Abstract
The tissue factor (TF)–initiated coagulation pathway plays important roles in hemostasis, inflammation, metastasis, and angiogenesis. Phosphorylation of the TF cytoplasmic domain is functionally relevant in metastasis. How TF cytoplasmic domain phosphorylation downstream of protein kinase C (PKC) activation is regulated in primary vascular cells remains poorly understood. Here, phosphorylation of Ser258, rather than the PKC consensus site Ser253, is identified as the major conformational switch required for recognition by a phosphorylation-specific antibody. With this novel reagent, we demonstrate that the TF cytoplasmic domain is primarily unphosphorylated in confluent endothelial cells. TF cytoplasmic domain phosphorylation can occur in the absence of the autologous TF transmembrane and extracellular domains but requires maturation of TF in the Golgi compartment and cell surface expression. Site-directed mutagenesis and 2-bromopalmitate treatment provide evidence that palmitoylation of the cytoplasmic Cys245 is a negative regulatory mechanism of Ser258 phosphorylation. Profiling with PKC-selective inhibitors identifies PKCα as important for TF cytoplasmic domain phosphorylation. Mutagenesis of protein kinase consensus sites are consistent with a model in which PKC-dependent phosphorylation of Ser253 enhances subsequent Ser258 phosphorylation by a Pro-directed kinase. Thus, cell surface location–dependent phosphorylation of the TF cytoplasmic domain is regulated at multiple levels.
Introduction
Tissue factor (TF) initiates arterial and venous thrombogenic cascades and thereby is a major pathogenic factor in cardiovascular disease.1 In addition, the TF initiation reaction has unique cell signaling properties mediated by the activation of protease-activated receptors (PARs).2,3 These cell signaling events likely contribute to inflammatory escalation in sepsis.4-7 TF also plays important roles in tumor cell metastasis and potentially in angiogenesis.8,9 Within the vasculature TF is found up-regulated in monocytes during inflammation and in endothelial cells in severe sepsis10 and angiogenesis.9 Extensive biochemical and structural data have yielded a detailed view of the extrinsic activation complex,11-13 but the structural features of the TF cytoplasmic domain are poorly defined.
A functional role for the TF cytoplasmic domain was first documented in hematogenous metastasis. TF-dependent metastasis14 involves both extracellular proteolysis and the presence of the TF cytoplasmic domain.15,16 In part, the metastatic function of TF depends on the cytoplasmic phosphorylation sites Ser253 and Ser258. Mutating all cytoplasmic Ser residues to Ala reduced metastasis, whereas charge conversion to Asp, which may mimic the phosphorylated state, did not reduce metastasis.15 These data in a Chinese hamster ovary metastasis model were confirmed in a human melanoma cell model.17 However, the procoagulant activity of TF is not influenced by cytoplasmic domain mutations,15 and regulation of proteolytic function of the TF-initiation complex by de-encryption also does not depend on the TF cytoplasmic tail.18,19 Furthermore, proteolytic signaling of the TF-VIIa complex, shown to involve PAR2,20,21 does not require the TF cytoplasmic domain in heterologous expression systems.22-24 Mice that lack the cytoplasmic domain of TF also do not display early embryonic lethality,25,26 as observed in the TF knock-out,27-29 consistent with the notion that neither initiation of coagulation nor protease-dependent cell signaling require the TF tail.
Conversely, phosphorylation of the TF cytoplasmic domain occurs in response to cell signaling. Protein kinase C (PKC) activation induces TF cytoplasmic domain phosphorylation in transfection models and tumor cells.30 TF phosphorylation involving PKC may occur at Ser253, which fits the consensus sequence for PKC phosphorylation. However, partial biochemical isolation of a kinase activity in glioblastoma cells demonstrated radioactive phosphate incorporation into multiple Ser residues in the TF cytoplasmic domain, and inhibitor studies suggested that in tumor cells a kinase other than PKC phosphorylates TF.31 Consistent with this notion, Ser258 in human TF is not a consensus PKC phosphorylation site but was suggested to be recognized by a Pro-directed kinase.30 Phosphorylation of TF in response to PMA (phorbol 12-myristate 13-acetate) stimulation may be explained by the activation of a Pro-directed kinase downstream of PKC. Phosphorylation of the TF cytoplasmic domain has been primarily analyzed in tumor cells, but little is known about the regulation of TF cytoplasmic domain phosphorylation in primary vascular cells. In the present study we address the following questions: Is TF constitutively phosphorylated in primary human endothelial cells? Does TF become phosphorylated on the cell surface or in intracellular compartments? How is phosphorylation of TF regulated?
Materials and methods
Proteins and antibodies
Recombinant factor VII and plasma X were purified as described.32,33 Chromogenic substrate Spectrozyme FXa was purchased from American Diagnostics (Greenwich, CT). Peptides encoding the TF cytoplasmic domain were synthesized as carboxyl amides, purified by reverse-phase high-performance liquid chromatography, and the peptide composition was confirmed by mass spectrometry. Kinase inhibitors and reagents for deglycosylation were purchased from Calbiochem (La Jolla, CA). Methyl-β–cyclodextrin and 2-bromopalmitate and the anti–interleukin 2 receptor α chain (anti–IL-2R) antibody for Western blotting (used at 1 μg/mL) were purchased from Sigma-Aldrich (St Louis, MO). Monoclonal and polyclonal antibodies to TF were previously characterized.34 7G7B6 monoclonal antibody to the small subunit of the IL-2R (American Type Culture Collection, Rockville, MD) was purified on immobilized protein A followed by ion exchange chromatography on MonoQ.
Antibodies to the TF cytoplasmic domain were generated by repeated immunization of rabbits with nonphosphorylated or phosphorylated peptides that were thioester coupled to keyhole limpet hemocyanin (KLH). Antisera were tested for reactivity with purified peptides in a dot blot assay. Antisera raised against wild-type and Ser253-phosphorylated peptides showed equal reactivity with nonphosphorylated and phosphorylated peptides. Antisera against Ser258 and Ser253/Ser258 phosphorylated peptides showed selective reactivity with phosphorylated TF cytoplasmic domain. However, the antiserum against Ser258 single-phosphorylated peptide showed increasing cross-reactivity with nonphosphorylated peptides in the course of the immunization protocol, suggesting that the conformation of the double-phosphorylated peptide is additionally stabilized by the phosphate group at Ser253. For further studies, immunoglobulin fractions were prepared from the antiserum against wild-type and Ser253/Ser258 double-phosphorylated peptides that were routinely used at 1 μg/mL for Western blot analysis. There was no difference in specificity between immunoglobulin G (IgG) or antiserum, based on dot blot assays with purified peptides. Phosphorylation-specific antibody was further affinity purified on Asp253/258 phosphorylation-mimicking peptides coupled to UltraLink Iodoacetyl beads (Pierce, Rockford, IL) for immunohistochemistry. A phosphorylation-specific antibody (anti–P-TFCD) was eluted with 0.1 M glycine, pH 2.7; neutralized immediately; and dialyzed against Tris-buffered saline (TBS) for storage. Specificity of the immunopurified antibody was confirmed by Western blotting with control samples of phosphorylated or nonphosphorylated TF expressed in endothelial cells.
Antibody specificity was tested by dot blot assay. Briefly, peptides were suspended in H2O and concentration was determined by absorbance (OD280) measurement. To demonstrate specificity of Ser phosphorylation, peptides were dephosphorylated by potato acid phosphatase in 100 mM MES (2-(N-morpholino) ethanesulfonic acid), pH 6.0, for 30 minutes at room temperature. One microliter each of different peptide concentrations (0.04-1 μg) were spotted on a nitrocellulose membrane that was hybridized with 1:1000 diluted rabbit antiserum or purified IgG at 1 to 2 μg/mL followed by our standard Western blotting hybridization technique.
Generation of adenoviral vectors
Chimeric receptors containing the extracellular and transmembrane domains of the small subunit of IL-2 receptor connected to the wild-type or mutant tissue factor cytoplasmic domains have been described previously.35 Mutants of the cytoplasmic domain of full-length TF were generated by site-directed mutagenesis of wild-type TF in CDM8 as described previously36 and were used for subcloning into pShuttle. The inserts containing the cytomegalovirus (CMV) promoter plus the chimeric receptor or TF sequence were excised with NruI + XhoI (for IL-2R chimera) or NruI + NotI (for TF) and subcloned into pShuttle. One microgram of the pShuttle construct was linearized with PmeI and combined with 0.1 μg of supercoiled pAdEasy-1 and electroporated into Escherichia coli (BJ5183) for homologous recombination. Confirmed recombinants were linearized with PacI for transfection into HEK 293 (human embryonic kidney 293) cells (American Type Culture Collection) using calcium phosphate precipitation. Virus plaque formation was observed after 7 to 10 days and cells were harvested with EDTA (ethylenediaminetetraacetic acid), suspended in 1 mL Dulbecco modified Eagle medium (DMEM), and lysed by freeze/thawing. Supernatants served as crude virus stock and were further purified by 2 rounds of plaque purification. High-titer stocks were obtained by CsCl gradient purification, dialyzed against 10% glycerol in 10 mM Tris-buffered saline, and stored at -80°C. The presence of the correct insert was confirmed by polymerase chain reaction analysis and sequencing.
Adenoviral transduction of endothelial cells
Human vascular endothelial cells (HUVECs) were obtained from Clonetics (San Diego, CA) or Cascade Biologics (Portland, OR) and were grown up to passage 6 in endothelial growth medium (EGM; Clonetics) supplemented with 2% to 5% fetal bovine serum (FBS), 1 mg/mL hydrocortisone, 10 μg/mL human endothelial growth factor (hEGF), and 3 mg/mL bovine brain extract. HUVEC cells were plated one day before infection. Virus was added to cells in complete medium for 3 hours, washed twice with phosphate-buffered saline (PBS), and incubated in fresh complete medium. Cell lysates were analyzed for expression of the appropriate transgene by Western blotting.
Immunoprecipitation and Western blotting
Antibodies were directly coupled to tosyl-activated paramagnetic Dyna-beads M-450 (DYNAL, Lake Success, NY). For immunoprecipitations, cells were washed once in ice-cold PBS and lysed in lysis buffer (20 mM Tris, pH 7.4; 150 mM NaCl; 10% glycerol; 0.2% NP-40 [nonidet P-40]; phosphatase inhibitors [50 mM NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate]; and protease inhibitor cocktail tablet [Roche Diagnostics, Indianapolis, IN]) for 20 minutes on ice. Lysates were cleared by centrifugation and incubated with beads for 4 hours at 4°C. Protein complexes were washed 3 times with lysis buffer and once with PBS, pH 7.4. For deglycosylation, the washed beads were resuspended in 15.5 μL H2O, 5 μL 5 × assay buffer (250 mM NaHPO4, pH 7.5 for N-glycosidase F or pH 5.5 for Endoglycosidase H [Endo H]) and 1.25 μL denaturation buffer (2% sodium dodecyl sulfate [SDS], 1 M β-mercaptoethanol). The reaction was boiled for 5 minutes and cooled down prior to addition of 1.25 μL Triton-X 100 and 2 μL N-glycosidase F or 1.25 μL H2O and 2 μL Endo H and incubated overnight at 37°C. Immunoprecipitates were resuspended in 2 × reducing sample buffer for Western blot analysis.
Cell surface biotinylation
Two days after adenovirus infection, HUVECs were rinsed 3 times with ice-cold PBS (pH 7.4) and labeled twice with 0.5 mg/mL sulfo-N-hydroxy-succinimide-LC-biotin (Pierce) in PBS at 4°C for 30 minutes each. The reaction was quenched with 20 mM Tris, washed extensively, and protein was immunoprecipitated as described in the previous paragraph. For precipitation of the fraction that was biotinylated, protein was eluted with 0.1% SDS, diluted 10-fold in lysis buffer, and incubated with streptavidin-coupled sepharose beads for 2 hours at 4°C. Beads were washed as for lysis buffer and resuspended in 2 × reducing sample buffer for Western blot analysis with specific antibody or streptavidin–horseradish peroxidase (SA-HRP) to detect the biotinylated cell surface fraction.
Confocal microscopy
For immunofluorescence, cells were grown on fibronectin-coated coverslips, transduced with adenovirus, and stimulated after 2 days. For staining, cells were washed in ice-cold TBS and stained with monoclonal anti–IL-2R antibody 7G7B6 in TBS, 5% bovine serum albumin (BSA) at 4°C. Cells were then fixed with 3.7% paraformaldehyde for 15 minutes at room temperature and permeabilized with 0.05% saponin in TBS for 10 minutes on ice. Cells were costained with anti–IL-2R (7G7B6 at 20 μg/mL) and rabbit anti–P-TFCD (10 μg/mL) for 1 hour on ice and counterstained with secondary antibodies (12.5 μg/mL fluorescein isothiocyanate [FITC]–labeled goat antimouse IgG and TRITC [tetramethylrhodamine-5-isothiocyanate]–labeled donkey antirabbit IgG) in TBS, 5% BSA for 1 hour on ice; washed extensively; and mounted in Slowfade for confocal laser scanning microscopy (MRC600; Biorad, Cambridge, MA).
Results
Generation of a phosphorylation-specific antibody to the TF cytoplasmic domain
We generated a phosphorylation-specific antibody in order to define whether the TF cytoplasmic domain is constitutively phosphorylated in primary endothelial cells and to characterize the regulation of TF cytoplasmic domain phosphorylation. Antibodies were raised in rabbits against KLH-coupled unphosphorylated or Ser253/Ser258 double-phosphorylated peptides corresponding to residues 245-263 of the TF cytoplasmic domain. Figure 1 demonstrates that the antiserum to nonphosphorylated peptide did not distinguish between phosphorylated and nonphosphorylated peptide. In contrast, the antiserum to the phosphorylated TF cytoplasmic domain (anti–P-TFCD) showed more than 50-fold stronger reactivity with the phosphorylated peptide. Individually phosphorylated peptides defined that phosphorylation of Ser258 is sufficient to induce the conformation recognized by anti–P-TFCD. To further demonstrate specificity of the antibody, phosphorylated peptides were dephosphorylated with potato acid phosphatase. Dephosphorylated peptides were no longer recognized by the phosphorylation-specific antibody, although reactivity was fully preserved with the antibody that was not phosphorylation specific. Rabbits were independently immunized with single-phosphorylated peptides. Antiserum to Ser253 single-phosphorylated peptide did not show phospho-specificity, whereas antiserum to Ser258 phosphorylated peptides initially yielded reactivity similar to the antiserum to double-phosphorylated peptide. Together, these data suggest a major role of Ser258 phosphorylation in inducing a conformational change in the TF cytoplasmic domain.
Specificity of TFCD and P-TFCD antibodies in dot blot assays. TF cytoplasmic domain peptides (nonphosphorylated, double-phosphorylated at Ser253 and Ser258 [P-Ser253, 258] or single-phosphorylated peptides (P-Ser253 or P-Ser258) with or without pretreatment with potato acid phosphatase [PAP]) were spotted as 1-μL dots at 3 different concentrations on a nitrocellulose membrane that was hybridized with 1:1000 diluted antiserum to the unphosphorylated (αTFCD, left) or the phosphorylated (αP-TFCD, right) TF cytoplasmic domain.
Specificity of TFCD and P-TFCD antibodies in dot blot assays. TF cytoplasmic domain peptides (nonphosphorylated, double-phosphorylated at Ser253 and Ser258 [P-Ser253, 258] or single-phosphorylated peptides (P-Ser253 or P-Ser258) with or without pretreatment with potato acid phosphatase [PAP]) were spotted as 1-μL dots at 3 different concentrations on a nitrocellulose membrane that was hybridized with 1:1000 diluted antiserum to the unphosphorylated (αTFCD, left) or the phosphorylated (αP-TFCD, right) TF cytoplasmic domain.
Golgi maturation mediated by the TF cytoplasmic domain in endothelial cells is independent of phosphorylation
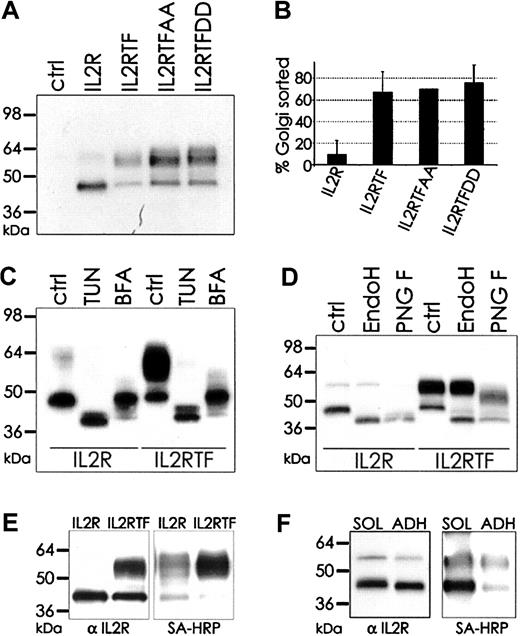
We first analyzed phosphorylation of the TF cytoplasmic domain in the absence of the autologous TF extracellular domain. To this end, a chimeric protein (IL-2RTF) consisting of the TF cytoplasmic domain fused to a cytoplasmic domain truncated form of the murine IL-2R was generated.35,37 The phosphorylation status of Ser253 and Ser258 in IL-2RTF was manipulated by either preventing phosphorylation by Ala replacement or by mimicking the negative charge by Asp mutation. Adenoviral gene delivery achieved efficient expression of the chimeric molecules in endothelial cells, as shown by Western blotting for the IL-2R extracellular domain (Figure 2A). Transduction with IL-2R yielded a major protein band consistent with the predicted molecular mass, whereas the chimeras with the 21-residue TF cytoplasmic domain migrated as a double band with a heterogenous high–molecular-weight form in addition to the sharper band of similar mobility as IL-2R. Mutation of Ser253 and Ser258 did not change the relative amount of the slower migrating form, based on densitometric quantitation of the Western blots (Figure 2B). IL-2R and IL-2RTF treatment with tunicamycin or brefeldin A, which block protein transport into the Golgi compartment, demonstrated that the size heterogeneity resulted from differences in Golgi-dependent modifications (Figure 2C). Consistent with addition of N-linked complex oligosaccharides in the Golgi, IL-2R and the low–molecular-weight form of IL-2RTF were sensitive to Endo H, which cannot cleave complex Golgi-modified oligosaccharides. In contrast, the high–molecular-weight form of IL-2RTF was Endo H insensitive and partially sensitive to N-glycosidase F, which cleaves some complex oligosaccharides (Figure 2D). This demonstrates that the presence of the TF cytoplasmic domain promotes Golgi-dependent maturation of N-linked carbohydrate modifications in endothelial cells.
TF cytoplasmic domain–mediated apical sorting in endothelial cells is independent of phosphorylation. (A) IL-2R, IL-2R fused with wild-type TF cytoplasmic domain (IL2RTF), IL-2R chimeras with Ser253/258 mutation to Ala in the TF cytoplasmic domain (IL2RTFAA), and IL-2R chimeras with Ser253/258 mutation to Asp in the TF cytoplasmic domain (IL2RTFDD) were transduced by adenovirus into HUVECs and protein expression after 48 hours was detected by Western blotting with αIL-2R antibody. Untransduced cells (ctrl) do not express the murine IL-2R. (B) High–molecular weight (Golgi matured) versus low–molecular weight (unmodified) protein was quantified by densitometry and blotted as percentage of Golgi-matured protein. Mean and standard deviation from 3 independent experiments is shown. (C) Mobility differences of IL-2R and IL-2RTF are due to N-linked glycosylation. Glycosylation was blocked by incubation with 10 μg/mL tunicamycin (TUN) or 10 μg/mL brefeldin A (BFA) for 48 hours. IL-2R in untreated (ctrl) and treated cells was detected with αIL-2R antibody. (D) Deglycosylation of immunoprecipitated protein with Endo H or N-glycosidase F (PNG F) was followed by Western blotting with αIL-2R antibody. (E) IL-2R–and IL-2RTF–transduced HUVECs were apically biotinylated and immunoprecipitated with αIL-2R antibody (7G7B6). Efficiency of precipitation was determined by Western blotting with αIL-2R (left) and biotinylation was detected by streptavidin-HRP (SA-HRP, right). (F) IL-2R–transduced cells were biotinylated as monolayer (ADH) or in solution (SOL), immunoprecipitated and resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) for subsequent Western blot analysis with αIL-2R (left) and SA-HRP (right).
TF cytoplasmic domain–mediated apical sorting in endothelial cells is independent of phosphorylation. (A) IL-2R, IL-2R fused with wild-type TF cytoplasmic domain (IL2RTF), IL-2R chimeras with Ser253/258 mutation to Ala in the TF cytoplasmic domain (IL2RTFAA), and IL-2R chimeras with Ser253/258 mutation to Asp in the TF cytoplasmic domain (IL2RTFDD) were transduced by adenovirus into HUVECs and protein expression after 48 hours was detected by Western blotting with αIL-2R antibody. Untransduced cells (ctrl) do not express the murine IL-2R. (B) High–molecular weight (Golgi matured) versus low–molecular weight (unmodified) protein was quantified by densitometry and blotted as percentage of Golgi-matured protein. Mean and standard deviation from 3 independent experiments is shown. (C) Mobility differences of IL-2R and IL-2RTF are due to N-linked glycosylation. Glycosylation was blocked by incubation with 10 μg/mL tunicamycin (TUN) or 10 μg/mL brefeldin A (BFA) for 48 hours. IL-2R in untreated (ctrl) and treated cells was detected with αIL-2R antibody. (D) Deglycosylation of immunoprecipitated protein with Endo H or N-glycosidase F (PNG F) was followed by Western blotting with αIL-2R antibody. (E) IL-2R–and IL-2RTF–transduced HUVECs were apically biotinylated and immunoprecipitated with αIL-2R antibody (7G7B6). Efficiency of precipitation was determined by Western blotting with αIL-2R (left) and biotinylation was detected by streptavidin-HRP (SA-HRP, right). (F) IL-2R–transduced cells were biotinylated as monolayer (ADH) or in solution (SOL), immunoprecipitated and resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) for subsequent Western blot analysis with αIL-2R (left) and SA-HRP (right).
To test whether Golgi-dependent modifications are related to apical sorting of TF, confluent endothelial cells were surface biotinylated by addition of N-hydroxy-succinimide-biotin to the top of the confluent monolayer. Immunoprecipitation with IL-2R antibody and streptavidin blots showed preferential modification of the high–molecular-weight form of the wild-type TF chimera and also revealed a minor fraction of a highly modified IL-2R form (Figure 2E). The faster-migrating forms of IL-2RTF and IL-2R were equally recovered in the anti–IL-2R immunoprecipitate but showed substantially less modification with biotin. Comparison of surface biotinylation of IL-2R in adherent versus suspended cells confirmed surface expression of the nonmatured form in suspended cells (Figure 2F). Surface expression was further supported by flow cytometry of nonpermeabilized cells that demonstrated similar levels of IL-2R and IL-2RTF (data not shown). These data excluded that the forms without Golgi modifications were retained predominantly in intracellular compartments. Thus, the TF cytoplasmic domain enhances Golgi-dependent modification of N-linked carbohydrates in a phosphorylation-independent manner and supports apical targeting.
Golgi transport is required for TF cytoplasmic domain phosphorylation
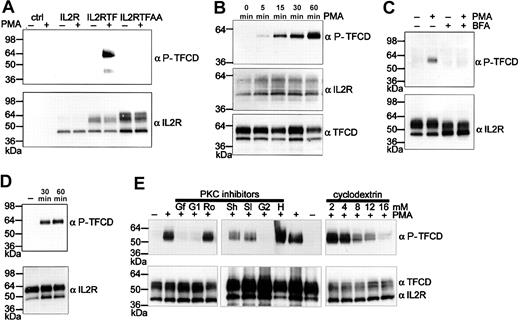
PMA stimulation is known to induce phosphorylation of the TF cytoplasmic domain.30,31 In Figure 3A, cells transduced with different IL-2R chimeras were stimulated with PMA for 1 hour. No reactivity was seen in cells that expressed Ser253/Ser258–mutated chimera, demonstrating that the antibody does not cross-react with nonphosphorylated TF cytoplasmic domain expressed in cells. In IL-2RTF–expressing cells no constitutive Ser258 phosphorylation was detectable. However, phosphorylation was observed upon PMA stimulation, demonstrating that the TF cytoplasmic domain can be phosphorylated in the absence of the autologous extracellular domain. Notably, only chimeric receptor with Golgi-matured N-linked carbohydrates became phosphorylated, which parallels findings for Ser phosphorylation of β secretase.38 Time course experiments (Figure 3B) demonstrated a gradual accumulation of phosphorylated TF, but the cytoplasmic domain of chimera without Golgi-modification was not phosphorylated at any time. Note that the antibody to the unphosphorylated TF tail confirmed the presence of the cytoplasmic domain of TF in the non Golgi-targeted form. Furthermore, interruption of protein transport to the Golgi compartment with brefeldin A (Figure 3C) prevented TF cytoplasmic domain phosphorylation. In order to test whether phosphorylation of the chimera leads to rapid internalization, cell surface pools of the chimera were detected by biotinylation. IL-2RTF–transduced endothelial cells were treated for 1 hour with PMA at 37°C and subsequently surface biotinylated on ice to prevent further internalization. IL-2RTF was sequentially immunoprecipitated with anti–IL-2R followed by immobilized streptavidin (Figure 3D). Similar amounts of IL-2RTF were recovered from unstimulated and stimulated cells and the recovered chimera showed prominent Ser258 phosphorylation. Thus, TF cytoplasmic phosphorylation is not sufficient to rapidly reduce surface expression of IL-2RTF.
Phosphorylation of the TF cytoplasmic domain in the absence of the autologous extracellular domain requires Golgi sorting. (A) Endothelial cells were untransduced (ctrl) or transduced 48 hours earlier with IL-2R, IL-2RTF, or IL-2RTFAA. Cells were stimulated with 20 ng/mL PMA for 1 hour and lysed with sample buffer for Western blotting with 1 μg/mL phosphorylation-specific anti-TF cytoplasmic domain antibody (αP-TFCD) and with αIL-2R antibody as loading control. (B) Time course experiment of PMA-stimulated endothelial cells expressing IL-2RTF. Loading was controlled by Western blotting with αTFCD antibody to the unphosphorylated TF cytoplasmic domain. (C) Disruption of Golgi transport by BFA prevents TF cytoplasmic domain phosphorylation. Note the difference in glycosylation of IL-2RTF in BFA-treated cells. (D) Phosphorylated TF cytoplasmic domain is expressed on the apical surface of endothelial cells. IL-2RTF–transduced HUVECs were stimulated with PMA for the indicated times. After surface biotinylation at 4°C, IL-2RTF was sequentially precipitated with αIL-2R antibody and streptavidin beads followed by Western blot analysis with αIL-2R or αP-TFCD antibodies. (E) PMA-induced phosphorylation of the TF cytoplasmic domain is dependent on PKCα and membrane composition. Cells were pretreated with the indicated concentrations of the cholesterol-depleting reagent methyl-β–cyclodextrin (cyclodextrin) or with the indicated kinase inhibitors for 30 minutes. PKC inhibitors were used at the following concentrations: 100 nM GF109203X (Gf), 1 μMGö6976 (G1), 6 μM Rottlerin (Ro), 2 μM Hispidin (H), 1 μMGö6983 (G2), and different concentrations of Safingol at 50 μM (Sl) or 200 μM (Sh). After PMA induction for 1 hour, cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αIL-2R antibodies. In the middle panel, αTFCD antibody was used as loading control instead of αIL-2R.
Phosphorylation of the TF cytoplasmic domain in the absence of the autologous extracellular domain requires Golgi sorting. (A) Endothelial cells were untransduced (ctrl) or transduced 48 hours earlier with IL-2R, IL-2RTF, or IL-2RTFAA. Cells were stimulated with 20 ng/mL PMA for 1 hour and lysed with sample buffer for Western blotting with 1 μg/mL phosphorylation-specific anti-TF cytoplasmic domain antibody (αP-TFCD) and with αIL-2R antibody as loading control. (B) Time course experiment of PMA-stimulated endothelial cells expressing IL-2RTF. Loading was controlled by Western blotting with αTFCD antibody to the unphosphorylated TF cytoplasmic domain. (C) Disruption of Golgi transport by BFA prevents TF cytoplasmic domain phosphorylation. Note the difference in glycosylation of IL-2RTF in BFA-treated cells. (D) Phosphorylated TF cytoplasmic domain is expressed on the apical surface of endothelial cells. IL-2RTF–transduced HUVECs were stimulated with PMA for the indicated times. After surface biotinylation at 4°C, IL-2RTF was sequentially precipitated with αIL-2R antibody and streptavidin beads followed by Western blot analysis with αIL-2R or αP-TFCD antibodies. (E) PMA-induced phosphorylation of the TF cytoplasmic domain is dependent on PKCα and membrane composition. Cells were pretreated with the indicated concentrations of the cholesterol-depleting reagent methyl-β–cyclodextrin (cyclodextrin) or with the indicated kinase inhibitors for 30 minutes. PKC inhibitors were used at the following concentrations: 100 nM GF109203X (Gf), 1 μMGö6976 (G1), 6 μM Rottlerin (Ro), 2 μM Hispidin (H), 1 μMGö6983 (G2), and different concentrations of Safingol at 50 μM (Sl) or 200 μM (Sh). After PMA induction for 1 hour, cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αIL-2R antibodies. In the middle panel, αTFCD antibody was used as loading control instead of αIL-2R.
PKC inhibitors and methyl-β–cyclodextrin treatment reduce TF cytoplasmic domain phosphorylation
In previous studies, PMA-induced phosphorylation was abolished by staurosporine, which inhibits PKC and other protein kinases. Ser253 is located within the consensus sequence of a PKC phosphorylation site, but Ser258 is probably the target for a Pro-directed kinase. Whether PKC regulates Ser258 phosphorylation remained unclear from the previous studies that measured incorporation of radioactive phosphate into the TF cytoplasmic domain. Endothelial cells express different isoforms of PKC. The general PKC inhibitor GF109203X (Figure 3E; Gf) and inhibitors for conventional PKCs (PKCα, β, and γ), Gö6976 (G1) or Gö6983 (G2), but not the PKCδ inhibitor Rottlerin (Ro), inhibited TF cytoplasmic domain phosphorylation. PKCμ is excluded by the fact that it is inhibited by Gö6976 but not Gö6983. The PKCβ inhibitor Hispidin (H) did not block phosphorylation, whereas increasing concentrations of the PKCα-inhibitor Safingol (50 μM [Sl] or 200 μM [Sh]) clearly reduced phosphorylation. Taking into consideration that PKCγ is not expressed in endothelial cells, these data suggest that TF cytoplasmic domain phosphorylation is predominantly dependent on PKCα activation in endothelial cells. Because PKCα is enriched in cholesterol- and glycosphingolipid-rich microdomains,39,40 we tested whether TF cytoplasmic domain phosphorylation is sensitive to drugs that are known to deplete cells of cholesterol. Pretreatment of endothelial cells for 30 minutes with methyl-β–cyclodextrin dose dependently decreased TF cytoplasmic domain phosphorylation without influencing overall expression levels of the IL-2RTF chimera. In addition, even at the highest concentration of methyl-β–cyclodextrin, cell viability by trypan blue exclusion was more than 95%. Nonspecific cell toxicity is therefore an unlikely explanation of the decreased phosphorylation levels. TF cytoplasmic domain phosphorylation thus appears to be dependent on intact cholesterol-containing microdomains.
Phosphorylation of the TF cytoplasmic domain in full-length TF
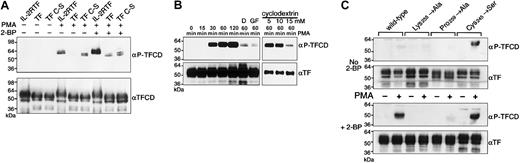
TF cytoplasmic domain phosphorylation was also analyzed in the context of the autologous transmembrane and extracellular domain with adenoviral constructs. Figure 4A shows that PMA-induced phosphorylation of full-length TF cytoplasmic domain in endothelial cells was barely detectable, which contrasts with the high levels of phosphorylation of the cytoplasmic domain in the IL-2RTF chimera. Note that both proteins were loaded at the same concentration, based on Western blotting for the nonphosphorylated TF cytoplasmic domain. We reasoned that either extracellular interactions or thioacylation of the cytoplasmic Cys245 could cause the low levels of phosphorylation of full-length TF. Bach et al41 have previously shown that the majority of TF isolated from brain is thioester bonded to either stearate or palmitate. Elimination of palmitoylation by Cys245Ser mutation indeed increased phosphorylation upon PMA stimulation. The palmitic acid analog 2-bromopalmitate (2-BP) is an inhibitor of fatty acylation42 and is routinely used as an inhibitor of protein palmitoylation.43-45 The 2-BP treatment did not change the phosphorylation of Cys245-mutated TF but increased phosphorylation of wild-type TF to levels observed with the Cys245 mutant (Figure 4A). In addition, under nonreducing conditions we detected a minor fraction of TF as dimer specifically in 2-BP–treated cells transduced with wild-type TF (data not shown), indicating that under these conditions the cytoplasmic cysteine residues were not thioacylated and capable of forming disulfide-linked dimers. Together, these data provide strong evidence that thioesterification of Cys245 negatively regulates PMA-induced phosphorylation of the TF cytoplasmic domain.
PMA-induced cytoplasmic domain phosphorylation in full-length TF. (A) IL-2RTF, TF, and cytoplasmic domain–mutated TF (Cys245Ser, TF C-S) transduced HUVECs were either incubated with 2-BP as indicated (+) immediately after transduction or were incubated without 2-BP (-). Forty-eight hours after transduction cells were either left untreated (-) or were stimulated for 1 hour with 20 ng/mL PMA (+). Cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTFCD antibodies. (B) TF C-S–transduced cells were preincubated with the indicated concentrations of methyl-β–cyclodextrin (cyclodextrin) or 100 nM GF109203X (GF) for 30 minutes. D indicates desensitation with 20 ng/mL PMA for 24 hours before PMA induction for the indicated times. Cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTF antibodies. (C) Wild-type TF and cytoplasmic domain mutants of TF (Lys255Ala, Pro259Ala, and Cys245Ser) were expressed in HUVECs. Immediately after transduction cells were incubated with 2-BP (bottom) or not treated (top) for 48 hours. After PMA induction for 1 hour, cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTF antibodies.
PMA-induced cytoplasmic domain phosphorylation in full-length TF. (A) IL-2RTF, TF, and cytoplasmic domain–mutated TF (Cys245Ser, TF C-S) transduced HUVECs were either incubated with 2-BP as indicated (+) immediately after transduction or were incubated without 2-BP (-). Forty-eight hours after transduction cells were either left untreated (-) or were stimulated for 1 hour with 20 ng/mL PMA (+). Cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTFCD antibodies. (B) TF C-S–transduced cells were preincubated with the indicated concentrations of methyl-β–cyclodextrin (cyclodextrin) or 100 nM GF109203X (GF) for 30 minutes. D indicates desensitation with 20 ng/mL PMA for 24 hours before PMA induction for the indicated times. Cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTF antibodies. (C) Wild-type TF and cytoplasmic domain mutants of TF (Lys255Ala, Pro259Ala, and Cys245Ser) were expressed in HUVECs. Immediately after transduction cells were incubated with 2-BP (bottom) or not treated (top) for 48 hours. After PMA induction for 1 hour, cells were lysed in 2 × reducing sample buffer and Western blot analysis was performed with αP-TFCD and αTF antibodies.
However, 2-BP treatment also increased phosphorylation of IL2RTF, consistent with the notion that the chimera was regulated by palmitoylation as well. Control experiments were performed to exclude that the differential phosphorylation observed upon modulation of thioacylation of Cys245 is due to reduced antibody reactivity with palmitoylated TF. Samples of PMA-treated cells expressing IL-2RTF were analyzed under nonreducing conditions or reduced with 50 mM dithiothreitol (DTT; our standard condition) or with 50 mM β-mercapto-ethanol, which has previously been shown to liberate palmitic acid from TF.41 There was no evidence for differential reactivity of the phosphorylation-specific antibody, which is not unexpected, because the immunogenic peptides were coupled to KHL through the amino-terminal Cys245. Notably, IL-2RTF in the presence or absence of 2-BP consistently showed higher phosphorylation than full-length TF. These data suggest that in addition to palmitoylation of the cytoplasmic domain, interactions of the TF extracellular and/or transmembrane domain may contribute to the regulation of TF cytoplasmic domain phosphorylation. Similar to IL-2RTF, Cys245-mutated TF showed a progressive increase in phosphorylation following PMA stimulation (Figure 4B), which was blocked by PKC inhibitors and overnight PMA desensitation of the PKC pathway. Since IL-2RTF phosphorylation was reduced by treatment with the cholesterol-depleting drug cyclodextrin, we wanted to test whether the reduced phosphorylation of full-length TF is due to location outside of cholesterol-rich rafts. In this case, full-length TF phosphorylation may not be sensitive to methyl-β–cyclodextrin. However, Figure 4B shows that phosphorylation of Cys245-mutated TF was still sensitive to methyl-β–cyclodextrin treatment, demonstrating that full-length TF and the IL-2RTF chimera are similarly regulated.
Ser258 is not a PKC consensus phosphorylation site, raising the question whether PKC is upstream of a kinase that directly phosphorylated Ser258 or whether phosphorylation of Ser253 by PKC is required to subsequently trigger phosphorylation of Ser258. To address this question, the recognition motif for proline-directed kinases was destroyed by Pro259Ala mutation, or the PKC consensus basic residue Lys255 was mutated to Ala. Phosphorylation of both mutants was analyzed in untreated and 2-BP–treated cells (Figure 4C). As expected from the data with wild-type TF, phosphorylation of Lys255- or Pro259-mutated TF cytoplasmic domain was barely detectable without 2-BP treatment. In the presence of 2-BP, phosphorylation of the Pro259-mutated TF cytoplasmic domain was severely reduced compared with wild-type TF, consistent with the previously suggested involvement of a Pro-directed kinase in Ser258 phosphorylation.30 Surprisingly, mutation of Lys255 within the PKC recognition motif also resulted in very poor phosphorylation of the TF cytoplasmic domain, indicating that efficient phosphorylation of Ser258 requires prior phosphorylation of Ser253.
Phosphorylated TF is cell surface expressed
The phosphorylation-specific antibody was affinity purified for immunohistochemical visualization of phosphorylated TF in cells. In Western blotting, this affinity-purified antibody did not show cross-reactivity with unphosphorylated TF but strongly recognized Cys245-mutated TF from PMA-stimulated cells. Figure 5 shows the location of phosphorylated TF by staining with the immunopurified antibody and counterstaining for the extracellular domain of TF or IL-2R, respectively, as a control for expression levels. TF and IL-2RTF were predominantly expressed on the cell surface. No reactivity of the antibody was obtained in cells that express Ser253/Ser258-mutated IL-2RTF, which cannot be phosphorylated. Unstimulated cells transduced with IL-2RTF were not stained with the antibody, consistent with the Western blot data that the TF cytoplasmic domain is not phosphorylated in unstimulated cells. Cells that were transduced with IL-2RTF uniformly showed TF cytoplasmic domain phosphorylation after PMA stimulation. Phosphorylation of wild-type TF was below the detection limit (data not shown), consistent with the extremely low levels of phosphorylation detected by Western blotting. In PMA-stimulated cells expressing Cys245-mutated TF, only some of the cells stained positive with the phosphorylation-specific antibody, a possible explanation for the reduced overall phosphorylation detected in Western blot analysis. However, similar to the IL-2R chimera, phosphorylated TF was cell surface localized. These data confirm the biochemical evidence that TF cytoplasmic domain phosphorylation occurs at the cell surface in endothelial cell monolayers and that phosphorylation is not sufficient to induce internalization of TF.
Phosphorylated TF is localized at the cell membrane. HUVECs were seeded on fibronectin-coated coverslips and transduced with IL-2RTF, IL-2RTF SS/AA (Ser253Ala/Ser258Ala), or the cytoplasmic domain mutant of full-length TF virus C-S TF (Cys245Ser). Forty-eight hours after transduction the cells were either left untreated or incubated for 1 hour with 20 ng/mL PMA (+PMA) prior to staining with directly labeled αTF 10H10 F(ab′)2-TRITC (TF) or mouse αIL-2R (7G7B6) followed by αmouse IgG-TRITC and rabbit αP-TFCD followed by αrabbit IgG-FITC antibodies for confocal laser scanning microscopy. Original magnification, × 60.
Phosphorylated TF is localized at the cell membrane. HUVECs were seeded on fibronectin-coated coverslips and transduced with IL-2RTF, IL-2RTF SS/AA (Ser253Ala/Ser258Ala), or the cytoplasmic domain mutant of full-length TF virus C-S TF (Cys245Ser). Forty-eight hours after transduction the cells were either left untreated or incubated for 1 hour with 20 ng/mL PMA (+PMA) prior to staining with directly labeled αTF 10H10 F(ab′)2-TRITC (TF) or mouse αIL-2R (7G7B6) followed by αmouse IgG-TRITC and rabbit αP-TFCD followed by αrabbit IgG-FITC antibodies for confocal laser scanning microscopy. Original magnification, × 60.
Discussion
In the present study, we provide novel insight into the regulation of TF phosphorylation in endothelial cells. The characterization of phosphorylation-specific antibodies to the TF cytoplasmic domain provided evidence that phosphorylation of Ser258, rather than Ser253, results in a unique conformation of the TF cytoplasmic domain that gives raise to the distinct phosphorylation specificity of the antibody. Antibodies raised to the nonphosphorylated TF cytoplasmic domain recognized both phosphorylated and nonphosphorylated peptides. The antibodies were raised against synthetic peptides but the antibodies displayed similar specificity in Western blotting, when the TF cytoplasmic domain was phosphorylated in endothelial cells by PMA stimulation. In addition, affinity-purified, phosphorylation-specific antibody was suitable to visualize phosphorylated TF in stimulated endothelial cells.
Our data demonstrate that the TF cytoplasmic domain is primarily nonphosphorylated in endothelial cells. Although our experiments employed heterologous expression of TF, we had also explored whether endogenous TF induced by tumor necrosis factor α (TNFα) stimulation is phosphorylated in endothelial cells. We did neither detect baseline phosphorylation nor phosphorylation upon PMA stimulation, which is consistent with the presented data showing highly inefficient phosphorylation of full-length TF and previous findings that the majority of TF is thioacylated in vivo.41 Previous studies found that endogenous TF in polarized endothelial cells is sorted apically.46,47 Heterologous expression of the TF cytoplasmic domain recapitulated the apical targeting of endogenous TF, as demonstrated by the finding that the TF cytoplasmic domain promoted apical targeting and Golgi-dependent maturation of N-linked carbohydrate of the heterologous IL-2R extracellular domain. Most significantly, only chimeras with Golgi-matured N-linked carbohydrate were phosphorylated, linking Golgi targeting to agonist-dependent phosphorylation of the TF cytoplasmic domain. Although the TF extracellular domain also carries complex N-linked carbohydrates,48 the glycosylation pattern is more complex due to the presence of 3 glycosylation sites49 versus 2 sites found in IL-2R. However, a faster migrating form of full-length TF can be detected (eg, Figure 4A) and this form was not phosphorylated, arguing that Golgi sorting is required for phosphorylation of full-length TF as well. The Golgi plays an important role in cholesterol transport and consistent with the involvement of Golgi transport, treatment with cyclodextrin, which depletes cells of cholesterol, reduced phosphorylation of the TF cytoplasmic domain.
Mutation of Cys245 and treatment with 2-BP provides concordant evidence that palmitoylation of the TF cytoplasmic domain suppressed phosphorylation. Palmitoylation may interfere with phosphorylation in several ways. Palmitoylation may position the TF cytoplasmic domain closer to the membrane and thus restrict access of the relevant kinases, as previously suggested for phosphorylation of the β-adrenergic receptor.50 Palmitoylation of the TF cytoplasmic domain may result in the constitutive association of a phosphatase with TF. However, this possibility is considered less likely because inhibition of potential Ser phosphatases with okadaic acid did not increase phosphorylation of wild-type TF (data not shown). Palmitoylation has been shown to regulate Golgi-dependent trafficking of certain transmembrane proteins,51 but palmitoylation of caveolin is not required for Golgi association and targeting to caveolae.52-54 However, palmitoylation of caveolin has recently been shown to regulate the Golgi trafficking of glycosyl-phosphatidylinositol (GPI)–anchored proteins.55 Palmitoylation of TF may similarly favor the association with caveolin-containing relative to other glycosphingolipid- and cholesterol-rich microdomains. As a consequence, relevant TF kinases, including PKCα, are more efficiently inhibited by the scaffolding domain of caveolin,56,57 resulting in inefficient phosphorylation of the TF cytoplasmic domain.
PKCα triggers an important pathway that induces TF cytoplasmic domain phosphorylation in endothelial cells. PMA stimulation recruits PKCα to caveolae,58 but PKCα membrane localization is not restricted to caveolae.40 TF is recruited to caveolae upon complex formation with TF pathway inhibitor (TFPI), but a fraction of TF can be detected in caveolae and in glycosphingolipid-rich microdomains in the absence of complex formation.34,59 While the requirement for Golgi targeting and the negative effect of cholesterol-depleting drugs suggest that raft localization is necessary for TF cytoplasmic domain phosphorylation, the dynamic trafficking of TF makes it difficult to define whether TF phosphorylation occurs in caveolae itself. Our data provide evidence that PMA-induced phosphorylation of TF in the absence of specific ligands occurs on the cell surface. However, phosphorylation-regulated internalization and/or recycling of TF may occur when physiologically relevant complexes are assembled with TF. Internalization and recycling of TF has been characterized and found to be independent of the TF cytoplasmic domain60 but these data do not necessarily exclude that phosphorylation plays a role in trafficking of TF. Domain deletions have been shown to sometimes eliminate both positive and negative regulatory functions, for example in the opioid receptor.61
There are possible routes by which PKC can be activated by signaling complexes specifically associated with TF. PKCα can be activated and recruited to syndecan-4,62 which is a relevant receptor for the TFPI on endothelial cells.63 Alternatively, TF-bound proteases can activate PARs, which through G-protein–coupled signaling recruit PKC following phospholipase C–dependent phosphatidylinositol hydrolysis. Importantly, depalmitoylation can be induced by G-protein receptor signaling,64 which may provide a mechanism to overcome the suppression of TF cytoplasmic domain phosphorylation by constitutive thioacylation of TF. The role of PKC in TF cytoplasmic domain phosphorylation has been debated.31 Indeed, mutation of Pro259 significantly reduced TF cytoplasmic domain phosphorylation, arguing in favor of a Pro-directed kinase as the relevant Ser258 kinase. However, mutation of the PKC consensus residue Lys255 also abolished TF cytoplasmic domain phosphorylation, consistent with a pathway in which phosphorylation of Ser253 by PKC is followed by Ser258 phosphorylation. PMA is a potent stimulator of PKC, but it remains an open question whether the Ser258 kinase is activated indirectly downstream of PKC or simultaneously activated by PMA. Our data provide novel information that palmitoylation of the TF cytoplasmic domain is a crucial regulator of a PKC-dependent pathway that leads to TF cytoplasmic domain phosphorylation.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-04-1149.
Supported by National Institutes of Health (NIH) grants HL-16411 and HL-60742. A.D. was the recipient of a fellowship from the Austrian Academy of Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pablito Tejada, Jennifer Royce, Dave Revak, and Cindi Biazak for excellent technical assistance and Barbara Parker for the preparation of figures. Dan Von Seggern kindly provided the viral backbone plasmids for adenovirus constructs.

![Figure 1. Specificity of TFCD and P-TFCD antibodies in dot blot assays. TF cytoplasmic domain peptides (nonphosphorylated, double-phosphorylated at Ser253 and Ser258 [P-Ser253, 258] or single-phosphorylated peptides (P-Ser253 or P-Ser258) with or without pretreatment with potato acid phosphatase [PAP]) were spotted as 1-μL dots at 3 different concentrations on a nitrocellulose membrane that was hybridized with 1:1000 diluted antiserum to the unphosphorylated (αTFCD, left) or the phosphorylated (αP-TFCD, right) TF cytoplasmic domain.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1149/6/m_h82335302001.jpeg?Expires=1769129513&Signature=XlUfZTorhxOi-T~Od~9o0skIra6MCAbr0u5x0JJmSmn4Z47UKKUFAW0zJfQSb8zajRbQhWNtq7OuW37fSMhaBXw17fGCp-vPONazbMwsndqcxHsCb1Lw5dXt9dtNf5mfJHV3oOBn0LXUMKRfVIiOQdqdcc8MTvOGzGVNQqVKzPGXTHpFRRMD4-kDkKjXQOsJKdqBbe2z8-s5V999bvR0Rh2UZyNRUkXzAxaRzyru0tiN6Bf8zrogV6YxaBrAek1a0XLP4FB1696h3GAxhxGawhxksMHsS-ln98nMxZGSvH0z46NmxzJovlh0Zuddta2FAyH3hrnQVn41fGea2mv~uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal