Abstract
Patients with systemic mastocytosis (SM) can suffer from disabling symptoms related to mast cell mediator release or mast cell infiltration, requiring mast cell eradication. In the present absence of any curative therapy, a recent case report describing the efficacy of cladribine showed promising results. In a pilot study, the efficacy of cladribine (0.10-0.13 mg/kg in a 2-hour infusion, days 1-5; repeated at 4-8 weeks until 6 cycles) was studied. Ten patients with SM with severe symptoms were treated. Four patients were classified as having indolent or smoldering mastocytosis, 3 as having aggressive systemic mastocytosis, and 3 as having SM with an accompanying hematologic malignancy. Nine patients received 6 courses, 1 patient stopped because of toxicodermia. All responded concerning signs, symptoms, and mast cell parameters (serum tryptase and urinary histamine metabolite excretion), although none achieved a complete remission. Prolonged follow-up is required, as response is ongoing in most cases. One patient relapsed within 11 months and showed a second response. Side effects were mainly related to bone marrow suppression. Single-agent cladribine is an effective and relatively safe treatment for severe systemic mastocytosis. The optimal dose and schedule need to be explored. (Blood. 2003;102:4270-4276)
Introduction
Systemic mast cell disease or systemic mastocytosis is characterized by an abnormal proliferation of mast cells in bone marrow, spleen, liver, and/or lymph nodes.1-3 Signs and symptoms are mainly related to the release of mast cell mediators, causing flushing, itching, headache, gastrointestinal symptoms, fatigue, osteoporosis, and even syncope or anaphylactic attacks. Moreover, the mast cell infiltration by itself can cause skin lesions (eg, urticaria pigmentosa), organomegaly, organopathy, and pancytopenia because of bone marrow infiltration. Finally, the presence of an associated non-mast cell hematologic disorder can dominate the clinical picture.
In the absence of any curative option, therapy of systemic mastocytosis is generally symptomatic, using H1 and H2 antihistamine blockade,4 oral disodium cromoglycate, and incidentally corticosteroids. Although many patients harbor only indolent forms of systemic mastocytosis for which intensive therapy is not justified, others can suffer from serious disease with aggressive behavior demanding mast cell eradication. Several cytostatic drugs have been applied, frequently because of an associated hematologic disorder for which a chemotherapeutic approach was considered useful. However, such therapies were generally not successful for the mast cell disease.4 Even high-dose bone marrow ablative therapy followed by stem cell transplantation has usually not been successful, with persistence of the mast cell infiltration4,5
Because of its similarity with myeloproliferative disorders, patients have been treated with interferon-alfa (IFN-alfa).6 For the first time, this intervention resulted in a decrease of mast cell infiltration with clearing of the skin lesions, decrease of bone marrow infiltration, decrease of hepatosplenomegaly, and accompanying diminishment of mast cell mediator-related symptoms.6 Several series of patients have been described since, generally with most of the patients responding.4,6-13 However, not all patients respond on IFN-alfa, side effects can be severe, and long-term duration of therapy is required.
Recently, Tefferi et al14 described the efficacy of 2-chlorodeoxy-adenosine, cladribine, in a patient with interferon-alfa-resistant systemic mastocytosis. Six cycles of 5 days of cladribine (0.13 mg/kg in 2-hour infusion) induced an almost complete disappearance of the mast cell infiltration in skin and bone marrow. Therefore, we decided to treat a series of 10 patients at various centers to verify the positive results of this new treatment modality.
Patients, materials, and methods
Patients
All patients were diagnosed with systemic mastocytosis (with or without associated non-mast cell hematologic disorder). The severity of the disease was such that according to the treating physician cytoreductive therapy was indicated. Diagnosis was confirmed by tissue biopsy of involved sites, and patients were classified according to the World Health Organization (WHO) proposal,3 based on the presence or absence of B and C findings, and an associated clonal hematologic non-mast cell lineage disease. B findings are defined as (1) bone marrow biopsy showing more than 30% infiltration by mast cells and/or serum total tryptase level more than 200 ng/mL; (2) signs of dysplasia or myeloproliferation in non-mast cell lineage, but insufficient criteria for definitive diagnosis of a hematopoietic neoplasm, with normal or slightly abnormal blood counts; and (3) hepatomegaly without impairment of liver function, and/or palpable splenomegaly without hypersplenism, and/or palpable or visceral lymphadenopathy. C (clinical) findings are defined by the following mastocytosis-related symptoms: (1) leukocytopenia (< 1000/μL); (2) anemia (hemoglobin [Hb] < 10 g/dL); (3) thrombocytopenia (< 100 000/μL); (4) hepatomegaly with ascites; (5) abnormal liver tests with elevated enzyme levels or (6) with hypalbuminemia; (7) portal hypertension; (8) palpable splenomegaly with hypersplenism, eg, thrombocytopenia; (9) malabsorption with hypalbuminemia or with (10) weight loss; (11) huge osteolysis and/or severe osteoporosis with pathologic fractures. According to these findings, the classification indolent systemic mastocytosis (ISM) was based on the absence of B and C findings; within this category smoldering systemic mastocytosis (SSM) could be recognized with criteria of systemic mastocytosis and the presence of 2 or more B findings but no C findings. Likewise, aggressive mastocytosis (ASM) consisted of systemic mastocytosis with at least one or more C findings. Finally, SM with associated clonal hematologic non-mast cell lineage disease (SM-AHNMD) would meet SM criteria and any other hematologic malignancy meeting the criteria for a distinct entity in the WHO classification. Staging prior to the start of cladribine consisted of skin examination by a dermatologist, skin biopsy of involved skin, bone marrow biopsy and aspirate with tryptase staining on the biopsy by routine immunohistochemistry, and toluidine blue staining on the aspirate. Hepatosplenomegaly was confirmed by ultrasound or computerized tomography (CT) scan of the abdomen. Standard questionnaires for histamine-related complaints were used. The patients were asked to score these complaints before, after the third and sixth cladribine cycle, and 3 and 6 months after the treatment period. All parameters that related to mast cell disease or associated hematologic disorder were reevaluated at the same time points mentioned earlier.
Laboratory tests
Laboratory tests consisted of complete hemograms, chemistry panel, serum tryptase using the B12 assay with Pharmacia UniCAP tryptase reagents and the Pharmacia Unicap100 analysis device (Pharmacia and Upjohn, Uppsala, Sweden),15 and the urinary excretion of the histamine metabolites N-methylhistamine and N-methylimidazoleacetic acid,16-18 preferably collected in the fasting state in the morning after voiding of the first portion. For serum tryptase, reference values for healthy individuals are those reported by Pharmacia and Upjohn, showing a geometric mean level of 5.6 μg/L and an upper 95th percentile of 13.5 μg/L (129 apparently healthy children and adults). For N-methylhistamine and N-methylimidazoleacetic acid excretion, mean values (ranges) of an apparently healthy population are 101 (50-154) μmol/mol and 1.3 (0.9-1.9) mmol/mol creatinine, respectively, in urine collected after an overnight fast.19 The c-kit mutation was assessed by polymerase chain reaction (PCR) on cDNA on mast cell lesions from bone marrow aspirates as described,20,21 using 6% melting point (mp) agarose for detection.
Therapy protocol
All patients were treated by cladribine infusions, aiming at a daily dose of 0.13 mg/kg, 5 days a week in 2-hour infusions. The cycles were repeated after 4 to 6 weeks. The intervals were enlarged in case of bone marrow suppression. The dose was adapted if recovery took more than 6 weeks. Premedication consisted of H1-blockade (usually clemastine 1-2 mg intravenously) and corticosteroids (either 5 mg dexamethasone or 25 mg di-adreson-F, both intravenously). All patients were protected with oral fluconazole and co-trimoxazole because of CD4 lymphocytopenia. Several patients also received valacyclovir as prophylaxis. Blood products had to be irradiated until recovery of the CD4 counts above 200 × 106/L.
Response criteria
Responses were assessed following the recently proposed criteria by Valent et al,22 defining a major, partial, or no response and using C (clinical) findings for the definition of the diverse mastocytosis-related symptoms. These response definitions have been made only for patients with aggressive systemic mastocytosis (ASM). A major response was defined by the complete resolution of at least one C finding without progression in other C findings. Major responses were subdivided into (1) complete response (disappearance of mast cell infiltrates and surrogate laboratory markers in follow-up investigations), (2) incomplete remission (incomplete regression of mast cell infiltrates and surrogate markers), and (3) a pure clinical response (disappearance of all C findings without change in mast cell infiltrates).
A partial response consisted of a measurable response: (1) good partial response if more than 50% and (2) minor response if less than 50% in one or more C findings without progression in other C findings. No response reflected stable C findings or even progressive clinical course. In the presence of improvement of one C finding and simultaneous progression of another C finding, the final response was scored as progressive disease.
A paired, 2-sided t test was used to compare the laboratory tests before and after treatment with cladribine.
The protocol was approved by the research ethics board of University Medical Center Utrecht and subsequently all other participating hospitals. Informed consent was provided according to the Declaration of Helsinki.
Results
Demographic data, signs, and symptoms from all patients are presented in Table 1. The 10 patients consisted of 6 women and 4 men with a median age of 53 years (range, 32-75 years). Two patients had received cytoreductive treatment before, consisting of interferon-alfa, and showed progressive disease later. According to the new WHO classification,3,22 3 patients had indolent systemic mastocytosis (ISM), but with severe histamine-related symptoms that were not controlled with H1 and H2 blockers. One patient (case I) fulfilled the criteria of smoldering SM and needed intensive care monitoring because of persistent anaphylaxis; 3 patients had systemic mastocytosis with associated clonal hematologic non-mast cell lineage disease (SM-AHNMD), consisting of myelodysplastic syndrome (MDS) with trisomy 8 (case D), atypical chronic myeloid leukemia (aCML) with trisomy 8 (case F), or with normal cytogenetics (case H); 3 patients had aggressive systemic mastocytosis (Table 1).
Demographic and clinical data of 10 patients with systemic mastocytosis
. | A . | B . | C . | D . | E . | F . | G . | H . | I . | J . |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex, age, y | M, 61 | F, 57 | F, 48 | M, 54 | M, 51 | M, 64 | F, 32 | F, 44 | F, 75 | F, 46 |
| Year of diagnosis of systemic mastocytosis | 1999 | 1996 | 2002 | 1996 | 2001 | 2002 | 1999* | 2002 | 1998 | 2000 |
| Previous cytoreductive therapy† | No | No | No | No | No | No | IFN, PRD | Hydrea | IFN, PRD | No |
| Skin symptoms | + (P, D) | + (P, D) | + (P, D) | + (P, D) | — | + (P, D) | + (P, D) | — | + (P, D) | + (P, D) |
| Urticaria pigmentosa | + | + | + | + | + | — | +* | — | + | + |
| Flushing | + | + | + | + | + | — | + | — | + | + |
| Syncope/anaphylaxis | + | — | — | + | — | — | + | — | + | — |
| Gastric/abdominal complaints | + | + | + | — | + | — | + | + | + | + |
| Diarrhea | — | — | + | + | + | + | — | — | + | + |
| Bone symptoms/osteoporosis | + | + | + | — | + | + | — | — | — | — |
| Weight loss | + | — | — | + | + | — | — | + | — | + |
| Severe fatigue | + | + | + | — | + | + | + | + | + | + |
| Anemia | — | — | — | — | + (9.4 g/dL) | — | + (9.9 g/dL) | + (8.5 g/dL) | — | — |
| Thrombocytopenia | — | — | — | — | + (117) | — | — | — | — | — |
| Bone marrow infiltration | 15% | 10% | 10% | 20% | 75% | 15% | 60% | 1%-15% | >60% | 35% |
| Hepatosplenomegaly | — | — | — | — | + | + | + | + | — | — |
| Lymphadenopathy | — | — | — | — | + | — | — | — | — | + |
| WHO classification3 | ISM* | ISM | ISM | SM-AHNMD* | ASM* | SM-AHNMD | ASM | SM-AHNMD | SSM* | ASM |
| Tryptase, N < 13.5 μg/L) | 120 | 34 | 200 | 100 | 497 | 270 | > 200 | 170 | 92 | 165 |
| Urinary M-histamine, N < 150 μM/M creatine | 489 | 447 | 354 | 1478 | 4686 | 1220 | 4094 | 4652 | 6506 | 1928 |
| c-kit mutation | NT* | NT | NT | NT | + | Absent | + | + | NT | NT |
| Associated hematologic disorder | — | — | — | MDS, +8 | Atyp MKC | Atyp CML, +8 | — | Atyp CML | — | — |
. | A . | B . | C . | D . | E . | F . | G . | H . | I . | J . |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex, age, y | M, 61 | F, 57 | F, 48 | M, 54 | M, 51 | M, 64 | F, 32 | F, 44 | F, 75 | F, 46 |
| Year of diagnosis of systemic mastocytosis | 1999 | 1996 | 2002 | 1996 | 2001 | 2002 | 1999* | 2002 | 1998 | 2000 |
| Previous cytoreductive therapy† | No | No | No | No | No | No | IFN, PRD | Hydrea | IFN, PRD | No |
| Skin symptoms | + (P, D) | + (P, D) | + (P, D) | + (P, D) | — | + (P, D) | + (P, D) | — | + (P, D) | + (P, D) |
| Urticaria pigmentosa | + | + | + | + | + | — | +* | — | + | + |
| Flushing | + | + | + | + | + | — | + | — | + | + |
| Syncope/anaphylaxis | + | — | — | + | — | — | + | — | + | — |
| Gastric/abdominal complaints | + | + | + | — | + | — | + | + | + | + |
| Diarrhea | — | — | + | + | + | + | — | — | + | + |
| Bone symptoms/osteoporosis | + | + | + | — | + | + | — | — | — | — |
| Weight loss | + | — | — | + | + | — | — | + | — | + |
| Severe fatigue | + | + | + | — | + | + | + | + | + | + |
| Anemia | — | — | — | — | + (9.4 g/dL) | — | + (9.9 g/dL) | + (8.5 g/dL) | — | — |
| Thrombocytopenia | — | — | — | — | + (117) | — | — | — | — | — |
| Bone marrow infiltration | 15% | 10% | 10% | 20% | 75% | 15% | 60% | 1%-15% | >60% | 35% |
| Hepatosplenomegaly | — | — | — | — | + | + | + | + | — | — |
| Lymphadenopathy | — | — | — | — | + | — | — | — | — | + |
| WHO classification3 | ISM* | ISM | ISM | SM-AHNMD* | ASM* | SM-AHNMD | ASM | SM-AHNMD | SSM* | ASM |
| Tryptase, N < 13.5 μg/L) | 120 | 34 | 200 | 100 | 497 | 270 | > 200 | 170 | 92 | 165 |
| Urinary M-histamine, N < 150 μM/M creatine | 489 | 447 | 354 | 1478 | 4686 | 1220 | 4094 | 4652 | 6506 | 1928 |
| c-kit mutation | NT* | NT | NT | NT | + | Absent | + | + | NT | NT |
| Associated hematologic disorder | — | — | — | MDS, +8 | Atyp MKC | Atyp CML, +8 | — | Atyp CML | — | — |
IFN indicates interferon-alfa; PRD, prednisone; Hydrea, hydroxyurea; P, pruritus; D, Darier sign; ISM, indolent systemic mastocytosis; SM-AHNMD, systemic mastocytosis with associated hematologic non-mast cell disease; ASM, aggressive systemic mastocytosis; SSM, smoldering systemic mastocytosis; NT, not tested; MDS, myelodysplastic syndrome; +8, trisomy 8; Atyp MKC, atypical megakaryocytes; Atyp CML, atypical chronic myeloid leukemia; and—, symptom or sign not present.
In 1989 extensive urticaria pigmentosa (UP) was present, which disappeared after IFN-alfa therapy.
H1 and H2 blocking and cromoglycate were excluded.
Therapy schedule
Between August 2001 and June 2003, 9 patients have received the full 6 cycles of cladribine, each cycle consisting of 5 days each. In case H, the therapy was terminated after the third cycle because of side effects. Most patients did not succeed in receiving one cycle every 4 weeks because of bone marrow suppression. After the observation of persisting pancytopenia in case D who also suffered from accompanying MDS, a longer treatment interval was planned for all patients.
Responses
According to the proposed criteria by Valent et al,22 all patients with aggressive systemic mastocytosis showed a response (Table 2). All other patients showed improvement of signs and symptoms: the main complaints (anaphylactic attacks, collapse, flushes, abdominal pain, bone pain, weight loss, vomiting, and fatigue) improved or even did not occur any more. In none of the patients, new mast cell mediator-related symptoms were reported.
Response of all 10 patients on cladribine therapy
. | A . | B . | C . | D . | E . | F . | G . | H . | I . | J . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean daily dose of cladribine mg/kg* | 0.12 | 0.12 | 0.12† | 0.12 | 0.13 | 0.10 | 0.10 | 0.13 | 0.13 | 0.10 |
| Tryptase, μg/L | ||||||||||
| Before | 120 | 34 | 200 | 100 | 497 | 270 | 224 | 170 | 92 | 163 |
| Best response | 44 | 16 | 124 | 55 | 171 | 153 | 144 | 12 | 70 | 43 |
| Urinary MH, μM/M creatinine | ||||||||||
| Before | 489 | 447 | 453 | 1478 | 4868 | 1220 | 4094 | 4652 | 6506 | 1928 |
| Best response | 166 | 96 | 125 | 269 | 340 | 537 | 880 | 248 | 184 | 124 |
| Urinary MIMA, μM/M creatinine | ||||||||||
| Before | 34.3 | 4.7 | 5.2 | 9.5 | 39.4 | 10.9 | 23.7 | 30.6 | 58.6 | NT |
| Best response | 5.1 | 1.7 | 4.1 | 7.3 | 7.5 | 10.1 | 20 | 4.3 | 2.5 | NT |
| Skin % UP | ||||||||||
| Before | > 50 | > 50 | > 50 | > 50 | 50 | 0 | 0 | 0 | > 50 | > 50 |
| Best response | < 5 | 10 | 10 | < 5 | < 5 | NA | NA | NA | < 5 | < 5 |
| Bone marrow | ||||||||||
| Before | 15% | 10% | 10% | 20% | >75% | 15% | 60% | 1%-15% | > 60% | 35% |
| Best response | 10% | 0% | 0% | < 5% | 20% | 5% | 30% | 1%-5% | Too early | < 5% |
| Main complaint | Collapse | Abd pain | Itching | Collapse | Weight loss | Fatigue | Flushes | — | Anaphylaxis | Diarrhea |
| Before | ++++ | ++++ | ++++ | ++++ | −11 kg | ++++ | +++ | — | Requiring IC | +++ |
| Best response | + | ++ | + | + | +12.5 kg | ++ | 0 | Under control | 0 | |
| Main complaint | Flushing | Bone pain | Fatigue | Diarrhea | Pancytopenia | Fever | Fatigue | — | Cardiomegaly; AF | Malabsorption |
| Before | ++++ | ++++ | ++++ | ++++ | Hb, 9.4; Thr, 117 | ++ | +++ | — | Therapy resistant | Gl mast cells |
| Best response | + | + | + | 0 | Hb, 13.6; Thr, 217 | 0 | + | Heart size decreased, AF controlled | Resolved; weight increased 10 kg | |
| Main complaint | Abd pain | Headache | Abd pain | Flushing | Fatigue | Splenomegaly | Splenomegaly | — | Fatigue | Skin symptoms |
| Before | ++++ | ++ | ++++ | ++++ | +++ | 17 cm | 17 cm | — | +++ | +++ |
| Best response | + | + | + | 0 | 0 | 16.5 cm | 15 cm | 0 | 0 | |
| Main complaint | — | — | — | — | Lymphadenopathy | — | — | — | — | Lymphadenopathy |
| Before | — | — | — | — | Paraaortic | — | — | — | — | Mesenterial |
| Best response | Resolved | Resolved | ||||||||
| Response22 | NA | NA | NA | NA | Major response | NA | Good PR | NA | NA | Major, incomplete |
| Time to best response | 9-12 mo | 6-9 mo | 6-9 mo | 6-9 mo | 6 mo | 3 mo | 3-6 mo | 3 mo | 2-3 mo | 8 mo |
| Response associated hematologic disorder | NA | NA | NA | Allogeneic SCT for MDS and pancytopenia | Normal hemogram, persistent abnormal MKC | Persistent Atypical CML with +8 | NA | Persistent atypical CML | NA | NA |
| Follow-up since end of therapy | Continuing response (+15 mo) | Continuing response (+5 mo) | Continuing response (+5 mo) | Not applicable, allogeneic SCT after 9 mo | Progression after 11 mo; 2nd response on re-treatment | Not applicable; change to IFN + Hydrea | No change | NA (only 3 cycles received) | Too early | Continuing response (+3 mo) |
| Adverse events | FUO; anemia requiring transfusion | None | None | Persistent pancytopenia requiring many transfusions | None | None | FUO; HZV; anemia requiring transfusions | Sweet syndrome/toxicodermia | None | Vena jugularis interna thrombosis |
. | A . | B . | C . | D . | E . | F . | G . | H . | I . | J . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean daily dose of cladribine mg/kg* | 0.12 | 0.12 | 0.12† | 0.12 | 0.13 | 0.10 | 0.10 | 0.13 | 0.13 | 0.10 |
| Tryptase, μg/L | ||||||||||
| Before | 120 | 34 | 200 | 100 | 497 | 270 | 224 | 170 | 92 | 163 |
| Best response | 44 | 16 | 124 | 55 | 171 | 153 | 144 | 12 | 70 | 43 |
| Urinary MH, μM/M creatinine | ||||||||||
| Before | 489 | 447 | 453 | 1478 | 4868 | 1220 | 4094 | 4652 | 6506 | 1928 |
| Best response | 166 | 96 | 125 | 269 | 340 | 537 | 880 | 248 | 184 | 124 |
| Urinary MIMA, μM/M creatinine | ||||||||||
| Before | 34.3 | 4.7 | 5.2 | 9.5 | 39.4 | 10.9 | 23.7 | 30.6 | 58.6 | NT |
| Best response | 5.1 | 1.7 | 4.1 | 7.3 | 7.5 | 10.1 | 20 | 4.3 | 2.5 | NT |
| Skin % UP | ||||||||||
| Before | > 50 | > 50 | > 50 | > 50 | 50 | 0 | 0 | 0 | > 50 | > 50 |
| Best response | < 5 | 10 | 10 | < 5 | < 5 | NA | NA | NA | < 5 | < 5 |
| Bone marrow | ||||||||||
| Before | 15% | 10% | 10% | 20% | >75% | 15% | 60% | 1%-15% | > 60% | 35% |
| Best response | 10% | 0% | 0% | < 5% | 20% | 5% | 30% | 1%-5% | Too early | < 5% |
| Main complaint | Collapse | Abd pain | Itching | Collapse | Weight loss | Fatigue | Flushes | — | Anaphylaxis | Diarrhea |
| Before | ++++ | ++++ | ++++ | ++++ | −11 kg | ++++ | +++ | — | Requiring IC | +++ |
| Best response | + | ++ | + | + | +12.5 kg | ++ | 0 | Under control | 0 | |
| Main complaint | Flushing | Bone pain | Fatigue | Diarrhea | Pancytopenia | Fever | Fatigue | — | Cardiomegaly; AF | Malabsorption |
| Before | ++++ | ++++ | ++++ | ++++ | Hb, 9.4; Thr, 117 | ++ | +++ | — | Therapy resistant | Gl mast cells |
| Best response | + | + | + | 0 | Hb, 13.6; Thr, 217 | 0 | + | Heart size decreased, AF controlled | Resolved; weight increased 10 kg | |
| Main complaint | Abd pain | Headache | Abd pain | Flushing | Fatigue | Splenomegaly | Splenomegaly | — | Fatigue | Skin symptoms |
| Before | ++++ | ++ | ++++ | ++++ | +++ | 17 cm | 17 cm | — | +++ | +++ |
| Best response | + | + | + | 0 | 0 | 16.5 cm | 15 cm | 0 | 0 | |
| Main complaint | — | — | — | — | Lymphadenopathy | — | — | — | — | Lymphadenopathy |
| Before | — | — | — | — | Paraaortic | — | — | — | — | Mesenterial |
| Best response | Resolved | Resolved | ||||||||
| Response22 | NA | NA | NA | NA | Major response | NA | Good PR | NA | NA | Major, incomplete |
| Time to best response | 9-12 mo | 6-9 mo | 6-9 mo | 6-9 mo | 6 mo | 3 mo | 3-6 mo | 3 mo | 2-3 mo | 8 mo |
| Response associated hematologic disorder | NA | NA | NA | Allogeneic SCT for MDS and pancytopenia | Normal hemogram, persistent abnormal MKC | Persistent Atypical CML with +8 | NA | Persistent atypical CML | NA | NA |
| Follow-up since end of therapy | Continuing response (+15 mo) | Continuing response (+5 mo) | Continuing response (+5 mo) | Not applicable, allogeneic SCT after 9 mo | Progression after 11 mo; 2nd response on re-treatment | Not applicable; change to IFN + Hydrea | No change | NA (only 3 cycles received) | Too early | Continuing response (+3 mo) |
| Adverse events | FUO; anemia requiring transfusion | None | None | Persistent pancytopenia requiring many transfusions | None | None | FUO; HZV; anemia requiring transfusions | Sweet syndrome/toxicodermia | None | Vena jugularis interna thrombosis |
MH indicates N-methylhistamine; MIMA, N-methylimidazoleacetic acid; %UP, percentage of body covered with urticaria pigmentosa; Abd, abdominal; IC, intensive care continuous monitoring; Thr, thrombocytes; AF, uncontrollable atrial fibrillation, finally requiring amiodarone; NA, not applicable (response criteria can only be applied to ASM patients); SCT, stem cell transplantation; MDS, myelodysplastic syndrome; MKC, megakaryocytes; CML, chronic myeloid leukemia; +8, trisomy 8; IFN, interferon-alpha; Hydrea, hydroxyurea; FUO, fever of unknown origin; HZV, herpes zoster varicella; and—, not applicable.
Cladribine was given in 6 cycles of 5 days each; per day 0.10 to 0.13 mg/kg was administered.
This patient had a weight of 135 kg. The dose was calculated for a body weight of 100 kg.
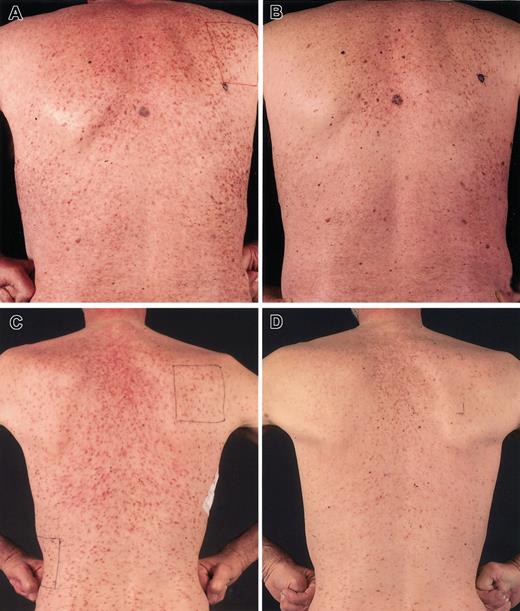
All 7 patients with urticaria pigmentosa showed a reduction of the characteristic red-brown maculae from more than 50% skin involvement to almost disappearance (scored as < 5% left) (Figure 1). Brown remnants of lesions were still seen after 6 months, consisting of predominantly melanocytes with hardly any mast cells present, confirmed in skin biopsies. Bone marrow involvement decreased in all patients, sometimes very remarkably (Table 2). Of note, given the mostly focal involvement in many cases, these measurements are not very precise. Two patients (F and H) showed the combination of bone marrow mastocytosis and atypical CML. During the cladribine therapy the leukocytosis decreased in both from more than 50 × 109/L to 10 to 20 × 109/L (case F) and from more than 20 × 109/L to 1 to 4 × 109/L (case H). However, the white blood cell count increased rapidly within weeks after the last cycle of cladribine. Still, in case F, symptoms of severe fatigue improved very much, and his performance improved from WHO grades 3-4 (> 50% bedridden) to grade 1-2. Case H had few symptoms before the start of cladribine and could, therefore, not show improvement in this regard.
Decrease of urticaria pigmentosa on cladribine. Remarkable reduction of urticaria pigmentosa lesions in patient A before treatment (A) and after 6 cycles of cladribine (B) and in patient D before treatment (C) and after 6 cycles of cladribine (D). Brown remnants of lesions are still seen after 6 months, consisting of predominantly melanocytes with hardly any mast cells present, confirmed in skin biopsies.
Decrease of urticaria pigmentosa on cladribine. Remarkable reduction of urticaria pigmentosa lesions in patient A before treatment (A) and after 6 cycles of cladribine (B) and in patient D before treatment (C) and after 6 cycles of cladribine (D). Brown remnants of lesions are still seen after 6 months, consisting of predominantly melanocytes with hardly any mast cells present, confirmed in skin biopsies.
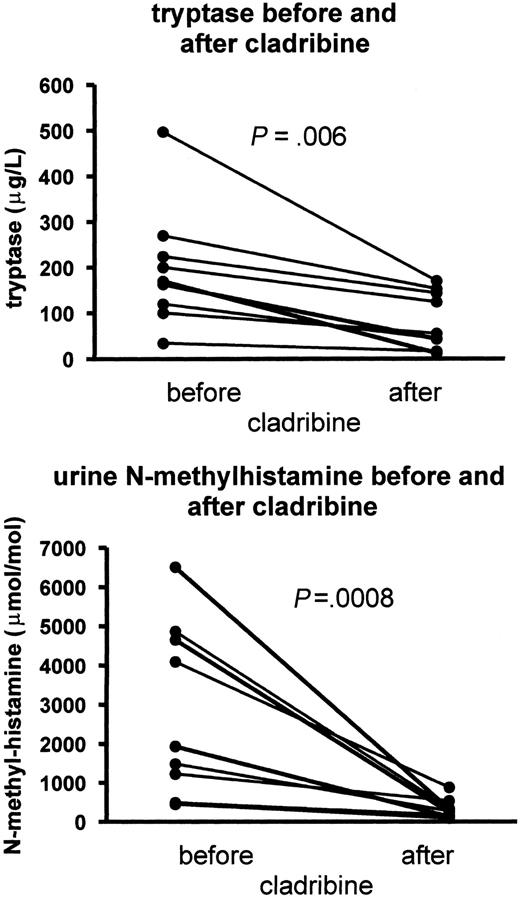
In all patients mast cell parameters such as serum tryptase and urinary histamine metabolite excretion decreased markedly (Figure 2), but in none a complete normalization of all 3 markers simultaneously was seen (Table 2). In most patients the response was remarkably fast with a steep decrease of serum tryptase and urinary N-methylhistamine and N-methylimidazoleacetic acid secretion already after the first cladribine cycle. The mean serum tryptase and urinary N-methylhistamine and N-methylimidazoleacetic acid concentrations, respectively, dropped from 198 μg/L before treatment to 85 μg/mL (P = .006) after treatment and from 2614 μmol/mol before treatment to 312 μmol/mol after treatment (P = .008) (Figure 2). N-methylhistamine decreased to a larger extend than N-methylimidazoleacetic acid (P = .019 after the third cycle). Moreover, most patients simultaneously experienced a marked improvement in their histamine-related symptoms. The median time to the best response was around 6 months, and in most of the patients the response is ongoing. However, patient E developed a relapse of his disease after an initial impressive response. This patient was suffering from severe fatigue, daily vomiting because of gastric complaints with 12-kg weight loss during the past 6 months, hepatosplenomegaly, retroperitoneal lymphadenopathy, anemia, and thrombocytopenia because of more than 75% mast cell involvement in the bone marrow. Already after the first cycle of cladribine he improved markedly, and his fatigue and gastric complaints vanished. He rapidly gained weight and resumed full-time work and sporting activities after the fourth cycle. He was without symptoms after the sixth cycle for about 6 months, until he experienced vague gastric complaints in parallel with an increase in the urinary histamine metabolite excretion and serum tryptase levels. Because of a further increase of symptoms and laboratory abnormalities, the cladribine was resumed 3 months later, immediately followed by a second response. More very impressive reactions were seen, such as in the eldest patient (case I), who was on intensive care because of severe anaphylactic reactions, cardiomegaly, and therapy-resistant atrial fibrillation. She responded immediately with a decrease of urinary methylhistamine secretion from 6506 to 184 μM/M creatinine and N-methylimidazoleacetic acid from 58.6 to 7.2 mmol/mol creatinine already after the first 5 days of cladribine. Presently having just finished the sixth course, she feels well and does not experience anaphylactic attacks anymore, the heart size decreased, atrial fibrillation was controlled with amiodarone, which could be stopped later on, and her skin showed a complete clearance of the urticaria pigmentosa.
Serum tryptase and urinary methylhistamine responses on cladribine. Levels of serum tryptase and urinary methylhistamine excretion before and after 6 cladribine courses. Normal upper level of tryptase = 13.5 μg/L; N-methylhistamine = 150 μmol/mol creatinine.
Serum tryptase and urinary methylhistamine responses on cladribine. Levels of serum tryptase and urinary methylhistamine excretion before and after 6 cladribine courses. Normal upper level of tryptase = 13.5 μg/L; N-methylhistamine = 150 μmol/mol creatinine.
Adverse events
Pancytopenia with WHO grades 3-4 neutropenia, thrombocytopenia, and anemia requiring transfusions was observed in cases A, D, and G. In case D allogeneic stem cell transplantation was performed, not only because of persisting pancytopenia but also because of an accompanying MDS with trisomy 8. Two patients experienced neutropenic fever, one patient a herpes zoster infection (she was not taking valacyclovir), one patient a thrombosis of the vena jugularis interna, and one patient a severe toxicodermia after the second and third cycles, simulating Sweet syndrome and leading to discontinuation of the trial therapy. Toxicodermia because of co-trimoxazole was excluded, and the patient was successfully treated with prednisone.
Discussion
We have shown in a series of 10 patients with 3 different subtypes of systemic mastocytosis that cladribine is an effective cytoreductive drug. Given the heterogeneity of the disease, here only 3 patients per group were enrolled. Until now there is no curative option for systemic mastocytosis.4,13,22 Generalized therapy guidelines for this disease cannot be given, because of the wide clinical disease heterogeneity frequently asking for an individually tailored approach. In most patients, indolent mastocytosis is usually controlled with drugs that counteract mediator effects or mediator production in those who suffer from mediator-related symptoms. Evidently, the beneficial effects of cytoreductive therapy have to be carefully balanced against the risks and adverse events. In our study, cytoreductive therapy was applied because all other drugs did not work in controlling serious, often life-threatening symptoms. Although IFN-alfa is probably the preferred drug for most patients in whom cytoreduction of the mast cell infiltrates is warranted, the side effects and required long duration can hamper application and continuation of this drug in many patients.23 The report by Tefferi et al14 advocating the use of cladribine seemed, therefore, an attractive alternative. We could, in a multicenter setting, confirm these results and observed an impressive positive effect of cladribine in all patients treated, although in none a definite complete remission was seen. Most patients showed a rapid decrease of mast cell infiltration and activation, already after the first 5 days of cladribine, if the biochemical parameters of mast cell activity were taken into account. The excretion of N-methylhistamine fell more than that of N-methylimidazaoleacetic acid (the major metabolite of histamine), which suggests an increase in the conversion from N-methylhistamine to N-methylimidazaoleacetic acid. In parallel with this biochemical decrease, symptoms of anaphylactic attacks, flushes, fatigue, and gastrointestinal complaints also responded rapidly, frequently after the first course of chemotherapy.
Several patients in this trial did not fulfill the criteria of aggressive mastocytosis and should according to proposed guidelines,22 therefore, not have been candidates for cytoreductive chemotherapy. However, the histamine-related symptoms were considered therapy resistant, causing much suffering and interference with daily life because of recurrent collapses, invalidating fatigue, abdominal complaints, and diarrhea. All these symptoms responded remarkably well on the cladribine therapy. Remarkably, all 3 patients with aggressive mastocytosis also had urticaria pigmentosa, whereas these patients often present without skin lesions.22 One of the 3 patients presented with skin symptoms for more than 10 years before the final diagnosis of systemic mastocytosis was made.
Despite the adverse events, cladribine was remarkably well tolerated in half of the patients. Because of the known T-cell lymphocytopenia, all patients received co-trimoxazole, antifungal, and later on also antiviral drugs prophylactically. Side effects were mainly related to bone marrow suppression, which probably could have been prevented if cladribine had been given at longer intervals. In particular the patient who developed bone marrow aplasia should have been offered a longer interval between the cladribine courses, because he was not fully recovered before the next cycle was given. Tefferi et al14 gave the drug at 6-month intervals. In contrast, we initially selected a more frequent (monthly) scheme as has been applied for Langerhans histiocytosis.24 A favorable toxicity profile was observed in these patients, with neutropenia in 7 of 13 patients, but with only one documented infection (dermal herpes zoster). Severe anemia and thrombocytopenia were not reported. The bone marrow aplasia observed in our study is, therefore, remarkable and could be caused by mast cell infiltrates in the bone marrow (all patients) or by the associated clonal hematologic non-mast cell lineage disease (3 patients). Bone marrow involvement in Langerhans histiocytosis is very rare.
Given the fact that in several patients the response is continuing, more time is probably required for further reduction of the mast cell load, similarly to the late responses seen after cladribine therapy in hairy cell leukemia. However, one patient with aggressive disease experienced a rapid relapse. Evidently, experience with the dose-related (adverse) effects of this drug is required to find an optimal treatment scheme. In addition, the combination of cladribine with or followed by interferon-alfa needs to be explored. Moreover, the mechanism of the beneficial effect of cladribine needs to be studied. It is tempting to speculate that the cladribine-induced T-cell reduction plays a role in the elimination of important mast cell growth factors, as interleukin 3 (IL-3) and IL-4 are important cytokines for mast cell growth.6,25
In conclusion, cladribine seems effective in most patients with systemic mastocytosis, both with the indolent form, the form with associated clonal hematologic non-mast cell lineage disease (SM-AHNMD), and the aggressive form. Cladribine should be recommended for patients with aggressive systemic mastocytosis, but it may not be an ideal drug for those with indolent forms of the disease. For these categories, the risk of adverse events needs to be balanced against the assumable benefits. These first results justify a larger trial, preferably in an international setting. The new initiative for a European Competence Network on Mastocytosis could offer an ideal setting for the development of better dose schemes and other new drugs for patients with severe forms of systemic mastocytosis.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1699.
Supported by the Noarber Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Dr Philip Kluin (Department of Pathology, University Hospital Groningen) for the bone marrow analyses; Dr Ido Kema and Mr Henk Breukelmans (Central Laboratory, University Hospital Groningen) for their help in the c-kit mutation, the tryptase, and histamine metabolite measurements; Dr T. van Maanen for referral of one of the patients; and Ms Chantal Rison for her help with the data analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal