Abstract
Cytomegalovirus (CMV) has been a major cause of morbidity and mortality after allogeneic stem cell transplantation (SCT). The importance of the recipient's serologic status is paramount. However, the importance of the donor's serologic status in CMV-seropositive recipients is controversial. We analyzed the influence of the donor's CMV status in a large cohort of patients. A total of 7018 patients seropositive for CMV reported to the European Group for Blood and Marrow Transplantation (EBMT) were included; 5910 patients had undergone HLA-identical sibling SCT and 1108 patients had undergone unrelated donor SCT. Univariate and multivariate proportional hazards models were constructed for survival, event-free survival, transplant-related mortality, and relapse incidence. Patients receiving grafts from CMV-seropositive HLA-identical sibling donors had the same survival as patients grafted from seronegative donors (hazard ratio [HR], 1.04; P = .37; 95% confidence interval [CI], 0.95-1.14). However, unrelated donor stem cell (SC) transplant recipients receiving grafts from CMV-seropositive donors had an improved 5-year survival (35% versus 27%; HR = 0.8; P = .006), an improved event-free survival (30% versus 22%; HR = 0.8; P = .01), and a reduced transplant-related mortality (49% versus 62%; HR = 0.7; P < .001). There was no influence on the relapse incidence. The effects of donor CMV status remained in multivariate analyses. The effect of donor status was different among different disease categories. In patients with chronic myelogenous leukemia (CML), T-cell depletion abrogated the beneficial effect of donor status, suggesting that the effect is mediated through transfer of donor immunity. Our data suggest that donor CMV status influences outcome of unrelated SCT. For a CMV-seropositive patient, a seropositive donor might be preferable. (Blood. 2003;102:4255-4260)
Introduction
Cytomegalovirus (CMV) has for many years been an important cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (SCT). There have been major advances in antiviral prophylactic strategies1-3 and development of new and more sensitive diagnostic techniques that allow better monitoring of patients and early intervention by antiviral therapy.4-6 These advances have reduced the risk of CMV as a direct cause of mortality in HLA-identical sibling transplant recipients, although it is still substantial in patients receiving transplants from HLA-mismatched or unrelated donors. However, the patient's CMV serologic status still has a strong influence on outcome.7 The influence of the donor's serologic status on outcome of transplantation of a CMV-seropositive patient has been controversial.8,9
The aim of this analysis was, therefore, to analyze the influence of donor seropositivity on outcome of allogeneic stem cell transplantation in a large population of CMV-seropositive patients.
Patients and methods
Patients
The European Group for Blood and Marrow Transplantation (EBMT) has collected data on patients since the 1970s. Patients were selected from the EBMT megafile, who were seropositive for CMV and for whom the donor CMV status was known (n = 7895). Syngeneic (n = 87) and non-HLA-identical family donor transplantations (n = 627) were excluded as were 50 patients for whom the donor type was unknown, incorrectly coded, or coded as multiple donor. Thus, 7018 patients were included in the study; 5910 patients had HLA-identical sibling donors and 1108 had unrelated donors. Patient characteristics of the study population are shown in Tables 1 and 2.
Patient characteristics of HLA-identical sibling transplantations (n = 5905)
Characteristic . | Donor CMV-seropositive (%) N = 4451 . | Donor CMV-seronegative (%) N = 1454 . | P* . |
|---|---|---|---|
| Year of transplantation | .41 | ||
| 1992 or before | 1697 (38.1) | 581 (40.0) | |
| 1993-1996 | 1506 (33.8) | 468 (32.2) | |
| 1997 and after | 1248 (28.1) | 405 (27.8) | |
| Age, y | 32 (14) | 30 (14) | <.01 |
| Patient sex | .31 | ||
| Male | 2536 (57.0) | 805 (55.4) | |
| Female | 1915 (43.0) | 649 (44.6) | |
| Stem cell source | <.01 | ||
| Bone marrow | 3562 (80.0) | 1185 (81.5) | |
| Peripheral blood | 731 (16.4) | 187 (12.9) | |
| Other | 158 (3.6) | 82 (5.6) | |
| Diagnosis | <.01 | ||
| Acute leukemia | 2207 (49.6) | 803 (55.2) | |
| CML | 1135 (25.5) | 203 (14.0) | |
| Other | 1109 (24.9) | 398 (27.4) | |
| Stage | .44 | ||
| Early stage | 2479 (77.7) | 789 (78.9) | |
| Advanced stage | 710 (23.3) | 211 (21.1) | |
| T-cell depletion and ATG | <.01 | ||
| T-cell depletion | 721 (16.2) | 214 (14.7) | |
| ATG | 48 (1.0) | 10 (0.7) | |
| Neither | 2470 (55.5) | 940 (64.6) | |
| Missing data | 1212 (27.3) | 290 (20.0) |
Characteristic . | Donor CMV-seropositive (%) N = 4451 . | Donor CMV-seronegative (%) N = 1454 . | P* . |
|---|---|---|---|
| Year of transplantation | .41 | ||
| 1992 or before | 1697 (38.1) | 581 (40.0) | |
| 1993-1996 | 1506 (33.8) | 468 (32.2) | |
| 1997 and after | 1248 (28.1) | 405 (27.8) | |
| Age, y | 32 (14) | 30 (14) | <.01 |
| Patient sex | .31 | ||
| Male | 2536 (57.0) | 805 (55.4) | |
| Female | 1915 (43.0) | 649 (44.6) | |
| Stem cell source | <.01 | ||
| Bone marrow | 3562 (80.0) | 1185 (81.5) | |
| Peripheral blood | 731 (16.4) | 187 (12.9) | |
| Other | 158 (3.6) | 82 (5.6) | |
| Diagnosis | <.01 | ||
| Acute leukemia | 2207 (49.6) | 803 (55.2) | |
| CML | 1135 (25.5) | 203 (14.0) | |
| Other | 1109 (24.9) | 398 (27.4) | |
| Stage | .44 | ||
| Early stage | 2479 (77.7) | 789 (78.9) | |
| Advanced stage | 710 (23.3) | 211 (21.1) | |
| T-cell depletion and ATG | <.01 | ||
| T-cell depletion | 721 (16.2) | 214 (14.7) | |
| ATG | 48 (1.0) | 10 (0.7) | |
| Neither | 2470 (55.5) | 940 (64.6) | |
| Missing data | 1212 (27.3) | 290 (20.0) |
Early stage indicates first complete remission, second complete remission, or first chronic stage.
Chi-square test is used for categorical variables; if ordered, a trend version was used; t test was used for continuous variables.
Patient characteristics of unrelated donor transplantations (n = 1108)
Characteristic . | Donor CMV-seropositive (%) N = 566 . | Donor CMV-seronegative (%) N = 542 . | P* . |
|---|---|---|---|
| Year of transplantation | .86 | ||
| 1992 and before | 61 (10.8) | 54 (10.0) | |
| 1993-1996 | 208 (36.7) | 204 (37.6) | |
| 1997 and after | 297 (52.5) | 284 (52.4) | |
| Age, y | 26 (15) | 27 (15) | .27 |
| Patient sex | .56 | ||
| Male | 310 (54.8) | 308 (56.8) | |
| Female | 256 (45.2) | 234 (43.2) | |
| Stem cell source | .05 | ||
| Bone marrow | 494 (87.3) | 445 (82.1) | |
| Peripheral blood | 35 (6.2) | 45 (8.3) | |
| Other | 37 (6.5) | 52 (9.6) | |
| Diagnosis | .21 | ||
| Acute leukemia | 261 (46.1) | 268 (49.4) | |
| CML | 183 (32.3) | 149 (27.5) | |
| Other | 122 (22.6) | 125 (33.1) | |
| Stage | .17 | ||
| Early stage | 271 (66.4) | 241 (61.8) | |
| Advanced stage | 137 (33.6) | 149 (38.2) | |
| HLA A, B, DRβ1 | .10 | ||
| Match | 474 (83.7) | 433 (79.9) | |
| Mismatch | 92 (16.3) | 109 (21.1) | |
| T-cell depletion and ATG | .91 | ||
| T-cell depletion | 148 (26.2) | 147 (27.1) | |
| ATG | 57 (11.1) | 59 (10.9) | |
| Neither | 294 (51.9) | 281 (51.8) | |
| Missing data | 67 (11.8) | 55 (10.2) |
Characteristic . | Donor CMV-seropositive (%) N = 566 . | Donor CMV-seronegative (%) N = 542 . | P* . |
|---|---|---|---|
| Year of transplantation | .86 | ||
| 1992 and before | 61 (10.8) | 54 (10.0) | |
| 1993-1996 | 208 (36.7) | 204 (37.6) | |
| 1997 and after | 297 (52.5) | 284 (52.4) | |
| Age, y | 26 (15) | 27 (15) | .27 |
| Patient sex | .56 | ||
| Male | 310 (54.8) | 308 (56.8) | |
| Female | 256 (45.2) | 234 (43.2) | |
| Stem cell source | .05 | ||
| Bone marrow | 494 (87.3) | 445 (82.1) | |
| Peripheral blood | 35 (6.2) | 45 (8.3) | |
| Other | 37 (6.5) | 52 (9.6) | |
| Diagnosis | .21 | ||
| Acute leukemia | 261 (46.1) | 268 (49.4) | |
| CML | 183 (32.3) | 149 (27.5) | |
| Other | 122 (22.6) | 125 (33.1) | |
| Stage | .17 | ||
| Early stage | 271 (66.4) | 241 (61.8) | |
| Advanced stage | 137 (33.6) | 149 (38.2) | |
| HLA A, B, DRβ1 | .10 | ||
| Match | 474 (83.7) | 433 (79.9) | |
| Mismatch | 92 (16.3) | 109 (21.1) | |
| T-cell depletion and ATG | .91 | ||
| T-cell depletion | 148 (26.2) | 147 (27.1) | |
| ATG | 57 (11.1) | 59 (10.9) | |
| Neither | 294 (51.9) | 281 (51.8) | |
| Missing data | 67 (11.8) | 55 (10.2) |
Early stage indicates first complete remission, second complete remission, or first chronic stage.
Chi-square test was used for categorical variables; if ordered, a trend version was used; t test was used for continuous variables.
Statistics
These data were analyzed by univariate and multivariate proportional hazards models. In all cases the proportionality assumption was verified for the final models. All stepwise model fitting procedures were carried out with a backward approach. Main effects with a log-rank (LR) test P greater than .05, and interaction terms with a LR test P greater than .10 were removed from the models except for the main effects (interaction terms, respectively) under study. Models were chosen with the specific goal of confirming or rejecting a plausible biologic mechanism and enhancing an understanding of the data. Hence the explicit incorporation of the main factors “donor CMV serologic status” and, when appropriate, the main term “T-cell depletion” as well as the interaction term “donor CMV serologic status”*“T-cell depletion.” “Age,” “calendar year of transplantation,” “donor sex,” “stage,” and “mismatch” were used to minimize their confounding effects on the risk factors under study. Stage in acute leukemia was defined as “CR1 (first complete remission) or CR2” versus “other” and in CML as “CP1 (chronic phase 1)” versus “other.” To verify whether the effect of CMV donor seropositivity has itself changed over the years, we added an interaction term “calendar year”*“donor CMV serologic status” to all models.
Separate models were constructed for patients who received transplants for acute leukemia (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL]; n = 529), chronic myelogenous leukemia (CML; n = 332), and other diseases (n = 247). This construction was due to the finding that the effect of donor CMV serologic status differed so that inclusion of the disease category in the models would have resulted in a violation of the proportional hazard assumption.
Information regarding donor age was available for only 457 patients. Because in many of the patients the donor age was not known, a yes/no variable was created, indicating whether or not donor age was known in a patient. For all outcomes this variable was added to the Cox models evaluated. A confounding effect by selection bias is assessed by comparing the hazard ratio (HR) of donor CMV serologic status and other risk factors before and after adjustment for this dichotomous variable. Each model was fitted once more using the actual donor age on the subset of patients with a known donor age.
The causes of death in the EBMT database are coded as multiple response variables, allowing analysis of more than one contributing causes of death. For this study, the contributing causes of death were combined into viral causes, other infectious causes, and noninfectious causes of death, leading to a 3-group classification. The risk of chronic graft-versus-host disease (GVHD) development was assessed in patients alive at day 100 after transplantation by logistic regression.
Results
HLA-identical sibling donor transplant recipients
Univariate analysis. Donor CMV serologic status had no significant effect on the outcome after HLA-identical sibling SCT (HR for death, 1.04; 95% confidence interval (CI), 0.95-1.14; P = .37). The estimated 10-year survival (Kaplan-Meier) was 42% (± 3%) for patients with CMV-positive donors and 46% (± 3%) for patients with CMV-seronegative donors (Figure 1). There was no effect of donor CMV status on the risk of acute GVHD, whereas patients alive at day 100 with CMV-positive donors had a higher risk of chronic GVHD than patients with seronegative donors (38.5% versus 33.3%; P = .01).
Kaplan-Meier estimates of overall survival in patients undergoing HLA-identical sibling SCT with CMV-seropositive or -seronegative donors.
Kaplan-Meier estimates of overall survival in patients undergoing HLA-identical sibling SCT with CMV-seropositive or -seronegative donors.
Multivariate analyses. The hazard ratio for death comparing CMV-seropositive and -seronegative donors was 1.04 (95% CI, 0.95-1.14; P = .40) after adjusting for age, calendar year of transplantation, donor sex, and diagnostic group. Similarly, the HR for transplantation-related mortality (TRM) was 1.04 (95% CI, 0.93-1.17; P = .46). In the different diagnostic subgroups the HRs for death were 1.01 (95% CI, 0.90-1.14; P = .83) for acute leukemia, 1.11 (95% CI, 0.89-1.39; P = .34) for CML, and 1.07 (95% CI, 0.89-1.29; P = .50) for patients with other diseases.
Patients with CML with CMV-seropositive donors who had survived for more than 100 days had significantly higher risk of chronic GVHD (odds ratio [OR], 1.7; 95% CI, 1.2-2.4; P < .05), whereas there was no difference in patients with acute leukemia (OR, 1.0; 95% CI, 0.8-1.3). However, this finding did not result in any no survival difference in either group.
Unrelated donor transplant recipients
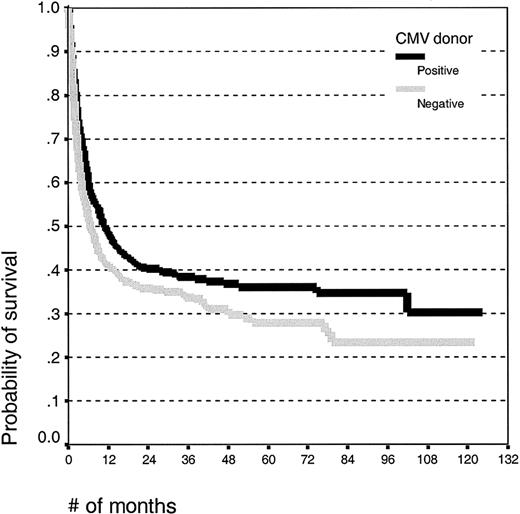
Univariate analysis. There was no difference in the mean age between patients with CMV-seropositive (26.2 ± 15.2 years) and -seronegative donors (27.2 ± 15.2 years). Patients receiving grafts from seropositive donors had improved survival (Figure 2; log rank, P = .006), and the 5-year survival estimates were 35% (± 5%) for CMV-seropositive donors and 27% (± 5%) for CMV seronegative donors.
Kaplan-Meier estimates of overall survival in patients undergoing unrelated donor SCT with CMV-seropositive or -seronegative donors.
Kaplan-Meier estimates of overall survival in patients undergoing unrelated donor SCT with CMV-seropositive or -seronegative donors.
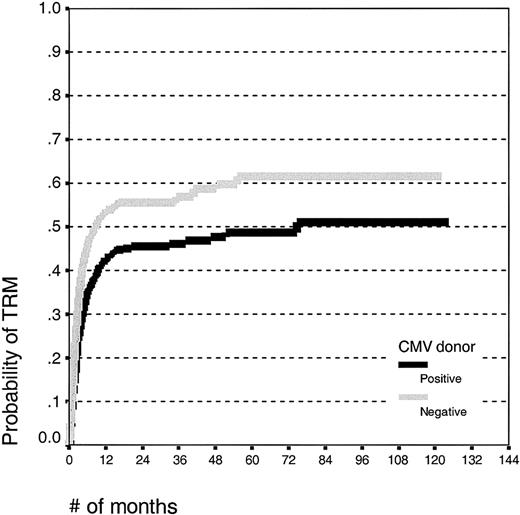
Patients receiving grafts from CMV-seropositive donors had an improved event-free survival (log rank, P = .01; survival estimates 30% ± 5% and 22% ± 5%) and a decreased treatment-related mortality (Figure 3; log rank, P < .001; estimates 49% ± 5% and 62% ± 6%, respectively) compared with patients receiving grafts from CMV-seronegative donors. There was no effect of the donor CMV serostatus on the relapse incidence (log rank, P = .50; estimates 62% ± 7% and 63% ± 8%, respectively). There was no effect of the donor CMV serostatus on the risk of acute GVHD or, in patients alive at day 100, for chronic GVHD.
Kaplan-Meier estimates of transplantation-related mortality in patients undergoing unrelated SCT with CMV-seropositive or -seronegative donors.
Kaplan-Meier estimates of transplantation-related mortality in patients undergoing unrelated SCT with CMV-seropositive or -seronegative donors.
Multivariate analyses. A multivariate model was constructed that included donor CMV serologic status, age, calendar year of transplantation, donor sex, and mismatched grafts (A, B, DRβ1 match versus mismatch; Table 3). Patients receiving a graft from a CMV-seropositive donor had a lower risk of death, resulting in improved overall survival. The hazard ratio for death was 0.82 (95% CI, 0.70-0.97). Similarly, patients receiving grafts from CMV-seropositive donors had a lower risk of transplantation-related mortality (HR, 0.74; 95% CI, 0.61-0.89). Graft type (bone marrow versus peripheral stem cells) had no affect on either the survival or the transplantation-related mortality.
Risk factors for survival, transplantation-related mortality, and relapse in patients receiving unrelated donor transplants
. | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Overall survival | |||
| Donor CMV status | 0.82 | 0.70-0.96 | .02 |
| Patient age | 1.01 | 1.00-1.02 | .001 |
| Year of transplantation | 0.95 | 0.92-0.97 | .001 |
| Donor sex | 0.99 | 0.84-1.16 | .87 |
| Mismatch graft | 1.29 | 1.04-1.60 | .02 |
| Transplantation-related mortality | |||
| Donor CMV status | 0.74 | 0.61-0.89 | .001 |
| Patient age | 1.01 | 1.01-1.02 | <.001 |
| Year of transplantation | 0.92 | 0.88-0.95 | <.001 |
| Donor sex | 1.03 | 0.85-1.23 | .77 |
| Mismatch graft | 1.33 | 1.03-1.71 | .03 |
. | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Overall survival | |||
| Donor CMV status | 0.82 | 0.70-0.96 | .02 |
| Patient age | 1.01 | 1.00-1.02 | .001 |
| Year of transplantation | 0.95 | 0.92-0.97 | .001 |
| Donor sex | 0.99 | 0.84-1.16 | .87 |
| Mismatch graft | 1.29 | 1.04-1.60 | .02 |
| Transplantation-related mortality | |||
| Donor CMV status | 0.74 | 0.61-0.89 | .001 |
| Patient age | 1.01 | 1.01-1.02 | <.001 |
| Year of transplantation | 0.92 | 0.88-0.95 | <.001 |
| Donor sex | 1.03 | 0.85-1.23 | .77 |
| Mismatch graft | 1.33 | 1.03-1.71 | .03 |
There was no effect of donor CMV serologic status on the risk of relapse (HR, 1.08; P = .76). There was no confounding effect of the stage of disease on the effect of CMV donor seropositivity. CMV donor status had no influence on the risk of developing chronic GVHD in patients alive at day 100 (OR, 1.4; 95% CI, 0.9-2.1; P = .13). Donor CMV status did not have any effect on survival in patients alive at day 100.
Causes of death
HLA-identical sibling transplant recipients. Of 4451 patients, 1860 died in the CMV-seropositive donor group compared with 589 of 1454 in the CMV-seronegative donor group. We found that 721 (16.1%) patients in the CMV-seropositive donor group and 223 (15.3%) in the CMV-seronegative donor group died of infectious causes (P = NS).
Unrelated donor transplant recipients. Analysis shows that 294 patients died in the CMV-seropositive donor group compared with 303 in the CMV-seronegative donor group. We found that 218 patients died of transplantation-related causes and 76 (26%) of relapse in the CMV-seropositive donor group compared with 244 of transplantation-related causes and 59 (20%) of relapse in the CMV-seronegative donor group. Patients who died in the CMV-seronegative donor group were, therefore, more likely to have died of transplantation-related causes than those having received grafts from CMV-seropositive donors (P = .06). An analysis of the different transplantation-related causes showed that patients receiving grafts from CMV-seronegative donors were more likely to die of infections (150 [27.6%] of 542 versus 130 [23.3%] of 556; P = .07). There was no difference in the risk of a viral cause of death (68 of 542 [12.5%] versus 55 of 566 [9.7%]; P = .15) or of death in interstitial pneumonia (29 [5.3%] of 542 versus 38 [6.7%] of 566; P = .37).
When the patients were divided into diagnostic subgroups (acute leukemia, CML, other diseases), there was a significant increase in the risk of death from noninfectious causes (relapse of original disease, venoocclusive disease, GVHD) in patients with acute leukemia compared with CML (233 of 529 versus 124 of 332; P = .05), but there was no difference between the groups regarding infectious causes of death (data not shown).
Effects of original disease. Donor CMV serologic status had no independent effect in patients who received transplants for acute leukemia either on survival (HR, 1.03; 95% CI, 0.82-1.30; P = .70) or TRM (HR, 0.91; 95% CI, 0.68-1.21; P = .60). Factors with significant influence on the survival and the TRM were age (P < .001) and calendar year of transplantation (P < .001). In contrast, donor CMV serologic status was independently associated with survival (HR, 0.65; 95% CI, 0.48-0.89; P = .006) and TRM (HR, 0.61; 95% CI, 0.44-0.86; P = .004) in patients who received transplants for CML. Donor CMV serologic status was also independently associated with survival (HR, 0.69; 95% CI, 0.49-0.96; P = .028) and TRM (HR, 0.60; 95% CI, 0.41-0.88; P = .009) in the group that included patients who received transplants for other diseases. There was no effect on the relapse incidence in the groups of patients who received transplants for acute leukemia or CML. The protective effect of donor CMV serologic status was significant in patients with CML in chronic phase, but a similar trend was seen also in more advanced stages.
HRs associated with the variable that indicated whether donor age was unknown or known were close to 1.0 for survival in acute leukemia (HR = 1.2, P = .12) and CML (HR = 0.9; P = .50) but significant for the patient group with other diseases (HR = 1.5; P = .02). However, adjusting for donor age known/unknown did not change the HRs of any other risk factor in the model by more than a few percent. Thus, the effect of donor CMV serologic status was not biased by the absence of information on donor age in a large group of the patients. In the subgroup of patients with known donor ages, we included donor age in all Cox models as a continuous covariate. A significant detrimental effect of donor age on survival and TRM can be detected in the data for the patients with CML, but the HR of donor CMV serologic status was almost identical whether we adjusted for donor age.
Effects of T-cell depletion. One possible hypothesis for the effect of donor CMV serologic status on outcome is that the effect is mediated by CMV-specific T cells. If this hypothesis is correct, T-cell depletion would reduce the favorable effect of donor CMV seropositive status. This possibility was analyzed in patients with CML through construction of a multivariate model that added “T-cell depletion, ATG (antithymocyte globulin), or neither” as a discrete covariate. Apart from the main effect, interaction terms were added to test for effect modification (ie, a possible dependence of the effect of donor CMV seropositivity on the presence or absence of T cells or ATG). The overall interaction effect was significant (P = .02), indicating that the effect of donor CMV serologic status is not the same in the 3 categories. The HR for the donor CMV serologic status was different in the T-cell-depleted subgroup compared with the other 2 groups. Moreover, the hazard ratios among the neither and ATG group did not differ significantly. These data show an estimate for the HR for overall survival of donor CMV serologic status of 0.49 (95% CI, 0.32-0.75) in the neither plus ATG subgroup and a HR of 1.22 (95% CI, 0.75-2.0) in the T-cell-depleted group. Hence, the positive effect of CMV seropositivity was completely abrogated by T-cell depletion, both concerning the nontreated subgroup and the ATG patients. Similar results were found for TRM. The HR was 0.43 (95% CI, 0.27-0.69; P < .01) for neither plus ATG subgroup, and it was 1.3 (95% CI, 0.75-2.2; P = .37) in the T-cell-depleted group.
Discussion
CMV has been a major cause of transplant-related mortality in allogeneic SC transplant recipients.10-12 One of the key risk factors has been patient CMV serologic status, especially in unrelated donor transplant recipients.7,8,13-15 CMV-seropositive patients have a poorer outcome than CMV-seronegative patients despite improvement in preventive strategies against CMV disease such as antiviral prophylaxis and preemptive therapy.7,15 CMV is immunosuppressive and in solid organ transplant recipients has been associated with an increased risk of bacterial and fungal infections,16 and prevention of CMV can result in a lower risk of bacterial and fungal infections16,17 and decreased mortality in infectious complications after SCT.2
The influence of the donor CMV serologic status to a CMV-seropositive patient has been controversial. An early study in T-cell-depleted patients receiving grafts from HLA-identical donors suggested that outcome could be improved by the use of a CMV-seropositive donor.9 This finding has not been verified in other studies.13 In this study, we found no influence by CMV serologic status on overall survival and transplantation-related mortality in patients receiving grafts from HLA-identical donors. However, there was a strong influence by donor CMV serologic status on outcome in unrelated donor transplantations. Patients receiving grafts from CMV-seropositive unrelated donors had improved survival, event-free survival, and reduced transplantation-related mortality. That a possible effect would be stronger in unrelated donor transplant recipients than in HLA-identical sibling graft recipients fits with the results showing that CMV-associated mortality and the risk of other infectious causes of transplant-related mortality were higher after unrelated than HLA-identical sibling transplantation.10,14,18 Disease stage was, of course, a highly significant predictor of mortality. Other factors significant in the multivariate analysis were patient age, calendar year of SCT, and the use of HLA-mismatched unrelated donors. These factors are well recognized from earlier studies.
How was this effect by donor CMV status mediated? The most likely explanation would be death in CMV disease by itself in patients receiving grafts from CMV-seronegative donors. However, although there was a slight trend in this direction with lower risks of death in interstitial pneumonia, this cannot be the entire explanation. CMV has been shown to be immunosuppressive and to increase the risk of bacterial and fungal infections in transplant recipients. Indeed, by preventing CMV replication, a reduction in mortality as a result of all types of infections can be achieved.2 This hypothesis is supported by our data because there was a strong trend for an increased risk of death from infections in patients receiving grafts from CMV-seronegative donors.
We have not looked at acute GVHD's effect on outcome in this study because the aim was to evaluate factors known before transplantation, thereby allowing a choice of the best donor. However, we could find no effect by CMV donor status on the risk of acute GVHD. Analyses of CMV donor status on the risk of chronic GVHD gave different results. There was no effect in patients who received transplants from unrelated donors, but we could find a significant increase of the risk of chronic GVHD in patients with CML who received transplants from CMV-seropositive sibling donors. In neither group, landmark analysis of patients alive at day 100 showed any influence of donor CMV status on survival. Thus, the effect on survival and TRM occurs before day 100 at the time when most infectious deaths do occur and is independent of acute and chronic GVHD.
Recently, Kollman et al19 in a very large study from the National Marrow Donor Program reported that increasing donor age was a significant risk factor for decreased survival in unrelated donor transplantations but no effect of CMV donor serologic status could be found. There was a difference in how disease categories were analyzed in the 2 studies. We found that the CMV donor status effect was different in patients who received transplants for different diseases; therefore, it was not possible to adjust for disease categories as a covariate because the adjustment resulted in a violation of the proportional hazard assumption, whereas Kollman et al19 adjusted for diagnosis. However, to allow comparison with the study by Kollman et al,19 we reran all models in a similar manner as in their study. It should be stressed that this is, of course, statistically incorrect. However, with this limitation, as well as the limitation of missing data regarding donor age, having a CMV-seropositive donor still had a positive effect on survival also when diagnosis was entered into the models.
In our different disease models, there were strong CMV donor status effects in patients with CML and in a mixed group of patients who received transplants mainly for myelodysplastic syndrome (MDS), aplastic anemia, and lymphoma, whereas there was no significant effect in patients with acute leukemia. The effects were mediated through a lower TRM in both groups. It should be noted that there was a trend for lower TRM also in patients with acute leukemia who had CMV-seropositive donors, but this trend did not translate into an improved survival. There are several different possible explanations to these different effects in different patient categories. Patients with acute leukemia are more likely to die of relapse; therefore, the effect of reducing transplantation-related mortality is less. A second possibility is that patients with acute leukemia as a result of previous chemotherapy might be more prone to die of transplantation-related causes unrelated to infections. A third possibility for the different effect in patients who receive transplants for acute leukemia and CMV is the large difference in age between patients with acute leukemia (mean age, 23.9 years) and CML (mean age, 33.7 years). This age difference is mainly due to the presence of many young children in the acute leukemia group. The likely mechanism for a negative effect by using a CMV-seronegative donor is the lack of transfer of mature CMV-specific effector T cells or the transfer of T-cell precursors able to multiply and reconstitute T-cell immunity. A key element in immune reconstitution after transplantation is thymic function, and children have a better posttransplantation thymic function than adults20-22 and might, therefore, better be able to mount a CMV-specific immune response.20,23 All 3 explanations might contribute to the different effect of donor CMV serologic status in the different diagnostic subgroups, although none of the possibilities can alone explain the differences.
One way to look at the influence of specific immunity would have been to look at the rates of CMV replication in patients receiving grafts from CMV-seropositive and -seronegative donors preferably with measurements of the viral load by quantitative methods.5 This approach unfortunately was not possible because of a limitation of such data in our database. Furthermore, the monitoring techniques have varied over time and at different centers. We, therefore, choose to analyze the influence of T-cell depletion. The results support the hypothesis of specific T cells as the key controlling factor. The protective effect by a CMV-seropositive donor was abrogated by T-cell depletion so that the overall survival and the risk of transplantation-related mortality became the same in patients receiving grafts from T-cell-depleted CMV-seropositive or -seronegative donors.
Our results indicate that a CMV-seropositive donor might improve outcome of a CMV-seropositive patient undergoing an unrelated donor transplantation. That the proportion of seronegative versus seropositive donors differs significantly between CMV-seropositive patients who receive transplants with identical sibling donors and unrelated donors suggests that instead the selection of a seronegative donor has been favored by transplantation physicians. Although our study leaves some unresolved questions, it at least questions this practice and rather suggests that, if possible, a CMV-seropositive donor should be chosen for a CMV-seropositive patient.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2002-10-3263.
Supported by the Swedish Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
P.L. designed the study and wrote the paper. R.B. performed the statistical analyses. H.E., F.F., D.N., and C.C. gave intellectual input into the paper regarding analysis and interpretation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal