Abstract
The mechanisms that underpin the intriguing capacity of Fas ligation on dendritic cells (DCs) to induce maturation and activation, rather than apoptosis, remain unclear. In the present study we confirm that Fas signaling induces both phenotypic and functional maturation of murine DCs, and we demonstrate that phenotypic maturation is associated with phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, activation of caspase-1, and secretion of interleukin-β (IL-1β). Specific inhibition of ERK1/2 diminished Fas ligation-induced caspase-1 activation, IL-1β secretion, and ensuing up-regulation of developmental markers, whereas treatment with neutralizing anti-IL-1β antibody abrogated phenotypic and functional maturation, indicating that IL-1β mediates Fas ligation-induced DC maturation in an autocrine manner. NF-κB activation was responsible for maintaining DC viability after Fas ligation. Inhibiting NF-κB did not affect either IL-1β secretion or phenotypic maturation but rather sensitized DCs to Fas-mediated apoptosis. In conclusion, positive signals originating from Fas are transduced through at least 2 different intracellular pathways in DCs, promoting not only survival but also an increase in maturation that correlates with increased antigen-presentation capability. (Blood. 2003;102:4441-4447)
Introduction
A member of the tumor necrosis factor (TNF) receptor superfamily, Fas (CD95/Apo-1), was originally identified through its role in mediating cell death. Fas-mediated apoptosis is triggered when Fas on target cells binds its ligand, FasL, and is considered to play essential roles in the maintenance of immune homeostasis and immune privilege in brain, eye, and testis and as a mechanism of immune escape by some tumor cells.1,2 More recently, Fas has been shown to transduce proliferative and activating signals in a variety of cell types and to contribute to inflammatory responses,3 mirroring the pleiotropic functions of TNF-α4 or TNF-related apoptosis-inducing ligand (TRAIL).5,6 Fas can stimulate the proliferation of human T lymphocytes,7,8 human chronic B lymphocytic leukemia cells,9 and diploid fibroblasts,10 and it can induce the secretion of cytokines by human fibroblasts (interleukin-6 [IL-6])11 and peritoneal exudate cells (IL-1β).12 To date, the mechanisms by which Fas mediates nonapoptotic signaling remain poorly defined.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) with a key role in initiating immune responses.13-16 During antigen uptake and presentation, DCs interact with various cells types, including tumor cells17-19 and activated T cells,20 which may express levels of FasL sufficient to induce apoptosis in sensitive cells. DC resistance to Fas-mediated apoptosis is thus crucial for the fulfillment of DC antigen-presenting functions. It was formerly proposed that mature DCs, but not immature DCs, were resistant to Fas-induced apoptosis21 ; however, recent reports demonstrate that bone-marrow-derived DCs (BMDCs) express Fas on their surfaces but are resistant to Fas-mediated apoptosis, regardless of their maturation state.22 The resistance of DCs to apoptosis through the Fas pathway may be the result of increased expression of the antiapoptotic protein FLICE-inhibitory protein (FLIP).23-25 Moreover, Rescigno et al26 have shown that the ligation of Fas on DC not only fails to trigger cell death, it induces phenotypic and functional changes consistent with maturation in immature DCs and enhances cytokine secretion by mature DCs. The latter findings were confirmed in our experiments.
Why does Fas ligation induce DC maturation and survival instead of cell death? We wondered whether nonapoptotic Fas signals were transduced by an alternative signaling pathway rather than the classical apoptotic signaling pathway in DCs. Fas ligation by the agonistic anti-Fas monoclonal antibody (mAb) Jo-2 induced phenotypic and functional maturation of DCs. Fas-mediated DC maturation was IL-1β-dependent and associated with activation of the extracellular signal-regulated kinase (ERK) signaling pathway, whereas DC survival was IL-1β-independent and relied on NF-κB activation.
Materials and methods
Reagents
Recombinant mouse granulocyte macrophage colony-stimulating factor (rmGM-CSF) and IL-4 were purchased from PeproTech (London, United Kingdom). Recombinant mouse IL-1β (rmIL-1β), recombinant mouse FasL (rmFasL), anti-mIL-1β, mouse anti-6 × histidine, and anti-mCD40 were sourced from R&D Systems (Minneapolis, MN). Anti-mFas antibody Jo-2 (15400D), isotype antibody (11150D), anti-mIab-fluorescein isothiocyanate (FITC), anti-mCD86-FITC, anti-mCD40-FITC, and isotype antibody were purchased from PharMingen (San Diego, CA). Anti-caspase-1 p20 (sc-1780), anti-NFκB p65 (sc-109), anti-phospho-ERK (sc-7383), antimouse immunoglobulin G horseradish peroxidase (IgG-HRP), and antigoat IgG HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IκB-α-P was produced by Biolabs (Beverly, MA). Pyrrolidinecarbodithoic acid (PDTC), an inhibitor of NF-κB; PD98059, an inhibitor of MEK1; and Ac-YVAD-CMK (40012), a relatively specific inhibitor of caspase-1, were obtained from Calbiochem (Darmstadt, Germany). Lipopolysaccharide (LPS) (Escherichia coli, O26:B6) and recombinant mouse interferon-γ (IFN-γ) were purchased from Sigma (St Louis, MO). Fas-deficient B6.MRL-Tnfrsf6lpr mice were purchased from JAX Mice (Bar Harbor, ME).
DC stimulation and phenotype analysis
BMDCs from normal C57BL/6J mice or B6.MRL-Tnfrsf6lpr mice were generated as previously described.27 Unless otherwise mentioned, DCs from C57BL/6J mice were used. After 7 days of culture with 10 ng/mL GM-CSF and 1 ng/mL IL-4, DCs were harvested as immature DCs. To produce mature DCs, immature DCs were stimulated with 500 ng/mL LPS for 24 hours. Then 5 × 105 immature DCs were resuspended in 1 mL complete culture medium in the presence of 10 ng/mL GM-CSF and 1 ng/mL IL-4 and were stimulated with Jo-2 for 24 hours at the indicated concentrations. In some experiments, cells were concurrently stimulated with 1 ng/mL rmIL-1β or were pretreated with 0.5 μg/mL anti-mIL-1β antibody or isotype antibody for 30 minutes before Jo-2 treatment. After stimulation, culture supernatants were collected for cytokine production analysis, and DCs were collected for phenotypic analysis. DCs were incubated with FITC-labeled antibodies specific for mouse Iab, CD86, CD40, or isotype antibodies (each used at 5 μg/mL) for 30 minutes at 4°C. Staining was carried out in the presence of 2.4 G2 to block Fc receptor binding. Cells were analyzed by flow cytometry using a FACScalibur flow cytometer and Cell Quest software (Becton Dickinson, Mountain View, CA). For signaling inhibition studies, DCs were pretreated at 37°C with MEK inhibitor PD98059, NF-κB inhibitor PDTC, caspase-1 inhibitor Ac-YVAD-CMK, or protein synthesis inhibitor cycloheximide (CHX) for 30 minutes, at the indicated concentrations, before they were stimulated with Jo-2, isotype antibody, or LPS.
Cytokine assays
TNF-α, IL-12p70, IL-6, and IL-1β levels were assayed using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (R&D Systems).
Detection of cell death
After the indicated 8-hour treatment with Jo-2 or isotype antibody, the externalization of phosphatidyl serine on DCs and thymocyte membranes was determined by incubating cells with FITC-Annexin V (PharMingen) for 30 minutes at 4°C, then adding 5 μg/mL propidium iodide (PI; Sigma) immediately before FACS analysis. Because the loss of mitochondrial membrane potential is a characteristic of apoptosis, mitochondrial membrane potential was determined by incubating cells with 1 μg/mL R123 (Molecular Probes, Eugene, OR) for 20 minutes at 37°C and then washing twice with cold phosphate-buffered saline (PBS). Cells were analyzed by flow cytometry as described.
Allogeneic mixed-leukocyte reaction
Allogeneic mixed-leukocyte reaction (MLR) was performed as previously described.28 Briefly, 2 × 105 allogeneic spleen T cells (H-2Kd) were incubated for 5 days with 2 × 104 DCs (H-2Kb) that had been treated as indicated and then γ-irradiated (30 Gy cyanocobalamin Co 60) before coculture. [3H]-thymidine (0.5 μCi (0.0185 MBq)/well; Amersham Pharmacia Biotech, Amersham, United Kingdom) was added to each well for the final 18 hours. Cell-associated radioactivity was determined by direct beta counting (Wallac1409; Wallac, Turku, Finland), and results were expressed as the mean of triplicate assays.
Western blot analysis of ERK and caspase-1 activation
ERK activation was detected using procedures described previously by us.29 For the detection of caspase-1 activation, blots were probed for 1 hour with anti-caspase1 p20 (1:1000) and then were incubated with 1:5000 diluted HRP-conjugated antigoat IgG for 1 hour at room temperature. Proteins were visualized using SuperSignal West Femto Maximum Sensitivity Substrate, as instructed by the manufacturer (Pierce, Rockford, IL).
Assessment of IκB phosphorylation and NF-κB nuclear translocation
Cytoplasmic and nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce), and the protein concentration was determined by BCA-200 protein assay (Pierce). Phosphorylated IκB in cytoplasmic extracts and NF-κB subunit p65 in nuclear extracts were detected by Western blot using specific antibodies.
Reverse transcription-polymerase chain reaction analysis of IL-1β expression
Total RNA was isolated from stimulated DCs with TRIzol (Gibco BRL, Carlsbad, CA), according to the manufacturer's directions. cDNA was synthesized from 1 μg total RNA by extension with oligo(dT)18 primer and SuperScript II (200 U; Gibco BRL. Primers used for polymerase chain reaction (PCR) amplification were 5′-GCA TCC TCA CCC TGA AGT AC-3′ and 5′-TTC TCC TTAATG TCA CGC AC-3′ (β-actin), and 5′-GCA GCT ATG GCA ACT GTT CCT-3′ and 5′-GGT GGG TGT GCC GTC T-3′ (IL-1β). PCR amplification from cDNA was performed in a final volume of 50 μL containing 2.5 mM magnesium dichloride, 1.25 U Ex Taq polymerase (TaKaRa, Dalian, China), and 1 μM specific primers. Cycling conditions were 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 60 seconds (GeneAmp 9600 PCR System; Perkin-Elmer, Wellesley, MA). The optimum cycle number was 30 cycles for mIL-1β and 25 cycles for β-actin. All PCR products were resolved by 2% agarose gel electrophoresis and visualized by staining with ethidium bromide.
Statistical analysis
Statistical comparisons between experimental and control groups were performed using Student t test analysis, with P less than .05 regarded as a statistically significant difference.
Results
Murine BMDCs are resistant to Fas-induced apoptosis
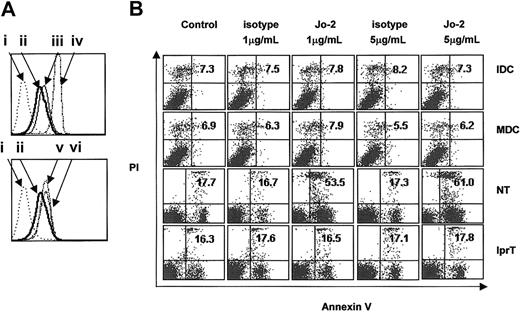
Fas expression on the surfaces of immature (7-day) and mature (LPS-stimulated immature) DCs was determined by fluorescence-activated cell sorter (FACS) analysis. Results showed that DCs at both stages of maturation expressed Fas; however, expression was weaker than that of murine thymocytes. Stimulation with anti-mCD40, rmIFN-γ, or LPS did not significantly increase the expression of Fas (Figure 1A). Ligation of Fas with 1 μg/mL Jo-2 for 8 hours effectively induced apoptosis of mouse thymocytes but failed to induce apoptosis of thymocytes from lpr mice, confirming that Jo-2 could ligate Fas on the cell surface and induce apoptosis of sensitive cells (Figure 1B). Immature and mature DCs were resistant to apoptosis, even at high concentrations of Jo-2. Apoptosis was not observed even when DCs were stimulated with 5 μg/mL Jo-2 for 24 hours (data not shown).
Murine DCs express Fas but are resistant to Fas-ligation-induced apoptosis. (A) Flow cytometric analysis of Fas expression on the surfaces of C57BL/6J thymocytes and DCs stimulated with different agents. The top panel compares Fas expression in DCs at both stages (ii shows immature DCs; iii, LPS-matured DCs) and in thymocytes (iv). The bottom panel compares Fas expression in immature DCs (ii) and in immature DCs stimulated with anti-mCD40 (vi) or IFN-γ (v). i indicates control. (B) Immature DCs (IDC) and mature DCs (MDC) from normal C57BL/6J mice and thymocytes from normal C57BL/6J mice (NT) or lpr mice (lprT) were treated with 1 μg/mL or 5 μg/mL of either Jo-2 or isotype antibody for 8 hours. The percentage of apoptotic cells was quantified by staining with Annexin V-FITC and PI. Annexin V single-positive cells, PI single-positive cells, and Annexin V and PI double-positive cells were considered apoptotic. Plots are labeled with the percentages of gated cells that expressed either or both apoptotic markers.
Murine DCs express Fas but are resistant to Fas-ligation-induced apoptosis. (A) Flow cytometric analysis of Fas expression on the surfaces of C57BL/6J thymocytes and DCs stimulated with different agents. The top panel compares Fas expression in DCs at both stages (ii shows immature DCs; iii, LPS-matured DCs) and in thymocytes (iv). The bottom panel compares Fas expression in immature DCs (ii) and in immature DCs stimulated with anti-mCD40 (vi) or IFN-γ (v). i indicates control. (B) Immature DCs (IDC) and mature DCs (MDC) from normal C57BL/6J mice and thymocytes from normal C57BL/6J mice (NT) or lpr mice (lprT) were treated with 1 μg/mL or 5 μg/mL of either Jo-2 or isotype antibody for 8 hours. The percentage of apoptotic cells was quantified by staining with Annexin V-FITC and PI. Annexin V single-positive cells, PI single-positive cells, and Annexin V and PI double-positive cells were considered apoptotic. Plots are labeled with the percentages of gated cells that expressed either or both apoptotic markers.
Fas ligation induces secretion of IL-1β by DCs
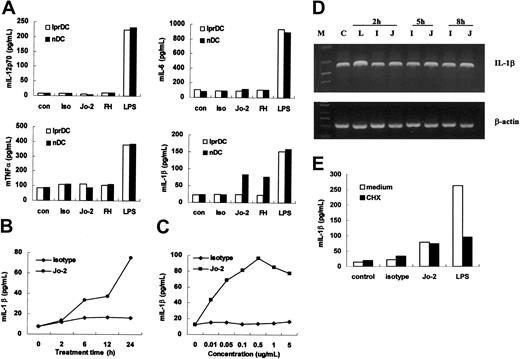
DCs derived from either lpr (lprDC) or normal C57BL/6J mice (nDC) were incubated with 1 μg/mL Jo-2 or 5 μg/mL rmFasL in the presence of anti-6 × histidine antibody (FH) for 24 hours. Culture supernatants were then assayed for the presence of proinflammatory cytokines. Compared with the high levels of IL-1β triggered by LPS stimulation, Fas ligation by either Jo-2 or rmFasL induced moderate IL-1β production in DCs from normal C57BL/6J mice. Neither reagent could elicit IL-1β secretion from lprDC. This demonstrates that Jo-2 and rmFasL have equivalent effects on cross-linking Fas on the surfaces of DCs; however, because the Jo-2 system is stabler and simpler than the rmFasL system, we chose to use Jo-2 to stimulate DCs in subsequent experiments. Fas ligation did not induce the secretion of IL-12p70, IL-6, or TNF-α (Figure 2A). Even very low concentrations of Jo-2 (0.01 μg/mL) could induce IL-1β secretion, indicating that Fas ligation is efficient at inducing IL-1β release (Figure 2B). Fas ligation rapidly induces IL-1β secretion by DCs; dynamic analysis showed that IL-1β could be detected after just 2 hours of Jo-2 stimulation (Figure 2C). This rapid secretion of IL-1β after Jo-2 stimulation indicated that it might not require de novo gene expression. Reverse transcription (RT)-PCR results showed that Jo-2 stimulation had little effect on IL-1β messenger RNA (mRNA) expression in DCs (Figure 2D), indicating that IL-1β secretion was independent of gene transcription. To confirm this, DCs were incubated with 0.5 μg/mL CHX for 30 minutes before stimulation with Jo-2, isotype, or LPS. As expected, the level of IL-1β induced by Jo-2 stimulation remained unchanged, whereas LPS-induced IL-1β secretion was decreased significantly (Figure 2E). This result further demonstrated that IL-1β secretion induced by Fas ligation is independent of de novo protein synthesis. Boiled Jo-2 could not induce IL-1β secretion (data not shown), and, given that the endotoxin level in the NA/LE format of Jo-2 is 0.01 ng/μg protein or less, the possibility of contaminating endotoxin in the antibody preparation triggering IL-1β could also be excluded. The observations that Jo-2 had no effect on DCs from Fas-deficient lpr mice and that similar effects could be elicited using rmFasL and cross-linking antibody confirmed that Fas ligation by Jo-2 is responsible for the DC responses detected.
Induction of IL-1β production, but not IL-12, IL-6, or TNF-α, by Fas ligation. (A) Cytokine production by DCs from normal C57BL/6J mice or lpr mice was measured using ELISA after DCs were stimulated with 1 μg/mL Jo-2 (Jo-2), isotype antibody (Iso), and 5 μg/mL rmFasL in the presence of 10 μg/mL anti-6 × histidine antibody (FH) or 0.2 μg/mL LPS (LPS) for 24 hours. Unstimulated DCs were used as control (con). (B) Production of IL-1β by immature DCs after stimulation with 1 μg/mL Jo-2 for various lengths of time. (C) IL-1β production by immature DCs after stimulation with various concentrations of Jo-2 (0.1 μg/mL-10 μg/mL). (D) IL-1β mRNA expression in DCs stimulated with medium alone (C), Jo-2 (J), isotype antibody (I) or LPS (L) at indicated time points. Results are representative of 3 independent experiments. (E) Effect of cycloheximide (CHX) on Jo-2-induced IL-1β secretion. DCs were stimulated with Jo-2, isotype antibody or LPS, for 24 hours in the presence or absence of 0.5 μg/mL CHX, and then IL-1β levels in supernatant were measured by ELISA.
Induction of IL-1β production, but not IL-12, IL-6, or TNF-α, by Fas ligation. (A) Cytokine production by DCs from normal C57BL/6J mice or lpr mice was measured using ELISA after DCs were stimulated with 1 μg/mL Jo-2 (Jo-2), isotype antibody (Iso), and 5 μg/mL rmFasL in the presence of 10 μg/mL anti-6 × histidine antibody (FH) or 0.2 μg/mL LPS (LPS) for 24 hours. Unstimulated DCs were used as control (con). (B) Production of IL-1β by immature DCs after stimulation with 1 μg/mL Jo-2 for various lengths of time. (C) IL-1β production by immature DCs after stimulation with various concentrations of Jo-2 (0.1 μg/mL-10 μg/mL). (D) IL-1β mRNA expression in DCs stimulated with medium alone (C), Jo-2 (J), isotype antibody (I) or LPS (L) at indicated time points. Results are representative of 3 independent experiments. (E) Effect of cycloheximide (CHX) on Jo-2-induced IL-1β secretion. DCs were stimulated with Jo-2, isotype antibody or LPS, for 24 hours in the presence or absence of 0.5 μg/mL CHX, and then IL-1β levels in supernatant were measured by ELISA.
Fas ligation induces maturation of DCs in an IL-1β autocrine manner
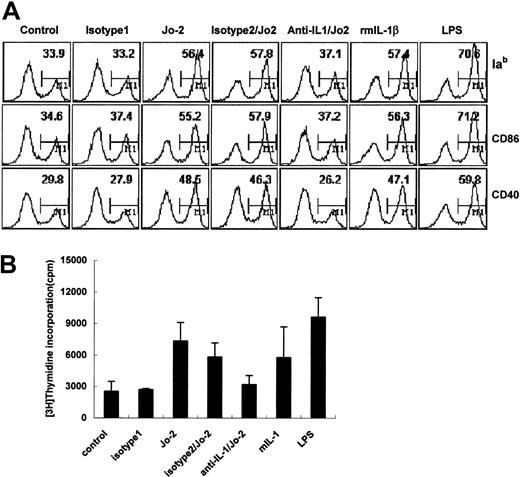
DCs were incubated with 1 μg/mL Jo-2 for 24 hours, and phenotypic changes were analyzed by FACS. Fas ligation induced the up-regulated expression of major histocompatibility complex (MHC) class II and costimulatory molecules CD86 and CD40 on DCs (Figure 3A). Jo-2-stimulated DCs also elicited significant T-cell proliferation in allo-MLR, indicating that Fas ligation can also induce the functional maturation of DCs (Figure 3B). Because IL-1β can mediate the maturation of murine epidermal Langerhans cells30 and enhance T-cell-dependent immune responses by amplifying DC function,31 we investigated whether Fas ligation-induced DC maturation was induced by IL-1β. As shown in Figure 3, recombinant IL-1β induces the phenotypic and functional maturation of DCs. Neutralizing anti-IL-1β antibody almost completely abrogated these Fas-ligation-induced effects. Taken together with the DC IL-1β secretion observed in Figure 2, it appears likely that IL-1β secreted in response to Fas ligation functions in an autocrine manner to mediate DC maturation.
DC maturation is induced by Fas ligation and recombinant mouse IL-1β (A) DCs were treated with Jo-2, isotype antibody (isotype 1), or recombinant mouse IL-1β for 24 hours or were pretreated with anti-mIL-1β antibody or isotype antibody (isotype 2) for 30 minutes, then treated with Jo-2 or isotype 1 for 24 hours. DCs were then stained with specific antibodies against MHC class II, CD86, or CD40 and were analyzed by flow cytometry. Plots are labeled with the percentages of mature DC. (B) Proliferation of allogeneic T cells induced by Jo-2-stimulated DCs. Data represent mean ± SD.
DC maturation is induced by Fas ligation and recombinant mouse IL-1β (A) DCs were treated with Jo-2, isotype antibody (isotype 1), or recombinant mouse IL-1β for 24 hours or were pretreated with anti-mIL-1β antibody or isotype antibody (isotype 2) for 30 minutes, then treated with Jo-2 or isotype 1 for 24 hours. DCs were then stained with specific antibodies against MHC class II, CD86, or CD40 and were analyzed by flow cytometry. Plots are labeled with the percentages of mature DC. (B) Proliferation of allogeneic T cells induced by Jo-2-stimulated DCs. Data represent mean ± SD.
Fas ligation induces activation of caspase-1
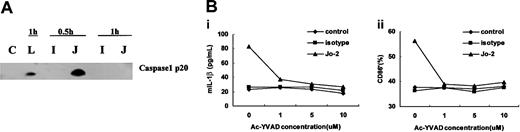
Because caspase-1 (ICE) is essential for cleaving pro-IL-1β into its mature 17-kDa bioactive form,32-36 we determined whether Fas ligation led to the activation of caspase-1 in DCs and whether the inhibition of caspase-1 influenced IL-1β secretion. As shown in Figure 4A, the caspase-1 precursor is cleaved into the active form during early stages of Jo-2-stimulated DC activation, which presumably leads to the processing of pro-IL-1β to its secreted form. Inhibition of capase 1 by the relatively specific inhibitor Ac-YVAD led to a decrease in IL-1β secretion levels and prevented Fas-induced maturation (Figure 4B). These data suggest that caspase-1 activation is essential for Fas-ligation-induced IL-1β secretion by DCs.
Caspase-1 is activated by Fas ligation and is required for Fas-ligation-induced IL-1β secretion and phenotypic maturation of DCs. (A) Western blot analysis of caspase-1 activation in DCs cultured in medium alone (C) or after stimulation with anti-Fas antibody Jo-2 (J), isotype antibody (I), or LPS (L) for the indicated times. (B) Inhibition of Fas-ligation-induced IL-1β secretion (i) and maturation marker CD86 expression in DCs (ii) by the relatively specific caspase-1 inhibitor Ac-YVAD. Comparable results were obtained from 3 independently conducted experiments.
Caspase-1 is activated by Fas ligation and is required for Fas-ligation-induced IL-1β secretion and phenotypic maturation of DCs. (A) Western blot analysis of caspase-1 activation in DCs cultured in medium alone (C) or after stimulation with anti-Fas antibody Jo-2 (J), isotype antibody (I), or LPS (L) for the indicated times. (B) Inhibition of Fas-ligation-induced IL-1β secretion (i) and maturation marker CD86 expression in DCs (ii) by the relatively specific caspase-1 inhibitor Ac-YVAD. Comparable results were obtained from 3 independently conducted experiments.
Fas ligation induces activation of ERK1/2 in DCs
FasL has been shown to activate ERK, but not c-Jun N-terminal kinase (JNK) or P38 MAP kinases, in human fibroblasts,11 and the ERK MAP kinases, but not the JNK or P38 MAP kinases, are the major MAP kinase targets of LPS signaling in the DC cell line D1.37 We investigated whether Fas ligation could activate ERK in DCs. Western blot analysis showed that stimulating DCs with Jo-2 led to an increase in ERK1/2 phosphorylation, indicative of activation. As shown in Figure 5A, activation of ERK1/2 in DCs could be detected 5 minutes after Jo-2 stimulation with ERK1/2 phosphorylation decreasing after 30 minutes, suggesting that ERK1/2 activation is an early event triggered by Fas ligation of DCs.
Activation of ERK1/2 by Fas ligation and its relationship with caspase-1 activation and DC maturation. (A) DCs were cultured in medium alone as a control (C) or were stimulated with anti-Fas antibody Jo-2 (J), isotype antibody (I), or LPS (L) for the indicated times. Whole-cell lysates were then electrophoresed and probed with phospho-ERK1/2, followed by an appropriate secondary antibody. (B) DCs were pretreated with different doses of PD98059 for 30 minutes, then stimulated with Jo-2, isotype antibody or LPS for 24 hours. The levels of IL-1β in the supernatants were determined using ELISA, and cells were harvested and analyzed for CD86 expression by flow cytometry. (C) DCs were pretreated with different doses of PD98059 for 30 minutes and stimulated with Jo-2 or isotype antibody for 8 hours. Cells were then stained with R123 and analyzed by FACS analysis. (D) DCs were pretreated with different doses of PD98059 for 30 minutes, then stimulated with Jo-2 for 30 minutes. ERK1/2 activation and caspase-1 activation were examined by immunoblotting of cell lysates with anti-phospho-ERK1/2 antibody or anti-caspase-1 P20 subunit antibody.
Activation of ERK1/2 by Fas ligation and its relationship with caspase-1 activation and DC maturation. (A) DCs were cultured in medium alone as a control (C) or were stimulated with anti-Fas antibody Jo-2 (J), isotype antibody (I), or LPS (L) for the indicated times. Whole-cell lysates were then electrophoresed and probed with phospho-ERK1/2, followed by an appropriate secondary antibody. (B) DCs were pretreated with different doses of PD98059 for 30 minutes, then stimulated with Jo-2, isotype antibody or LPS for 24 hours. The levels of IL-1β in the supernatants were determined using ELISA, and cells were harvested and analyzed for CD86 expression by flow cytometry. (C) DCs were pretreated with different doses of PD98059 for 30 minutes and stimulated with Jo-2 or isotype antibody for 8 hours. Cells were then stained with R123 and analyzed by FACS analysis. (D) DCs were pretreated with different doses of PD98059 for 30 minutes, then stimulated with Jo-2 for 30 minutes. ERK1/2 activation and caspase-1 activation were examined by immunoblotting of cell lysates with anti-phospho-ERK1/2 antibody or anti-caspase-1 P20 subunit antibody.
Phosphorylation of ERK1/2 is responsible for caspase-1 activation and IL-1β secretion and maturation, but not for DC survival
To examine the role of ERK1/2 activation in DCs induced by Fas ligation, we pretreated DCs with PD98059, an inhibitor of MEK1, the kinase that phosphorylates ERK1/2, before Jo-2 stimulation. As shown in Figure 5B, the secretion of IL-1β by DCs induced by Jo-2 stimulation was inhibited by pretreatment with PD98059. PD98059 effectively inhibited the ability of Jo-2 stimulation to up-regulate CD86 expression on DCs, indicating that inhibiting the ERK1/2 pathway could block DC maturation induced by Fas ligation. The results also confirmed our conclusion that Fas-ligation-induced IL-1β was the main mediator of DC maturation. Although PD98059 could partially inhibit IL-1β secretion induced by LPS stimulation, it did not alter phenotypic maturation, demonstrating that the activation of ERK is not responsible for LPS-induced DC maturation. This finding is consistent with results previously obtained by other groups,37 suggesting that the secretion of IL-1β might play different roles in Jo-2 and LPS-induced DC maturation. Different from maturation, as shown in Figure 5C, Fas-ligation-induced survival of DCs was not affected by the ERK inhibitor PD98059, indicating that the ERK signal transduction pathway is not responsible for DC survival.
Because caspase-1 plays an important role in the production of IL-1β, we wondered whether inhibiting ERK activation could affect caspase-1 activation in DCs. We assayed caspase-1 activation in DCs after Jo-2 stimulation in the presence of PD98059 and found that the ERK inhibitor obstructed caspase-1 activation in a dose-dependent manner (Figure 5D). These data show, for the first time, that there may be a direct or an indirect relationship between the activation of caspase-1 and the activation of ERK in DCs, and they indicate that the ERK signal pathway may be upstream of caspase-1 activation.
Fas ligation stimulates the phosphorylation of IκB and the nuclear translocation of NF-κBinDCs
It is well documented that the transcription factor NF-κB controls antiapoptotic gene expression, which is important in cell types that are resistant to apoptosis induced by death receptors.38,39 Moreover, nonapoptotic Fas signaling is often accompanied by the activation of NF-κB.11,40 NF-κB consists of a heterodimeric complex, usually composed of the p50 and 65-kDa RelA subunits. In its inactive state, the complex is sequestered in the cytosol by an inhibitory subunit, IκB. On stimulation, IκB is phosphorylated and degraded, which allows NF-κB to translocate to the nucleus and to activate target genes.41 We tested whether the NF-κB signaling pathway was activated in DCs after Fas ligation. As shown in Figure 6A, IκB phosphorylation peaked at 30 minutes after Jo-2 stimulation, then decreased to basal levels. Nuclear translocation of NF-κB was detected in DCs 30 minutes after Jo-2 stimulation and continued to increase for the following 2 hours. These data confirm that Fas ligation activates the NF-κB signaling pathway in DCs.
Fas ligation activation of NF-κB in DCs and its involvement in DC survival. (A) Determination of Fas ligation-induced phosphorylation of IκB and nuclear translocation of NF-κB. Cytoplasmic or nuclear extracts from DCs cultured in medium alone (C) or stimulated with Jo-2 (J), isotype antibody (I), or LPS (L) were prepared, blotted, and probed with phospho-IκB- and NF-κB-specific antibodies, respectively. (B) DCs were pretreated with different doses of PDTC, an inhibitor of NF-κB activation, for 30 minutes, then stimulated with Jo-2, isotype antibody or LPS, for 24 hours. Levels of IL-1β in supernatants were determined by ELISA, and DCs were harvested for CD86 staining and analysis by flow cytometry. (C) Detection of Fas ligation-induced DC death. After pretreatment with the indicated dose of PDTC, DCs were stimulated with Jo-2 or isotype antibody for 8 hours, then stained with R123 and analyzed by flow cytometry. Data are presented as the percentage of cells that were apoptotic (R123low).
Fas ligation activation of NF-κB in DCs and its involvement in DC survival. (A) Determination of Fas ligation-induced phosphorylation of IκB and nuclear translocation of NF-κB. Cytoplasmic or nuclear extracts from DCs cultured in medium alone (C) or stimulated with Jo-2 (J), isotype antibody (I), or LPS (L) were prepared, blotted, and probed with phospho-IκB- and NF-κB-specific antibodies, respectively. (B) DCs were pretreated with different doses of PDTC, an inhibitor of NF-κB activation, for 30 minutes, then stimulated with Jo-2, isotype antibody or LPS, for 24 hours. Levels of IL-1β in supernatants were determined by ELISA, and DCs were harvested for CD86 staining and analysis by flow cytometry. (C) Detection of Fas ligation-induced DC death. After pretreatment with the indicated dose of PDTC, DCs were stimulated with Jo-2 or isotype antibody for 8 hours, then stained with R123 and analyzed by flow cytometry. Data are presented as the percentage of cells that were apoptotic (R123low).
NF-κB is involved in DC survival, but not maturation, induced by Fas ligation
NF-κB is also known to be a mediator of inflammatory gene expression, so we investigated whether the NF-κB signaling pathway could influence the Fas-ligation-induced production of IL-1β and the subsequent maturation of DCs. DCs were incubated with the NF-κB inhibitor PDTC for 30 minutes, then stimulated with Jo-2. After 24 hours, IL-1β secretion levels were determined, and phenotypic changes of DCs were analyzed. Inhibition of NF-κB activation had no effect on DC maturation and IL-1β secretion (Figure 6B). Although PDTC did not inhibit LPS-induced IL-1β secretion, it could significantly inhibit DC maturation, demonstrating that NF-κB plays a role in the maturation of DCs induced by LPS, consistent with previous reports.37 DCs pretreated with PDTC before Jo-2 stimulation were harvested and stained with R123 to detect apoptosis. The result showed that Fas ligation combined with NF-κB inhibition could induce DC apoptosis (Figure 6C). However, fewer DCs underwent apoptosis compared with thymocytes induced by Fas ligation. The heterogeneity of DCs studied may provide an explanation for this phenomenon, with the NF-κB signaling pathway possibly playing different roles in different DC subsets. PDTC may thus only make some subsets of DCs sensitive to Fas-induced apoptosis. Therefore, it appears that NF-κB signaling influences the intrinsic survival pathway of DCs induced by Fas ligation and plays a pivotal role in regulating susceptibility of DCs to Fas-induced apoptosis.
Discussion
Autocrine feedback of IL-1β appears to be an important mechanism for Fas-ligation-induced DC maturation; Fas ligation induces DCs to secrete IL-1β rapidly, DCs mature in response to recombinant IL-1β, and neutralizing anti-IL-1β antibody almost completely abrogates Fas-ligation-induced phenotypic and functional DC maturation. We show here that Fas ligation leads to the activation of ERK1/2 in DCs, which is responsible for maturation, and that Fas-ligation-induced nuclear translocation of NF-κB determines the sensitivity of DCs to Fas-mediated apoptosis. Most interestingly, we have demonstrated for the first time that Fas-ligation-induced activation of ERK1/2 in DCs results in the activation of caspase-1, with caspase-1 activation obstructed by the ERK inhibitor PD98059 in a dose-dependent manner. This finding sheds light on the largely unknown apical activation pathway for proinflammatory caspases.
Resistance to Fas-induced apoptosis is of great biologic significance for DCs in their role as professional APCs. During antigen uptake, processing, and presentation, DCs may interact with cells that express FasL. According to our data, interaction of FasL with Fas on the DC surface may promote DCs to secrete IL-1β, which acts in an autocrine manner to induce DC maturation, thus enhancing allogeneic T-cell-stimulatory capacity. It has been shown that autocrine IL-1β activity not only acts as an important second signal in T-cell proliferation, it can also enhance T-cell-dependent immune responses by amplifying DC function.35,36,42,43 Therefore, the ligation of Fas on DCs, through the release of IL-1β, enhances the capability of DCs to prime T-cell proliferation and, as a result, positively regulates the immune response. The proinflammatory cytokine IL-18 is also cleaved by caspase-1, but we could not detect increased IL-18 secretion after the Fas ligation of DCs, despite the triggering of caspase-1 activity (data not shown). In addition, even LPS stimulation was unable to elicit an increase in IL-18 secretion by DCs, consistent with the findings of other groups.44,45 Therefore, we speculate that IL-18 may not play an important role in the effects induced by Jo-2 ligation. Furthermore, evidence shows that FasL functions as a costimulatory receptor for peripheral CD8+ and CD4+ T cells,46-48 indicating that costimulatory interaction between Fas expressed on APCs and FasL expressed on T cells plays an essential role in fine-tuning T-cell reactivity.
Recent work has shown that Fas signaling is involved in inflammation. Forced expression of FasL by tumor cells49,50 and nontransformed cell populations51 elicited a vigorous inflammatory response within subcutaneous tissues,49 peritoneum,12 or pancreas,51 ultimately resulting in the rapid destruction and rejection of cells by infiltrated neutrophils and the destruction of not only FasL-expressing cells but also bystander cells that did not express FasL.49 This destruction or rejection is T-cell- and B-cell-independent.12,49 Taken together with our results, it appears that Fas-stimulated DCs may be involved in these inflammatory reactions. Forcibly expressed FasL may ligate Fas on the DC surface, leading to the production of IL-1β, which might act indirectly as a stimulator, promoting adjacent cells to secrete neutrophil-attractant chemokines, or directly as a proinflammatory mediator to activate neutrophils.12
The molecular mechanisms of the non-apoptosis-inducing function of Fas ligation are not well defined. The ERK1/2 and NF-κB pathways have been shown to act as important modulators of various apoptosis-inducing signals in different systems.52 Our results demonstrated that Fas ligation leads to the activation of ERK1/2, which is consistent with the observation that treating serum-starved GM6112 fibroblasts with soluble FasL results in a rapid and transient phosphorylation of ERK1/2.11 In DCs, inhibiting ERK1/2 activation leads to the failure of caspase-1 activation and subsequent IL-1β secretion, preventing Fas-ligation-induced DC maturation, as we observed. How Fas ligation is linked to ERK1/2 activation remains unknown. A possible key factor may be c-FADD-like IL-1β converting enzyme-inhibitory protein (c-FLIP), a caspase-8-like molecule that lacks catalytic activity and can act as a caspase-8 inhibitor by competing for Fas-associated death domain (FADD)-binding. c-FLIP, regarded as an essential mediator of DC resistance to Fas-mediated apoptosis,23-25 is up-regulated after the incubation of DCs with FasL.26 In a study in which the proliferation of CD3-activated human T cells was augmented by recombinant FasL, FLIP was shown to be recruited by Fas ligation and to interact with TNF-receptor-associated factors 1 and 2, RIP, and Raf-1, resulting in the activation of ERK and NF-κB.53 We detected the phosphorylation of IκB and the nuclear translocation of NF-κB in DCs, consistent with the reported activation of NF-κB in some tumor cell lines54,55 and cortical neuroblasts,56 and we found that NF-κB activation was required for DC survival after Fas ligation. This finding is contrary to the general view that, unlike TNF-α stimulation, Fas signaling is unable to trigger detectable NF-κB activation. A possible explanation was that autocrine IL-1β, rather than direct Fas signaling, induced NF-κB activation. In our experiments, blocking NF-κB inhibited neither IL-1β secretion nor DC maturation mediated by IL-1β; rather, it made DCs more sensitive to Fas-induced apoptosis. These data suggest that Fas-ligation-induced NF-κB signaling is independent of IL-1β and that the survival of DC is IL-1β-independent.
Of great interest was our demonstration, for the first time, that there might be a direct or an indirect association between the activation of ERK1/2 and caspase-1. It has been previously proposed that a link between the mitogen-activated protein kinase (MAPK) signal transduction pathway and ICE-like caspases may exist.57 Although there was a controversial opinion on the relationship between TNF receptor signaling and ceramide generation,58 it was generally accepted that the NSD domain of the cytoplasmic portion of the 55-kDa TNF-α receptor might mediate proliferation and inflammation by activating Raf-1 and the ERK cascade.59,60 As a homolog of the TNF receptor, Fas may indeed have a cytoplasmic domain homologous to NSD, which mediates inflammation and proliferation through the activation of ERK1/2. Because caspase-1 plays a pivotal role in the regulation of proinflammatory networks, the link between ERK1/2 and caspase-1 appears reasonable.
Originally identified as a cell surface receptor that triggered the death of lymphocytes and tumor cells, it is now recognized that Fas has distinct functions in the life and death of different cell types in the immune system. The various signal transduction pathways triggered by Fas underpin these cellular outcomes. Because DCs play a crucial role in the initiation of immune responses, a better understanding of the function of Fas on DC membranes and its underlying mechanisms could have important immunotherapeutic applications for the treatment of tumors and transplant rejection.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2002-11-3420.
Supported by grants from the National Natural Science Foundation of China (30121002) and the National Key Basic Research Program of China (2001CB510002).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rui Zhang, Chunfang Luo, Minggang Zhang, and Naisong Lin for technical assistance; Drs Wenya Wang, Hongmei Xu, Runzi Qi, and Jun Zhou for their helpful discussion; and Dr Jane Rayner for review of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal