Abstract
Antithymocyte globulins (ATGs), the immunoglobulin G (IgG) fraction of sera from rabbits or horses immunized with human thymocytes or T-cell lines, are used in conditioning regimens for bone marrow transplantation, in the treatment of acute graft-versus-host disease, in the prevention or treatment of acute rejection in organ transplantation, and in severe bone marrow aplasia. In nonhuman primates, ATGs induce rapid, dose-dependent, T-cell depletion in peripheral lymphoid tissues, where apoptotic cells can be demonstrated in T-cell zones. We show here that increasing ATG concentrations in vitro resulted in reduced lymphocyte proliferative responses, associated with a rapid increase in the percentage of apoptotic cells. Apoptosis did not require prior exposure to interleukin-2, nor did it result in CD178/CD95 or tumor necrosis factor/tumor necrosis factor receptor (TNF/TNF-R) interactions; it was therefore clearly different from activation-induced cell death. Cytochrome c release, caspase-9, and caspase-3 activation were not implicated, excluding a direct involvement of the intrinsic mitochondrial pathway. The cysteine protease inhibitor E64d and cathepsin-B-specific inhibitors conferred significant protection, whereas apoptosis was associated with the release of active cathepsin B into the cytosol. These data demonstrate a role for cathepsin B in T-cell apoptosis induced by ATGs at concentrations achieved during clinical use. (Blood. 2003; 102:3719-3726)
Introduction
Polyclonal antithymocyte globulins (ATGs) are the purified immunoglobulin G (IgG) fraction of sera from rabbits, horses, or, more rarely, goats immunized with human thymocytes or T-cell lines. Antilymphocyte globulins were first studied in various animal species by Metchnikoff1 at the end of the 19th century, then in the context of allograft rejection,2,3 and then they were introduced in the clinic for the treatment of corticosteroid-resistant rejection in organ transplantation. Human thymocytes are the most common source of antigen for the preparation of ATGs. Clinical indications for ATGs include prevention (induction therapy) of acute rejection in organ transplantation,4-6 rescue treatment of acute rejection, conditioning for hematopoietic stem cell transplantation from unrelated HLA-matched7 or haploidentical donors,8 treatment of graft-versus-host disease,9 and treatment of severe aplastic anemia.10 The doses used in bone marrow transplantation are usually higher than those used in organ transplantation.
Because ATGs contain many different antibody specificities,11-13 they are expected to induce a variety of biologic activities. For instance, we recently demonstrated that ATGs contain functional antibodies against several integrins and their ligands and against the chemokine receptors CXCR4, CCR5, and CCR7.14 However, the most common effect of ATG administration is the depletion of peripheral blood T cells, which progressively reverses after treatment ceases but may be long-lasting in the CD4+ subset in adults.15 Our experiments in monkeys showed that T-cell depletion was not restricted to the blood compartment.16 Indeed, T-cell apoptosis was readily demonstrated in spleen and lymph nodes but not in the thymus. The kinetics and magnitude of apoptosis were positively correlated with the dose of ATG.16 From these observations it was possible to delineate 2 broad categories of biologic effects for ATGs: T-cell depletion and functional effects on cells that escape depletion (eg, modulation, anergy).
Several mechanisms could account for the T-cell depletion induced by ATGs. Complement-dependent lysis occurs only at high ATG concentrations (100 to 1000 μg/mL) in rabbits and horses, and complement consumption could be demonstrated in sera from patients treated with horse (10-15 mg/kg/d) but not with rabbit (1 mg/kg/d) ATG.17 Antibody-dependent, cell-mediated cytotoxicity (ADCC) may be considered, but only activated T cells (T-cell blasts) are susceptible to ADCC at low concentrations of rabbit ATG (0.1-1 μg/mL); nonactivated lymphocytes were resistant.17 Furthermore, ADCC is achieved over a narrow range of low ATG concentrations (approximately 1 μg/mL), and only with rabbit but not with horse ATG.17 Activation-induced cell death (AICD) is another mechanism that could lead to T-cell apoptosis. Indeed, nonactivated T cells express CD178 (CD95L or FasL) mRNA within 6 hours of exposure to ATG (10-100 μg/mL)17 and could then trigger apoptosis of CD95+ target cells if such cells become susceptible to the CD95 apoptotic pathway.18 Despite the fact that we could document the role of CD95 by using the antagonist blocking CD95 monoclonal antibody (mAb) ZB4, which fully inhibited apoptosis, it is important to note that CD95+ T cells only become sensitive to this apoptotic pathway several days after activation in vitro or in vivo.18
Hence, ADCC and AICD are likely to contribute to ATG-induced T-cell depletion only after the time interval required for activated T cells to become susceptible to these cytotoxic pathways in vivo. Given that peripheral T-cell apoptosis occurs early during ATG treatment, starting at the first ATG infusion, other mechanisms of apoptosis should be sought. In the present study we report a novel type of T-cell apoptosis that occurs on activation with supraoptimal mitogenic concentrations of ATGs. We found that this apoptosis involves a previously unknown effect of ATGs—the release of cathepsin B from the lysosomes into the cytosol.
Materials and methods
Antibodies and reagents
The rabbit polyclonal ATG preparation used in this study was thymoglobulin (batch number 98THY0120; Sangstat, Lyon, France), a ready-to-use liquid solution stored in darkness at 2°C to 8°C and containing 5 mg/mL purified rabbit IgG; the horse ATG used was lymphoglobulin (batch number 99LPG0020; Sangstat, Lyon, France). The IgG fraction of a nonimmune rabbit serum (NRS), prepared according to the manufacturer's procedures, was used as a negative control. An F(ab′)2 fraction of ATG was prepared by pepsin digestion, and a previously described17 nonmitogenic ATG (number 5, which lacks mitogenic activity and CD2, CD3, and CD5 specificities) was also used. Two other rabbit ATGs used in clinical organ transplantation and previously used for comparison purposes (Tecelac Biotest and ATG-Fresenius) were introduced in proliferation and apoptosis assays (data not shown).14,19 Anti-Flag mAb (clone M2), rapamycin (RPM), staurosporine (STS), and pepstatin A were purchased from Sigma (St Quentin, Fallavier, France). Cyclosporin A (CsA) was kindly supplied by Novartis Pharma (Basel, Switzerland). Purified anti-CD95 mAb agonist (IgM, clone 7C11) and antagonist (IgG1, clone ZB4) were purchased from Immunotech (Marseilles, France). Tumor necrosis factor-receptor (TNF-R) p55 immunoglobulin fusion protein was kindly provided by Prof H. Waldmann (Oxford, United Kingdom), and TNF-α was obtained from R&D Systems (Abingdon, United Kingdom). Recombinant soluble human CD178 was obtained from Alexis (San Diego, CA). The broad-spectrum caspase inhibitor benzyloxycarbonyl (Cbz)-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk) and L-trans-Epoxysuccinyl-Leu-3-methylbutylamide ethyl ester (E-64d), Z-Phe-DL-Ala-fluoromethylketone (zFA-fmk), and Z-Phe-Lys-2,4,6-trimethylbenzoyloxy-methylketone (zFK-mbmk) were purchased from Bachem (Voisins-Le-Bretonneux, France). N-Benzyloxycarbonyl-L-leucylnorleucinal (Calpeptin) and N-Acetyl-Leu-Leu-Methional (calpain inhibitor 2) were obtained from Tebu (Le Perray-en-Yvelines, France), and PD150606 was obtained from Calbiochem (Meudon, France).
Cell preparation and culture
Peripheral blood lymphocytes (PBLs) were purified from healthy blood donors with their informed consent and ethics committee approval from the Etablissement Français du Sang. Blood was defibrinated, and mononuclear cells were isolated by centrifugation on a layer of Histopaque (Dutcher, Brumath, France). Those suspensions contained 74.4% ± 2.0% T lymphocytes, 7.5% ± 1.2% B lymphocytes, 16.1% ± 1.9% natural killer (NK) cells, and 0.8% ± 0.4% monocytes as defined by the expression of CD3, CD20, CD56, and CD14, respectively. PBLs were resuspended in RPMI 1640 (Sigma) supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL) and were cultivated in a humid atmosphere containing 5% CO2. PBLs were activated in 96-well tissue culture plates with various concentrations of ATGs, F(ab′)2 fragments of ATG, or nonmitogenic ATG. Activated T lymphocytes were obtained by culture of PBLs for 3 days with phytohemagglutinin (PHA) at a concentration of 5 μg/mL. At this stage, dead cells were removed and viable cells (106/mL) were treated with different apoptotic stimuli such as STS, anti-CD95, and CD178. After 3 days of stimulation with PHA, cells were further incubated for 11 days with IL-2 (50 U/mL). In these conditions, T lymphoblasts were susceptible to apoptosis induced by TNF-α.20 For proliferation assay, cells were pulsed during the indicated time with (methyl-3H) thymidine (3[H]TdR) (Amersham France SA, Les Ullis, France) at 0.5 μCi/well (0.0185 MBq/well). 3[H]TdR uptake was measured using a Packard direct β counter (Packard, Meriden, CT) after harvesting.
Measurement of apoptosis
Phosphatidylserine (PS) exposure was quantified by surface binding of annexin V.21 Two hundred thousand cells were resuspended in annexin V binding buffer containing fluorescein isothiocyanate (FITC)-conjugated annexin V for 15 minutes according to the instructions of the manufacturer (Bender MedSystems, Vienna, Austria). Propidium iodide (PI) (1 μg/mL) was then added, and cell suspension was immediately analyzed by flow cytometry using a FACScalibur and CellQuest software (BD Biosciences). Single-stranded DNA fragmentation was detected using F7-26 mAb from Alexis Corp (Apostain, Laufelfingen, Switzerland), according to the manufacturer's instructions.
Western blot analysis
Treated cells were washed with phosphate-buffered saline (PBS), and pellets were resuspended in appropriate reaction buffers: caspase-8 lysis buffer (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Triton X-100, 10 mM EDTA [ethylenediaminetetraacetic acid], 10 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg/mL aprotinin); total lysis buffer (62.5 mM Tris HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 0.72 M β-mercaptoethanol, 7% glycerol) for cleavage of caspase-3, -7, -9, polyadenosyl ribose polymerase (PARP), and FLIP. To measure cytochrome c and cathepsin release in the cytosol, cytosolic fractions (S-100) were prepared as described by Nakayama et al22 with minor modifications. Pellets were resuspended in an appropriate extraction buffer (200 mM mannitol, 68 mM sucrose, 50 mM PIPES-KOH [pH 7.4], 50 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol [DTT], 1 mM pefablock, 10 μg/mL leupeptin). After 30 minutes on ice, cells were lysed with a glass dounce homogenizer with 100 strokes of the B pestle, followed by centrifugation at 750g for 10 minutes at 4°C to remove the nuclei and unbroken cells. Supernatants (enucleated whole-cell lysates) were then centrifuged at 15 000g for 30 minutes at 4°C to remove lysosomes and mitochondria. The resultant supernatants were further centrifuged at 100 000g for 1 hour at 4°C, and the final supernatants (cytosolic fractions) were collected. Thirty micrograms protein was separated on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Blots were blocked for 2 hours at room temperature in PBS, 0.1% Tween, containing 5% nonfat dried milk and were incubated overnight at 4°C with anti-caspase-3, anti-caspase-7, anti-cytochrome c (mAb 7H8.2C12; BD Biosciences), anti-caspase-9 (New England BioLabs, Beverly, MA), anti-PARP mAb C-2-10 (Biomol, Plymouth Meeting, PA), anti-cytochrome oxidase (Molecular Probes, Eugene, OR), anti-actin (Sigma), anti-caspase-8, or anti-FLIP antibodies. Anti-caspase-8 and anti-FLIP antibodies were kindly provided by Prof P. Krammer (Heidelberg, Germany). Detection was achieved with the appropriate secondary antibodies coupled to horseradish peroxidase (HRP), followed by enhanced chemiluminescence (ECL) Western blot analysis (Amersham).
Measurement of cytosolic cathepsin activity
To measure cathepsin activity in the cytosol, cytosolic fractions were prepared as described above. Cathepsin B activity was estimated as described by Foghsgaard et al23 on 30 μg cytosolic protein by adding 50 μM Z-Arg-Arg-AMC (zRR-AMC) (Bachem) in cathepsin B reaction buffer (50 mM sodium acetate, 4 mM EDTA, 8 mM DTT, 1 mM pefablock [pH 6.0]). The release of 7-amido-4-methylcoumarin (AMC) (excitation 380 nm, emission 442 nm) was measured at indicated times at 37°C with a fluorometer.
Immunofluorescence and confocal laser scanning microscopy
Treated cells were seeded on glass coverslips by the cytospin method, fixed in paraformaldehyde 3.7%, permeabilized with 0.2% Triton X-100, and stained with anti-CD8 mAb (20 μg/mL) and anti-CD4 mAb (5 μg/mL; Sigma), or anti-cytochrome c (mAb 6H2.B4, 1:50; BD Biosciences), or rabbit anti-cathepsin B antibody (1:50; Oncogen Research Products, Darmstadt, Germany), and secondary antibodies Alexa Fluor 488 donkey antirabbit IgG (Molecular Probes), or Cy 5-labeled antimouse (Jackson Immunoresearch), or FITC-labeled antimouse (Jackson Immunoresearch). Cells were observed under a laser scanning confocal microscope (LSM 510; Zeiss, Oberkochen, Germany), and images were processed with Adobe Photoshop software 6.0 (Adobe Systems, San Jose, CA).
Transmission electron microscopy
After 2 washes with PBS, cells were fixed in 2% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4), dehydrated, and embedded in Epon. Thin sections were cut and following lead to citrate and uranyl acetate contrasting, were observed in a JEOL 100CS electron microscope (Peabody, MA).
Results
High concentrations of mitogenic ATGs induce apoptosis of T lymphocytes
Previous studies had shown that the proliferative response to ATGs, assessed by 3H-thymidine incorporation, followed a bell-shaped curve with a decreasing response at high ATG concentrations.24 We checked that this profile was a common feature of horse and rabbit ATGs (Figure 1A). Furthermore, in keeping with previous reports,24 we observed that F(ab′)2 fragments from a rabbit ATG were as efficient as whole IgG in triggering lymphocyte proliferation, with a similar bell-shaped response (Figure 1A). The 2 other rabbit ATGs gave similar results: the dose responses of Tecelac and thymoglobulin were similar, whereas those of ATG-Fresenius were reduced by a factor of 5 (data not shown).
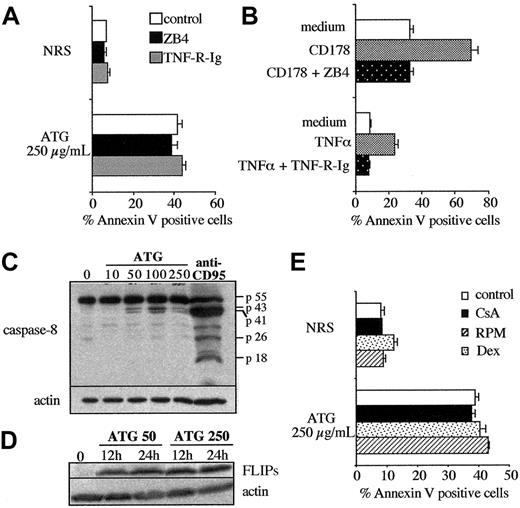
High concentrations of ATG induce apoptosis of PBL. (A-B) PBLs were incubated with rabbit (▪) or horse (•) ATGs, F(ab′)2 fragments of ATG (□), or nonmitogenic ATG number 5 (○) over a range of concentrations. In panel A, [3H]TdR uptake was measured after 72 hours of culture. In panel B, apoptosis was measured by surface binding of annexin V after 24 hours of culture. (C) Kinetics of ATG-induced apoptosis. PBLs were incubated with NRS alone (○), rabbit ATGs (250 μg/mL; ▪), or horse ATGs (500 μg/mL; •), and apoptosis was measured by surface binding of annexin V. (A-C) Results are expressed as the mean ± SEM from triplicate measurements of one experiment representative of several independent experiments. (D) PBLs were incubated for 24 hours in the presence of NRS (control) or ATG (250 μg/mL). Anti-CD95 (7C11; 1 μg/mL) was added to 3-day-activated lymphoblasts during 24 hours. DNA fragmentation was analyzed by flow cytometry staining using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. Results shown are representative of 4 independent experiments. (E) Morphologic features of cells after treatment with NRS or ATG were observed using transmission electron microscopy. Original magnification, × 10 000.
High concentrations of ATG induce apoptosis of PBL. (A-B) PBLs were incubated with rabbit (▪) or horse (•) ATGs, F(ab′)2 fragments of ATG (□), or nonmitogenic ATG number 5 (○) over a range of concentrations. In panel A, [3H]TdR uptake was measured after 72 hours of culture. In panel B, apoptosis was measured by surface binding of annexin V after 24 hours of culture. (C) Kinetics of ATG-induced apoptosis. PBLs were incubated with NRS alone (○), rabbit ATGs (250 μg/mL; ▪), or horse ATGs (500 μg/mL; •), and apoptosis was measured by surface binding of annexin V. (A-C) Results are expressed as the mean ± SEM from triplicate measurements of one experiment representative of several independent experiments. (D) PBLs were incubated for 24 hours in the presence of NRS (control) or ATG (250 μg/mL). Anti-CD95 (7C11; 1 μg/mL) was added to 3-day-activated lymphoblasts during 24 hours. DNA fragmentation was analyzed by flow cytometry staining using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. Results shown are representative of 4 independent experiments. (E) Morphologic features of cells after treatment with NRS or ATG were observed using transmission electron microscopy. Original magnification, × 10 000.
To test whether the lack of proliferation at high ATG concentrations was caused by cell death, we measured 2 parameters of apoptosis: surface exposure of PS quantified by annexin V binding and DNA fragmentation. The percentage of apoptotic cells increased in a dose-dependent manner, reaching approximately 40% after 24-hour treatment with 500 μg/mL ATG (Figure 1B). Interestingly, a similar dose-response (in μg/mL) was observed with F(ab′)2 fragments, suggesting that Fc-dependent mechanisms, including as an example ADCC, play little or no role in the apoptotic response observed at high ATG concentrations. The kinetics of apoptosis showed a progressive increase in the percentage of annexin-V-positive cells, starting at 6 hours of culture, with a delayed increase in PI-positive cells (Figure 1C and data not shown). Apoptosis was also unequivocally proven by the occurrence of DNA fragmentation, demonstrable at 24 hours in the presence of ATG (250 μg/mL). In this experiment we used T cells preactivated for 3 days and an anti-CD95 agonist mAb as positive control (Figure 1D). Finally, cells treated with high concentrations of ATG displayed the typical features of apoptosis in transmission electron microscopy, including condensed chromatin (Figure 1E).
In a previous study we had used ATG number 5, a horse ATG containing most specificities present in other ATGs (including CD11a/CD18) but lacking CD2, CD3, and CD5 specificities.11 This ATG number 5 was not mitogenic at concentrations ranging from 1 to 1000 μg/mL and did not induce AICD.17 This ATG was added at increasing concentrations (up to 1 mg/mL) to PBL cultures, and results confirm the lack of mitogenic activity of ATG number 5 and show that it does not induce an apoptotic response (Figure 1A-B). Therefore, ATG number 5 is devoid of mitogenic and proapoptotic activities, supporting the view that the antibody specificities accounting for these 2 biologic activities were both absent from ATG number 5 but present in all the other ATG preparations studied.
Apoptosis induced at high ATG concentrations differs from AICD
AICD involves interactions between ligands and death receptors such as CD178/CD95 and TNF/TNF-R.18,25-28 We showed previously that ATG could induce AICD, using as target cells T cells activated over 3 days by PHA, anti-CD3 mAb, or ATG. In these conditions apoptosis was inhibited in the presence of the antagonist CD95 mAb ZB4.17 To determine whether AICD was similarly involved in apoptosis observed at high ATG concentrations, the experiments were performed in the presence of ZB4 and soluble TNF receptor (TNF-R-Ig). No decrease of apoptosis was observed in these conditions, though ZB4 inhibited CD178-triggered apoptosis and TNF-R-Ig TNF-induced apoptosis, respectively (Figure 2A-B).
Apoptosis of T lymphocytes at high concentrations of ATG is not the consequence of an AICD process. (A) PBLs were activated by rabbit ATG at the indicated concentration in the presence or absence of ZB4 (2.5 μg/mL) and TNF-R-Ig (20 μg/mL), and apoptosis was measured at 24 hours. (B) Activated lymphoblasts were treated for 24 hours with either medium, CD178 (100 ng/mL) in the presence or absence of ZB4 (2.5 μg/mL), or TNF-α (50 ng/mL) in the presence or absence of TNF-R-Ig (20 μg/mL). (A-B) Apoptosis was measured by surface binding of annexin V. (C) Analysis of caspase-8 processing in the presence of rabbit ATG. PBLs were treated with ATG for 24 hours. As positive control, activated lymphoblasts were treated with anti-CD95 (7C11; 1 μg/mL) for 6 hours. (D) Kinetics of FLIPs expression in PBLs treated with rabbit ATG (50 or 250 μg/mL). (C-D) Cells were lysed after a wash with PBS, and proteins were loaded and separated on 12% SDS-PAGE followed by Western blotting with the anti-caspase-8 mAb (C) or the anti-FLIP mAb (D). Amounts of loaded proteins have been controlled for homogeneity by probing membranes with an anti-β-actin mAb. Results from one experiment representative of 2 independent experiments showing similar results. (E) PBLs were incubated with NRS or ATG (250 μg/mL) for 24 hours in the presence of CsA (1 μg/mL), RPM (500 nM), or dexamethasone (1 μM). Apoptosis was measured by surface binding of annexin V, and results shown are representative of 2 independent experiments.
Apoptosis of T lymphocytes at high concentrations of ATG is not the consequence of an AICD process. (A) PBLs were activated by rabbit ATG at the indicated concentration in the presence or absence of ZB4 (2.5 μg/mL) and TNF-R-Ig (20 μg/mL), and apoptosis was measured at 24 hours. (B) Activated lymphoblasts were treated for 24 hours with either medium, CD178 (100 ng/mL) in the presence or absence of ZB4 (2.5 μg/mL), or TNF-α (50 ng/mL) in the presence or absence of TNF-R-Ig (20 μg/mL). (A-B) Apoptosis was measured by surface binding of annexin V. (C) Analysis of caspase-8 processing in the presence of rabbit ATG. PBLs were treated with ATG for 24 hours. As positive control, activated lymphoblasts were treated with anti-CD95 (7C11; 1 μg/mL) for 6 hours. (D) Kinetics of FLIPs expression in PBLs treated with rabbit ATG (50 or 250 μg/mL). (C-D) Cells were lysed after a wash with PBS, and proteins were loaded and separated on 12% SDS-PAGE followed by Western blotting with the anti-caspase-8 mAb (C) or the anti-FLIP mAb (D). Amounts of loaded proteins have been controlled for homogeneity by probing membranes with an anti-β-actin mAb. Results from one experiment representative of 2 independent experiments showing similar results. (E) PBLs were incubated with NRS or ATG (250 μg/mL) for 24 hours in the presence of CsA (1 μg/mL), RPM (500 nM), or dexamethasone (1 μM). Apoptosis was measured by surface binding of annexin V, and results shown are representative of 2 independent experiments.
The major pathway of AICD uses CD178/CD95 and TNF/TNF-R interactions, but involvement of other death receptors belonging to the TNF receptor family, such as TNF-related apoptosis-induced ligand (TRAIL) receptors or DR3, could not be excluded. Apoptosis mediated by these death receptors requires the formation of an active death-inducing signaling complex (DISC) in which caspase-8 or caspase-10 are activated in the absence of FLIP.29 Therefore, caspase-8 processing and FLIP expression from PBLs treated with high concentrations of ATG were analyzed by Western blotting in our model. As shown in Figure 2C, cleavage products of caspase-8 p43/p41 were detected at mitogenic concentrations of ATG (50 and 100 μg/mL) but only slightly at the high concentration (250 μg/mL) that triggers apoptosis. However, the p18 active subunit, which was readily cleaved from caspase-8 in the presence of CD95 mAb (positive control), was never detected (Figure 2C). Furthermore, FLIPs expression was up-regulated in cells cultured with high and mitogenic concentrations of ATG (Figure 2D). All these results suggested that apoptosis of T cells induced by high concentrations of ATG is independent of death receptor/ligand interactions.
Several other characteristics distinguish AICD triggered by ATG from the apoptosis at high ATG concentrations. The main differences lie in the ATG concentrations required to trigger cell death in the 2 models and the kinetics of the reaction. As reported earlier, susceptibility to AICD and down-regulation of FLIP expression usually take several days.18,30 Furthermore, this process requires interleukin-2 (IL-2) and can be blocked in vitro by adding calcineurin inhibitors (CsA or FK506), anti-IL-2 receptor CD25 antibodies, RPM,31,32 or corticosteroids.33 Conversely, apoptosis induced by ATG at high concentrations follows rapid kinetics (Figure 1C), starting at 6 to 12 hours, and does not require progression beyond the G1 phase of the cell cycle, as shown by the lack of interference of CsA and RPM (Figure 2E). Furthermore, it is not inhibited in the presence of dexamethasone (Figure 2E).
Cytochrome c release is a late event in ATG-induced apoptosis
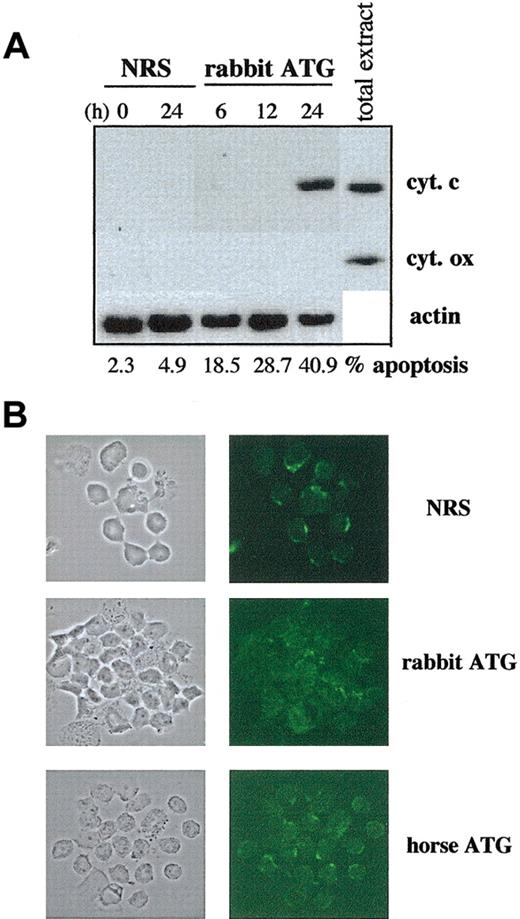
Several apoptogenic agents, including UV irradiation and lymphokine withdrawal, act directly on the mitochondrion and induce apoptosis by activating the intrinsic apoptotic pathway.34 In this context, some proteins, in particular cytochrome c and apoptosis-inducing factor (AIF), are released from the intermembrane space into the cytosol.35,36 To determine whether high concentrations of ATG triggered initially this intrinsic apoptotic pathway, the kinetics of cytochrome c release were studied by Western blotting in cytosolic extracts. As shown in Figure 3A, cytochrome c was released in the cytosol of PBLs treated by high concentrations of ATG only after 24 hours, whereas apoptosis measured by PS exposure was detectable as soon as 6 hours (18.5% of apoptotic cells vs 2.3% in the NRS-treated condition). We also confirmed by immunofluorescence the massive cytochrome c release observed after 24-hour treatment by high concentrations of horse or rabbit ATG (Figure 3B). These results indicate that cytochrome c release in the cytosol is a consequence rather than a cause of ATG-induced apoptosis.
Kinetics of cytochrome c release during ATG-induced apoptosis of T cells. PBLs were incubated with NRS, rabbit ATG (250 μg/mL), or horse ATG (250 μg/mL). (A) At the indicated times, cytosolic extracts were prepared and proteins were separated on 15% SDS-PAGE followed by Western blotting with the anti-cytochrome c mAb or the anti-cytochrome oxidase mAb. Anti-cytochrome oxidase served as a marker of mitochondrial contamination of the extracts. Amounts of loaded proteins have been controlled for homogeneity by probing membranes with an anti-β-actin mAb. Apoptosis (%) was measured by surface binding of annexin V at the indicated times. (B) After 24-hour treatment, cytochrome c release was visualized by immunofluorescence staining. Results are from one representative experiment of 2 independent experiments showing similar results. Original magnification, × 63.
Kinetics of cytochrome c release during ATG-induced apoptosis of T cells. PBLs were incubated with NRS, rabbit ATG (250 μg/mL), or horse ATG (250 μg/mL). (A) At the indicated times, cytosolic extracts were prepared and proteins were separated on 15% SDS-PAGE followed by Western blotting with the anti-cytochrome c mAb or the anti-cytochrome oxidase mAb. Anti-cytochrome oxidase served as a marker of mitochondrial contamination of the extracts. Amounts of loaded proteins have been controlled for homogeneity by probing membranes with an anti-β-actin mAb. Apoptosis (%) was measured by surface binding of annexin V at the indicated times. (B) After 24-hour treatment, cytochrome c release was visualized by immunofluorescence staining. Results are from one representative experiment of 2 independent experiments showing similar results. Original magnification, × 63.
Role of caspases in apoptosis induced by high concentrations of ATG
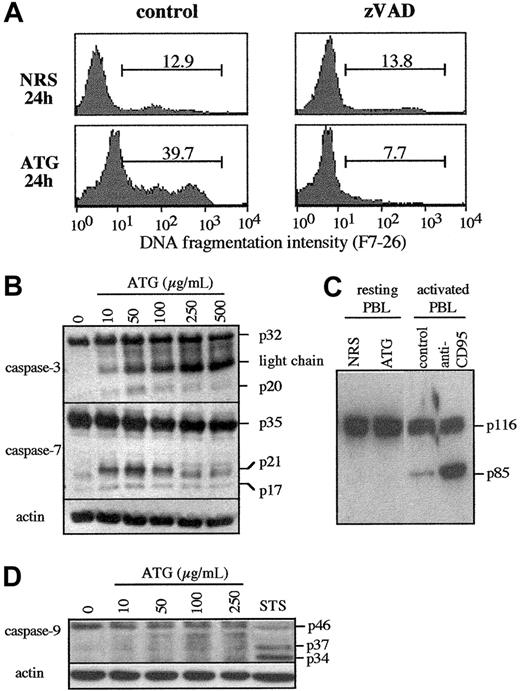
As a first approach to assess the role of caspases in apoptosis induced by high concentrations of ATG, we examined the effect of a pan-caspase inhibitor zVAD-fmk on DNA fragmentation. The percentage of cells that exhibited fragmented DNA was measured in PBLs treated using 250 μg/mL ATG at the indicated time. As shown in Figure 4A, zVAD-fmk strongly inhibited the generation of ATG-induced DNA fragmented cells (7.7% vs 39.7%). These results indicate that DNA fragmentation induced by high concentrations of ATG involves zVAD-fmk-sensitive proteases.
Role of caspases in apoptosis induced by high concentrations of ATG. (A) Effect of zVAD-fmk on DNA fragmentation induced by ATG. The caspase inhibitor zVAD-fmk (100 μM) was added to PBLs for 1 hour, and then cells were incubated with NRS or ATG (250 μg/mL). DNA fragmentation was analyzed at the indicated time by using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. (B) Caspase-3 and -7 processing. PBLs were treated with rabbit ATG at indicated concentrations and lysed at 24 hours, and proteins were separated on SDS-PAGE. (C) Analysis of PARP cleavage. PBLs were incubated with NRS or ATG (250 μg/mL) and preactivated T cells with anti-CD95 (7C11 1 μg/mL). At 24 hours, cells were lysed and proteins were separated on SDS-PAGE. The p85 band corresponds to the caspase-3 cleavage product of PARP. (D) Caspase-9 cleavage. Cells were treated with rabbit ATG and lysed at 24 hours, and proteins were separated on SDS-PAGE and analyzed by Western blotting with an anti-caspase-9 antibody that recognizes the proform (p46) and the cleaved forms (p37/p34) of caspase-9. As positive control, 3-day-activated PBLs were treated with STS (0.5 μM) for 12 hours. Amounts of loaded proteins were controlled for homogeneity by probing membranes with anti-β-actin mAb. All data are from one representative experiment of 2 independent experiments showing similar results.
Role of caspases in apoptosis induced by high concentrations of ATG. (A) Effect of zVAD-fmk on DNA fragmentation induced by ATG. The caspase inhibitor zVAD-fmk (100 μM) was added to PBLs for 1 hour, and then cells were incubated with NRS or ATG (250 μg/mL). DNA fragmentation was analyzed at the indicated time by using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. (B) Caspase-3 and -7 processing. PBLs were treated with rabbit ATG at indicated concentrations and lysed at 24 hours, and proteins were separated on SDS-PAGE. (C) Analysis of PARP cleavage. PBLs were incubated with NRS or ATG (250 μg/mL) and preactivated T cells with anti-CD95 (7C11 1 μg/mL). At 24 hours, cells were lysed and proteins were separated on SDS-PAGE. The p85 band corresponds to the caspase-3 cleavage product of PARP. (D) Caspase-9 cleavage. Cells were treated with rabbit ATG and lysed at 24 hours, and proteins were separated on SDS-PAGE and analyzed by Western blotting with an anti-caspase-9 antibody that recognizes the proform (p46) and the cleaved forms (p37/p34) of caspase-9. As positive control, 3-day-activated PBLs were treated with STS (0.5 μM) for 12 hours. Amounts of loaded proteins were controlled for homogeneity by probing membranes with anti-β-actin mAb. All data are from one representative experiment of 2 independent experiments showing similar results.
It was recently demonstrated that some caspases were activated during T-lymphocyte activation and proliferation.37,38 In this context, we investigated the activation of caspase-3, -7, and -9 in PBLs treated with ATG at increasing concentrations during 24 hours. We first used antihuman caspase-3 and caspase-7 antibodies, which recognize the pro-form and the processed forms of these caspases. The p20 subunit of caspase-3 and the p21/p17 subunits of caspase-7 were detected at mitogenic concentrations of ATG (10-50 μg/mL) and then decreased and were almost not detectable at higher concentrations of ATG (Figure 4B). In contrast to caspase-7, the p17-active cleaved form of caspase-3 was never detected (Figure 4B). To assess whether caspase-3 was active in these conditions, cleavage of PARP, a caspase-3 substrate, was studied by Western blotting in cell lysates of PBLs treated with high concentrations of ATG after 24 hours of culture. The p85 cleavage product of PARP was detected in anti-CD95-treated cells, but never in ATG-treated PBLs (Figure 4C). Taken together, these results suggest that caspase-3 was only partially cleaved but was never activated during apoptosis induced by high concentrations of ATG. In contrast to caspase-3 and caspase-7, caspase-9 was never cleaved into p37/p34 in cells treated with a dose range of ATG, whereas these cleaved forms were strongly detectable in lysates from STS-treated cells used as positive control of apoptosis (Figure 4D). Although caspase-3 and caspase-7 were found to be cleaved into p20 and p21/p17, respectively, this phenomenon seems to be associated with T-cell activation rather than apoptosis because these cleavage products were detected at mitogenic concentrations of ATG but were decreased at the higher concentrations that trigger cell death (Figure 4B).
Cathepsin B is released and active in the cytosol during ATG-induced apoptosis
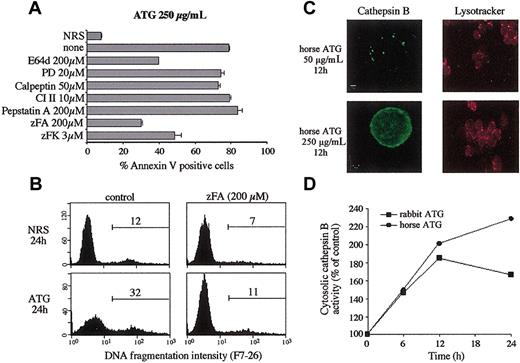
Because we could not detect the activation of caspase-3, -7, and -9 specifically associated with apoptosis induced at high concentrations of ATG, we examined whether the protective effect of zVAD-fmk could be attributed to the inhibition of proteases other than caspases. For instance, zVAD-fmk has been shown to inhibit lysosomal cysteine proteases,39 which have been recently reported to contribute to TNF-induced apoptosis.40 Thus, we studied the effect of a panel of protease inhibitors on ATG-induced apoptosis (Figure 5A). Compounds reported to inhibit lysosomal cysteine proteases (E64d) and those more specific for cathepsin B (zFA-fmk and zFK-mbmk) conferred significant protection against ATG-induced apoptosis in T cells, whereas inhibitors of calpains (PD 150606, calpeptin, and CI II) or aspartic proteases, including cathepsin D inhibitor (pepstatin A), had no significant effect on apoptosis measured by surface binding of annexin V (Figure 5A). We further tested the effect of zFA-fmk on DNA fragmentation induced by high concentrations of ATG; as shown in Figure 5B, DNA fragmentation was completely inhibited in the presence of this inhibitor.
Cathepsin B is translocated from lysosomes into the cytosol during ATG-induced apoptosis. (A-B) PBLs were treated by NRS alone or ATG (250 μg/mL) in the presence or absence of indicated protease inhibitors. (A) The percentage of apoptotic cells was measured 24 hours later by surface binding of annexin V. Results are mean ± SEM from a triplicate experiment and are representative of 3 independent experiments. (B) DNA fragmentation was analyzed at 24 hours by using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. (C) Visualization of cathepsin B release from lysosomes into cytosol in PBLs treated by horse ATG (50 or 250 μg/mL). Staining with lysotracker red (acidic organelle-selective probe) was used to characterize lysosomal membrane alterations. (D) Cells were treated with rabbit or horse ATG (250 μg/mL), and cytosolic extracts were prepared at indicated times. Equal amounts of proteins were analyzed for cathepsin B activity using the fluorogenic protease substrate zRR-AMC. Results are mean ± SEM from a triplicate experiment.
Cathepsin B is translocated from lysosomes into the cytosol during ATG-induced apoptosis. (A-B) PBLs were treated by NRS alone or ATG (250 μg/mL) in the presence or absence of indicated protease inhibitors. (A) The percentage of apoptotic cells was measured 24 hours later by surface binding of annexin V. Results are mean ± SEM from a triplicate experiment and are representative of 3 independent experiments. (B) DNA fragmentation was analyzed at 24 hours by using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. (C) Visualization of cathepsin B release from lysosomes into cytosol in PBLs treated by horse ATG (50 or 250 μg/mL). Staining with lysotracker red (acidic organelle-selective probe) was used to characterize lysosomal membrane alterations. (D) Cells were treated with rabbit or horse ATG (250 μg/mL), and cytosolic extracts were prepared at indicated times. Equal amounts of proteins were analyzed for cathepsin B activity using the fluorogenic protease substrate zRR-AMC. Results are mean ± SEM from a triplicate experiment.
To determine whether the effect of cathepsin B inhibitors on apoptosis may be associated with a release of this cathepsin from the lysosome into the cytosol, confocal microscopy was used to visualize directly the cellular redistribution of cathepsin B. In cells treated with mitogenic concentrations of horse ATG (50 μg/mL), the distribution of cathepsin B was localized in granules (Figure 5C). After treatment with high concentrations of horse ATG, fluorescence was diffusely distributed in the cytoplasm, suggesting that cathepsin B was released from the lysosomes into the cytosol (Figure 5C). Interestingly, lysosomes of ATG-treated cells retained the ability to accumulate an acidic organelle-selective probe, the lysotracker red (Figure 5C), suggesting that the cytosolic-lysosomal pH gradient was not altered and the lysosomal membranes were, at least, not completely disrupted. Thus, these results are consistent with translocation of cathepsin B from a vesicular compartment into the cytosol during exposure to high concentrations of ATG.
We next addressed whether cathepsin B was active in the cytosol of cells treated with ATG at high concentrations. Cathepsin B activity was measured in cytosolic extracts using the fluorogenic protease substrate zRR-AMC. Cytosolic cathepsin B activity was detected as soon as 6 hours for both ATGs, and it reached a maximum after 12 hours of treatment with rabbit ATG, whereas it still increased until 24 hours for horse ATG (Figure 5D). These results indicate that cathepsin B is released and is active in the cytosol of cells treated with high concentrations of ATG.
Discussion
We report here that ATGs trigger T-cell death in short-term cultures in the absence of an exogenous source of complement. Cell death was associated with the characteristic features of apoptosis, including externalization of membrane PS, preceding membrane permeability to PI, condensation of the chromatin, and DNA fragmentation. The triggering of apoptosis appears to be a general property of ATGs. It was indeed observed with all rabbit and horse ATGs tested, with one exception discussed below. The kinetics of apoptosis were rapid, starting at 6 to 12 hours and completed at 48 hours. ATG concentrations required to achieve apoptosis vary from one manufacturer to the other, in parallel with the total antilymphocyte antibody content as determined by indirect fluorescence or complement-dependent lysis.19 Those concentrations were higher with horse ATG than rabbit ATG. Apoptosis was moderate at mitogenic concentrations of ATGs and increased rapidly with a symmetrical drop in proliferative responses when ATG concentrations were raised. F(ab′)2 fragments of a rabbit ATG were fully active in inducing T-cell proliferation and apoptosis, thus ruling out the contribution of any Fc-dependent mechanism to cell death, such as, for instance complement-dependent lysis or ADCC.
ATG-induced T-cell apoptosis is likely to reflect the effect of activating (mitogenic) antibodies and to represent one of the mechanisms of T-cell depletion achieved by ATG treatment. By using the horse ATG number 5 devoid of CD2, CD3, and CD5 specificities,11 and lacking mitogenic activities,17 we could show that, in parallel, this ATG did not trigger T-cell apoptosis. Furthermore, ATG number 5 did not trigger apoptosis in the presence of optimal mitogenic concentrations of other ATGs (data not shown). Therefore, the hypothesis of 2 populations of antibodies, one accounting for T-cell activation and the other for T-cell apoptosis, is unlikely, though it cannot be formally excluded.
During thymoglobulin treatments at 2.5 mg/d, the trough levels of rabbit IgG (24 hours after the onset of ATG infusion) are approximately 100 μg/mL,19 and the peak levels are approximately 5 times higher (this represents a dynamic equilibrium with rapid changes). Those dosages are applicable to thymoglobulin and Tecelac. Fresenius ATG is given at doses 4 to 5 times higher, because of lower antilymphocyte antibody content per milligram IgG.19 During lymphoglobuline treatments of aplastic anemia, the peak levels of horse ATG measured by enzyme-linked immunosorbent assay (ELISA) were in the range of 100 to 1000 μg/kg in 10 patients who received 15 mg/kg/d.41 Thus, supramitogenic ATG concentrations are readily achieved during ATG treatments for bone marrow transplantation7,8 (2.5 mg/kg or more for 2-4 days) and during the recently introduced short-term induction treatments in kidney transplantation6 but sufficiently high ATG concentrations are unlikely to be achieved during treatment with thymoglobulin at 1 mg/kg/d (for 8-10 days) or their equivalent with ATGs from other manufacturers.
ATG-induced apoptosis occurs rapidly (6-12 hours) after the binding of ATG to T cells. Such kinetics are compatible with those of T-cell depletion, which is already maximal at the third day of treatment (possibly before) at the highest dose administered in cynomolgus monkeys.16 Most of the antibody specificities contained in ATGs are not T-cell-lineage specific. Some transient decreases in B and NK cell counts may be observed at high ATG doses, probably reflecting complement-dependent lysis, but the bulk of depletion concerns T cells, supporting a role for ATG-induced T-cell apoptosis. Interestingly, concomitant immunosuppression with corticosteroids, calcineurin inhibitors, or rapamycin does not impair ATG-induced apoptosis, in contrast to the blocking effect of these drugs on AICD. Despite the documented capacity of ATG to induce CD178 expression and to trigger CD95-dependent apoptosis of preactivated T cells,17 we could formally exclude a contribution of AICD, whatever the death receptor involved, in ATG-induced apoptosis during the first days of treatment. Indeed, the inhibitory protein FLIP was rapidly up-regulated during the 24-hour incubation with ATG, and caspase-8 was not cleaved in its active form.
ATG-induced apoptosis appears to use a novel apoptotic signaling mechanism, different from any described in other current models of apoptosis. The “intrinsic” mitochondrial pathway involves cytochrome c release from mitochondria, resulting in the formation of a ternary complex of cytochrome c, APAF-1, and caspase-9, referred to as apoptosome.42 This complex triggers the autocatalytic activation of caspase-9 and by cascade of the executioner caspases such as caspase-3 and -7. Our data show that caspase-9 is not processed during ATG-induced apoptosis (Figure 4), whereas cytochrome c is released after the externalization of PS has already occurred (Figure 3A).
In agreement with previous reports37,38 demonstrating that caspase-3, -6, -7, and -8 were processed during activation through the T-cell receptor (TCR), we observed that p20 of caspase-3 and p17 of caspase-7 were detectable in lysates from T cells exposed 24 hours at mitogenic (10-50 μg/mL) ATG concentrations, but no longer or to a lower extent at higher ATG concentrations. However, it is noteworthy that activation of these caspases remained incomplete because the p17-active cleaved form of caspase-3 was never detectable, whereas PARP, a caspase-3 substrate, was not cleaved (Figure 4B-C). The fact that zVAD-fmk still inhibits DNA fragmentation of T cells treated with high concentrations of ATG is intriguing. We cannot exclude that the inhibition of caspases could interfere with T-cell activation and subsequent apoptosis or, alternatively, that zVAD-fmk could interfere with other proteases (eg, cathepsin B).39
Strong evidence supports a critical role for cathepsin B in ATG-induced apoptosis. The specific inhibitor zFA-fmk markedly reduces the percentage of annexin-V-positive cells and suppresses ATG-induced DNA fragmentation. The enzyme is released from the lysosomes into the cytosol as an enzymatically active form, with a peak at 12 hours (Figure 5), and this process does not entail a complete disruption of lysosomal membranes as demonstrated by staining with lysotracker red. Recent evidence is emerging regarding the ability of cathepsins, including the cathepsins D43 and B,23 to induce all the classical features of apoptosis in the absence of caspase activation. Cathepsin B, for instance, is directly connected to proteins endowed with endonuclease activity,44 which accounts for DNA fragmentation.45
In conclusion, we report here that high concentrations of ATG trigger apoptosis, which is associated with the release of cathepsin B from the lysosomes into T-cell cytosol. By manipulating this process in the clinic, it is possible to tailor ATG regimens to achieve greater T-cell depletion without increasing the cumulative dose of ATG, such as by using short treatment courses with high doses (eg, 2-3 mg/kg thymoglobulin). This could ultimately permit more aggressive conditioning regimens, such as in haploidentical bone marrow transplantation. The need for more aggressive T-cell depletion is no longer restricted to bone marrow transplantation because, in kidney and in kidney plus pancreas allografts, several ongoing clinical trials are evaluating regimens without calcineurin inhibitors and without corticosteroids after intensive initial T-cell depletion by ATGs46 or by CDw52 mAb (alemtuzumab [Campath-1]). Such escalation, however, requires a careful assessment of the long-term effects of T-cell depletion. Reconstitution occurs primarily through intrathymic differentiation of T-cell precursors,47 a process that progressively declines with aging, making children, adolescents, and young adults more resistant to depletion than elder adults. In this respect ATGs, which do not penetrate the thymic tissue in vivo,16 may be less toxic than other T-cell-depleting agents.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-04-1075.
Supported by INSERM (grant 98 046) and the Rhône-Alpes region (grant 00816945). M.-C.M. is a recipient of a fellowship from the Ministère de l'Education Nationale et de la Recherche.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jean-Pierre Rouault for measuring cytosolic cathepsin activity by fluorometry, Simone Peyrol for helping with electronic microscopy, Prof H. Waldmann for the kind gift of TNF-R-Ig, and Prof P. Krammer for anti-caspase-8 and anti-FLIP antibodies. We also thank Dr N. Whitaker and Dr R. Buffet for careful editing of the manuscript.
We dedicate this work to Prof Jean-Pierre Revillard, who died on June 2, 2003.
Author notes
Jean-Pierre Revillard died on June 2, 2003.

![Figure 1. High concentrations of ATG induce apoptosis of PBL. (A-B) PBLs were incubated with rabbit (▪) or horse (•) ATGs, F(ab′)2 fragments of ATG (□), or nonmitogenic ATG number 5 (○) over a range of concentrations. In panel A, [3H]TdR uptake was measured after 72 hours of culture. In panel B, apoptosis was measured by surface binding of annexin V after 24 hours of culture. (C) Kinetics of ATG-induced apoptosis. PBLs were incubated with NRS alone (○), rabbit ATGs (250 μg/mL; ▪), or horse ATGs (500 μg/mL; •), and apoptosis was measured by surface binding of annexin V. (A-C) Results are expressed as the mean ± SEM from triplicate measurements of one experiment representative of several independent experiments. (D) PBLs were incubated for 24 hours in the presence of NRS (control) or ATG (250 μg/mL). Anti-CD95 (7C11; 1 μg/mL) was added to 3-day-activated lymphoblasts during 24 hours. DNA fragmentation was analyzed by flow cytometry staining using the F7-26 mAb. The percentage of cells with fragmented DNA is indicated for each histogram. Results shown are representative of 4 independent experiments. (E) Morphologic features of cells after treatment with NRS or ATG were observed using transmission electron microscopy. Original magnification, × 10 000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-04-1075/6/m_h82235245001.jpeg?Expires=1769116053&Signature=w6RPrN5DAkP8BB33~z3NN7Co84ot1NQUxBI8JVF488RmqibsfjapuEFXiuLnmvULJ9HH5Eip~Kf25azyn5xsP6zegjjc936B7kETj-qT6a12G~Oq~BDfziLpm6mTNBrAhJ4jblBpIpU-xniHG~sa-3GKuFwLuItBi621eJ6kosntlK1fPeHWs5LZjSVy-A-L54KI3Js79-1RrTPirJ8yHWgeTqbYoIsdFBsKdtXOPn48Y6RKECegy9LqTKcBqLGqwA94UScdtbM0Nrf9GTLPRPp--Ta7ctBp~rpxYpfLKFHrOOo-wAz~RPE9n6tld2~cBW0gKfB2MWbmV5IC-UQecg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal