Abstract
Kaposi sarcoma (KS), the most common neoplasm in patients with AIDS, typically presents with multiple skin lesions characterized by “spindle cells,” the vast majority of which are infected with KSHV (Kaposi sarcoma herpes virus, also named HHV-8). In patients with AIDS, the presence of cell-associated KSHV DNA in blood is predictive of subsequent KS development, but the mechanisms by which circulating KSHV-infected cells contribute to AIDS-KS pathogenesis are unclear. Here, we show that the chemokine stromal-derived factor-1 (SDF-1), which is constitutively expressed by skin capillary endothelium and displayed on the endothelial cell surface in association with heparan sulfate, can trigger specific arrest of KSHV-infected cells under physiologic shear flow conditions. Moreover, in the presence of soluble SDF-1 gradients, SDF-1 expressed on the endothelial barrier can promote transendothelial migration of KSHV-infected cells. By triggering specific adhesion of circulating KSHV-infected cells and favoring their entry into the extravascular cutaneous space, endothelial cell–associated SDF-1 in cutaneous capillaries may dictate the preferential occurrence of KS in the skin.
Introduction
Kaposi sarcoma (KS), a rare malignancy in the general population, is the most common neoplasm in patients with AIDS.1 Typically, AIDS-associated KS presents with simultaneous multiple skin lesions that often involve the lower extremities.2 Less frequently, KS can also arise in lymph nodes, the gastrointestinal mucosa, and the lung.2 Histologically, KS lesions comprise “spindle cells,” the majority of which are infected with KSHV (Kaposi sarcoma herpes virus, also named HHV-8), inflammatory cells, and vascular slits.1,3 The spindle cells can express markers for smooth muscle cells, vascular endothelium, macrophages, and dendritic cells, suggesting that they are either heterogeneous or represent different stages of differentiation from a pluripotent cell.4 Spindle cells have been identified belonging to the lymphatic endothelial cell lineage.5,6
KSHV, which consistently infects KS lesions, is believed to play an essential role in the pathogenesis of KS through its expression of viral latency genes that directly or indirectly promote cell growth, cytokine and chemokine expression, and angiogenesis.1,3,7 A proportion of HIV-infected individuals harbor in the circulation KSHV-infected cells, which have been identified as B lymphocytes or monocytes.8-10 Detection of cell-associated KSHV DNA in the peripheral blood is predictive of subsequent KS development, whereas the detection of free KSHV DNA is not.8,11 However, the mechanisms by which circulating cells infected with KSHV contribute to AIDS-KS pathogenesis are currently unclear.
A number of studies have implicated stromal-derived factor-1 (SDF-1), a chemokine produced by stromal cells and other cells in various tissues,12-16 as a critical regulator of cell recruitment from the bloodstream to specific tissues by promoting transendothelial migration through chemokine gradients across the endothelium. Thus, hematopoietic cell movement to and from the bone marrow17-24 ; plasma cell homing to secondary organs and the bone marrow25 ; thymocyte emigration from the thymus26 ; breast cancer cell metastasis to the liver, bone marrow, lymph node27 ; dendritic cell recruitment to ovarian tumors28 ; and melanoma cell localization to the lung29 have been linked to the interaction between CXC chemokine receptor-4 (CXCR-4)–expressing cells and SDF-1 gradients produced in the homing tissue.
Recently, we observed that SDF-1 is constitutively expressed by vascular endothelial cells, and that VEGF (vascular endothelial growth factor) can enhance SDF-1 expression in endothelial cells.30 Here, we have examined the potential contribution of endothelial cell–associated SDF-1 to the recruitment of KSHV-infected cells from the circulation to sites of KS development.
Materials and methods
Cells and cell cultures
HUVECs (human umbilical vein endothelial cells) obtained from the umbilical vein were derived and cultured as previously described.30 HDMECs (human dermal microvascular endothelial cells) were obtained from neonatal foreskin and cultured as previously described.29 Early-passage (passage 1-4) HUVECs or HDMECs were used throughout, except when noted. Late-passage HUVECs were from passage 16 to 18. The KSHV-infected primary effusion lymphoma cell lines BC-1 (a gift from Dr Y. Chang, Columbia University, New York, NY) and the Burkitt lymphoma cell line BL41 and the erythroleukemia cell line K562 (ATCC, Rockville, MD) were propagated by standard techniques.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting
Cell and tissue lysates were diluted in tricine sodium dodecylsulfate (SDS) sample buffer, boiled, and run (50 μg/lane) through 10% to 20% tricine gels (Novex, San Diego, CA), as described.30 After transfer, Immobilon-P membranes were incubated overnight with rabbit anti–SDF-1 (PeproTech, Rocky Hill, NJ), rabbit anti–CXCR-4 (Santa Cruz Biotechnology, Santa Cruz, CA), or goat immunoglobulin G (IgG) anti-actin antibodies (Santa Cruz Biotechnology), followed by affinity-purified, peroxidase-linked, donkey antirabbit IgG (Amersham Pharmacia Biotech, Piscataway, NJ) or donkey antigoat antibodies (Santa Cruz Biotechnology) and a chemiluminescence detection system (ECL kit, Amersham Pharmacia Biotech).
Immunohistochemistry
Tissue sections from patients with AIDS, collected with consent and institutional approvals, were retrieved from the files of the Laboratory of Pathology (National Cancer Institute, Bethesda, MD). KSHV-LANA (latency-associated nuclear antigen) staining was performed as reported previously.31 SDF-1 immunostaining was performed as previously described on formalin-fixed and paraffin-embedded specimens30 by using mouse monoclonal anti–SDF-1 antibody (clone 79018.111, dilution 1:50; R&D Systems, Minneapolis, MN), followed by a biotin-conjugated secondary antibody formulation for recognition of mouse immunoglobulins (Ventana Medical Systems, Tucson, AZ). Double staining for KSHV-LANA/SDF-1 used DAKO EnVision doublestain System (Carpinteria, CA), in which SDF-1 staining was visualized by DAB (diaminobenzidine; brown) and LANA by Fast Red (red) chromogens. CXCR-4 immunostaining was performed as previously described14 on frozen tissue specimens by using mouse monoclonal anti–CXCR-4 antibody (clone 44716, IgG2B 4 μg/mL; R&D Systems), followed by biotinylated horse antimouse antibodies and avidin-biotin-peroxidase complex (ABC; Elite ABC kit; Vector Labs, Burlingame, CA). The mouse monoclonal antibody S12 directed at latent membrane protein-1 (LMP-1; IgG2B 4 μg/mL; a gift from Dr F. Wang, Harvard Medical School, Cambridge, MA) was used as a control. Sections were counterstained with hematoxylin.
Flow cytometry
HUVECs were detached from culture plates with 2 mM EDTA (ethylenediaminetetraacetic acid) in phosphate-buffered saline (PBS). Removal of cell-surface glycosaminoglycans was performed as previously described.32 Briefly, HUVECs (106 cells) were washed and incubated for 90 minutes at 37°C with 1 mU/mL heparinase I, heparinase III, or chondroitinase (all from Sigma Chemical, St Louis, MO). After washing, the cells were stained for surface antigens, as described.30 Surface SDF-1 was revealed by mouse monoclonal anti–SDF-1α (clone MAB310; R&D Systems) followed by fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled goat antimouse F(ab′)2 fragment (30 minutes at 4°C; Jackson Immuno Research, West Grove, PA). Surface CXCR-4 was revealed by PE-labeled mouse monoclonal anti–CXCR-4 (clone 12G5; Pharmingen, San Diego, CA; 5 μg/mL for 45 minutes at 4°C). Surface CD31 was revealed by FITC-labeled anti-CD31 antibody (Pharmingen). Data were collected using FACScalibur cytofluorometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed by using CELLQuest software (Becton Dickinson).
Analysis of cell attachment under shear flow
Cell adhesion assays under flow were performed as previously described.29,33 HUVECs and HDMECs (passage 2-4) were grown to confluency onto 35 × 10–mm tissue-culture plates coated with gelatin (Sigma Chemical; 0.2% in PBS). The monolayers were used untreated or after exposure to 200 ng/mL SDF-1α (R&D Systems) for 15 minutes at 20°C in binding buffer (cation-free Hanks balanced salt solution containing 10 mM HEPES [N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)] at pH 7.4 and 2 mg/mL bovine serum albumin supplemented with 1 mM Ca2+ and Mg2+; all from Sigma). Unbound chemokine was washed away. For SDF-1 neutralization, confluent endothelial cells were treated with mouse monoclonal anti–SDF-1 neutralizing antibody (IgG1 clone 79014; R&D Systems) or control mouse IgG1 (clone 11711) for 30 minutes at 20°C in binding buffer. Unbound antibody was washed away. To block Gi-protein–mediated signaling, BC-1 and BL-41 cells were incubated with pertussis toxin (PTX; Sigma Chemical; 200 ng/mL, for 1 hour at 37°C), washed, and suspended in binding buffer. Enzymatic removal of cell-surface glycosaminoglycans from HUVEC and HDMEC monolayers was achieved as described earlier. After enzyme treatment, endothelial cell monolayers were washed. BL-41, BC-1, and K562 cells were labeled with calcein-am (Molecular Probes, Eugene, OR), suspended in binding medium, and then injected (2.5 × 105/mL) at 1.5 dyne/cm2 into a parallel plate flow chamber (Glycotech, Gaithersburg, MD) containing confluent monolayers of HUVECs or HDMECs. Two minutes later, flow was stopped to enable the calcein-labeled cells to interact with HUVECs or HDMECs in the absence of shear stress. After 5 minutes, shear was re-established at 1.5 dyne/cm2 to detach unbound cells. Adherent cells present in 5 random fields (each field measuring 1.18 mm2) were digitally photographed with excitation at 488 nm and emission at 513 nm using approximately 1-second exposure time to allow distinction between nonmoving and moving cells (moving cells appear as dim streaks). The number of cells per field was counted by using IPlab software (Scanalytics, Fairfax, VA).
Analysis of cell migration under static conditions
Transendothelial migration assays were performed by using HUVEC-coated polycarbonate filters (pore size 5 or 8 μm) of transwells separating the upper and lower chamber of 24-well plates (Costar, Cambridge, MA). Early (1-4) or late (16-18) passage HUVECs (4 × 104 cells/filter) were seeded in complete HUVEC culture medium on transwell filters 1 to 2 days before use to achieve confluency. Enzymatic removal of cell-surface glycosaminoglycans was achieved as described earlier. Medium alone or medium supplemented with SDF-1 (100 ng/mL) was added to the bottom chamber. BL-41 or BC-1 cells (1 × 106 cells/well) were placed in the upper chamber, and the plates were incubated at 37°C for 4 hours. Viable cells in the lower chamber were collected and counted.
Statistical analysis
The significance of group differences was determined by 2-sided, parametric, Student t test using STATview (SAS Institute, Cary, NC).
Results
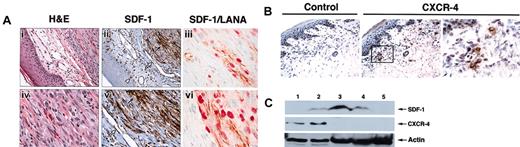
In patients with AIDS, KS occurs most frequently in the skin, and occasionally in lymph nodes, lung, and gastrointestinal tract.2 Initially, we evaluated SDF-1 and CXCR-4 expression in AIDS-KS lesions. By immunohistochemistry, cutaneous KS lesions expressed SDF-1 at high levels (Figure 1A, representative KS lesion). Within KS lesions, the spindle cells that are KSHV infected, as demonstrated by their expression of LANA-1 (latent nuclear antigen 1),1 also expressed SDF-1 (Figure 1A). By contrast, KS spindle cells generally did not express CXCR-4 (Figure 1B), the SDF-1 receptor.13 Within the overlaying epidermis (Figure 1A), endothelial cells lining the blood capillaries and some dendritic cells were SDF-1 positive, whereas scattered mononuclear cells and dermal fibroblasts stained for CXCR-4 (Figure 1B). By immunoblotting, expression of SDF-1, but not CXCR-4, was confirmed in tissue extracts from KS lesions (Figure 1C, lanes 3-4).
SDF-1 and CXCR-4 expression in cutaneous KS lesions. (A) Parallel sections from a formalin-fixed biopsy of an AIDS-KS skin lesion stained with hematoxylin and eosin (H&E) for SDF-1 and double stained for KSHV-LANA/SDF-1. H&E staining reveals normal epidermis overlaying a spindle cell proliferation consistent with cutaneous involvement with KS. SDF-1 immunostaining reveals intensely positive spindle cells within the KS lesion (dark arrow); endothelial cells lining skin capillaries (open arrow) and dendritic cells (arrowhead) in the skin overlaying the KS lesion are also SDF-1 positive. Original magnifications, × 40 (i,ii) and × 60 (iv,v). (iii,vi) Double staining for KSHV-LANA (red) and SDF-1 (brown) reveals that spindle cells confined to the KS lesion are positive for both LANA and SDF-1 (original magnification, × 100). (B) Parallel sections from a frozen biopsy of an AIDS-KS skin lesion stained with antihuman CXCR-4 monoclonal antibody or control monoclonal antibody. CXCR-4–specific immunostaining is confined to scattered cells within superficial dermis. Original magnifications, × 20 (left and middle panels) and × 100 (right panel). (C) Western blot analysis of SDF-1 and CXCR-4 expression in tissues and cell cultures detected by specific antibodies against SDF-1 and CXCR-4. Cell lysates were obtained from BL41 cells (lane 1), HUVECs (lane 2), KS tissue from 2 patients (lanes 3-4), and normal skin (lane 5). Protein loading was evaluated by reprobing the membrane with anti-actin antibodies.
SDF-1 and CXCR-4 expression in cutaneous KS lesions. (A) Parallel sections from a formalin-fixed biopsy of an AIDS-KS skin lesion stained with hematoxylin and eosin (H&E) for SDF-1 and double stained for KSHV-LANA/SDF-1. H&E staining reveals normal epidermis overlaying a spindle cell proliferation consistent with cutaneous involvement with KS. SDF-1 immunostaining reveals intensely positive spindle cells within the KS lesion (dark arrow); endothelial cells lining skin capillaries (open arrow) and dendritic cells (arrowhead) in the skin overlaying the KS lesion are also SDF-1 positive. Original magnifications, × 40 (i,ii) and × 60 (iv,v). (iii,vi) Double staining for KSHV-LANA (red) and SDF-1 (brown) reveals that spindle cells confined to the KS lesion are positive for both LANA and SDF-1 (original magnification, × 100). (B) Parallel sections from a frozen biopsy of an AIDS-KS skin lesion stained with antihuman CXCR-4 monoclonal antibody or control monoclonal antibody. CXCR-4–specific immunostaining is confined to scattered cells within superficial dermis. Original magnifications, × 20 (left and middle panels) and × 100 (right panel). (C) Western blot analysis of SDF-1 and CXCR-4 expression in tissues and cell cultures detected by specific antibodies against SDF-1 and CXCR-4. Cell lysates were obtained from BL41 cells (lane 1), HUVECs (lane 2), KS tissue from 2 patients (lanes 3-4), and normal skin (lane 5). Protein loading was evaluated by reprobing the membrane with anti-actin antibodies.
To evaluate SDF-1 expression in the vascular endothelium within tissues not involved with KS, we obtained biopsy and autopsy specimens from patients with AIDS and systematically performed immunohistochemical staining for SDF-1. The vascular endothelium was identified either by its expression of the endothelial cell marker CD31 or morphologically as the inner lining of channels containing red blood cells. Within tissues not involved with KS, SDF-1 was restricted to the blood capillaries in the skin, the sinusoids of the bone marrow, and the high endothelial venules in lymph nodes (not shown). The capillary vascular endothelium from kidneys, heart, liver, skeletal muscle, lower intestine, lung, adrenal glands, thyroid, testis, and brain did not stain for SDF-1 (not shown). Selected specimens displayed SDF-1 staining in the capillaries of the small intestinal wall (not shown). This pattern of SDF-1 expression in the capillary vascular endothelium of patients with AIDS is similar to that reported for normal tissues from individuals without AIDS.14,15,30
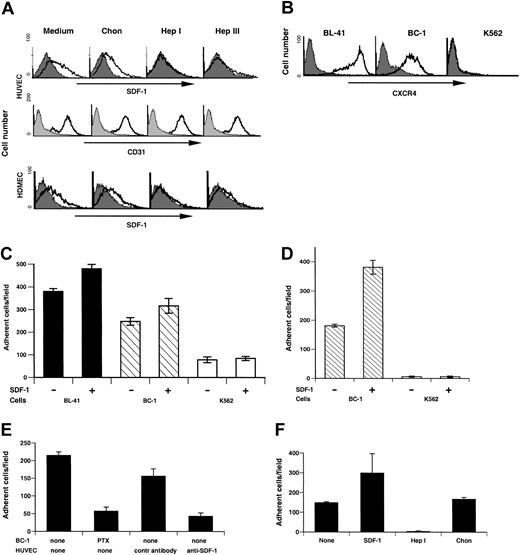
A proportion (37%) of primary human umbilical vein endothelial cells (HUVECs) constitutively express surface SDF-1 during early passage in culture (Figure 2A), as does the endothelium lining the umbilical vein from which they are derived.30 Similarly, a proportion (34%) of early passage human dermal microvascular endothelial cells (HDMECs) express surface SDF-1, as does the capillary endothelium in the skin (Figure 2A).30
Endothelial cell surface SDF-1 expression regulates specific cell arrest under conditions of shear flow. (A) Flow cytometric analysis of surface SDF-1 and CD31 expression in HUVECs (passage 3) and surface SDF-1 expression in HDMECs prior to or after treatment with the enzymes chondroitinase (Chon), heparinase I (Hep I), or heparinase III (Hep III). Filled histograms indicate background staining; open histograms indicate specific staining. (B) Flow cytometric analysis of surface CXCR-4 expression in the Burkitt cell line BL-41, the KSHV-infected primary effusion lymphoma cell line BC-1, and the erythroleukemia cell line K562. (C) BL-41, BC-1, and K562 cells accumulated on HUVEC monolayers either untreated or overlaid with SDF-1 under conditions of physiologic shear stress (1.5 dyne/cm2 for 5 minutes). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 5 performed is shown. (D) Accumulation of BC-1 and K562 cells on HDMEC monolayers either untreated or overlaid with SDF-1 under conditions of shear flow (1.5 dyne/cm2 for 5 minutes). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. (E) Effects of BC-1 cell treatment with pertussin toxin (PTX) and effects of HUVEC treatment with either anti–SDF-1 mouse monoclonal neutralizing antibody or control mouse IgG1 on the arrest of BC-1 cells under conditions of shear flow. The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 3 performed is shown. (F) BC-1 cell arrest under conditions of shear flow on HUVEC monolayers untreated, loaded with SDF-1, treated with heparinase I (Hep I), or treated with chondroitinase (Chon). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 4 performed is shown.
Endothelial cell surface SDF-1 expression regulates specific cell arrest under conditions of shear flow. (A) Flow cytometric analysis of surface SDF-1 and CD31 expression in HUVECs (passage 3) and surface SDF-1 expression in HDMECs prior to or after treatment with the enzymes chondroitinase (Chon), heparinase I (Hep I), or heparinase III (Hep III). Filled histograms indicate background staining; open histograms indicate specific staining. (B) Flow cytometric analysis of surface CXCR-4 expression in the Burkitt cell line BL-41, the KSHV-infected primary effusion lymphoma cell line BC-1, and the erythroleukemia cell line K562. (C) BL-41, BC-1, and K562 cells accumulated on HUVEC monolayers either untreated or overlaid with SDF-1 under conditions of physiologic shear stress (1.5 dyne/cm2 for 5 minutes). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 5 performed is shown. (D) Accumulation of BC-1 and K562 cells on HDMEC monolayers either untreated or overlaid with SDF-1 under conditions of shear flow (1.5 dyne/cm2 for 5 minutes). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. (E) Effects of BC-1 cell treatment with pertussin toxin (PTX) and effects of HUVEC treatment with either anti–SDF-1 mouse monoclonal neutralizing antibody or control mouse IgG1 on the arrest of BC-1 cells under conditions of shear flow. The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 3 performed is shown. (F) BC-1 cell arrest under conditions of shear flow on HUVEC monolayers untreated, loaded with SDF-1, treated with heparinase I (Hep I), or treated with chondroitinase (Chon). The results reflect the mean number (± SEM) of adherent cells from 5 randomly selected fields per condition. Representative experiment of 4 performed is shown.
The enzymes heparinase I and heparinase III can similarly depolymerize heparan sulfate into oligosaccharides.34 Treatment of HUVECs and HDMECs with these enzymes markedly reduced surface SDF-1 on these cells (HUVECs, 0.4% and 3.22% positive; HDMECs, 2.3% and 5.8% positive after heparinase I and heparinase III treatment, respectively). This enzymatic treatment did not reduce levels of CD31 staining in HUVECs (positive cells ranged from 94% to 96%; Figure 2A,) and HDMECs (not shown). Chondroitinase ABC (Figure 2A), which degrades chondroitin sulfate, had little effect on surface SDF-1 expression in HUVECs (37% positive cells) and in HDMECs (22% positive cells). These results are consistent with SDF-1 being secreted by endothelial cells and then retained on the cell surface in association with proteoglycan molecules containing heparan sulfate.32
Skin is a preferred site for KS, and this fact prompted us to examine whether surface SDF-1 expressed by cutaneous endothelial cells may play a role in the local recruitment of KSHV-infected cells, which would then initiate the development of KS lesions. Because KSHV-infected cells from the peripheral blood of virus-infected individuals cannot be isolated because of their low frequency,8 we used the primary effusion lymphoma cell line BC-135 as a prototype for a circulating KSHV-infected B cell. Because of their coinfection with Epstein-Barr virus (EBV), BC-1 cells may reflect the close association of KSHV and EBV in peripheral blood mononuclear cells from patients with KS36 and in cells underlying KHSV-associated germinotropic lymphoproliferative disorder (GLD).37
We tested BC-1 cells for surface CXCR-4 expression and compared them with Burkitt lymphoma BL-41 cells known to express high levels of CXCR-4 and to the erythroleukemia K562 cells known not to express CXCR-4.38 Both BL-41 and K562 are EBV and KSHV negative. By flow cytometry, 99% BC-1 cells and 93% BL-41 cells expressed surface CXCR-4; the erythroleukemia K562 cells were only minimally positive for CXCR-4 (< 1% cells positive; Figure 2B). We examined the ability of BC-1, BL-41, and K562 to bind to HUVECs (that bear surface SDF-1) under conditions of physiologic shear flow (1.5 dyne/cm2). A significantly greater proportion of BL-41 and BC-1 cells arrested onto the endothelial monolayer compared with K562 cells (P < .001) that displayed minimal arrest (Figure 2C). We then examined whether loading SDF-1 onto the endothelial monolayer could enhance cell arrest. When the endothelium was preincubated with SDF-1 (200 ng/mL) under conditions known to enhance levels of surface SDF-1 on HUVECs33,39 (not shown), a slight but significant increase (P < .05) in the number of BC-1 and BL-41 cells arresting onto the endothelium was observed (Figure 2C). By contrast, the number of K562 cells that arrested remained minimal (Figure 2C). We also tested the ability of BC-1 cells to arrest, under conditions of physiologic shear flow (1.5 dyne/cm2) onto HDMECs, which, like HUVECs, display surface SDF-1 (Figure 2A). As shown (Figure 2D), a significantly greater proportion of BC-1 cells arrested onto HDMECs either untreated or preincubated with SDF-1 (200 ng/mL) as opposed to K562 cells (P < .001, all comparisons), providing evidence that SDF-1–positive dermal vascular endothelium can promote specific arrest of CXCR-4–positive cells under conditions of physiologic shear flow.
Because pertussis toxin (PTX) can block Gi-protein–mediated CXCR-4 signaling through adenosine diphosphate (ADP) ribosylation of the α subunit of Gi-proteins,40 we tested its effects on cell arrest under conditions of shear flow. Pretreatment of BC-1 (Figure 2E) and BL-41 (not shown) cells with pertussis toxin (PTX; 200 ng/mL incubation for 1 hour) significantly (P < .001) reduced cell arrest onto HUVECs, providing evidence for a requirement of a Gi-protein–mediated process.
To test for the contribution of endothelial cell surface–associated SDF-1 to cell arrest, we used a neutralizing antibody directed at SDF-1. Pretreatment of HUVECs with anti–SDF-1 neutralizing antibody, but not a control antibody, significantly (P < .001) reduced BC-1 (Figure 2E) and BL-41 (not shown) cell arrest onto the endothelial cells. Furthermore, pretreatment of HUVECs with heparinase I under conditions that reduce cell surface SDF-1 expression on HUVECs (Figure 2F) virtually abolished BC-1 cell arrest onto HUVECs under conditions of shear flow (P < .001). By contrast, treatment of HUVECs with chondroitinase ABC had little effect on BC-1 cell arrest (P > .05; Figure 2F). Similar results were observed with BL-41 cells (not shown). These results provide evidence that CXCR-4–expressing BC-1and BL-41 cells can arrest under conditions of shear flow onto endothelial cell monolayers that constitutively express surface SDF-1. This process is dependent on the presence of surface SDF-1 on the endothelium and signaling through a Gi-protein–coupled receptor in the arresting B cells.
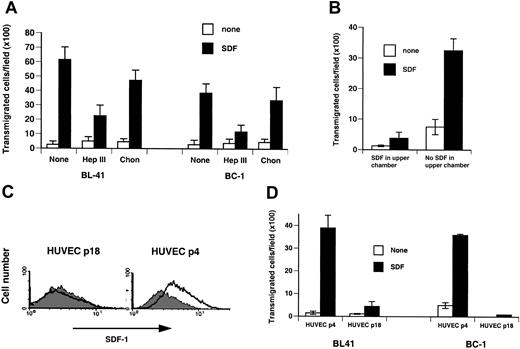
An in vitro transendothelial migration assay using a monolayer of SDF-1–expressing HUVECs or HDMECs was used to assess whether cells arrested onto SDF-1–bearing endothelium would transmigrate across the endothelial barrier. In the absence of a soluble SDF-1 gradient across the HUVEC barrier, minimal cell transmigration was observed with BC-1 and BL-41 cells (Figure 3A). In the presence of soluble SDF-1 (100 ng/mL) across the HUVEC barrier, BC-1 and BL-41 cell transmigration was markedly enhanced (P < 0.05; Figure 3A). Similar results were obtained for BC-1 cell transmigration across a monolayer of SDF-1–expressing HDMECs (Figure 3B). Treatment of the HUVEC endothelial cell barrier with heparinase III prior to addition of BC-1 or BL-41 cells to the upper chamber reduced significantly (P < .05) cell transmigration (Figure 3A). Only a modest reduction was noted after treatment of the endothelial cell barrier with chondroitinase ABC (P > .05; Figure 3A). Addition of SDF-1 (200 ng/mL) to the upper chamber reduced significantly (P < .05) BC-1 cell transmigration across the HDMEC monolayer, confirming a requirement for soluble SDF-1 gradients across the endothelial barrier for transmigration to occur.
Endothelial cell surface SDF-1 regulates specific cell transmigration under static conditions. (A) BL-41 and BC-1 cell migration across HUVEC monolayers in the presence or absence of SDF-1 in the lower chamber of transwells. HUVEC monolayers were either untreated or treated with heparinase III (Hep III) or chondroitinase (Chon). The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber over 4 hours from 3 replicate wells per condition. Representative experiment of 5 performed. (B) BC-1 cell migration across HDMEC monolayers in the presence or absence of SDF-1 in the lower and upper chamber of transwells. The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber over 4 hours from 3 replicate wells per condition. Representative experiment of 3 performed. (C) Surface SDF-1 expression in early (passage 4) and late (passage 18) passage HUVECs detected by flow cytometry. (D) BL-41 and BC-1 cell transmigration as a function of HUVEC passage in culture. Early (passage 4) or late (passage 18) passage HUVECs were used to generate monolayers separating the upper and lower chambers of transwells. Medium alone or with SDF-1 was placed in the lower chamber. The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber from 9 replicate wells per condition. Representative experiment of 3 performed is shown.
Endothelial cell surface SDF-1 regulates specific cell transmigration under static conditions. (A) BL-41 and BC-1 cell migration across HUVEC monolayers in the presence or absence of SDF-1 in the lower chamber of transwells. HUVEC monolayers were either untreated or treated with heparinase III (Hep III) or chondroitinase (Chon). The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber over 4 hours from 3 replicate wells per condition. Representative experiment of 5 performed. (B) BC-1 cell migration across HDMEC monolayers in the presence or absence of SDF-1 in the lower and upper chamber of transwells. The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber over 4 hours from 3 replicate wells per condition. Representative experiment of 3 performed. (C) Surface SDF-1 expression in early (passage 4) and late (passage 18) passage HUVECs detected by flow cytometry. (D) BL-41 and BC-1 cell transmigration as a function of HUVEC passage in culture. Early (passage 4) or late (passage 18) passage HUVECs were used to generate monolayers separating the upper and lower chambers of transwells. Medium alone or with SDF-1 was placed in the lower chamber. The results reflect the mean (± SEM) number of cells transmigrated to the lower chamber from 9 replicate wells per condition. Representative experiment of 3 performed is shown.
To further evaluate the contribution of cell-associated SDF-1, we compared cell transmigration across endothelial monolayers that differ in the levels of cell-associated SDF-1 they express. Early passage (passage 2-4) HUVECs express surface SDF-1, whereas HUVECs from a later passage (passage 18) do not (Figure 3C). The cells are otherwise indistinguishable morphologically, in the levels of surface CD31, CD34, and von Willebrand factor they express, and their ability to form extracellular matrix-dependent tubes. In the presence of a soluble SDF-1 gradient across the endothelium, BC-1 and BL-41 cells transmigrated significantly (P < .05) better across the endothelial monolayer composed of early passage, SDF-1–positive HUVECs as opposed to the later passage, SDF-1 weakly positive HUVECs (Figure 3D). Together, these results show that surface SDF-1 constitutively expressed by vascular endothelial cells in selected tissues can promote CXCR-4–mediated cell arrest and transmigration across endothelial monolayers.
Discussion
A number of studies have implicated SDF-1 and CXCR-412,13 as critical regulators of cell recruitment from the bloodstream to specific tissues by promoting transendothelial migration through chemokine gradients across the endothelium.41 Thus, hematopoietic cell movement to and from the bone marrow17,23 ; plasma cell homing to secondary organs and the bone marrow25 ; and cancer cell metastasis to the liver, bone marrow, and lymph node27,29 have been linked to the interaction between CXCR-4–expressing cells and SDF-1 gradients produced in the homing tissue. In these in vivo studies, high-level expression of SDF-1 in a target tissue is critical to the generation of a chemokine gradient across the endothelium, which then promotes transendothelial migration of SDF-1 receptor-expressing cells. Experiments in vitro have shown that SDF-1 could be “loaded” onto tumor necrosis factor α (TNF-α)–activated endothelial cells, themselves negative for SDF-1. Once present on the surface of endothelial cells, SDF-1 could support lymphocyte arrest onto the vascular endothelium.33 However, the homing potential of cell surface–bound SDF-1 constitutively expressed by selected capillary endothelium has not been previously explored.
Here, we show that surface SDF-1 constitutively expressed by selected vascular endothelium can promote specific arrest and transendothelial migration of cells that express the SDF-1 receptor CXCR-4 and suggest that endothelium-associated SDF-1 can play an important role in the recruitment of KSHV-infected cells to the skin. Cell arrest under conditions of shear flow is not simply dependent on mechanical adhesion between chemokine-coated surfaces and chemokine receptors on rolling cells.42 Rather, it constitutes a multistep cascade-type process that requires Gi-protein–linked signaling and is dependent on increased adhesiveness of integrins VLA-4 (very late activation antigen 4) and LFA-1 (lymphocyte function-associated antigen-1) to their respective endothelial cell ligands VCAM (vascular cell adhesion molecule) and ICAM-1 (intercellular adhesion molecule 1), in part because of integrin clustering to specific cell surface contact points.39,43,44
Circulating KSHV-infected cells have been identified as B lymphocytes or monocytes,8,10 cell types known to be CXCR-4 positive and, thus, would be expected to respond to SDF-1. The HIV-1 protein Tat can markedly enhance expression of CXCR-4 in lymphocytes.45 HIV-Tat can also activate the VEGF receptor Flk-1/KDR, acting as a pro-angiogenic factor.46,47 We have found that VEGF-A can promote SDF-1 expression in vascular endothelial cells.30 Recently, KSHV infection induced CXCR-4 expression in microvascular endothelial cells.48 Thus, circulating cells naturally infected with KSHV, particularly in HIV-infected individuals, would be expected to come to a stop and bind to the SDF-1–expressing vascular capillary network of the skin.
Once arrested at this site, KSHV-infected cells could migrate to the extravascular space by chemotaxis in the presence of a transendothelial SDF-1 gradient. Dermal Langerhans cells and fibroblasts constitutively express SDF-114 and, thus, could be a source of such an SDF-1 gradient. Quantitative analysis of SDF-1 expression in different human tissues has revealed that the skin has higher levels of expression compared with muscle and brain.27 Once established, KS lesions expressing high levels of SDF-1 could generate strong local SDF-1 gradients, explaining why KS lesions often occur in clusters. CXCR-4 expression was undetectable in KS lesions, suggesting that the spindle cells may be progeny of a CXCR-4–negative cell, which became infected with KSHV from infected circulating cells. Sustained contact of KSHV-infected cells with the endothelial cell lining or other cells in the extravascular space could favor transmission of virus through the α3β1 integrin receptor for KSHV entry.49
Less frequently than in the skin, KS lesions in AIDS involve the lymph node, and in advancedAIDS also the lung and the gastrointestinal tract.2 The high endothelial venules in lymph nodes are consistently SDF-1 positive,30 but only rarely did the capillaries from the lung and the gastrointestinal tract stain for SDF-1. However, because the proangiogenic factor VEGF-A30 and presumably the HIV-Tat protein which activates the VEGF-Areceptor Flk-1/KDR46,47 can induce SDF-1 expression on endothelial cells, the capillary vasculature of the lung and the gastrointestinal tract could also become SDF-1 positive, perhaps under hypoxic conditions.
The potential contribution of CXCR-4 expression on circulating KSHV-infected cells and SDF-1 expression on selected capillary endothelium to KS pathogenesis suggests that therapeutic manipulations designed to alter SDF-1/CXCR-4 interactions may prove useful in preventing KS or in reducing its dissemination.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-02-0641.
L.Y. and O.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Rich Little and Josh Farber for helpful discussions and Ms Angelica Vivero, Sabrina Villalba, and Kathy Wyvill for help in various aspects of this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal