Cells (2 × 105 cells/mL) were exposed to 0 to 40 μg/mL daunorubicin for 1 to 3 days in the presence and absence of verapamil (10 μg/mL) and the absorbance at 490 nm was analyzed for triplicate samples. Similarly, 2 × 105 cells/mL were exposed to 0 to 5 μM imatinib for 3 days prior to absorbance analysis. The HEL cell line was included as control line that expressed a high level of functional Pgp23,24 but was not influenced by imatinib.
Analysis of the apoptotic effects of imatinib
The same cell lines analyzed in the MTS assay were also analyzed for the level of apoptosis observed following treatment with imatinib as described previously.25 The cells were treated with various doses of imatinib for 48 hours, washed in cold phosphate-buffered saline (PBS), resuspended in 100 μL binding buffer (Hanks balanced salt solution plus 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] and 2.5 mM CaCl2) containing a 1:50 dilution of annexin V-FLUOS (Roche) and 10 μg/mL 7-amino-actinomycin D (7AAD; Molecular Probes, Eugene, OR), and incubated for 15 minutes at room temperature. After addition of 400 μL binding buffer the cells were analyzed for annexin V expression (FL1) or staining with 7AAD (FL3) on a FACScalibur flow cytometer (Becton Dickinson). Cells that were annexin V+ were considered apoptotic and those that stained with 7AAD were considered dead.
Western blot analysis
K562 cell lines were incubated in RPMI/10% FCS in the presence of the indicated concentrations of imatinib for 2 hours. Cells were harvested and lysed in the presence of protease and phosphatase inhibitors as described previously.25 Protein concentrations in the lysates were quantitated using the BCA protein assay reagent (Pierce, Rockford, IL) and 25-μg aliquots were separated on 10% sodium dodecyl sulfate-polyacrylamide gels. Gels were electroblotted to nitrocellulose membrane (Advantec MFS, Pleasanton, CA) and membranes were washed with Tris (tris(hydroxymethyl)aminomethane)-buffered saline (TBS) at pH 7.4. Blots were either blocked with TBS/0.1% Tween-20 (TBST) and probed with a cocktail of antiphosphotyrosine Mabs: 4G10 (Upstate Biotechnology, Lake Placid, NY) and PY20 (Transduction Laboratories, Lexington, KY) diluted in TBST, or blocked with 2.5% membrane blocking agent (Amersham Life Sciences, Buckinghamshire, United Kingdom) in TBST and probed with monoclonal anti-ABL (Oncogene Research, Boston, MA). Following washes with TBST, membranes were incubated with antimouse Ig-alkaline phosphatase conjugate (Silenus, Melbourne, Australia). Blots were incubated with enhanced chemifluorescence (ECF) substrate (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) and scanned using a 560-nm long-pass filter by a Typhoon 9410 Variable Mode Imager (Amersham Biosciences, Sunnyvale, CA).
Results
K562(Pgp+) cells express high surface levels of Pgp and are capable of functional drug efflux
The level of surface Pgp was determined in K562 parental cells, K562(Pgp+) and HEL using an immunofluorescence assay. Results indicated that K562 cells express little or no Pgp, and HEL cells express relatively high levels of Pgp (Figure 1A), in agreement with transcription levels of mdr-1 in these cell lines23 and earlier antibody-binding data.24 Surface expression of Pgp in K562(Pgp) was found to be approximately 10-fold higher than that of HEL, which was in turn high relative to primary myeloid leukemia specimens and normal CD34+ bone marrow cells24 indicating that Pgp expression in K562(Pgp) was greater than physiologic levels.
Functional expression of Pgp in K562 cells. (A) Expression of cell surface Pgp by HEL erythroleukemia cells, parental K562 cells, and sorted K562 infectants was measured by indirect immunofluorescence and flow cytometry using Mab U1C2. Neg refers to a control where an irrelevant isotype-matched Mab was used in place of U1C2. (B) Daunorubicin retention in the presence or absence of 3 μM verapamil was assayed by flow cytometry as described in “Materials and methods.” The rate of drug efflux is calculated as the slope of the line derived from the retention of daunorubicin over the assay period expressed as a percentage of that of inhibitor-treated cells for each time point (as described previously24 ). The relative rate of efflux was calculated to be 0.152 for K562 (none), 0.4222 for K562 (Pgp+), and 0.117 for K562 (Bcl-xL).
Functional expression of Pgp in K562 cells. (A) Expression of cell surface Pgp by HEL erythroleukemia cells, parental K562 cells, and sorted K562 infectants was measured by indirect immunofluorescence and flow cytometry using Mab U1C2. Neg refers to a control where an irrelevant isotype-matched Mab was used in place of U1C2. (B) Daunorubicin retention in the presence or absence of 3 μM verapamil was assayed by flow cytometry as described in “Materials and methods.” The rate of drug efflux is calculated as the slope of the line derived from the retention of daunorubicin over the assay period expressed as a percentage of that of inhibitor-treated cells for each time point (as described previously24 ). The relative rate of efflux was calculated to be 0.152 for K562 (none), 0.4222 for K562 (Pgp+), and 0.117 for K562 (Bcl-xL).
To determine if K562(Pgp+) cells were capable of functional drug efflux, an efflux assay with daunorubicin as substrate was used. Fluorescence of daunorubicin decreased significantly in K562(Pgp+) cells compared to parental cells or K562(Bcl-xL) cells (Figure 1B). The addition of verapamil to the cells inhibited efflux (Figure 1B), demonstrating that Pgp expressed in K562(Pgp+) cells was functional and that the level of expression was sufficient for drug efflux at a rate higher than that observed for HEL cells previously.24 The rate of daunorubicin efflux is always much lower than that of rhodamine 123 efflux commonly used in this assay because of the lower fluorescence of daunorubicin and the consequent higher levels necessary for detection.
To further demonstrate functional Pgp in K562(Pgp+) cells, we conducted a cell growth (MTS) assay with various doses of daunorubicin in the presence and absence of verapamil. The results are shown in Figure 2A and demonstrate that Pgp in K562(Pgp+) cells increased the dose of drug that results in 50% growth inhibition (IC50) of daunorubicin by 5- to 10-fold and this increase was substantially overcome by the presence of verapamil. Additionally this resistance was greater than that observed due to Bcl-xL overexpression in K562(Bcl-xL) cells, which was not influenced by the addition of verapamil. This assay was repeated at various days (1-3) and with a range of doses of daunorubicin (0-40 μg/mL), as well as following a 1-hour high-dose pulse with daunorubicin instead of continuous exposure as previously conducted by us.23 In each experiment a similar result was obtained.
Growth of cell lines in MTS assays following treatment with daunorubicin or imatinib. (A) Cell growth (survival and proliferation) after 2 days of culture in the presence of the indicated concentrations of daunorubicin was measured by colorimetric MTS assay in the presence or absence of 10 μg/mL verapamil. The absorbance is shown as a percentage of the absorbance for same cells cultured in the absence of daunorubicin. (B) Cell growth (survival and proliferation) after 3 days of culture in the presence of the indicated concentrations of imatinib was measured by the MTS assay. HEL erythroleukemia cells, which do not express BCR/ABL, were used as a control for parental and transduced K562 cells. The absorbance is shown as a percentage of the absorbance for same cells cultured in the absence of imatinib. The error bars show the SEM for triplicate samples at each dose of drug.
Growth of cell lines in MTS assays following treatment with daunorubicin or imatinib. (A) Cell growth (survival and proliferation) after 2 days of culture in the presence of the indicated concentrations of daunorubicin was measured by colorimetric MTS assay in the presence or absence of 10 μg/mL verapamil. The absorbance is shown as a percentage of the absorbance for same cells cultured in the absence of daunorubicin. (B) Cell growth (survival and proliferation) after 3 days of culture in the presence of the indicated concentrations of imatinib was measured by the MTS assay. HEL erythroleukemia cells, which do not express BCR/ABL, were used as a control for parental and transduced K562 cells. The absorbance is shown as a percentage of the absorbance for same cells cultured in the absence of imatinib. The error bars show the SEM for triplicate samples at each dose of drug.
Overexpression of Bcl-xL but not Pgp, in K562 cells confers a growth advantage following treatment with imatinib
A colorimetric growth assay measuring viable cell number was used to compare imatinib sensitivity among HEL, K562(none), K562(Pgp+), and K562(Bcl-xL) cells. Results (Figure 2B) showed that survival and proliferation of the BCR/ABL- cell line HEL was unaffected by the presence of up to 5 μM imatinib, whereas K562 lines exhibited a dose-dependent reduction of cell numbers that reached a plateau between 1 μM and 5 μM imatinib. Growth of K562(Pgp+) cells did not differ from control K562 cells, both having an IC50 value of 0.25 μM. In comparison, the IC50 of K562(Bcl-xL) cells was within the range of 0.5 to 1 μM. At high doses of imatinib (1-5 μM), growth of K562(Bcl-xL) was inhibited only by 50% compared to more than 80% for K562 parental cells and K562(Pgp+). The results were not significantly altered with the addition of the Pgp-specific inhibitor verapamil (data not shown). Together these results demonstrate that Pgp, unlike Bcl-xL, was not able to confer a substantial growth advantage in K562 cells treated with imatinib.
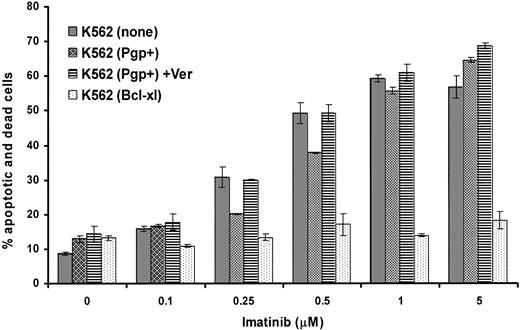
Effect of Bcl-xL or Pgp overexpression on the induction of apoptosis by imatinib
The same cell lines were analyzed for the total percentage of cells that were either annexin V+ (apoptotic) or annexin V+ and stained with 7AAD (dead) after 48 hours of exposure to varying concentrations of imatinib with and without the addition of verapamil. The results of a typical experiment (1 of 3) indicate that, as expected, Bcl-xL overexpression conferred a strong antiapoptotic effect in K562 cells following treatment even with high levels of imatinib (Figure 3). Overexpression of Pgp resulted in a slight but consistent reduction in apoptosis of K562 in response to low but not high levels of imatinib. This reduction in apoptosis was inhibited by verapamil, suggesting that the mild antiapoptotic effect observed was conferred by Pgp activity. Thus Pgp, when expressed at very high levels, appeared to provide only minor protection from the drug at low doses; however, this was insufficient to alter the overall growth inhibition of the cell population.
Effect of overexpression of Pgp or Bcl-xL on induction of apoptosis by imatinib. Parental or transduced K562 cells were cultured in the indicated concentrations of imatinib for 48 hours in the absence or presence of verapamil. The cells were assayed by flow cytometry for annexin V and 7AAD binding, which indicate apoptotic cells and dead cells, respectively. The average results ± SEM of triplicates for the total percentage of cells that were positive for expression of either annexin V or 7AAD or both are shown.
Effect of overexpression of Pgp or Bcl-xL on induction of apoptosis by imatinib. Parental or transduced K562 cells were cultured in the indicated concentrations of imatinib for 48 hours in the absence or presence of verapamil. The cells were assayed by flow cytometry for annexin V and 7AAD binding, which indicate apoptotic cells and dead cells, respectively. The average results ± SEM of triplicates for the total percentage of cells that were positive for expression of either annexin V or 7AAD or both are shown.
Overexpression of Pgp or Bcl-xL does not affect inhibition of BCR/ABL phosphorylation by imatinib in intact cells
The K562 cell lines were treated with various doses of imatinib for 2 hours, lysed, and analyzed by Western blot for the level of total BCR/ABL protein and for the level of phosphorylated BCR/ABL as shown in Figure 4A. The proportional decrease (compared to no drug) in the ratio of phosphorylated BCR/ABL relative to total BCR/ABL protein for each sample was calculated and is shown in Figure 4B. Bcl-xL serves as an additional negative control for this experiment because it acts downstream of receptor phosphorylation. The slight effect of both Bcl-xL and Pgp overexpression was considered to be within the experimental error of the method and the results indicate that Pgp overexpression did not have a detectable effect on the dose-dependent decrease in BCR/ABL phosphorylation induced by imatinib.
Effect of imatinib on BCR/ABL tyrosine phosphorylation in parental and transduced K562 cells. K562 cells overexpressing the indicated proteins were incubated with various concentrations of imatinib for 2 hours and then lysed and phosphorylation of BCR/ABL analyzed. (A) Whole-cell lysates were resolved by electrophoresis and probed by Western blotting with detection by chemifluorescence for p210 BCR/ABL using antibody to c-Abl, and for tyrosine phosphorylated p210 using antiphosphotyrosine Mabs. (B) Bands were quantitated using Typhoon and analyzed using ImageQuant software (Amersham Biosciences, Buckinghamshire, United Kingdom). The ratios of tyrosine phosphorylated and total p210 for each treatment were calculated after correction for background. Data are expressed relative to the value for each cell line in the absence of drug.
Effect of imatinib on BCR/ABL tyrosine phosphorylation in parental and transduced K562 cells. K562 cells overexpressing the indicated proteins were incubated with various concentrations of imatinib for 2 hours and then lysed and phosphorylation of BCR/ABL analyzed. (A) Whole-cell lysates were resolved by electrophoresis and probed by Western blotting with detection by chemifluorescence for p210 BCR/ABL using antibody to c-Abl, and for tyrosine phosphorylated p210 using antiphosphotyrosine Mabs. (B) Bands were quantitated using Typhoon and analyzed using ImageQuant software (Amersham Biosciences, Buckinghamshire, United Kingdom). The ratios of tyrosine phosphorylated and total p210 for each treatment were calculated after correction for background. Data are expressed relative to the value for each cell line in the absence of drug.
Discussion
Imatinib has been very successful as a specific anticancer drug therapy in the treatment of Ph+ leukemias and GISTs. An emerging problem associated with imatinib treatment is the acquisition of resistance to the drug by patients. Many studies have now shown that the main cause of resistance is due to the “reactivation” of BCR/ABL in patients by point mutations or via overexpression that overcomes the inhibitory effects of the drug. However, some patients display resistance to imatinib in a “BCR/ABL-independent” manner.9 A recent study has demonstrated that one such mechanism could be the activation of other tyrosine kinases such as Lyn.26
Many studies have demonstrated that Bcl-xL is up-regulated in BCR/ABL-expressing cells and it has been demonstrated that a decrease in Bcl-xL levels following imatinib treatment due to BCR/ABL inhibition results in apoptosis.19,20 Our results shown here confirm data by Horita et al19 that enforced overexpression of Bcl-xL, regulated independently of BCR/ABL can confer resistance to apoptosis. Additionally, we have demonstrated a growth advantage in K562(Bcl-xL) cells in vitro. This implies that Bcl-xL up-regulation, induced by a BCR/ABL-independent mechanism, may be able to contribute to resistance to imatinib.
Many reports have suggested that conventional drug resistance mechanisms such as Pgp may play a role in conferring resistance to imatinib (for reviews, see Buchdunger et al27 and Mauro et al28 ). One group has reported IC50 summary data suggesting that K562 cells expressing Pgp had a growth advantage over K562 cells when treated with imatinib (among various other drugs), which was decreased with the addition of the Pgp inhibitor verapamil, but even more so with their newly synthesized Pgp inhibitor JTV-519.29 However, in our cell growth assay, K562(Pgp+) cells were not resistant to imatinib although they showed markedly greater resistance than parental cells to daunorubicin. Conversely, K562(Bcl-xL) cells were resistant to imatinib but displayed less resistance to daunorubicin than K562(Pgp+) cells.
Hegedus et al15 reported interaction of imatinib with Pgp where the concentration of the drug for half-maximal interaction with Pgp was in the range of 2 to 20 μM for the different assays conducted. This must be compared with the IC50 of around 0.25 μM for inhibition of BCR/ABL kinase activity.30 Hence, it is possible that imatinib is a poor substrate for Pgp-mediated efflux, which is in accordance with our data indicating that high levels of Pgp can confer some resistance to apoptosis induced by low doses of imatinib. Additionally, a recent clinical report demonstrated that Pgp expression was not associated with lack of imatinib response in patients with CML in myeloid blast crisis.31 Our data presented here are in accordance with this clinical finding, suggesting that up-regulation of Pgp is unlikely to be a major mechanism of imatinib resistance, although it could have a minor potentiating effect when combined with other mechanisms.
In this study we have used K562 cells expressing high levels of functional Pgp as shown by enhanced growth in daunorubicin as well as daunorubicin efflux activity. The results from our cellular growth and apoptosis assays, as well as immunoblotting experiments, all indicate overexpression of functional Pgp had a very minimal effect on the sensitivity of K562 cells to imatinib, which was only observed at low doses. A recent report demonstrated “Pgp-specific” inhibition of imatinib effects in BCR/ABL-expressing human cell lines,32 which is in contrast to results shown here. One explanation for this discrepancy could be due to a higher level of intrinsic imatinib resistance in the parental K562 cells used in their study. They estimated an IC50 of 5 μM for K562 cells and 15 μM for doxorubicin-selected K562 cells expressing high Pgp.32 This is much higher than the IC50 of 0.25 μM for imatinib growth inhibition of the parental K562 cells used in our study. It is important to note that the IC50 previously published for imatinib inhibition of BCR/ABL kinase activity is approximately 0.25 μM.30 No Pgp overexpression was detected in 6 patients in CML blast crisis who were clinically resistant to imatinib,32 which is in accordance with our conclusion from the data presented here and with the previous clinical study.31
From all the studies taken together, we can conclude that at physiologic doses of imatinib overexpression of Pgp is unlikely to be an important mechanism of resistance in patients, mainly because imatinib is a poor substrate for Pgp.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-01-0083.
Supported by a National Health and Medical Research Council of Australia (NHMRC) project grant. M.J.F. is the recipient of an Australian Postgraduate Award. L.K.A. is an NHMRC Principal Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Imatinib used in this study was provided by Novartis (Basel, Switzerland).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal