Abstract
Fetal hemoglobin (HbF) decreases polymerization of sickle hemoglobin (HbS) and improves outcomes in sickle cell disease (SSD). Therefore, a therapeutic goal in SSD is pharmacologic reactivation of HbF. Silencing of the γ-globin (HbF) gene is associated with DNA methylation. The cytosine analog 5-aza-2′-deoxycytidine (decitabine) hypomethylates DNA by inhibiting DNA methyltransferase. We examined if subcutaneous decitabine could increase HbF levels and improve SSD pathophysiology without cytotoxicity. Eight symptomatic SSD patients resistant or intolerant of standard treatment with hydroxyurea received decitabine 0.2 mg/kg subcutaneously 1 to 3 times per week in 2 cycles of 6-week duration. Treatment decreased neutrophils and increased mean HbF (6.5% to 20.4%, P < .0001) and mean total hemoglobin (76 to 96 g/L [7.6 to 9.6 g/dL], P < .001). Features of vaso-occlusive crisis pathophysiology such as red cell adhesion, endothelial damage, and coagulation pathway activity significantly improved. γ-Globin gene promoter methylation decreased, and platelets and the proportion of megakaryocytes and erythroid cells in the marrow increased without a decrease in marrow cellularity, consistent with a DNA hypomethylating, noncytotoxic mechanism of action. Weekly subcutaneous decitabine produces cumulative increases in HbF and total hemoglobin through a noncytotoxic mechanism of action. Chronic dosing and sustained increases in hemoglobin F and total hemoglobin levels may be possible. Further studies in SSD and thalassemia are indicated.
Introduction
Fetal hemoglobin (HbF) decreases polymerization of sickle hemoglobin (HbS) and the complications of sickle cell disease (SSD).1-3 The current standard for pharmacologic reactivation of fetal hemoglobin in SSD is the ribonucleotide reductase inhibitor hydroxyurea (HU) which, when administered at cytotoxic doses, reduces crisis frequency.4 Improved survival with HU treatment correlates with higher induced HbF levels.5 Despite this significant advance for most patients, not all respond; some patients attain HbF levels in excess of 30% of total hemoglobin (tHb) while others have minimal response.6 Methylation of cytidine residues in DNA is associated with transcriptional repression or silencing of genes.7 5-Azacytidine and 5-aza-2′-deoxycytidine (decitabine) are analogs of cytidine that incorporate into DNA and inactivate the DNA methyltransferase enzymes (DNMT) that methylate DNA.8 By preventing DNA methylation these agents can effectively reactivate gene transcription. In animal studies and small patient series, 5-azacytidine and decitabine profoundly increased HbF.9-12 In addition to having a more favorable safety profile than 5-azacytidine, decitabine is a more potent inhibitor of DNMT. Therefore, we have pursued studies of decitabine to increase HbF levels in patients with SSD. Previously, in HU nonresponder patients, pulsed intravenous decitabine increased HbF from 2.28% ± 1.61% to 12.70% ± 1.81% of total hemoglobin (tHb).13 Building on this experience, we wished to assess the safety, efficacy, and mechanism of action of weekly subcutaneous low-dose decitabine to produce cumulative and sustained increases in HbF and tHb in symptomatic SSD patients who were resistant to or intolerant of HU therapy.
The pathophysiology of vaso-occlusive crises in SSD involves red blood cell (RBC) adhesion to endothelium, endothelial damage, and activation of coagulation and inflammatory cascades (reviewed by Bunn14 ). Therefore, as surrogate clinical end points, we measured RBC adhesion to thrombospondin (TSP) and laminin and measured markers of endothelial damage, coagulation system activation, and inflammatory activity. Finally, to gain insight into the mechanism of action of decitabine at these doses, we studied bone marrow morphology and DNA methylation changes.
Patients and methods
Patient population
This single-arm phase 1/2 study of subcutaneous decitabine in SSD was approved by the University of Illinois at Chicago Institutional Review Board and funded by SuperGen. To be eligible, patients had to be at least 18 years of age and have SSD (SS or S-β-thalassemia) that was symptomatic, defined as at least one of the following: 3 or more pain episodes per year requiring parenteral narcotics, 2 or more acute chest syndrome or pneumonia episodes, or chronic transfusion therapy with development of at least 2 iso-antibodies. Additionally, patients had to be either resistant to HU (no clinical improvement and increase in HbF less than 5 g/L [0.5 g/dL] above baseline after 3 months of therapy as per Multicenter Study of Hydroxyurea [MSH] trial guidelines) or intolerant to HU due to toxicity.
Between November 2001 and February 2002, 8 patients were enrolled into the study with informed consent. There were 5 females and 3 males; average age was 38 years with an age range between 22 and 61 years. All patients had multiple clinically significant complications of sickle cell disease. Three patients (unique patient numbers [UPNs] 1, 3, 5) had been on HU for more than 1 year in the MSH4 and failed to demonstrate a more than 5 g/L (0.5 g/dL) increase in HbF or decreased symptoms. Compliance had been documented by HU blood levels and pill counts. The remaining patients demonstrated increases in HbF and decreased frequency of their painful crises with HU. HU was discontinued for the following reasons: UPNs 2 and 4 developed lichen planus, which resolved upon discontinuation of HU; UPNs 6, 7, and 8 had leg ulcers that progressed on HU.
End points
Primary end points were (1) incidence and severity of local skin reactions and (2) successful induction of HbF defined as more than 80% of RBCs containing more than 20% HbF (%F-cells). Additional surrogate clinical end points were RBC adhesion to TSP and laminin, levels of D-dimers, thrombin-antithrombin (TAT) complexes, prothrombin fragments 1 and 2 (F1 + 2), C-reactive protein (CRP), soluble vascular cell adhesion molecule-1 (SVCAM-1), and von Willebrand factor propeptide (VWFpp).
Drug administration
Drug was administered subcutaneously in the thigh or upper arm (by the nurse in the outpatient clinic) no more than 30 minutes after reconstitution of lypholized drug with normal saline.
Decitabine was administered at 0.2 mg/kg 1 to 3 times per week in 2 cycles of 6-week duration with a 2-week interval between cycles. In cycle 1, drug was administered twice per week on 2 consecutive days. If the patient achieved an F-cell percentage (%F-cells) of at least 80% during cycle 1, the dose frequency was reduced to once per week in cycle 2. If the highest %F-cells during the cycle 1 was less than 80%, the dose frequency was increased to 3 times per week in cycle 2.
F-cell analysis
Peripheral blood samples were fixed and stained with fluorescein isothiocyanate–conjugated anti-HbF (Caltag, Burlingame, CA) as per the manufacturer's instructions. A normal adult negative control and cord blood–positive control were run with each analysis on the Becton Dickinson FACSCalibur (Sunnyvale, CA).
HbF quantification
The percentage of hemoglobin F as a proportion of tHb (%HbF) was measured by alkali denaturation as previously described.15
RBC adhesion studies
Blood samples from patients were collected in 3.8% buffered sodium citrate and stored at 4°C for up to 72 hours before being tested. Adhesion of RBCs to thrombospondin (TSP) (purified from human platelets16 ) and human laminin (Gibco, Gaithersburg, MD) was performed as previously described.17 RBCs stored in citrated whole blood at 4°C and tested within 2 hours after completion of the RBC wash have similar RBC adhesion (to TSP) results for up to 5 days following the blood draw under the conditions of this flow adhesion assay. All flow adhesion assays were performed on 2 separate blood draws 1 week apart for each patient at each sample time.
Measurements of coagulation activation, inflammation, and endothelial damage
Plasma aliquots immediately isolated from patient samples were stored at -80°C. The following kits were used per manufacturers' recommendations: Asserachrom D-Di (Diagnostica Stago, Parsippany, NJ) to measure D-dimers, EnzygnostTAT enzyme immunoassay kit (Dade Behring, Liederbach, Germany) to measure TAT complexes, EnzygnostF1 + 2 kit (Dade Behring) to measure F1 + 2, Human SVCAM-1 Immunoassay kit (R&D Systems, Minneapolis, MN) to measure SVCAM-1, and ABC kit (Vectastain; Vector Laboratories, Burlingame, CA) to measure VWFpp by double-sandwich enzyme-linked immunosorbent assay (ELISA) (standard curves were derived from serial dilutions of pooled normal plasma). CRP was measured by fixed-point immunorate using the Vitros Analyzer (Ortho-Clinical Diagnostics, Raritan, NJ).
Bone marrow morphology
Bone marrow aspirate smears were stained with Wright-Giemsa.
Analysis of γ-globin gene methylation
Methylation of the γ-globin promoter was measured by bisulfite sequence analysis18 and combined bisulfite restriction analysis (COBRA).19 For bisulfite sequence analysis the γ-globin promoter overlapping 5 CpG residues at -54, -51, + 5, +16, and +48 was amplified using nested polymerase chain reaction (PCR). PCR products were cloned in the PCR4 vector (TOPO TA cloning kits, Invitrogen, Carlsbad, CA). Miniprepped DNA from individual random clones was sequenced using an ABI Prism 300 Genetic Analyzer. For COBRA, DNA was subjected to bisulfite modification followed by 2 rounds of PCR using nested primers specific for the γ-globin promoter. Hinf I digestion was performed to distinguish between PCR products derived from methylated versus unmethylated DNA. The PCR product was cleaved by Hinf I if the DNA in the original sample was methylated at the -256 site. On each sample, COBRA was performed in triplicate and densitometry used to analyze the intensity of the band corresponding to methylated γ-globin promoter.
Statistical analysis
Two-tailed paired t tests were used to analyze changes in %F-cells, %HbF, tHb, RBC adhesion, D-dimers, TAT complexes, F1 + 2, soluble VCAM-1, CRP, and VWFpp levels. Regression analysis using the REG and GLM procedures in SAS version 8 were used to analyze correlations between each secondary end point (RBC adhesion to TSP and laminin, D-dimers, SVCAM-1, and VWFpp) and hematologic parameters (%F-cells, tHb, absolute reticulocyte count [ARC], and absolute neutrophil count [ANC]).
Results
Nonhematologic toxicity
National Cancer Institute (NCI) Common Toxicity Criteria were used to assess toxicity (NCI, Bethesda, MD). No local toxicity was documented at subcutaneous injection sites. No patients described nausea, vomiting, diarrhea, constipation, or decreased appetite.
Increase in HbF
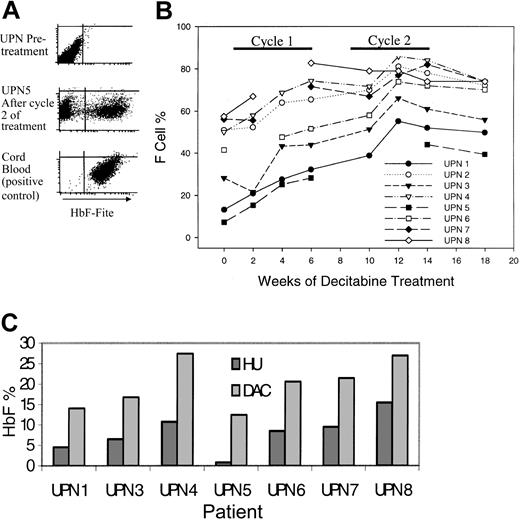
Ideally, any increase in HbF should be distributed uniformly among a large proportion of RBCs, thus increasing the proportion of RBCs resistant to HbS polymerization and sickling; therefore, the primary efficacy end point was the proportion of RBCs expressing HbF at levels that inhibit sickling (%F-cells) (Figure 1A). All patients demonstrated statistically significant increases in %F-cells (Figure 1B; Table 1). HU nonresponders (UPNs 1, 3, 5) had lower %F-cells at baseline but demonstrated a rate of increase similar to that seen in HU responders (Figure 1B). %F-cells increased by the second week after initiation and decreased by the second week after discontinuation of treatment. The primary end point of 80% F cells was achieved by UPN 8 during cycle 1 and UPNs 2, 4, and 7 during cycle 2 (Figure 1B). The maximum %HbF per F cell (%HbF/%F-cells × 100) was more than 20% in all patients.
Decitabine increased fetal hemoglobin levels (HbF) in all treated patients. (A) Flow cytometric analysis for F cells (RBCs expressing high levels of HbF). The F-cell percentage is the proportion of gated RBCs with HbF expression similar to that of the cord blood positive control. (B) Change in F-cell percentage with treatment. (C) The peak HbF values with decitabine treatment (light gray bars) were higher than those measured during previous treatment with hydroxyurea (dark gray bars) for between 4 and 36 months. Peak HbF levels were not measured in UPN 2 during hydroxyurea treatment.
Decitabine increased fetal hemoglobin levels (HbF) in all treated patients. (A) Flow cytometric analysis for F cells (RBCs expressing high levels of HbF). The F-cell percentage is the proportion of gated RBCs with HbF expression similar to that of the cord blood positive control. (B) Change in F-cell percentage with treatment. (C) The peak HbF values with decitabine treatment (light gray bars) were higher than those measured during previous treatment with hydroxyurea (dark gray bars) for between 4 and 36 months. Peak HbF levels were not measured in UPN 2 during hydroxyurea treatment.
Change in %F-cells and HbF as a percentage of total hemoglobin (%HbF) with treatment
Patients . | Pretreatment %F-cells . | Maximum %F-cells . | P . | Pretreatment %HbF . | Maximum %HbF . | P . |
|---|---|---|---|---|---|---|
| HU nonresponders | 16.2 ± 10.8 | 55.1 ± 11.1 | .002 | 2.4 ± 1.6 | 14.5 ± 2.2 | .0006 |
| HU responders | 51.3 ± 6.3 | 81.2 ± 4.4 | .0001 | 9.0 ± 2.5 | 23.9 ± 3.1 | < .0001 |
| All patients | 38.1 ± 7.6 | 71.4 ± 6.5 | < .0001 | 6.5 ± 1.4 | 20.4 ± 2.0 | < .0001 |
Patients . | Pretreatment %F-cells . | Maximum %F-cells . | P . | Pretreatment %HbF . | Maximum %HbF . | P . |
|---|---|---|---|---|---|---|
| HU nonresponders | 16.2 ± 10.8 | 55.1 ± 11.1 | .002 | 2.4 ± 1.6 | 14.5 ± 2.2 | .0006 |
| HU responders | 51.3 ± 6.3 | 81.2 ± 4.4 | .0001 | 9.0 ± 2.5 | 23.9 ± 3.1 | < .0001 |
| All patients | 38.1 ± 7.6 | 71.4 ± 6.5 | < .0001 | 6.5 ± 1.4 | 20.4 ± 2.0 | < .0001 |
Values are mean ± SD. P values determined by 2-tailed paired t test.
The %F-cells decreased between week 12 and week 14 despite ongoing therapy. The reason for this decrease may be loss of F cells produced during the early part of protocol treatment. During ongoing therapy, a plateau in the level of %F-cells may be reached when the rate at which newly formed F cells enter the circulation is matched by the rate at which old ones are destroyed.
The increase in the level of HbF as a percentage of tHb (%HbF) mirrored the increase in %F-cells (Table 1).
Because of noncompliance, UPN 4 and UPN 5 received 1 to 2 injections per week during cycle 2 (UPN 4: 12 injections; UPN 5: 9 injections; both patients found it difficult to return to the clinic 3 times per week because of work commitments; noncompliance was not a consequence of toxicity). Five patients received drug 3 times per week in cycle 2: increased dose frequency of 3 times per week did not significantly accelerate the rate of increase of %F-cells or %HbF (average %HbF increase with 2 times per week was 7; with 3 times per week, an additional 5.8).
Peak %HbF levels during decitabine treatment were higher than peak levels measured during previous HU treatment (Figure 1C). Peak levels were measured after 4 to 36 months of treatment with HU.
Increase in total hemoglobin/decreased hemolysis
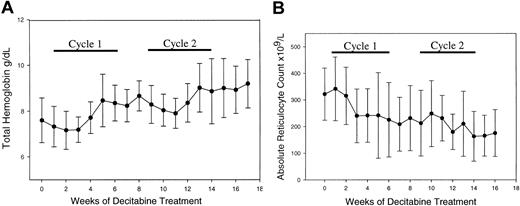
The tHb increased from 76 ± 20 g/L (7.6 ± 2 g/dL) to 96 ± 18 g/L (9.6 ± 1.8 g/dL) (mean ± 2 SD of pretreatment to peak Hb, paired t test P < .001) (Figure 2A).
Decitabine increased total hemoglobin (Hb) and decreased the absolute reticulocyte count. (A) Change in Hb: 76 ± 10 to 96 ± 9 g/L (7.6 ± 1 to 9.6 ± 0.9 g/dL) (mean ± SD of pretreatment to peak Hb, 2-tailed paired t test P < .001). (B) Absolute reticulocyte counts decreased significantly: 231 × 109/L ± 98 × 109/L to 163 × 109/L ± 93 × 109/L (mean ± SD pretreat ment to after cycle 2, 2-tailed paired t test P = .0006) (error bars represent SD). There was a significant inverse correlation between hemoglobin levels and the reticulo cyte count (P < .0001).
Decitabine increased total hemoglobin (Hb) and decreased the absolute reticulocyte count. (A) Change in Hb: 76 ± 10 to 96 ± 9 g/L (7.6 ± 1 to 9.6 ± 0.9 g/dL) (mean ± SD of pretreatment to peak Hb, 2-tailed paired t test P < .001). (B) Absolute reticulocyte counts decreased significantly: 231 × 109/L ± 98 × 109/L to 163 × 109/L ± 93 × 109/L (mean ± SD pretreat ment to after cycle 2, 2-tailed paired t test P = .0006) (error bars represent SD). There was a significant inverse correlation between hemoglobin levels and the reticulo cyte count (P < .0001).
Both the ARC (P = .0006) and total bilirubin (P = .01, 2-tailed paired t test) decreased during treatment (Figure 2B). The ARC correlated inversely with tHb (P < .0001), suggesting that the ARC decrease resulted from decreased hemolysis and increased hemoglobin.
Decreased RBC adhesion and correlation with hematologic parameters
Adhesion of RBCs to TSP and laminin (molecules on endothelium) is most likely triggered by distortion of the RBC membrane by HbS polymerization and consequent expression of adhesion molecules. By decreasing HbS polymerization, HbF should decrease RBC adhesion to TSP and laminin. RBC adhesion to both TSP and laminin decreased following cycle 1 (Table 2, P < .005). In multivariate analysis, a significant association was noted between %F-cells and RBC adhesion to TSP and laminin (P = .046 and P = .004, respectively). RBC adhesion to TSP also demonstrated a significant association with the ANC (P = .02).
Changes in secondary end points
. | Before therapy . | After cycle 1 . | P* . | After cycle 2 . | P† . | Normal range . | Correlation with hematologic parameters in multivariate regression analysis, P < .05 . |
|---|---|---|---|---|---|---|---|
| Measures of RBC adhesion to endothelium | |||||||
| Adhesion to TSP, RBCs/mm2 | 1570 ± 170 | 690 ± 150 | < .001 | 910 ± 160 | < .001 | < 60 | F-cell % and ANC |
| Adhesion to laminin, RBCs/mm2 | 3470 ± 500 | 1950 ± 300 | .004 | 1570 ± 210 | < .001 | < 250 | F-cell % |
| Measures of thrombin generation and fibrinolysis | |||||||
| D-dimer, ng/mL | 490 ± 90 | 320 ± 50 | .02 | 300 ± 50 | .03 | < 400 | Nil |
| TAT, μg/L | 7.0 ± 1.7 | 8.6 ± 2.3 | .15 | 5.2 ± 0.9 | .11 | 1.0-4.1 | — |
| F1 + 2, nM | 1.75 ± 0.22 | 1.56 ± 0.16 | .23 | 1.41 ± 0.15 | .051 | 0.04-1.1 | — |
| Measure of inflammation | |||||||
| CRP, mg/dL | 1.25 ± 0.27 | 1.19 ± 0.34 | .80 | 0.82 ± 0.26 | .18 | < 0.7 | — |
| Measures of endothelial cell damage | |||||||
| SVCAM, ng/mL | 1170 ± 140 | 930 ± 100 | .01 | 840 ± 100 | .02 | 395-714 | Total Hb |
| VWFpp, U/dL | 196 ± 26 | 156 ± 28 | .015 | 144 ± 13 | .049 | 74-153 | Absolute reticulocyte count |
. | Before therapy . | After cycle 1 . | P* . | After cycle 2 . | P† . | Normal range . | Correlation with hematologic parameters in multivariate regression analysis, P < .05 . |
|---|---|---|---|---|---|---|---|
| Measures of RBC adhesion to endothelium | |||||||
| Adhesion to TSP, RBCs/mm2 | 1570 ± 170 | 690 ± 150 | < .001 | 910 ± 160 | < .001 | < 60 | F-cell % and ANC |
| Adhesion to laminin, RBCs/mm2 | 3470 ± 500 | 1950 ± 300 | .004 | 1570 ± 210 | < .001 | < 250 | F-cell % |
| Measures of thrombin generation and fibrinolysis | |||||||
| D-dimer, ng/mL | 490 ± 90 | 320 ± 50 | .02 | 300 ± 50 | .03 | < 400 | Nil |
| TAT, μg/L | 7.0 ± 1.7 | 8.6 ± 2.3 | .15 | 5.2 ± 0.9 | .11 | 1.0-4.1 | — |
| F1 + 2, nM | 1.75 ± 0.22 | 1.56 ± 0.16 | .23 | 1.41 ± 0.15 | .051 | 0.04-1.1 | — |
| Measure of inflammation | |||||||
| CRP, mg/dL | 1.25 ± 0.27 | 1.19 ± 0.34 | .80 | 0.82 ± 0.26 | .18 | < 0.7 | — |
| Measures of endothelial cell damage | |||||||
| SVCAM, ng/mL | 1170 ± 140 | 930 ± 100 | .01 | 840 ± 100 | .02 | 395-714 | Total Hb |
| VWFpp, U/dL | 196 ± 26 | 156 ± 28 | .015 | 144 ± 13 | .049 | 74-153 | Absolute reticulocyte count |
Statistical analysis of changes in RBC adhesion to TSP and laminin, levels of D-dimers, thrombin-antithrombin (TAT) complexes, prothrombin fragments 1 and 2 (F1 + 2), C-reactive protein (CRP), soluble VCAM (sVCAM), and von Willebrand factor propeptide (VWFpp). Those variables that changed significantly with treatment were analyzed for correlations with the following hematologic parameters: %F-cells, absolute neutrophil counts, total hemoglobin levels, and reticulocyte counts (multivariate regression analysis); significant associations are shown in the last column. Values are mean ± SE. Statistical significance determined by paired 2-tailed t test. Nil indicates no significant relationship; and —, not analyzed.
P, significance of change from before therapy to after cycle 1
P, change from before therapy to after cycle 2
Changes in markers of coagulation activation, endothelial damage, and inflammation and correlation with hematologic parameters
In SSD, abnormal exposure of molecules such as phosphatidylserine on the RBC surface and adhesion of RBCs to endothelial cells/endothelial damage can trigger coagulation and inflammatory pathways. In agreement with previous reports,20-22 increased levels in markers of active coagulation, TAT complexes, F1 + 2, and D-dimers, were noted at baseline. Treatment decreased D-dimer levels, a measure of fibrinolysis of cross-linked fibrin (P < .04), while markers of thrombin generation, TAT complexes and F1 + 2, decreased but not to a statistically significant extent.
The adhesion molecule SVCAM-1 and VWFpp are released from damaged endothelial cells; levels of both molecules decreased with treatment (P < .05).
C-reactive protein, a marker of inflammation, was elevated at baseline; the decrease with therapy was not statistically significant (P = .18).
For those markers that decreased significantly with treatment (D-dimers, SVCAM-1, VWFpp), we looked for correlations with hematologic parameters (%F-cells, tHb, ARC, and ANC). SVCAM-1 levels inversely correlated with total hemoglobin (P = .002); VWFpp levels correlated with the ARC (P < .0001). There was no significant correlation between D-dimers and the hematologic parameters (Table 2).
Increase in platelet counts
Platelet counts increased in all patients during treatment. The highest platelet count was 877 × 109/L (877 000/mm3) (Figure 3A).
Decitabine increased megakaryocyte and erythroid commitment and decreased myeloid commitment. (A) Change in platelet counts with treatment, all patients. (B) Change in absolute neutrophil counts with treatment, all patients. (C) Inverse relationship between platelet and neutrophil counts illustrated by data from UPN 2 (the only patient to sustain grade 4 neutropenia; absolute neutrophil count [ANC] less than 0.5 × 109/L).
Decitabine increased megakaryocyte and erythroid commitment and decreased myeloid commitment. (A) Change in platelet counts with treatment, all patients. (B) Change in absolute neutrophil counts with treatment, all patients. (C) Inverse relationship between platelet and neutrophil counts illustrated by data from UPN 2 (the only patient to sustain grade 4 neutropenia; absolute neutrophil count [ANC] less than 0.5 × 109/L).
Decrease in absolute neutrophil counts
Most patients demonstrated a downward trend in the ANC with treatment (Figure 3B). UPN 2 had grade 4 (per NCI Common Toxicity Criteria) neutropenia (nadir ANC 0.4 × 109/L); UPN 6 and UPN 8 had grade 3 neutropenia (nadir ANC 0.8 × 109/L); and UPN 3 and UPN 4 had grade 2 neutropenia (nadir ANC 1.3 × 109/L and 1.0 × 109/L, respectively). In the 3 remaining patients, the ANC remained above 2.0 × 109/L. ANC nadirs occurred at the end of each cycle of treatment. In UPN 2, neutropenia with an ANC of less than 1.0 × 109/L persisted from week 14 to 16 (cycle 2 decitabine 3 times per week from week 9 to 14) and recovered to 1.7 × 109/L on week 17. There was an inverse relationship between platelet and neutrophil counts (Figure 3C).
Change in bone marrow morphology
Serial bone marrow aspirates were obtained in 4 patients who consented to this procedure at enrollment. There was no appreciable decrease in aspirate spicule cellularity with treatment. Treatment decreased the myeloid-erythroid ratio (ie, increased the proportion of erythroid cells) and increased megakaryocyte numbers in all patients (Table 3).
Change in bone marrow morphology with treatment
. | Pretreatment . | . | . | After cycle 1 . | . | . | After cycle 2 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID no. . | Cellularity . | M/E ratio . | MK/LPF . | Cellularity . | M/E ratio . | MK/LPF . | Cellularity . | M/E ratio . | MK/LPF . | ||||||
| UPN 1 | Hyper | 0.8 | 4 | Hyper | 0.7 | 10 | Hyper | 0.6 | 13 | ||||||
| UPN 3 | Hyper | 0.8 | 2-3 | — | — | — | Hyper | 0.3 | 6 | ||||||
| UPN 5 | Norm | 2 | 2 | Norm | 0.8 | 6 | Norm | 0.5 | 6 | ||||||
| UPN 7 | Hyper | 1 | 1 | Hyper | 0.3 | 8 | Hyper | 0.03 | 13 | ||||||
. | Pretreatment . | . | . | After cycle 1 . | . | . | After cycle 2 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID no. . | Cellularity . | M/E ratio . | MK/LPF . | Cellularity . | M/E ratio . | MK/LPF . | Cellularity . | M/E ratio . | MK/LPF . | ||||||
| UPN 1 | Hyper | 0.8 | 4 | Hyper | 0.7 | 10 | Hyper | 0.6 | 13 | ||||||
| UPN 3 | Hyper | 0.8 | 2-3 | — | — | — | Hyper | 0.3 | 6 | ||||||
| UPN 5 | Norm | 2 | 2 | Norm | 0.8 | 6 | Norm | 0.5 | 6 | ||||||
| UPN 7 | Hyper | 1 | 1 | Hyper | 0.3 | 8 | Hyper | 0.03 | 13 | ||||||
Cellularity indicates bone marrow aspirate cellularity; M/E ratio, myeloid to erythroid ratio; MK/LPF, number of megakaryocytes per low-power microscope field; and —, not done.
Decreased γ-globin promoter methylation
In UPN 1 and UPN 3, sufficient DNA was obtained from aspirated bone marrow nuclear cells to enable assessment of γ-globin promoter methylation before treatment, after cycle 1, and after cycle 2 of decitabine treatment using both bisulfite genome sequencing and COBRA. The methylation status of 5 CpG residues located at -54, -51, +5, +16, and +48 of the γ-globin promoter was analyzed using bisulfite genome sequencing of 20 individual clones from pretreatment and posttreatment marrow samples. In UPN 1, 91 of 100 total CpG sites analyzed were methylated before treatment, which decreased to 74 of 100 sites after treatment (χ test, P = .0002). In UPN 3, 98 of 100 sites were methylated before treatment, which decreased to 71 of 100 after treatment (χ test, P < .0001). The pattern of demethylation with treatment appeared to be random with no strong preference for specific sites within the γ-globin promoter. COBRA demonstrated a successive decrease in the mean level of methylation of the γ-globin promoter (as measured using densitometry) with each cycle of decitabine treatment (UPN 1: decreased from 97.6 to 78.2 to 65.7; UPN 3: decreased from 94.5 to 77.2 to 55.5).
Discussion
Safe and effective pharmacologic reactivation of HbF is likely to have a significant impact on the considerable morbidity and mortality of SSD. In this study of the DNA hypomethylating agent decitabine, our objectives were to assess the safety of subcutaneous administration, to produce cumulative and sustained increases in HbF and tHb by weekly administration, and to assess the mechanism of induced HbF production. The subcutaneous regimen was well tolerated with the only significant toxicity being neutropenia. HbF levels increased in all treated patients, even those with low baseline HbF levels who had not responded to previous treatment with HU. Increased HbF levels, by decreasing HbS polymerization, are expected to decrease RBC adhesion to endothelium and consequent endothelial cell damage, which are usual features of vaso-occlusive pathophysiology in SSD. Significant decreases in RBC adhesion to both TSP and laminin occurred with treatment, which correlated inversely with %F-cells. RBC adhesion to TSP also correlated with ANC, suggesting that some clinical improvement in decitabine- and HU-treated patients may result from treatment-related reduction in neutrophils (adhesion and activation of neutrophils by damaged endothelium contributes to vasoocclusive pathophysiology). In multivariate analysis, endothelial damage as indicated by VWFpp levels and SVCAM-1 levels correlated with the reticulocyte count and inversely correlated with tHb levels, respectively. The tHb may improve vaso-occlusive pathophysiology via improved oxygen delivery (insofar as the tHb increase does not increase blood viscosity and capillary transit time) and indirectly through decreased reticulocyte counts (reticulocytes are the most adhesive fraction of RBCs23 ). The correlation of these in vivo markers of endothelial damage with the ARC and hemoglobin levels indicates the importance of decreasing hemolysis, increasing hemoglobin, and decreasing reticulocytes as a therapeutic goal in SSD.
Platelet counts increased during treatment. This increase was of concern, because the coagulation pathway is likely to be involved in sickle cell disease pathophysiology. However, activation of the coagulation pathway as indicated by D-dimer levels decreased with decitabine treatment, despite the increased platelet counts. In the MSH study, 9 patients had platelet counts of more than 800 × 109/L (800 000/mm3), and 12 patients repeatedly exceeded 128 g/L (12.8 g/dL) of tHb; no morbidity was associated with these findings.4 Nonetheless, future clinical studies in sickle cell disease may require decitabine dose adjustments to prevent rheologically disadvantageous increases in tHb or platelets.
An increase in the platelet count and megakaryocyte numbers during treatment, maintenance of bone marrow cellularity, and progressive hypomethylation of the γ-globin promoter suggest a hypomethylating but noncytotoxic mechanism of action of decitabine at these doses. Although local hypomethylation at the γ-globin promoter may be contributory, it may not be the only basis for increased HbF with decitabine treatment. Epigenetic changes (DNA methylation and histone alterations) are a feature of hematopoietic differentiation.24 Therefore, global DNA hypomethylation by decitabine causing altered gene expression may shift hematopoietic differentiation toward erythroid and megakaryocytic lineages. F-cell increases may be one consequence of this altered hematopoietic differentiation. Consistent with our findings, treatment with 5-azacytidine has been shown to induce megakaryocytic differentiation and GATA-1 expression in the 416B myeloid cell line.25
Although this study suggests that decitabine can produce increases in HbF at doses that are not cytotoxic, the possibility remains that decitabine is genotoxic. Decitabine is incorporated into DNA, might impact genomic stability, and might have mutagenic effects,26,27 although in other studies decitabine did not show a reproducible, concentration-dependent increase in mutation frequency.28 Up-regulation of gene expression seems unlikely by itself to cause secondary malignancies. The histone deacetylase inhibitor phenylbutyrate is believed to up-regulate gene expression and has been used for many decades to treat children with inherited metabolic disorders without a reported increase in secondary malignancies. In some studies, decitabine has been able to decrease the rate of neoplastic transformation.29-31 Nonetheless, the possibility of secondary malignancy induction with decitabine cannot be ruled out with certainty except with longer follow-up of treated patients. Meanwhile, further studies of the molecular and cellular effects of decitabine can aid in risk assessment with long-term use.
Safe and effective pharmacologic reactivation of HbF, decreased hemolysis, and increased total hemoglobin are likely to bring significant clinical benefits to patients with SSD. Decitabine, either alone or in combination with other agents that alter those epigenetic processes that silence the γ-globin gene, could fulfill this role. Its mechanism of action differs from that of HU, the current standard for pharmacologic induction of HbF. Further studies are indicated to determine the effect of therapy on clinical sequelae of SSD.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1738.
Supported by SuperGen, the Illinois Department of Public Health through the University of Illinois Sickle Cell Center, the Sickle Cell Disease Association of America (Y.S.), and Public Health Service grants HL44612 and HL70981 (C.A.H.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge the assistance of Glenda Pendarvis, Marlene McGee, and Pamela Moore in the University of Illinois at Chicago Adult Sickle Cell Clinic.
The funding sources did not influence study design, collection, analysis or interpretation of data, writing of the report, or submission of the manuscript for publication.

![Figure 3. Decitabine increased megakaryocyte and erythroid commitment and decreased myeloid commitment. (A) Change in platelet counts with treatment, all patients. (B) Change in absolute neutrophil counts with treatment, all patients. (C) Inverse relationship between platelet and neutrophil counts illustrated by data from UPN 2 (the only patient to sustain grade 4 neutropenia; absolute neutrophil count [ANC] less than 0.5 × 109/L).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-05-1738/6/m_h82335317003.jpeg?Expires=1769085427&Signature=zdlNol72HjXkypXe1h5gcGZueTl3cxkFNK2bIX6vUFZGVHgjrlsGR8oJjDJc1xQpFsgCl4gW-D31xUdZoomLQdL3mU31VG8tx7dXPdI8DDWyknHs4jHBnPY8or--gbdpwS2ZD7QUH6L00~~9TDjgcyW84cSwpoizI9atyNfXbyd6gzDxCBB85oNIJcoaJB~Rz7fSVQfEMUXa~2GKb8~MIxpdyYiScjrfdHamh4iTxWoKFBfgMXPFy4bMXzim8iteBJ2unJ1TKKCRSUyOSKwp2-G3o3~vsx6KV6GcCIHeu~pZacbHIc4eytBqTXAqrHX~37kkBj7VU-MMc9ssnmUBTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal