Abstract
Fibrin(ogen) deficiency (Fg-/-) was shown previously to be compatible with rapid thrombus growth within injured arterioles, but platelet fibronectin content was increased and newly formed thrombi were unstable. To further define the role of fibrin(ogen) in thrombus formation and stabilization, platelet biology was examined in mice expressing a form of fibrinogen that clots normally but lacks the γ chain C-terminal binding site for αIIbβ3 (FgγΔ5). Thrombus growth within the arterioles of FgγΔ5 mice appeared faster than in wild-type mice despite a far greater emboli formation. Unlike Fg-/- mice, the emboli were relatively small and released from the top of thrombi, rather than by fracture at the vessel wall. The fibronectin content in FgγΔ5 platelets was also dramatically increased through a β3 integrin-dependent mechanism. The following has been concluded: (1) Fibrin formation contributes to, but is not sufficient for, the stabilization of arterial thrombi. Platelet receptor engagement of the C-terminal of the Fg γ chain contributes to the stable incorporation of platelets into thrombi. (2) Alternative ligands to fibrinogen can support efficient thrombus growth. (3) Fibrinogen is internalized through αIIbβ3 engagement of the fibrinogen γ chain element, and this interaction secondarily controls the fibronectin content of platelets. (Blood. 2003;102: 3609-3614)
Introduction
Arrest of bleeding at the site of injury is mediated by the adhesion and aggregation of platelets and the formation of the polymerized fibrin matrix. The same general process contributes to the generation of inopportune thrombi within atherosclerotic arteries. Thrombosis in coronary or cerebral arteries is a major cause of morbidity and mortality worldwide. It has been demonstrated that platelet membrane glycoprotein (GP) Ib complex and its ligand, von Willebrand factor (VWF), are involved in initiating platelet adhesion, particularly at high shear.1 Subsequent stable platelet adhesion and aggregation are mediated by several integrin receptors and their ligands, such as α2β1/collagen and αIIbβ3/fibrinogen (Fg) or VWF.1,2 The GPIb complex is also involved in platelet aggregation.3-5 In contrast to static or low shear conditions, where fibrinogen is necessary for platelet aggregation,6,7 at high shear efficient platelet aggregation can occur independently of Fg8-10 and even independently of both VWF and Fg.10 Under these conditions, fibronectin (Fn)11 and perhaps other molecules are able to support platelet aggregation. However, fibrinogen and the local conversion of fibrinogen to fibrin by thrombin are generally thought to be indispensable in stabilizing the thrombus.9,10
Fibrinogen is a 340-kDa glycoprotein dimer consisting of 2 sets of 3 polypeptide chains, termed the Aα, Bβ, and γ chains. Fibrinogen both provides the fundamental building block for assembly of provisional fibrin matrices and supports cell adhesion through specific integrin and nonintegrin receptor binding motifs. The C-terminal of the γ chain contains a critical binding site for the platelet integrin receptor, αIIbβ3 (GPIIbIIIa), which has been demonstrated to be required for platelet aggregation.12,13 Fg from FgγΔ5 mice, carrying the genetically modified form of the γ chain gene, which eliminates the last 5 residues (QAGDV) of the γ chain, maintains clotting function and factor XIIIa-mediated crosslinking but does not support platelet aggregation in vitro.13 FgγΔ5 mice uniformly develop to term and generally survive to adulthood, but the defect in platelet-fibrinogen interaction results in extended bleeding times following a surgical challenge and occasional spontaneous perinatal bleeding events.13 Arginyl-glycyl-aspartic acid (RGD) sequences, the most common binding motif for the integrin family,14,15 are present multiple times in the fibrinogen Aα subunit. However, the role of these RGDs in platelet aggregation has not yet been established. They may contribute to αIIbβ3-mediated clot retraction since β3 integrin,16 but not QAGDV from the γ subunit, is required for clot retraction.13
It is difficult to distinguish the role of Fg from fibrin in thrombus stabilization, but this information may be important for designing antithrombotic therapies. There are many agents that are able to block fibrin formation; most of them are thrombin inhibitors such as heparin, hirudin, and argatroban.17,18 However, thrombin inhibitors block not only fibrin formation but block also platelet activation through thrombin-mediated cleavage of protease-activated receptors (PAR-1, -3, and -4),17-20 which may affect thrombus growth and stabilization by inhibition of platelet granule release and possibly platelet aggregation through an Fg-independent pathway.10 Peptide GPRP, which is analogous to the N-terminal of both the α and β chains of fibrinogen, is able to inhibit fibrin polymerization, but it also inhibits platelet aggregation at high concentration in vitro and in vivo.21,22 In order to better dissect the importance of fibrinogen-platelet integrin interactions and fibrin polymer formation in thrombus formation and stability, we monitored thrombus growth in FgγΔ5 mice by using the intravital microscopy thrombosis model.10,23,24 We report here that, despite the preservation of fibrin polymer formation in FgγΔ5, these mice exhibit a distinct and significant defect in thrombus stability. In addition, consistent with the concept that ligand-αIIbβ3 interactions by resting platelets mediates fibrinogen/fibronectin internalization into α granules, we report that the FgγΔ5 mutation decreases the fibrinogen content of platelets and increases the fibronectin content of platelets.
Materials and methods
Mice
FgγΔ5 mice have been described.13 This strain has been backcrossed 9 times to C57BL/6J. To further minimize the influence of strain-dependent genetic differences, we produced the Fg+/+ and FgγΔ5 mice for this study by heterozygous crosses of Fg+/γΔ5 mice. Platelet donors were adult mice (> 5 weeks old). Recipient mice were young mice (23-30 days old, weighing 13-18 g). The experimental procedures were performed at the Center for Blood Research and approved by its Animal Care and Use Committee.
FgγΔ5/β3-/- mice were generated by crossing C57BL/6J background FgγΔ5 mice with the same background integrin β3-/- mice16 (kindly provided by Dr Richard O. Hynes, Massachusetts Institute of Technology, Cambridge). FNflox/FNflox; Mx-cre+/- mice25 were kindly provided by Dr Reinhard Fässler, Max Planck Institute for Biochemistry, Martinsried, Germany.
Flow cytometry
Blood samples from 3 mice of each genotype were pooled and platelets isolated on a Sepharose 2B column.10 For labeling αIIbβ3, platelets were first incubated 30 minutes at room temperature with 1:200 diluted rat monoclonal antibody D9, which specifically recognizes mouse αIIbβ3 (kindly provided by Dr A. K. Ng, University of Southern Maine, Portland). Fluorescein isothiocyanate (FITC) goat anti-rat immunoglobulin G (IgG; Cappel, Durham, NC) 1:500 was then added and incubated for another 30 minutes. For staining β1 integrins, 1:200 diluted FITC-hamster antirat CD29 antibody Ha2/5 (PharMingen, San Diego, CA), which cross-reacts with mouse CD29, was incubated with platelets at room temperature for 30 minutes. Platelets (10 000) were analyzed using a BD FACScaliber (Becton Dickinson, San Jose, CA). No alteration in the expression of either αIIbβ3 or β1 integrin has been found on platelets of FgγΔ5 mice (data not shown).
In vivo thrombosis model
The thrombosis model used in these studies was previously described in detail.10,23,24 Briefly, platelets were isolated from donor mice through a Sepharose 2B column, fluorescently labeled with calcein acetoxymethyl ester (1 μg/mL; Molecular Probes, Eugene, OR). Male mice (23-30 days old) were injected with fluorescently-labeled platelets (5 × 106/g) of matching genotype in the lateral tail vein. The mice were anesthetized, and the mesentery was exteriorized through a midline abdominal incision. Arterioles were visualized with a Zeiss Axiovert 135-inverted microscope (32 ×, 0.4NA; Zeiss, Oberkochen, Germany) and recorded on videotape. FeCl3 (30 microliters of a 250-mM solution) was applied to an approximately 2- to 5-mm in length section of arteriole by topical application, which induced local vessel injury and denudation of the endothelium.10,26 Vessels were monitored for 40 minutes after injury or until full occlusion occurred (blood flow stopped) and lasted for more than 10 seconds. In all experiments, one arteriole was chosen in each mouse based on quality of blood flow and exposure. A total of 14 wild-type (Fg+/+) and 18 FgγΔ5 recipient mice were studied. Shear rate in wild-type mice (1230 ± 30 s-1, n = 14) was similar to FgγΔ5 mice (1289 ± 35 s-1, n = 18) (P = .23). The method of shear rate measurement and the variation of shear rate during an experiment have been described before.10
Several parameters were applied to describe the characteristics of thrombus formation: (1) early single platelet deposition on the vessel wall, determined as the number of fluorescently labeled platelets that deposited on the vessel wall per minute after injury, (2) the time required for the formation of a thrombus of diameter larger than 20 μm, (3) the time required for 50% vessel occlusion (ie, diameter of thrombus was measured on videotape to be approximately 50% of the diameter of the vessel), (4) thrombus stability by determining the number of thrombi of diameter larger than 30 μm that embolized away from the viewing field before the vessel occluded, (5) occlusion time of vessel (ie, time required for blood to stop flowing), and (6) site of vessel occlusion (ie, same site of injury or downstream).
Western blot
Blood samples from 3 mice of each genotype were pooled and platelets were isolated on a Sepharose 2B column. Platelets (108) of each genotype were lysed in 45 microliters lysis buffer (0.1 M Tris [tris(hydroxymethyl)aminomethane]-Cl, pH 8.3, 0.2% sodium dodecyl sulfate [SDS], 10% glycerol, 4 mM EDTA [ethylenediaminetetraacetic acid], 2 mM N-methylmaleimide, 2 mM iodoacetic acid, 4 mM phenylmethylsulfonyl fluoride); 5 microliters 1 M dithiothreitol (DTT) was added and the sample was boiled for 10 minutes. Protein extracts from 2.5 × 106 platelets were separated in 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). This membrane was blocked with 20 mM Tris-Cl, pH 7.6, containing 137 mM NaCl, 0.1% Tween 20, and 5% nonfat milk, then incubated at room temperature for 2 hours with 1:2000 rabbit antimouse fibronectin or rabbit antihuman Fg, which cross-reacts with mouse Fg. Membrane was then incubated with 1:10 000 diluted peroxidase-conjugated affiniPure F(ab′)2 fragment donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The blots were developed with SuperSignal West Pico Chemiluminescent Substrate (PIERCE, Rockford, IL) and revealed with either X-ray film or a phosphorimager (Molecular Dynamics Storm 860) and analyzed by IMAGEQUANT (Molecular Dynamics, Sunnyvale, CA). To ensure protein was equally loaded, 1:1000 mouse antichicken vinculin, which cross-reacts with mouse vinculin (clone no. VIN-11-5; Sigma), was used as an internal control, and images were developed with horseradish peroxidase-conjugated purified goat anti-mouse IgG (Zymed Laboratories, South San Francisco, CA). To compare the plasma levels of fibronectin in Fg+/+, FgγΔ5, and FgγΔ5/β3-/- mice, proteins (0.1 microliters/well) of pooled plasma samples (3 mice/each genotype) were separated by SDS-PAGE gels and blots were developed by the chemiluminescence method following the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical analysis
Data are presented as means ± SEM. Statistical significance was assessed by unpaired Student t test or χ2 test as indicated.
Results
Early platelet adhesion was not affected in FgγΔ5 mice
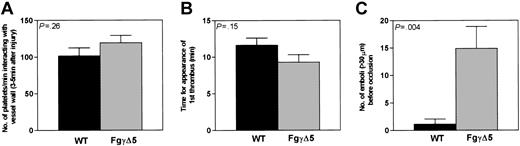
Fg-mediated platelet adhesion is known to be sensitive to shear rate27 and occurs mainly when the shear rate is less than 600 to 900 s-1. It is not clear whether Aα chain RGD sequences or the γ chain C-terminal sequence is involved in platelet adhesion and whether Fg/fibrin plays a role in platelet adhesion at high shear after tethering by VWF. Shortly after the endothelial injury in wild-type mice, platelets began to adhere to the vessel wall with interactions that were mostly transient (Figure 1). In FgγΔ5 mice expressing a mutant form of fibrinogen γ chain lacking the C-terminal QAGDV sequence recognized by the platelet integrin receptor, αIIbβ3, the numbers of single adherent platelets per minute, determined in the interval 3 to 5 minutes after the injury, were not significantly affected (Figure 2A). Thus, the C-terminal of γ subunit is not essential for the early platelet vessel wall adhesion at high shear rate. This finding is consistent with the prevailing view that VWF plays a predominant role in initial platelet adhesion under high shear stress.1
Thrombus growth in wild-type and FgγΔ5 mice. Times after FeCl3-induced injury are indicated in white. Blood flow was from left to right. In the wild-type (top row) and the FgγΔ5 (bottom row) mice, single fluorescent platelets are seen to adhere in the arterioles at 4 minutes after injury. In the wild-type vessel, stable thrombi grew (15 minutes) leading to complete vessel occlusion at 16 minutes. Several well-anchored small thrombi can be seen (15 minutes), but embolization was not observed. In the FgγΔ5 mouse, the thrombi grew faster (brighter and larger thrombi can be seen at 13 minutes). However, thrombi were not stable in blood flow, and emboli were frequently released by rupture through the center of thrombi (2 sequential panels taken within one second at 13 minutes). The arrows in these panels show 2 emboli formed in the first panel that translocated downstream. One is still seen in the second panel. The vessel was occluded at 14 minutes by an occlusive thrombus formed at the site of injury. The sequence of events is better visualized in the accompanying video clips on the Blood website; see the Supplemental Video link at the top of the online article.
Thrombus growth in wild-type and FgγΔ5 mice. Times after FeCl3-induced injury are indicated in white. Blood flow was from left to right. In the wild-type (top row) and the FgγΔ5 (bottom row) mice, single fluorescent platelets are seen to adhere in the arterioles at 4 minutes after injury. In the wild-type vessel, stable thrombi grew (15 minutes) leading to complete vessel occlusion at 16 minutes. Several well-anchored small thrombi can be seen (15 minutes), but embolization was not observed. In the FgγΔ5 mouse, the thrombi grew faster (brighter and larger thrombi can be seen at 13 minutes). However, thrombi were not stable in blood flow, and emboli were frequently released by rupture through the center of thrombi (2 sequential panels taken within one second at 13 minutes). The arrows in these panels show 2 emboli formed in the first panel that translocated downstream. One is still seen in the second panel. The vessel was occluded at 14 minutes by an occlusive thrombus formed at the site of injury. The sequence of events is better visualized in the accompanying video clips on the Blood website; see the Supplemental Video link at the top of the online article.
Quantitative analysis of formation of thrombi in wild-type (▪) and FgγΔ5 (▦) arterioles. (A) The number of fluorescent platelets deposited per minute was determined in the interval of 3 to 5 minutes after injury. Fg γ chain C-terminal sequence appeared not to influence significantly the early platelet interactions with the subendothelium. (B) Thrombus formation. The time required for the formation of the first thrombus larger than 20 μm in diameter was also similar in these 2 genotypes. (C) Embolization. The number of large emboli larger than 30 μm generated in the period before occlusion was determined. About 15 times more emboli formed in FgγΔ5 mice than in wild type (Mean ± SE. wild type: n = 14; FgγΔ5: n = 18).
Quantitative analysis of formation of thrombi in wild-type (▪) and FgγΔ5 (▦) arterioles. (A) The number of fluorescent platelets deposited per minute was determined in the interval of 3 to 5 minutes after injury. Fg γ chain C-terminal sequence appeared not to influence significantly the early platelet interactions with the subendothelium. (B) Thrombus formation. The time required for the formation of the first thrombus larger than 20 μm in diameter was also similar in these 2 genotypes. (C) Embolization. The number of large emboli larger than 30 μm generated in the period before occlusion was determined. About 15 times more emboli formed in FgγΔ5 mice than in wild type (Mean ± SE. wild type: n = 14; FgγΔ5: n = 18).
Platelet aggregation and embolus formation were increased in FgγΔ5 mice
Several minutes after the injury, platelets started to adhere more stably to the vessel wall and the first aggregates formed (Figure 1). For each mouse, we measured the time from injury to formation of a thrombus of more than 20 micrometers in diameter. We found the mean time required for initial thrombus formation was 2.3 minutes shorter in FgγΔ5 mice but statistical difference was not quite reached (wild type = 11.6 ± 1.3, FgγΔ5 = 9.3 ± 0.9 minutes, P = .1486) (Figure 2B). However, the time required to reach 50% vessel occlusion was significantly shorter in FgγΔ5 mice (Table 1). Thus, similar to previous observations using in vitro perfusion chambers with Fg-deficient blood,8,9 thrombi grew faster in FgγΔ5 mice. This counterintuitive finding is also consistent with our unpublished observation that thrombi were more rapidly formed in Fg-deficient mice than in wild-type mice (H.N. and D.D.W., 1998).
Comparison of thrombus growth and vessel occlusion time in wild-type and FgγΔ5 mice
. | 50% occlusion, min . | 100% occlusion, min . |
|---|---|---|
| Wild type, n = 14 | 16.8 ± 2.1 | 20.6 ± 2.8 |
| FgγΔ5, n = 18 | 11.8 ± 1.2 | 15.8 ± 1.7 |
| P | .0399 | .1329 |
. | 50% occlusion, min . | 100% occlusion, min . |
|---|---|---|
| Wild type, n = 14 | 16.8 ± 2.1 | 20.6 ± 2.8 |
| FgγΔ5, n = 18 | 11.8 ± 1.2 | 15.8 ± 1.7 |
| P | .0399 | .1329 |
The time required for 50% and 100% arterial occlusion was measured. Thrombus growth was faster in FgγΔ5 mice, but no significant difference was found in time required for complete occlusion.
This rapid thrombus growth was even more impressive given the most distinctive phenomenon observed in FgγΔ5 mice compared with wild-type animals—the persistent shedding of large numbers of emboli before vessel occlusion. Indeed, far higher numbers of embolic events (thrombi > 30 micrometers) were observed following vascular injury in FgγΔ5 mice than in the control cohort (14.9 ± 4 embolic events in FgγΔ5 mice vs 1.1 ± 1 in wild-type mice, P < .004) (Figure 2C). However, differing from the emboli formed in Fg-deficient mice,10 the majority of the emboli in FgγΔ5 mice were released by rupture through the center or upper portion of thrombi (Figure 1; see 13 minutes), whereas most of the emboli in Fg-/- mice were stripped from the interfaces of thrombi and vessel walls.10
FgγΔ5 vessels occluded at the site of injury without a significant delay
While platelet aggregation and thrombus growth were faster in FgγΔ5 mice and there was a tendency toward faster occlusion (mean occlusion time was 4.8 minutes shorter), in comparison with wild type, this difference was not statistically significant (Table 1). This may be due to the many emboli formed, which slowed down occlusive thrombi formation in the mutant mice. Interestingly, 88% (16/18) of the vessels in FgγΔ5 mice were able to occlude at the site of injury. This sharply differed from Fg-deficient mice10 in which 0% (0/17) of the vessels occluded at the site of injury and all occluded downstream by emboli (X2 = 19.7, P < .005). The emboli shed from thrombi in FgγΔ5 mice were usually smaller than emboli stripped from vessel walls in Fg-deficient mice,10 and this may explain why the frequency of downstream occlusion was less than in Fg-/- mice. The frequency of downstream occlusion in FgγΔ5 mice (2/18) was not statistically different from wild-type controls (0/14) (χ2 = 1.68, P > .1).
Increased Fn content in FgγΔ5 platelets
We previously observed that platelet Fn content was dramatically increased in mice lacking Fg.10 However, it was not clear from these early findings whether fibrinogen or fibrin controlled platelet Fn levels or whether the increased Fn content in platelets resulted from plasma Fn internalization or from up-regulation of Fn synthesis by megakaryocytes. Another lingering question was whether RGD sequences in the Fg Aα chain or the C-terminal sequence of the γ chain mediated platelet internalization of Fg and/or, secondarily, controlled the internalization of other platelet ligands.
Interestingly, we found that FgγΔ5 platelets, similar to Fg-/- platelets,10 contained large amounts of Fn but little Fg (Figure 3). The increased Fn content in FgγΔ5 platelets disappeared when FgγΔ5 mutation was combined with a deficiency of β3 integrin by breeding the 2 strains of animals (Figure 3). The combined mutation had no effect on plasma Fn level (data not shown). The Fn content in FgγΔ5/β3 double-mutant platelets was even less than in wild-type platelets (Figure 3), suggesting that the key platelet integrin receptor, αIIbβ3, is responsible not only for Fn internalization in FgγΔ5 mice and Fg-deficient animals but that it also promotes some Fn internalization in wild-type mice as well. These results indicate that the C-terminal of Fg γ chain mediates Fg internalization and inhibits excessive Fn internalization, most likely by competition for the αIIbβ3 integrin.
Increased fibronectin content in platelets of FgγΔ5 mice by fibronectin internalization mediated by β3 integrin. For each genotype, platelets were isolated from pooled blood of 3 mice. Gel-filtrated platelets (2.5 × 106) were lysed and analyzed by Western blot. Platelet fibronectin (Fn) content was dramatically increased but little fibrinogen (Fg) was detected in FgγΔ5 mice. The platelet fibronectin content in FgγΔ5 mice was similar to Fg-/- mice.10 The increased fibronectin content in FgγΔ5 platelets disappeared when the mice were crossed with β3-/- mice. Platelet fibronectin content of the FgγΔ5/β3 double-deficient mice was similar to that of plasma fibronectin-/- mice (ie, lower than wild-type [WT]).
Increased fibronectin content in platelets of FgγΔ5 mice by fibronectin internalization mediated by β3 integrin. For each genotype, platelets were isolated from pooled blood of 3 mice. Gel-filtrated platelets (2.5 × 106) were lysed and analyzed by Western blot. Platelet fibronectin (Fn) content was dramatically increased but little fibrinogen (Fg) was detected in FgγΔ5 mice. The platelet fibronectin content in FgγΔ5 mice was similar to Fg-/- mice.10 The increased fibronectin content in FgγΔ5 platelets disappeared when the mice were crossed with β3-/- mice. Platelet fibronectin content of the FgγΔ5/β3 double-deficient mice was similar to that of plasma fibronectin-/- mice (ie, lower than wild-type [WT]).
Discussion
The studies presented here further underscore the importance of Fg in thrombus formation and reveal that fibrinogen plays a role in thrombus stability by a mechanism that is distinct from fibrin polymer formation. Reported are 4 important findings related to the role of Fg in hemostasis and thrombosis. First, contrary to initial expectations, thrombi grew appreciably faster in vivo at high shear rate in FgγΔ5 mice in the absence of Fg γ chain C-terminal binding site for αIIbβ3 integrin. Second, despite the preservation of clotting function in FgγΔ5 mice, newly established thrombi were less stable and frequently embolized. Third, the γ chain motif recognized by αIIbβ3 was not critical for anchoring the thrombi to the vessel wall. Thus, the prevention of embolization by the Fg γ chain sequence occurred mainly within thrombi by strengthening the adhesion of newly recruited platelets. Finally, the Fg γ chain C-terminal QAGDV sequence was essential for αIIbβ3-mediated Fg internalization into platelets and this element also controlled internalization of Fn, apparently through competitive occupancy of the platelet αIIbβ3 integrin.
Thrombi grow faster in the presence of Fg lacking the C-terminal γ chain sequence
The fact that Fg is required for platelet aggregation has been documented over several decades based on results from in vitro aggregation assays at low shear conditions.6,7 However, the notion that Fg is essential to thrombus formation was challenged by recent observations made under high shear stress conditions in in vitro perfusion chambers8,9,28 and in in vivo studies with intravital microscopy.10 Interestingly, at high shear stress, thrombi grew even faster when Fg binding to platelets was genetically eliminated (Table 1). An attractive explanation for the maintenance of platelet aggregation in the absence of Fg was that VWF replaced the function of Fg to bridge platelets at high shear29 since VWF may assume a conformation with superior binding and accessibility to platelet receptors at high shear stress.27,30,31 However, even if largely correct, this explanation may not be entirely sufficient in that efficient platelet aggregation can still occur in mice lacking both Fg and VWF.10 Our recent work shows that Fn may also be able to support platelet aggregation and thrombus formation at high shear stress in vivo,11 and perhaps at low shear rates as well (H.N., D.D.W., unpublished results, 2001). Thus, the contribution of various platelet ligands to thrombus formation in vivo may be more sophisticated than initially perceived.
Given that αIIbβ3 appears to be required for any platelet aggregation in the thrombosis model used in these studies (P. Yuen, H.N., Q. Xiao, D.D.W., and R.O. Hynes, manuscript in preparation), one would not have anticipated that thrombi would grow faster in the FgγΔ5 mice. One explanation is that the alternative ligand(s) of β3 integrin such as VWF or Fn may more efficiently bridge activated platelets at the site of vascular injury at high shear. However, an attractive alternative explanation is that fibrinogen constitutes a superior bridging molecule in supporting the formation of tight junctionlike contacts (“tight contacts”) between opposing platelet surfaces that appear to contribute to the rapid formation of compact thrombi.32,33 Therefore, the loose and unstable thrombi formed in the absence of fibrinogen form a voluminous network that more readily captures incoming platelets. The rapidly changing shapes of these thrombi might also increase local blood turbulence within and around these thrombi that would recruit/activate more platelets to the thrombi. In addition, we cannot exclude the possibility that the high Fn content in the platelets of Fg-/-10 and FgγΔ5 mice (Figure 3) enhanced platelet aggregation in these mice.
Thrombus stabilization by the fibrinogen γ chain
Although Fg is not the only molecule able to support platelet aggregation, it plays an indispensable role in thrombus stabilization in vivo.10,34 A large number of emboli were formed in both the Fg-/-10 and FgγΔ5 mice. While available platelet ligands in FgγΔ5 mice were sufficient for anchoring thrombi to the vessel wall, the specific γ chain C-terminal αIIbβ3 binding site was necessary for preventing the splitting of newly formed bonds between platelets within thrombi. It remains uncertain whether platelets were passively stabilized at the level of the vessel wall by fibrin polymer, or whether platelet αIIbβ3 in thrombi actively bound to the fibrin of FgγΔ5 mice. Conformational changes that increase RGD accessibility in fibrinogen have been recorded,35 and alternate αIIbβ3 binding sites were suggested in FgγΔ5 clots in clot retraction assays.13 In addition, Fn released from platelets of FgγΔ5 mice may become crosslinked to fibrin. Platelets may then be anchored to fibrin through αIIbβ3-Fn-fibrin interactions. Thus, it is possible that platelet thrombi in FgγΔ5 mice may be fixed to the vessel by fibrin through an active anchor formed by either an αIIbβ3-RGD interaction, and/or an αIIbβ3-Fn-fibrin interaction.
It is worthwhile to note that the embolization observed in FgγΔ5 mice was also distinctive from that seen in plasma Fn-deficient mice.11 In the Fn-deficient mice, platelet recruitment and thrombi dissolution occurred simultaneously, whereas FgγΔ5 mice exhibited rapid platelet recruitment and significantly larger emboli. This suggests that plasma Fn may contribute in part to initial platelet recruitment, and Fg provides important stabilization to platelet aggregates prior to fibrin polymer formation. Clearly, even in the presence of Fn, the Fg/fibrin ligation is still required when shear forces are high and are escalated following the thrombus growth as we see in this study.
Platelet internalization of fibronectin and fibrinogen
α granule release is known to play an important role in platelet aggregation,36 and it has been proposed that platelet-released Fg may be a core adhesive ligand in supporting thrombus growth at high shear.37 Fg complexes, such as the Fg-Fn complex, may be particularly efficient in supporting platelet aggregation.
Fg internalization into platelet α-granules is mediated by the β3 integrin.16,38 We demonstrate in this study that some or all of the last 5 residues (QAGDV) of Fg γ subunit are necessary for this process. It is interesting that, reciprocally, the platelet Fn content in FgγΔ5 platelets, as in Fg-/- mice, was dramatically increased. This indicates that these 2 ligands are internalized by the same receptor (αIIbβ3 integrin). There is no evidence indicating that the C-terminal of Fg γ subunit binds to other receptors on platelets. α5β1 integrin, the receptor of Fn, does not appear to be important for Fn internalization. This may be due to the small amount of this receptor on platelets and/or a reduced ability of this integrin to support receptor-mediated uptake.
In this study, we demonstrated an important role for the C-terminal region of Fg γ in thrombus formation at high shear rates and in regulating platelet Fn and Fg content. In low shear conditions, the loss of Fg-αIIbβ3 interaction may also decrease platelet adhesion and aggregation. This would explain the bleeding tendency of FgγΔ5 mice.13 Our study shows that therapeutic strategies to specifically block the interaction between the C-terminal Fg γ chain and αIIbβ3 may have to be used with caution, as blocking this interaction may actually enhance thrombus growth at high shear rates and promote emboli formation.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-03-0850.
Supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grants R37HL41002 and P01HL56949 to D.D.W. and R01HL63194 to J.L.D. and the Heart and Stroke Foundation of Canada, of which H.N. was a fellow.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Lesley Cowan and Marybeth Cameron for help with preparation of the manuscript.


![Figure 3. Increased fibronectin content in platelets of FgγΔ5 mice by fibronectin internalization mediated by β3 integrin. For each genotype, platelets were isolated from pooled blood of 3 mice. Gel-filtrated platelets (2.5 × 106) were lysed and analyzed by Western blot. Platelet fibronectin (Fn) content was dramatically increased but little fibrinogen (Fg) was detected in FgγΔ5 mice. The platelet fibronectin content in FgγΔ5 mice was similar to Fg-/- mice.10 The increased fibronectin content in FgγΔ5 platelets disappeared when the mice were crossed with β3-/- mice. Platelet fibronectin content of the FgγΔ5/β3 double-deficient mice was similar to that of plasma fibronectin-/- mice (ie, lower than wild-type [WT]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-03-0850/6/m_h82235256003.jpeg?Expires=1769097747&Signature=SRHxg1YJw9R30HkZV3yZeS9NMOYPN~zq2LrPS2WaYFpsmDCV027pAsPzDp6cvY2j9KGb69AeqwyWLR4fjmrLXqD5gG5OUz3isB7Bg7DimaKZ3s0Hlrujzf2Wy45l6ShsgyJLYTRxXkNhZ2uF8Bbl5QmJ1Pf8DdtpV~xmia8yPzMk9E-LRcbUMxLlbqbhMvfDPOCuo3Deg1gKg1MsYeIdoObkY26rZ36gBZqqi11RA1cnRJ2juUutN6UDCIzCJtiG0m8uTCS2eEPhdulJgEr0B7CI6Ov45JOXkI3HuihzooneMjgMsLsQpB7bvdAcqmdMR-RZsKBFweNu1QEkKsOpYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal