Abstract
We report on the characteristics of 21 patients with hepatosplenic γδ T-cell lymphoma (HSγδTCL), an entity recognized since 1994 in the Revised European American Lymphoma (REAL) classification. Median age was 34 years. Patients had splenomegaly (n = 21), hepatomegaly (n = 15), and thrombocytopenia (n = 20). Histopathologic findings were homogeneous and showed the presence of medium-sized lymphoma cells within the sinusoids of splenic red pulp, liver, and bone marrow. Marrow involvement was usually mild but could be demonstrated by phenotyping in all patients. Cells were CD3+CD5-, expressed the γδ T-cell receptor, and had a nonactivated cytotoxic cell phenotype (TIA-1+, granzyme B-). Most patients were CD4-/CD8- (16 of 18); CD56+ (15 of 18), expressed the Vδ1epitope (Vd1+/Vd2-/Vd3-) (9 of 12); and were negative for Epstein-Barr virus (EBV) (18 of 20). Isochromosome arm 7q was documented in 9 of 13 patients. Eight patients had previously undergone kidney transplantation or had a history of systemic lupus, Hodgkin disease, or malaria. Prognosis was poor; median survival time was 16 months, and all but 2 patients ultimately died despite consolidative or salvage high-dose therapy. In conclusion, HSγδTCL is a disease with distinctive clinical, histopathologic, and phenotypic characteristics. Bone marrow biopsy with combined phenotyping is sufficient for diagnosis, and splenectomy is therefore unwarranted. Current treatment modalities appear to be ineffective in most patients. (Blood. 2003;102:4261-4269)
Introduction
Human γδ T lymphocytes represent a normal subset of postthymic T cells with cytotoxic functions and preferential homing in some epithelial-rich tissues and within sinusoidal areas of the splenic red pulp.1,2 In 1990, we proposed hepatosplenic γδ T-cell lymphoma (HSγδTCL) as a distinct entity among peripheral T-cell lymphomas recognized on the basis of clinical presentation, pattern of histologic involvement resulting from the sinusal/sinusoidal tropism of neoplastic cells, and expression of the γδ T-cell receptor (TCR) by tumor cells.3,4
Since our initial reports, several cases of hepatosplenic lymphoma expressing a γδ phenotype have been described.5-25 Among these, Cooke et al11 published findings on a series of 7 patients, 5 of whom had a proven γδ phenotype, providing further evidence that HSγδTCL is a distinct clinicopathologic entity. The identification of this lymphoma subtype, recognized as a provisional entity in the Revised European American Lymphoma (REAL) classification,26 has been further supported by its cytotoxic phenotype11,27 and its strong association with the isochromosome arm 7q cytogenetic abnormality.12,14,25,28-32
More recently, a few cases of HSTCL with sinusoidal infiltration and an αβ T-cell receptor phenotype have been reported.33-35 This is now considered an immunophenotypic variant of the same disease entity in the World Health Organization (WHO) classification.36
Because of the rarity of the disease and the difficulty in assessing the γδ T-cell origin, which relies on frozen-tissue immunophenotyping, most previous reports of HSγδTCL refer to sporadic cases or limited series with short follow-up and heterogeneous therapies, as recently reviewed by Weidman.37 This prompted us to analyze the clinicopathologic characteristics and outcome of 21 patients with hepatosplenic T-cell lymphoma displaying a γδ TCR phenotype as demonstrated on frozen material that we collected over a 20-year period. Our results confirm that HSγδTCL is a clinicopathologic entity of cytotoxic T-cell origin. We also show that bone marrow involvement is a constant finding with diagnostic value and that the disease, which may occur in patients with immunosuppression, has a poor prognosis.
Patients, materials, and methods
Patient selection
Patients with proven HSγδTCL diagnosed between 1981 and 2001 were selected from the files of the Department of Pathology (Hôpital Henri Mondor, Créteil, France). Selection criteria for this study were diagnosis of HSγδTCL established through histopathology and immunophenotyping of frozen or fresh material or both and availability of clinical information and follow-up data. Twenty-one patients fulfilled these criteria. For 13 patients, biopsy samples had been referred for consultation. Parts of immunomorphologic, genotypic, and cytogenetic features of some patients in this series have been reported previously.4,14,32,38,39
Clinical staging
The extent of disease was measured by physical examination, bone marrow biopsy or aspiration, cerebrospinal fluid examination, and computed tomography (CT) of the chest and abdomen. Staging was performed according to the Ann Arbor system. Performance status was based on the Eastern Cooperative Oncology Group scale (0 to 4). Serum lactate dehydrogenase (LDH) level was expressed as the ratio over the maximum normal value. Patients were retrospectively staged according to the age-adjusted international prognostic index.40
Histopathologic studies
Biopsy specimens from initial sites of involvement were reviewed, as were those from all initial bone marrow biopsies. Biopsy specimens at progression were also studied in 11 patients. Samples were fixed in buffered formalin or Bouin fluid. Paraffin-embedded tissue sections were stained with hematoxylin-eosin, periodic acid-Schiff (PAS), or Giemsa. In all but 1 patient, a portion of tumoral tissue obtained at diagnosis (spleen, 11 patients; liver, 9 patients; bone marrow, 8 patients) was snap-frozen in liquid nitrogen for phenotypic, genotypic, or cytogenetic studies. In 7 patients, including the patient without frozen tumoral tissue, fresh blood or bone marrow cells were also immunophenotyped by flow cytometry.
Immunohistochemical staining
Cryostat sections of spleen, liver, or bone marrow specimens were evaluated for T-, natural killer (NK)-, and B-cell differentiation antigens using the alkaline phosphatase/antialkaline phosphatase (APAAP) method.41 Mouse monoclonal antibodies were used to detect the following antigens: CD2, CD3, CD4, CD5, CD7, CD8 (Becton Dickinson, Mountain View, CA); CD30, LMP-1 (DAKO, Glostrup, Denmark); and CD19, CD56 (Coulter, Hialeah, FL). Expression of the TCR chains was analyzed using βF1, δTCR-1, δTCS-1, Vδ2, and Vδ3 monoclonal antibodies (T-cell Diagnosis, Woburn, MA). βF1 recognizes a nonpolymorphic epitope of the β chain of the αβ TCR heterodimer. δTCR-1 recognizes a nonpolymorphic epitope of the δ chain of the γδ TCR heterodimer. δTCS-1 is directed against a conformational epitope of the Vδ1/Jδ1 junction, whereas Vδ2 and Vδ3 antibodies recognize an epitope of the human Vδ2 and Vδ3 regions of the δ chain, respectively.
After appropriate antigen retrieval, deparaffinized tissue sections were also evaluated with a panel of antibodies including CD20, CD3ϵ (DAKO), CD5, and CD56 (Novocastra, Newcastle, United Kingdom) and for formalin-resistant epitopes of cytotoxic cell proteins such as T-cell intracellular antigen-1 (TIA-1; Coulter) and granzyme B (Monosan, Uden, The Netherlands). Expression of bcl-2 and p53 (DO7) (DAKO) proteins was also investigated in several patients.
In situ hybridization study
The presence of Epstein-Barr virus (EBV) RNA was analyzed by nonisotopic in situ hybridization (ISH) with EBV-encoded RNA (EBER) 1 and 2 oligonucleotide probes (DAKO) on deparaffinized tissue sections. Details of the procedure have been previously reported.42
Genomic study
DNA analysis was performed using Southern blot in the first 4 patients of our series, as previously reported.39 Subsequently, clonality was assessed by analyzing TCR-γ-chain gene rearrangements using a guanine-cytosine (GC) clamp multiplex polymerase chain reaction (PCR)/denaturing gradient gel electrophoresis (DGGE) procedure.43
Cytogenetic studies
In 9 patients, cytogenetic analysis of marrow, spleen, or blood cells was performed according to standard protocol. In addition, the status of chromosome 7 was investigated by interphase fluorescence in situ hybridization (FISH) in 1 of these 9 patients and on archival or fresh material of 4 other patients, as recently reported elsewhere.32
Results
Clinical findings at presentation
Patients had been referred because of splenomegaly and cytopenia. Presenting features also included B symptoms in 14 patients and abdominal pain in 5. Fifteen patients were male, and the median age was 34 years (range, 16 to 58 years). Median time from first discovery of these symptoms to diagnosis of HSγδTCL was 60 days (range, 15-180 days). All patients had splenomegaly, and 15 of them hepatomegaly. Physical examination findings were otherwise normal, showing no enlarged peripheral lymph nodes. CT disclosed no mediastinal or retroperitoneal lymphadenopathy.
As shown in Table 1, 20 patients had thrombocytopenia (platelet count range, 25-121 × 109/L), 15 had anemia (hemoglobin level range, 57-113 g/L [5.7-11.3 g/dL]), and 12 had leukopenia. Only 2 patients had elevated white blood cell counts with up to 5% detectable atypical lymphoid cells on blood smears. Retrospective examination of blood smears, however, revealed few atypical cells in 8 other patients, regardless of whether they had leukopenia. A low rate of circulating myeloid precursor cells was observed in 8 patients. Monocytosis greater than 1 × 109/L was found in 5 patients, 1 of whom was initially given a misdiagnosis of myelomonocytic leukemia. Liver test results were essentially normal, with slightly elevated levels of serum aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), or alkaline phosphatase activities in 8 patients. In 1 patient, a low level of fibrinogen associated with increased triglyceridemia and ferritinemia suggested hemophagocytic syndrome, which was confirmed by bone marrow examination. Serologic test results were negative for human T-lymphotrophic virus 1 (HTLV1), HIV, and hepatitis B virus (HBV) in all patients and for hepatitis C virus (HCV) in the 12 studied patients.
Presenting clinical and biologic features of patients with HSγδTCL
Patient . | Age, y/sex . | PS . | LDH . | Hemoglobin, g/L . | WBC count, × 109/L . | PBALC count, × 109/L . | Monocyte count greater than 1 × 109/L . | Circulating myeloid precursor cells . | Platelet count, × 109/L . | Previous history . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30/M | 2 | ND | 150 | 2.8 | 0 | — | — | 72 | Malaria |
| 2 | 32/M | 2 | n | 126 | 6.8 | 0 | Yes | — | 63 | — |
| 3 | 23/M | 2 | n | 127 | 5.4 | 0 | — | — | 30 | — |
| 4 | 39/F | 0 | n | 113 | 2.3 | 0.1 | — | — | 120 | — |
| 5 | 58/F | 1 | n | 102 | 3.6 | 0 | — | Yes | 78 | — |
| 6 | 19/M | 2 | 4n | 144 | 25.7 | 1.5 | Yes | Yes | 80 | — |
| 7 | 21/M | 2 | n | 112 | 8.5 | 0.3 | Yes | Yes | 46 | Malaria |
| 8 | 16/M | 1 | 2n | 84 | 3.2 | 0.2 | — | Yes | 68 | — |
| 9 | 43/M | 2 | 8n | 57 | 6.4 | 0.3 | — | Yes | 88 | Kidney transplantation |
| 10 | 34/M | 1 | 2n | 81 | 6.8 | 0 | — | — | 76 | Hodgkin disease |
| 11 | 48/M | 1 | n | 89 | 0.9 | 0.1 | — | Yes | 166 | Kidney transplantation |
| 12 | 16/F | 0 | 2n | 92 | 1.8 | 0 | — | — | 55 | Lupus erythematosus |
| 13 | 28/M | 1 | n | 74 | 1.2 | 0 | — | — | 121 | — |
| 14 | 41/M | 3 | 16n | 130 | 14.3 | 0.7 | — | Yes | 90 | — |
| 15 | 34/F | 1 | n | 124 | 7.9 | 0 | Yes | — | 84 | — |
| 16* | 44/M | 1 | n | 94 | 7.0 | 0 | Yes | — | 25 | — |
| 17 | 54/F | 1 | 2n | 97 | 2.7 | 0 | — | — | 116 | — |
| 18 | 27/M | 1 | 2n | 106 | 1.4 | 0.1 | — | — | 109 | Kidney transplantation |
| 19 | 50/M | 1 | 2n | 105 | 3.9 | 0 | — | — | 100 | — |
| 20 | 50/F | 1 | 2n | 98 | 0.9 | 0.3 | — | Yes | 90 | — |
| 21 | 36/M | 1 | 2n | 111 | 1.2 | 0.1 | — | — | 66 | Kidney transplantation |
Patient . | Age, y/sex . | PS . | LDH . | Hemoglobin, g/L . | WBC count, × 109/L . | PBALC count, × 109/L . | Monocyte count greater than 1 × 109/L . | Circulating myeloid precursor cells . | Platelet count, × 109/L . | Previous history . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30/M | 2 | ND | 150 | 2.8 | 0 | — | — | 72 | Malaria |
| 2 | 32/M | 2 | n | 126 | 6.8 | 0 | Yes | — | 63 | — |
| 3 | 23/M | 2 | n | 127 | 5.4 | 0 | — | — | 30 | — |
| 4 | 39/F | 0 | n | 113 | 2.3 | 0.1 | — | — | 120 | — |
| 5 | 58/F | 1 | n | 102 | 3.6 | 0 | — | Yes | 78 | — |
| 6 | 19/M | 2 | 4n | 144 | 25.7 | 1.5 | Yes | Yes | 80 | — |
| 7 | 21/M | 2 | n | 112 | 8.5 | 0.3 | Yes | Yes | 46 | Malaria |
| 8 | 16/M | 1 | 2n | 84 | 3.2 | 0.2 | — | Yes | 68 | — |
| 9 | 43/M | 2 | 8n | 57 | 6.4 | 0.3 | — | Yes | 88 | Kidney transplantation |
| 10 | 34/M | 1 | 2n | 81 | 6.8 | 0 | — | — | 76 | Hodgkin disease |
| 11 | 48/M | 1 | n | 89 | 0.9 | 0.1 | — | Yes | 166 | Kidney transplantation |
| 12 | 16/F | 0 | 2n | 92 | 1.8 | 0 | — | — | 55 | Lupus erythematosus |
| 13 | 28/M | 1 | n | 74 | 1.2 | 0 | — | — | 121 | — |
| 14 | 41/M | 3 | 16n | 130 | 14.3 | 0.7 | — | Yes | 90 | — |
| 15 | 34/F | 1 | n | 124 | 7.9 | 0 | Yes | — | 84 | — |
| 16* | 44/M | 1 | n | 94 | 7.0 | 0 | Yes | — | 25 | — |
| 17 | 54/F | 1 | 2n | 97 | 2.7 | 0 | — | — | 116 | — |
| 18 | 27/M | 1 | 2n | 106 | 1.4 | 0.1 | — | — | 109 | Kidney transplantation |
| 19 | 50/M | 1 | 2n | 105 | 3.9 | 0 | — | — | 100 | — |
| 20 | 50/F | 1 | 2n | 98 | 0.9 | 0.3 | — | Yes | 90 | — |
| 21 | 36/M | 1 | 2n | 111 | 1.2 | 0.1 | — | — | 66 | Kidney transplantation |
PS indicates performance status based on the Eastern Cooperative Oncology Group scale; WBC, white blood cell; PBALC, peripheral blood atypical lymphoid cell; n, normal value; ND, not done; and —, no.
Biologic features of hemophagocytic syndrome.
All patients had initial bone marrow involvement by lymphoma cells, as revealed by trephine biopsy in 19 patients and marrow aspirates in the 2 remaining patients (see “Pathologic findings at presentation”). On this basis, all patients had stage IV disease. Given the presence of an abnormal LDH level and of a performance status greater than 1 (Table 1), 15 of the 21 patients had 2 to 3 risk factors according to the age-adjusted international prognostic index40 and belonged to the high-risk group.
Several patients had significant medical histories (Table 1). Four patients received long-term immunosuppressive therapy—that is, steroid treatment with cyclosporin or azathioprine—for kidney transplantation performed 4, 5, 15, and 27 years before the diagnosis of HSγδTCL. A 16-year-old girl had been monitored for 5 years for systemic lupus erythematosus when lymphoma was diagnosed. Two African patients had falciparum malaria 4 and 15 years earlier. Finally, HSγδTCL rapidly developed in a 34-year-old patient with recently diagnosed stage III EBV-positive Hodgkin disease after he had completed a third course of mechlorethamine, Oncovin, procarbazine, prednisone/Adriamycin, bleomycin, vinblastine (MOPP/ABV) chemotherapy.
Pathologic findings at presentation
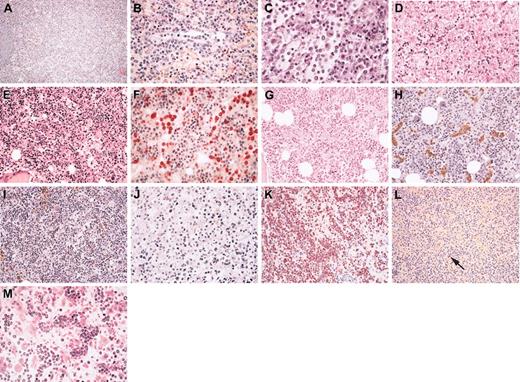
Splenectomy was performed in 12 patients for the purposes of diagnosis 8 of them or as part of the initial therapy in 4. In each patient, the spleen was enlarged (weight range, 780-6000 g) and was free of nodules on the cut surface. Histopathologic findings were similar in each patient. The general architecture was preserved, but there was marked hyperplasia of the red pulp and atrophy of the white pulp (Figure 1A). To a variable extent in each patient, atypical lymphoid cells infiltrated the cords and sinuses of the red pulp. In most patients, neoplastic cells tended to form clusters within the sinuses (Figure 1B). A number of histiocytes were also admixed within lymphoid infiltrates, disclosing features of hemophagocytosis in 3 patients (Figure 1C). In 4 patients, infracentrimetric hilar lymph nodes could be analyzed and, although their architecture was preserved, showed a mild sinusal and perisinusal infiltration by neoplastic cells, the detection of which was highlighted by immunohistochemistry.
Histopathology of the spleen, liver, and bone marrow in hepatosplenic γδ T-cell lymphoma. In the spleen, (A) the general architecture is preserved with marked hyperplasia of the red pulp; (B) at high magnification, medium-sized neoplastic cells are located within the cords and sinuses of the red pulp (patient 15), and (C) histiocytes are admixed (patient 12), disclosing some features of hemophagocytosis. In the liver (D), neoplastic lymphoid cells infiltrate variably dilated sinusoids (patient 1). In bone marrow biopsy (E-I), the marrow is usually hypercellular (E) and exhibits an elective sinusal infiltrate composed of medium-sized atypical lymphocytes (E-F), which is more obvious in panel E (patient 7) than in panel G (patient 17). In the latter, the mild infiltrate is strongly highlighted by immunostaining showing the CD3+CD5- phenotype of the neoplastic cells (H-I). In rare instances, atypical cytology was observed at initial examination (J), with presence of pleomorphic medium and large cells within the hepatic sinusoids (patient 14). Neoplastic cells display a nonactivated cytotoxic profile with strong granular cytoplasmic staining for TIA1 (K) but absence of granzyme B expression (L), as shown in the spleen (patient 2) (the arrow indicates the rare Granzyme-B-positive lymphocytes that act as internal positive control). Cytologic features of progression with blastic appearance were observed in the heavy infiltrated bone marrow of patient 1, at relapse (M); compare the cytology with that observed in the liver at initial examination, as seen in panel D.
Histopathology of the spleen, liver, and bone marrow in hepatosplenic γδ T-cell lymphoma. In the spleen, (A) the general architecture is preserved with marked hyperplasia of the red pulp; (B) at high magnification, medium-sized neoplastic cells are located within the cords and sinuses of the red pulp (patient 15), and (C) histiocytes are admixed (patient 12), disclosing some features of hemophagocytosis. In the liver (D), neoplastic lymphoid cells infiltrate variably dilated sinusoids (patient 1). In bone marrow biopsy (E-I), the marrow is usually hypercellular (E) and exhibits an elective sinusal infiltrate composed of medium-sized atypical lymphocytes (E-F), which is more obvious in panel E (patient 7) than in panel G (patient 17). In the latter, the mild infiltrate is strongly highlighted by immunostaining showing the CD3+CD5- phenotype of the neoplastic cells (H-I). In rare instances, atypical cytology was observed at initial examination (J), with presence of pleomorphic medium and large cells within the hepatic sinusoids (patient 14). Neoplastic cells display a nonactivated cytotoxic profile with strong granular cytoplasmic staining for TIA1 (K) but absence of granzyme B expression (L), as shown in the spleen (patient 2) (the arrow indicates the rare Granzyme-B-positive lymphocytes that act as internal positive control). Cytologic features of progression with blastic appearance were observed in the heavy infiltrated bone marrow of patient 1, at relapse (M); compare the cytology with that observed in the liver at initial examination, as seen in panel D.
Liver biopsy was performed in 15 patients, either during splenectomy or as part of the initial clinical staging in 3 patients. All patients had tumoral lymphoid infiltration in the sinusoids, which were variably dilated, whereas involvement of the portal tracts was mild or absent (Figure 1D).
Initial bone marrow biopsy performed in 19 patients disclosed hypercellular marrow that had been infiltrated by atypical lymphoid cells (Figure 1E-I). However, infiltration was often subtle (Figure 1G) and could be better demonstrated by careful histologic review together with CD3 immunostaining (Figure 1F-H). This subtle involvement was not immediately recognized in 6 patients, leading to misdiagnoses of reactive hypercellular marrow in 5 patients and of chronic myelomonocytic leukemia in another patient with overt monocytosis at initial examination. The pattern of lymphoid infiltration was peculiar because, in all patients except 1, it appeared to be located electively within variably dilated sinuses, resulting in Indian files or clusters of neoplastic cells. In only 1 patient (patient 21) was the mild sinusal involvement associated with a more diffuse pattern of infiltration. In 2 patients, significant numbers of histiocytes with features of hemophagocytosis were observed, paralleling the lesions observed in their spleens. In the 2 patients without initial bone marrow biopsy, involvement was demonstrated by the presence of 10% (patient 18) to 40% (patient 14) atypical lymphoid cells on marrow aspirate smears.
Overall, the cytologic aspect of lymphoma cells at presentation showed little variation from patient to patient. Indeed, regardless of the sites of involvement, neoplastic cells in all but 2 patients were monomorphic and had round or slightly irregular small to medium nuclei containing conspicuous nucleoli and moderately abundant cytoplasm with rare mitotic figures. Cell pleomorphism with the presence of medium to large cells (Figure 1J) was detected in only 2 patients (patients 12 and 14) at initial examination. In many patients, a proportion of neoplastic cells in the bone marrow or in the spleen had elongated shapes with dendritic projections, likely resulting from close contact with endothelial cells and subendothelial matrix. On bone marrow and blood smears, abnormal lymphoid cells were usually agranular except in 1 patient, disclosing cytoplasmic azurophilic granules in a few cells.
Finally, based on morphologic findings and phenotypic results described in “Immunophenotypic findings,” diagnoses were established on the basis of spleen specimens in 8 patients, bone marrow biopsy in 9 patients, and liver biopsy in 3 patients. In the remaining patient, diagnosis was based on morphologic and flow cytometric studies of the bone marrow aspirate. Median time from first discovery of cytopenia/splenomegaly to diagnosis of HSγδTCL was 60 days (range, 15-180 days).
Immunophenotypic findings
By definition, patients in the present study expressed γδ TCR (δTCR1+) and were negative for αβ TCR (βF1-), as demonstrated on frozen tissue material. As shown in Table 2, the neoplastic cells in all patients expressed the T-cell-associated markers CD2 and CD3 but were CD5-, whereas CD7 was detected in 9 of 19 patients. Sixteen patients were CD4-/CD8-, 2 patients expressed CD8, and the 3 remaining CD8- patients were not interpretable for CD4. CD56 NK-cell antigen was detected in 15 of 18 studied patients whereas CD16 was found in only 2 of the 7 patients studied by flow cytometry. Among the 12 patients who could be investigated using the 3 antibodies reacting with variable epitopes of the δ chain, 9 were shown to derive from the Vδ1 subset (δTCS1+), 2 expressed the Vδ2-encoded epitope (Vδ2+), and the remaining patient was negative. CD30 was negative in all patients, as were CD19 and CD20 B-cell-associated antigens.
Immunohistochemical and genomic data and EBV status of patients with HSγδTCL
Patient . | CD2 . | CD3 . | CD5 . | CD7 . | CD4 . | CD8 . | βF1 . | δTCR1 . | Vδ1 . | Vδ2 . | Vδ3 . | CD56 . | TiA1 . | Gr B . | EBV status* . | TCR-gene status . | Iso7q . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | NI | − | − | + | − | − | ND | + | + | − | − | ND | ND |
| 2 | + | + | − | − | − | − | − | + | + | − | − | − | + | − | − | R(Jδ1) G(Cβ) | −(CCs)† |
| 3 | + | + | − | − | − | − | − | + | + | − | − | − | + | − | − | R(Jδ1) R(Cβ) | ND |
| 4 | + | + | − | − | − | − | − | + | − | − | ND | + | + | − | − | R(Jδ1) G(Cβ) | +(FISH) |
| 5 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | − | R(Jδ1) G(Cβ) | +(FISH) |
| 6 | + | + | − | NI | NI | + | − | + | ND | ND | ND | NI | + | − | − | R(Jγ) | ND |
| 7 | + | + | NI | + | NI | − | − | + | ND | ND | ND | − | + | − | − | R(Jγ) | +(CCs) |
| 8 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | ND | +(CCs) |
| 9 | + | + | − | ND | − | + | − | + | ND | ND | ND | + | ND | ND | − | R(Jγ) | +(CCs) |
| 10 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | R(Jγ) | +(CCs) |
| 11 | + | + | − | + | − | − | − | + | ND | ND | ND | + | + | − | − | R(Jγ) | −(CCs) |
| 12 | + | + | − | + | − | − | − | + | − | + | − | + | + | − | − | R(Jγ) | ND |
| 13 | + | + | − | − | − | − | − | + | − | ND | ND | + | + | − | + | R(Jγ) | +(FISH) |
| 14 | + | + | − | + | − | − | − | + | − | + | − | + | + | + | − | ND | ND |
| 15 | + | + | − | − | − | − | − | + | ND | ND | ND | NI | + | NI | + | ND | ND |
| 16 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | ND | −(CCs) |
| 17 | + | + | − | − | NI | − | − | + | − | ND | ND | NI | + | − | − | R(Jγ) | +(FISH)‡ |
| 18 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | ND | R(Jγ) | +(FISH) |
| 19 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | GL(Jγ) | ND |
| 20 | + | + | − | + | − | − | − | + | − | − | − | + | + | − | − | ND | ND |
| 21 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | − | R(Jγ) | −(CCs) |
Patient . | CD2 . | CD3 . | CD5 . | CD7 . | CD4 . | CD8 . | βF1 . | δTCR1 . | Vδ1 . | Vδ2 . | Vδ3 . | CD56 . | TiA1 . | Gr B . | EBV status* . | TCR-gene status . | Iso7q . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | NI | − | − | + | − | − | ND | + | + | − | − | ND | ND |
| 2 | + | + | − | − | − | − | − | + | + | − | − | − | + | − | − | R(Jδ1) G(Cβ) | −(CCs)† |
| 3 | + | + | − | − | − | − | − | + | + | − | − | − | + | − | − | R(Jδ1) R(Cβ) | ND |
| 4 | + | + | − | − | − | − | − | + | − | − | ND | + | + | − | − | R(Jδ1) G(Cβ) | +(FISH) |
| 5 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | − | R(Jδ1) G(Cβ) | +(FISH) |
| 6 | + | + | − | NI | NI | + | − | + | ND | ND | ND | NI | + | − | − | R(Jγ) | ND |
| 7 | + | + | NI | + | NI | − | − | + | ND | ND | ND | − | + | − | − | R(Jγ) | +(CCs) |
| 8 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | ND | +(CCs) |
| 9 | + | + | − | ND | − | + | − | + | ND | ND | ND | + | ND | ND | − | R(Jγ) | +(CCs) |
| 10 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | R(Jγ) | +(CCs) |
| 11 | + | + | − | + | − | − | − | + | ND | ND | ND | + | + | − | − | R(Jγ) | −(CCs) |
| 12 | + | + | − | + | − | − | − | + | − | + | − | + | + | − | − | R(Jγ) | ND |
| 13 | + | + | − | − | − | − | − | + | − | ND | ND | + | + | − | + | R(Jγ) | +(FISH) |
| 14 | + | + | − | + | − | − | − | + | − | + | − | + | + | + | − | ND | ND |
| 15 | + | + | − | − | − | − | − | + | ND | ND | ND | NI | + | NI | + | ND | ND |
| 16 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | ND | −(CCs) |
| 17 | + | + | − | − | NI | − | − | + | − | ND | ND | NI | + | − | − | R(Jγ) | +(FISH)‡ |
| 18 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | ND | R(Jγ) | +(FISH) |
| 19 | + | + | − | − | − | − | − | + | + | − | − | + | + | − | − | GL(Jγ) | ND |
| 20 | + | + | − | + | − | − | − | + | − | − | − | + | + | − | − | ND | ND |
| 21 | + | + | − | + | − | − | − | + | + | − | − | + | + | − | − | R(Jγ) | −(CCs) |
+ indicates neoplastic cells positive; −, neoplastic cells negative; ND, not done; NI, not informative; CCs, conventional cytogenetic study; R, rearranged; and GL, germline configuration. Gr B refers to granzyme B.
EBV status determined by in situ hybridization with EBER probes.
Conventional cytogenetic study showed a monosomy of chromosomes 6 and 10.
Conventional cytogenetic study gave a normal result.
In addition to studies on frozen sections, immunohistochemistry on routinely fixed paraffin-embedded tissue sections clearly demonstrated the common CD3+/CD5-/CD56+ phenotype of the lymphoma cells and highlighted their sinusal distribution. On routinely fixed material, all investigated patients but 1 (8 of 9) were shown to be negative for p53 (less than 5% tumor cells positive) whereas bcl-2 protein was negative or weakly expressed. All 20 patients investigated for cytotoxic molecules demonstrated strong granular cytoplasmic staining for the granule-associated protein TIA-1. By contrast, neoplastic cells were found positive for granzyme B in only 1 patient (Figure 1K-L).
EBV status
In situ hybridization studies with EBER probes demonstrated the absence of EBV genome in neoplastic cells, except for the 2 described patients with pleomorphic cytologic features. Expression of the EBV-encoded latent membrane protein-1 (LMP-1), as studied by immunohistochemistry, was negative in all patients (Table 2).
Genomic study
The results of DNA genotyping performed in the 4 first patients of this series have been previously reported.3,39 These 4 patients showed a biallelic rearrangement of the δ gene that could be ascribed to Vδ1Jδ1 joining regions in 3 of them. One of these patients had an unproductive TCR β gene rearrangement. A clonal rearrangement of TCR γ gene was demonstrated by PCR analysis in 10 of the 11 subsequently studied patients (Table 2).
Cytogenetic studies
As shown in Table 2, conventional cytogenetic analysis was performed in 9 patients. Karyotype was normal in 4 patients and showed monosomy of chromosomes 6 and 10 in 1 patient, whereas isochromosome arm 7q was found in 4 patients that was associated with trisomy 8 in 3 of them. In addition, the 5 patients studied using FISH showed isochromosome arm 7q.
Treatment and outcome
Table 3 summarizes the results of therapy. Patients were given multiagent chemotherapy by cyclophosphamide, hydroxydaunomycin/doxorubicin, Oncovin, and prednisone (CHOP), CHOP-derived (19 patients), or platinum-cytarabine-based (2 patients) regimens as first-line treatment. Three patients died within 2 months, before completing induction treatment, because of disease progression. Four other patients failed to respond to induction and, despite salvage therapy including high-dose therapy (HDT) with autologous transplantation in 1, died within 6 to 16 months of diagnosis.
First-line treatments and outcome
| . | Induction phase . | . | Consolidation phase . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Regimen . | Response . | Regimen . | Response . | Relapse, mo . | Survival, mo . | Status . | ||
| 1spl | CHOP | CR | Chemotherapy | CR | 16 | 37 | DOD | ||
| 2spl | CHOP-like | CR | Chemotherapy | CR | 16 | 44 | DOD | ||
| 3spl | CHOP-like | CR | Auto BMT | CR | 8 | 24 | DOD | ||
| 4spl | CHOP-like | CR | Auto BMT | CR | 15 | 33 | DOD | ||
| 5spl | CHOP-like | CR | Chemotherapy | CR | 13 | 36 | DOD | ||
| 6spl | CHOP-like | CR | Allo BMT | CR | 15 | 25 | DOD | ||
| 7 | CHOP-like | PR | Auto PBSC | CR | 4† | 19 | DOD | ||
| 8 | CHOP-like | PR | Auto PBSC | Failure | — | 13 | DOD | ||
| 9 | CHOP-like | CR | Chemotherapy | CR | 3 | 10 | DOD | ||
| 10spl | CHOP-like | Failure | — | — | — | 1 | DOD | ||
| 11 | CHOP | Failure | — | — | — | 6 | DOD | ||
| 12spl | CHOP-like | Failure* | — | — | — | 11 | DOD | ||
| 13spl | CHOP-like | CR | Allo BMT | NE | — | 6 | TRD | ||
| 14spl | CHOP | Failure | — | — | — | 1 | DOD | ||
| 15spl | CHOP-like | CR | Chemotherapy | CR | 3* | 33 | DOD | ||
| 16 | CHOP-like | PR | Allo BMT | NE | — | 9 | TRD | ||
| 17 | Platinum-Ara-C based | PR | Auto PBSC | CR | — | 52 | Alive | ||
| 18 | CHOP | Failure | — | — | — | 2 | DOD | ||
| 19spl | Platinum-Ara-C based | PR | Auto PBSC | CR | — | 42 | Alive | ||
| 20 | CHOP-like | Failure | — | — | — | 16 | DOD | ||
| 21 | CHOP-like | Failure | — | — | — | 7 | DOD | ||
| . | Induction phase . | . | Consolidation phase . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Patient . | Regimen . | Response . | Regimen . | Response . | Relapse, mo . | Survival, mo . | Status . | ||
| 1spl | CHOP | CR | Chemotherapy | CR | 16 | 37 | DOD | ||
| 2spl | CHOP-like | CR | Chemotherapy | CR | 16 | 44 | DOD | ||
| 3spl | CHOP-like | CR | Auto BMT | CR | 8 | 24 | DOD | ||
| 4spl | CHOP-like | CR | Auto BMT | CR | 15 | 33 | DOD | ||
| 5spl | CHOP-like | CR | Chemotherapy | CR | 13 | 36 | DOD | ||
| 6spl | CHOP-like | CR | Allo BMT | CR | 15 | 25 | DOD | ||
| 7 | CHOP-like | PR | Auto PBSC | CR | 4† | 19 | DOD | ||
| 8 | CHOP-like | PR | Auto PBSC | Failure | — | 13 | DOD | ||
| 9 | CHOP-like | CR | Chemotherapy | CR | 3 | 10 | DOD | ||
| 10spl | CHOP-like | Failure | — | — | — | 1 | DOD | ||
| 11 | CHOP | Failure | — | — | — | 6 | DOD | ||
| 12spl | CHOP-like | Failure* | — | — | — | 11 | DOD | ||
| 13spl | CHOP-like | CR | Allo BMT | NE | — | 6 | TRD | ||
| 14spl | CHOP | Failure | — | — | — | 1 | DOD | ||
| 15spl | CHOP-like | CR | Chemotherapy | CR | 3* | 33 | DOD | ||
| 16 | CHOP-like | PR | Allo BMT | NE | — | 9 | TRD | ||
| 17 | Platinum-Ara-C based | PR | Auto PBSC | CR | — | 52 | Alive | ||
| 18 | CHOP | Failure | — | — | — | 2 | DOD | ||
| 19spl | Platinum-Ara-C based | PR | Auto PBSC | CR | — | 42 | Alive | ||
| 20 | CHOP-like | Failure | — | — | — | 16 | DOD | ||
| 21 | CHOP-like | Failure | — | — | — | 7 | DOD | ||
Relapse values represent time from end of first-line treatment. Survival values represent time from diagnosis. CR indicates complete response; DOD, died of disease; BMT, bone marrow transplantation; PR, good partial response; PBSC, peripheral blood stem cell; —, not applicable; NE, nonevaluable because of cerebral toxoplasmosis; TRD, transplantation-related death; and spl, splenectomy. *Salvage treatment by autotransplantation. †Salvage treatment by allotransplantation.
Overall, 14 (67%) patients responded to induction treatment, 9 with complete response and 5 with good partial response. Five of them, in complete response after finishing consolidative sequential chemotherapy, had relapses at various times and died within 10 to 44 months, despite HDT with autotransplantation in 1 patient. In accordance with the policy of some institutions, 6 other patients who responded to induction treatment received up-front consolidative HDT followed by autologous bone marrow (2 patients) or peripheral blood stem cell (4 patients) transplantation. Two remain in first complete remission at 42 and 52 months from diagnosis, whereas 4 experienced disease progression and died within 13 to 33 months of diagnosis, despite salvage allogeneic transplantation in 1 patient. Three other responding patients who underwent up-front consolidative allogeneic bone marrow transplantation died, 2 from early cerebral toxoplasmosis and 1 from disease progression. Finally, the median survival time of this series is 16 months, and only 2 patients are alive at the time of analysis.
Pathologic findings at progression
In all patients, progression remained in the initial sites, resulting in splenomegaly, hepatomegaly, bone marrow involvement, or blood involvement. Of note, recurrent and severe thrombocytopenia was observed in all patients with progressive disease. Before death, 1 patient appeared to have leukemia because of the high number of atypical lymphocytes (96 × 109/L) with blastic features. Additional sites of involvement (skin, oral mucosa, or kidney) were observed in only 3 patients. Among 11 patients for whom material could be histologically reviewed, 8 had cytologic features of progression in bone marrow, liver, or spleen with either blastic (2 patients) (Figure 1M) or pleomorphic large cell (6 patients) appearance. Paralleling these cytologic changes, lymphoma cells in the bone marrow tended to extend outside sinuses, blending hematopoietic cells. In the 7 studied patients, neoplastic cells retained their original CD3+, TIA1+, granzyme B- phenotype, as demonstrated on paraffin-embedded tissue sections. In 2 of the 4 patients with available frozen material, however, lymphoma cells had lost detectable TCR γδ expression.
Discussion
We report on the clinical, morphologic, phenotypic, and genetic characteristics of 21 patients with HSγδTCL, an entity we initially described in 1990 in 2 patients.3 Subsequently, a number of reports have been published, mainly as single cases or short series.4-25 In the present study, we show that this lymphoma has a uniform clinicopathologic presentation, may occur in immunocompromised patients, and is characterized by poor outcome. In accordance with Cooke et al,11 we also show a phenotype consistent with its derivation from nonactivated cytotoxic γδ T cells. Our findings further confirm that, among peripheral T-cell lymphomas, HSγδTCL is a rare and distinct clinicopathologic entity that can be recognized by its characteristic sinusal pattern of infiltration on routine bone marrow biopsy.
In our series, the disease confirmed a predilection to develop most often in young men and usually manifested in all patients with splenomegaly without lymphadenopathy and with hepatomegaly. Irrespective of the latter or of abnormal function test results, liver involvement by lymphoma was documented in all biopsies performed at initial examination. Notably, during disease progression, HSγδTCL remained preferentially localized within the spleen, liver, and bone marrow, without lymph node enlargement. Other sites of involvement were observed at progression in the skin, oral mucosa, and kidney in only 3 of our patients.
Variable degrees of hematologic abnormalities were observed in all patients. As in previous reports, thrombocytopenia was the most striking finding in all but 1 of our patients, and it was associated with anemia and leukopenia in more than half of the patients. The mechanism of pancytopenia remains unclear. It could result at least in part from the presence of marked splenomegaly, but, as suggested by others,44,45 cytokine secretion by neoplastic γδ T cells, such as interferon-γ, might also contribute to the suppression of hematopoiesis. The observation that recurrent thrombocytopenia paralleled disease progression, even in patients who underwent splenectomy, favors the second hypothesis. Circulating myeloid precursor cells, monocytosis, or both were observed at initial examination in several of our patients, and to our knowledge this has not been reported before. Such abnormalities are most likely unrelated to the degree of bone marrow infiltration, which was mild in all our patients, but may reflect some regulatory functions of γδ T cells known to promote macrophage activation.46 The latter might also explain our observation in spleen and bone marrow of numerous histiocytes admixed within the neoplastic γδ T cells, resulting in features of hemophagocytosis in occasional patients, as also reported by others.17 For clinical purposes, the presence of circulating myeloid precursor cells, monocytosis, or both in patients with splenomegaly may be confusing. This was illustrated in 1 of our patients initially given a misdiagnosis of chronic myelomonocytic disorder. In agreement with our findings, abnormal lymphocytosis at initial examination has been infrequently reported.11,47 However in 8 patients in our series, careful examination of peripheral blood smears revealed a minor population of abnormal lymphoid cells identical to those seen in the bone marrow, in accordance with a recent report.47 A frank leukemic picture, a rarely reported event,24 was observed in only 1 patient with progressive disease in the present series.
According to the literature, bone marrow involvement in HSγδTCL is found in approximately two thirds of patients at diagnosis.37 We show here that it can be regarded as a constant feature at presentation and that careful histologic and immunohistologic examination of a trephine biopsy specimen appears to be the adequate and easily feasible procedure enabling the recognition of lymphoma infiltration. The discrepancy between our findings and those reported in the literature is likely the result of the peculiar sinusal distribution of tumor cells that, at initial examination, is often subtle and therefore difficult to recognize without immunohistochemistry. This infiltration was initially not diagnosed in 6 patients of the present series but could be easily documented by histologic review together with appropriate immunostaining. Thus, in our initial experience, as in several previous reports reviewed by Weidmann,37 it appears that the underestimation of bone marrow involvement has led to splenectomy for diagnostic purposes. Improvement in recognizing discrete marrow involvement using immunophenotypic studies48 has resulted in a change in our diagnostic strategy in HSγδTCL, as shown in the present series in which no splenectomies were performed for diagnosis after 1995.
We also show that immunohistochemistry in routinely fixed bone marrow biopsy specimens is a satisfactory approach for the identification of the neoplastic lymphocytes, by demonstrating clusters of CD3+CD5- lymphocytes, usually with a CD8-, TIA1+/granzyme B- nonactivated cytotoxic phenotype and highlighting their sinusal distribution. The latter phenotype, however, also characterizes the recently reported cases of HSTCL, which share the same clinicopathologic features but express αβ TCR33-35 and are considered a variant of the disease, now referred as hepatosplenic T-cell lymphoma in the current WHO classification.36 Therefore, immunophenotyping on frozen tissue biopsy samples is required to determine the expression of γδ TCR, as shown in the present study. Flow cytometric study on cell suspensions might be an alternative when frozen tissue samples are unavailable, although it may be hampered by the low percentage of neoplastic γδ cells in marrow aspirates.11,47
Although 16 cases of HSTCL expressing αβ chains have been described in the past few years,33-35 the relative frequency and the prognostic relevance of the γδ versus the αβ phenotype in HSTCL remain unknown. In our institution, HSTCL with the αβ phenotype was observed in only 3 patients during this study period and thus likely represents a rare condition. It should be noted, however, that a recent report indicated that the γδ phenotype is an adverse prognostic factor among cutaneous T-cell lymphomas.49
In view of their common nonactivated cytotoxic profile and their similar tissue distribution, it is tempting to speculate that the αβ and γδ variants of HSTCL could represent proliferation of NK/T cells, which participate with NK cells in the innate immune system.50 Indeed, normal NK/T cells comprise subsets of γδ and αβ T cells with similar cytolytic properties involving cell-surface receptor molecules, referred to as killer immunoglobulin-like receptors (KIRs), able to recognize major histocompatibility complex (MHC) class I determinants on target cells.51 It would be of interest to further investigate whether αβ and γδ variants of HSTL express KIR molecules, as recently reported in 1 patient with HSγδTCL.52
Normal human γδ T cells comprise 2 major subsets according to the use of Vδ1 or Vδ2 chains, which have distinct patterns of tissue distribution and bear different functions.53 In the present study, we extended our preliminary results39 showing that 9 of 12 investigated cases of HSγδTCL use the Vδ1 gene, a finding in agreement with a recent report.54 Interestingly, it has been established that normal γδ T cells that reside in spleen predominantly express the Vδ1 gene.53 The observed Vδ1 usage in HSγδTCL contrasts with the predominant expression of the Vδ2 gene by cells of nonhepatosplenic γδ TCL, which develop in skin and mucosal tissue.55
Eight of our patients had remarkable medical histories. Four had received long-term immunosuppressive therapy for kidney transplantation, an observation in accordance with previous reports of HSγδTCL as a late-onset posttransplantation lymphoproliferative disorder7,14,15,19,22,24,56,57 of host origin.20 Two other patients had falciparum malaria, 1 was monitored for systemic lupus erythematosus, and 1 was found to have HSγδTCL during therapy delivered for EBV-positive Hodgkin disease. Interestingly, all these conditions have been associated with the expansion of γδ T cells, presumably as a result of chronic antigenic stimulation.58 Increase levels of γδ T cells have been observed based on peripheral blood59 and kidney biopsies60 in recipients of renal allografts, possibly as a result of an alloreactive response to MHC class II antigens,61 cytomegalovirus infection, or both.62 In addition, a potential role for γδ T cells in autoimmune disorders has been proposed in view of the accumulation of these cells in rheumatoid arthritis, celiac sprue, polymyositis, multiple sclerosis, and systemic lupus erythematosus.63-68 The depletion of γδ T cells has proved to dramatically reduce the severity of rheumatoid arthritis in a mouse model.58 Furthermore, Plasmodium falciparum exoantigens have been shown to stimulate the proliferation of γδ T-lymphocytes in patients after primary infection,69 and accumulations of these cells have been observed in the spleens and peripheral blood of patients with P falciparum malaria.2,70 Finally, γδ T cells, which are known to participate in the immune response to several viruses,71 can respond in vitro to EBV-infected human Burkitt cell lines.72 Conversely, the CD30 antigen expressed by Reed-Sternberg cells can trigger in vitro the proliferation of a γδ T-cell clone.73
It has been shown that the Vδ1 T-cell subset is increased in organ allograft recipients74 and is induced in vitro by EBV-infected Burkitt cell lines,72 whereas expansion of the Vδ2 subset has been observed in autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus.75,76 Interestingly, in accordance with a previous report,20 the γδ neoplastic cells in the 2 investigated kidney recipients of the present series had a Vδ1 phenotype, as did the malignant cells in the patient with EBV-positive Hodgkin lymphoma. Similarly, the Vδ2 subset was used by cells of our HSγδTCL patient after systemic lupus erythematosus. Therefore, in the context of antigen-driven stimulation of reactive γδ T cells, it is tempting to speculate that neoplastic transformation could result from a multistep process involving impairment of the immune system (as in the patients receiving immunosuppressive therapy) or additional genetic alterations such as isochromosome arm 7q. The latter aberration was present in 9 of 13 documented cases, further confirming the association between HSγδTCL and isochromosome arm 7q, which was reported as a hallmark not only of HSγδTCL12,14,25,28-32 but also of its unusual αβ immunophenotypic variant.33,35 Although the mechanism by which it might contribute to the pathogenesis of HSTCL is unknown, its previously reported accumulation in forms with features of cytologic progression suggests that it benefits the outgrowth of malignant clones.32 In the present series, however, 5 patients were thought to be negative for the presence of isochromosome arm 7q using conventional cytogenetics. Because this was studied on bone marrow samples with subtle involvement, it cannot be excluded that the karyotype reflects the normal rather than the neoplastic cell population, in agreement with our finding that in 1 patient isochromosome arm 7q was detected using FISH, whereas the karyotype appeared normal on conventional cytogenetic study. Alternatively, isochromosome 7q-negative HSTCL has been occasionally detected using FISH, which may indicate that different molecular genetic pathways are involved in the pathogenesis of this lymphoma.32
Despite a satisfactory response to induction treatment in two thirds of our patients, the long-term therapeutic results are poor. Relapses occurred early, median survival was 16 months, and only 2 patients survived at the time of analysis. These results are in accordance with those previously reported.9,11 In our series, regardless of whether prior splenectomy had been performed, patients treated with CHOP or CHOP-like first-line regimens ultimately died, despite consolidative or salvage high-dose therapy in many of them. Thus, therapeutic strategies that have proved to be efficient in other subtypes of aggressive lymphoma, such as diffuse large cell lymphoma, have failed in HSγδTCL, and new treatment modalities are needed. In this respect, 2′-deoxycoformycin has been recently shown to display an in vitro selective cytotoxic effect on γδ tumoral T cells77 and to induce complete response in 2 patients, but these reports lacked long-term follow-up.78,79 The 2 patients of our series who received a platinum-cytarabine-based induction regimen are in continuous complete remission at 42 and 52 months. Such a regimen has previously proved to be effective in aggressive lymphoma in relapse.80-82 Our observation should encourage the use of such a potentially synergistic combination in patients with HSγδTCL.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1675.
Supported in part by Association pour la Recherche Thérapeutique, Génétique et Immunologique dans les Lymphomes receiving grants from Amgen (Neuilly-sur-Seine, France) and Roche France (Neuilly-sur-Seine, France).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marine Diviné, Corinne Haioun, Michel Tulliez (CHU Henri Mondor), Jose-Luis Pico (Institut Gustave Roussy), and Iwona Wlodarska (University of Leuven) for providing information on these patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal