Abstract
Granulocyte colony-stimulating factor (G-CSF) is the principal cytokine regulating granulopoiesis. G-CSF receptor-deficient mice (G-CSFR-/-) are neutropenic but have only a modest reduction of committed myeloid progenitors. Since it is likely that compensatory mechanisms are induced by the severe neutropenia present in G-CSFR-/- mice, a competitive repopulation assay was performed. These data show that under basal conditions, G-CSF drives nearly all of granulopoiesis through multiple mechanisms. Most importantly, G-CSFR signals regulate the production and/or maintenance of committed-myeloid progenitors. Surprisingly, G-CSFR signals also play a significant role in the regulation of primitive multipotential progenitors in vivo. The contribution of G-CSFR-/- cells to the hematopoietic stem cell compartment is modestly reduced. Moreover, a marked decrease in the contribution of G-CSFR-/- cells to other progenitors in the myeloid pathway, including erythroid and megakaryocytic progenitors, is observed. In contrast, relative to the hematopoietic stem cell compartment, the contribution of G-CSFR-/- cells to the lymphoid lineages is increased. These data suggest that G-CSFR signals may play a role in directing the commitment of primitive hematopoietic progenitors to the common myeloid lineage. Thus, regulation of G-CSF levels may provide a mechanism for directing primitive hematopoietic progenitors into the common myeloid lineage in response to environmental stresses. (Blood. 2003; 102:3562-3568)
Introduction
The role that external factors, in particular cytokines, play in the regulation of hematopoiesis is not fully defined. It is well established that cytokines play an important role in regulating individual hematopoietic lineages. For example, in response to anemia, expression of erythropoietin often increases, leading to the specific stimulation of erythropoiesis. Likewise, the serum level of granulocyte colony-stimulating factor (G-CSF) is often elevated in response to neutropenia or infectious stress, leading to the specific stimulation of granulopoiesis.1,2 Whether similar environmental cues regulate more primitive multipotent progenitors is less clear.
As a model for hematopoietic growth factors in general, the contribution of G-CSF to the regulation of hematopoiesis has been extensively studied. G-CSF is the principal growth factor regulating granulopoiesis. The biologic effects of G-CSF are mediated through the G-CSF receptor (G-CSFR), a member of the hematopoietic (class I) cytokine receptor family.3 The G-CSFR is expressed on multipotential hematopoietic progenitors and on more differentiated cells in the myeloid lineage.4,5 The role of G-CSF in basal granulopoiesis has been confirmed by the severe, but not absolute, neutropenia present in G-CSF-deficient and G-CSFR-deficient (G-CSFR-/-) mice.6,7 Interestingly, the number of granuloctye-macrophage colony-forming units (CFU-GMs) is minimally reduced in these mice, suggesting that G-CSFR signals are not required for commitment to the myeloid lineage.
A potential confounding problem of loss of function mouse models is the induction of compensatory mechanisms that may mask an important phenotype. It is likely that compensatory mechanisms are induced by the severe neutropenia that is present in G-CSFR-/- mice. To circumvent this problem, a competitive repopulation assay with G-CSFR-/- and wild-type bone marrow cells was performed. Here, evidence is provided that under basal conditions, G-CSFR signals drive nearly all of granulopoiesis. Surprisingly, G-CSFR signals are also required for the normal production of progenitors in the common myeloid pathway, including erythroid and megakaryocytic progenitors. In contrast, the loss of G-CSFR signals results in enhanced production of lymphoid cells. These data suggest that G-CSFR signals may play a role in directing commitment of primitive hematopoietic progenitors to the common myeloid lineage.
Materials and methods
Wild-type and G-CSFR-/- mice
Wild-type C57BL/6 mice and a congenic strain of C57BL/6 mice (B6.SJL-Ptprc* Pep3b BoyJ) that have the Ly5.1 gene were obtained from Jackson Laboratory (Bar Harbor, ME). G-CSFR-/- mice, generated as previously described,7 were backcrossed 10 generations onto a C57BL/6 background. All mice were housed in a specific pathogen-free environment and examined daily by veterinary staff for signs of illness.
Competitive repopulation assay
Wild-type (Ly5.1) and G-CSFR-/- (Ly5.2) bone marrow cells were harvested from strain- and sex-matched mice. A total of 5 million wild-type and G-CSFR-/- bone marrow cells were mixed at the indicated ratios and injected into the tail vein of lethally irradiated wild-type mice (Ly5.1). Recipient mice were conditioned with 900 cGy from a 137Cesium source at a rate of approximately 95 cGy per minute at 24 hours prior to transplantation. Prophylactic antibiotics (trimethoprim-sulfamethoxazole; Alpharma, Baltimore, MD) were given during the initial 2 weeks after transplantation. Mice were analyzed 3 to 6 months after transplantation. Three independent groups of mice received transplants; mice were analyzed separately, and the results pooled.
Peripheral blood and bone marrow analysis
Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA (ethylenediaminetetraacetic acid). Complete blood counts were determined by means of a Hemavet automated cell counter (CDC Technologies, Oxford, CT). Bone marrow was harvested by flushing both femoral bones with alpha-minimum essential medium (α-MEM) containing 10% fetal bovine serum. Manual leukocyte differentials were performed on Wright-stained blood smears or cytospin preparations of bone marrow mononuclear cells.
Flow cytometry
Red blood cells in peripheral blood and bone marrow cell preparations were lysed in Tris (tris(hydroxymethyl)aminomethane)-buffered ammonium chloride (pH 7.2) buffer, and the remaining cells were incubated with the indicated antibody at 4°C for 1 hour in phosphate-buffered saline (PBS) containing 0.1% sodium azide and 0.2% bovine serum albumin (fluorescence-activated cell sorter [FACS] buffer) to block nonspecific binding. Incubation with a secondary reagent was done for 20 minutes at 4°C following 2 washes with FACS buffer. The following monoclonal antibodies were used (all from Pharmingen, San Diego, CA, unless otherwise stated): Gr-1 (RB6-8C5), B220 (RA3-6B2), CD11b (M1/70), CD3e (145-2C11), CD45.2 (104), CD45.1 (A20), and F4/80 (CI:A3-1; Serotec, Raleigh, NC). Streptavidin/R-phycoerythrin-cyanin 5 (RPE-Cy5) (no. C0050; Dako, Carpinteria, CA) was used as a secondary reagent to visualize biotinylated antibodies. Appropriate isotype controls were used to set gate parameters. Peripheral blood cells were analyzed on a FACScan flow cytometer.
Progenitor analysis was done using a separate protocol. Cells were stained with Cy5-PE (Tricolor)-conjugated rat antibodies specific for the following lineage markers: CD3 (CT-CD3), CD4 (CT-CD4), CD8 (CT-CD8a), B220 (RA3-6B2), Gr-1 (RB6-8C5), and CD19 (6D5) (Caltag). Cells were then stained with phycoerythrin (PE)-conjugated anti-CD45.1 (Ly5.1), biotinylated anti-CD45.2 (Ly5.2), fluorescein isothiocyanate (FITC)-conjugated anti-CD34 (RAM34) or anti-Sca-1 (E13-161-7), PE-Texas Red-conjugated anti-FcγRII/III (2.4G2) and allophycocyanin (APC)-conjugated anti-c-Kit (2B8) monoclonal antibodies. For myeloid progenitor analysis, anti-Sca-1 antibodies were included in the lineage cocktail, and biotinylated anti-CD45.2 antibodies were visualized with avidin-APC-Cy7 (Caltag). Myeloid progenitors were sorted as Lin-Sca-1-c-Kit+CD34+FcγRII/IIIlo (CMPs), Lin-Sca-1-c-Kit+CD34+FcγRII/IIIhi (GMPs), and as Lin-Sca-1-c-Kit+CD34-FcγRII/IIIlo (MEPs) as described previously. HSCs were sorted as Lin-Sca-1hic-Kithi population. Cells were sorted using a double laser (488 nm/350 nm Enterprise II + 647 nm Spectrum) high-speed FACS (Moflo-MLS; Cytomation, Fort Collins, CO).
Hematopoietic progenitor assays
Bone marrow cells from each individual mouse were enumerated by means of an automated cell counter and plated in 1 mL methylcellulose medium (MethoCult M3230; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with GM-CSF (granulocyte/macrophage colony-forming unit [CFU-GM]) or with erythropoietin and stem cell factor (erythroid burst-forming unit [BFU-E]). For mixed colony-forming unit (CFU-mixed) and pre-B colony-forming unit (CFU-pre-B) cultures, cells were plated in 1 mL MethoCult M3434 or MethoCult M3630, respectively (both from Stem Cell Technologies). Cells were plated at the following cell concentrations: 2 × 104/mL for CFU-GM and CFU-mixed cultures, and 3 × 105/mL for CFU-pre-B and BFU-E cultures. Colonies with more than 30 cells were counted on days 10 through 14. Benzidine staining was used to identify erythroid cells in BFU-E and CFU-mixed colonies.9,10 Recombinant cytokines were used at the following concentrations: murine GM-CSF, 20 ng/mL; stem cell factor, 100 ng/mL; erythropoietin, 4 U/mL (all from R&D Systems, Minneapolis, MN). Geneticin (G418; Invitrogen, Carlsbad, CA) was added to specific cultures at a final concentration of 1 mg/mL. This dose of geneticin was empirically chosen to kill nearly all wild-type hematopoietic progenitor cells with minimal effect on the growth of G-CSFR-/- hematopoietic progenitor cells (“Results”).
Tritiated thymidine suicide assay
The percentage of CFU-GM progenitors in S-phase was determined by means of a tritiated thymidine suicide assay, as previously described.11 Bone marrow cells were incubated at 37°C for 20 minutes with 50 μL 3H-thymidine (1 mCi/mL [37 MBq/mL]; specific activity 50 Ci/mmol [1850 MBq/mmol]) in the presence or absence of 500 μg unlabeled thymidine. After incubation, cells were washed with 2000 μg thymidine in iced medium, washed twice with iced medium alone, and plated in MethoCult M3230 to enumerate CFU-GMs. The percentage of CFU-GMs in S-phase was calculated by dividing the difference in colony number (between cultures initiated with cells exposed to 3H-thymidine alone or coincubated with excess unlabeled thymidine) by those coincubated with excess unlabeled thymidine.
Long-term culture-initiating cell (LTC-IC) assay
LTC-ICs in the bone marrow were quantified according to the manufacturer's instructions in murine long-term culture medium (MyeloCult M5300; Stem Cell Technologies) supplemented with hydrocortisone, with the following modifications: a feeder layer of irradiated AFT024 stromal cells (gift from Dr Ihor Lemischka, Princeton University, NJ) was first established in 96-well plates. Four different concentrations of bone marrow cells (4, 2, 1, and 0.5 × 104 cells per well) were plated onto the feeder layer. Cultures were maintained at 33°C for 5 weeks with weekly half-media exchanges. Cells were harvested from each well with the use of trypsin and plated in MethoCult M3434 medium to enumerate colony-forming cells. L-Calc software (Stem Cell Technologies) was used to calculate the progenitor cell frequency with a standard error of the mean (SEM).
In experiments with the mixed chimeras, the LTC-IC assay was modified as follows: 3 × 104 bone marrow cells per well were plated onto the feeder layer. After 5 weeks of culture, the contents of each well were harvested, divided into 2 equal parts, and plated in MethoCult M3434 medium with and without 1 mg/mL G418.
Homing assay
A total of 10 million wild-type and G-CSFR-/- bone marrow cells were mixed at a 1:1 ratio and transplanted into lethally irradiated hosts, as described. Mice were killed 24 hours after transplantation and bone marrow cells harvested from both femurs. Wild-type and G-CSFR-/- CFU-GMs were quantified as described, except that 2 × 105 cells per dish were cultured. The percentage of G-CSFR-/- CFU-GMs was compared before and after transplantation to determine homing efficiency relative to wild-type progenitors.
Statistical analysis
Data are presented as the mean ± the standard deviation (SD), unless otherwise stated. Statistical significance was assessed by means of a 2-sided Student t test.
Results
Inbred G-CSFR-/- mice display impaired granulopoiesis and subtle defects in other hematopoietic lineages
Hematopoiesis was characterized in G-CSFR-/- mice backcrossed 10 generations onto a C57BL/6 background. Similarly to G-CSFR-/- mice on an outbred background,7 no difference in peripheral blood counts or bone marrow cellularity was observed compared with wild-type mice. The numbers of B cells, T cells, granulocytes, and monocytes were assessed by flow cytometry and morphologic analysis. The only peripheral blood abnormality in the inbred G-CSFR-/- mice is neutropenia, with the level of circulating neutrophils 16% that of wild-type mice (P < .001). In the bone marrow, granulocytic precursors were present at 50% of the level found in wild-type bone marrow (P < .001), and B cells were increased at 120% of the level found in wild-type bone marrow (P < .05).
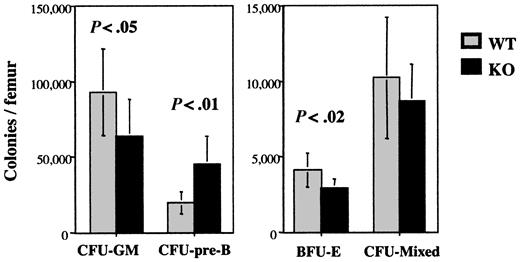
The number and cytokine responsiveness of lineage-committed and multipotential progenitors in the bone marrow of the mice were next examined. As reported previously for outbred G-CSFR-/- mice, a modest but significant decrease in the number of CFU-GMs was observed in inbred G-CSFR-/- mice relative to wild-type mice (Figure 1). Surprisingly, a significant decrease in erythroid committed progenitors (BFU-Es) and a trend to decreased progenitors with mixed granulocyte/macrophage and erythroid potential (CFU-mixed) was also observed. In contrast, the number of B-lymphoid progenitors (CFU-pre-Bs) was significantly increased.
Hematopoietic progenitor assays. Bone marrow cells from wild-type (WT) or G-CSFR-/- (KO) mice were plated in methylcellulose-containing media supplemented with the appropriate cytokines for each progenitor type (“Materials and methods”). Hematopoietic colonies containing more than 30 cells were counted after 7 to 10 days and reported as the number of colonies per mouse femur. Data represent the mean ± SD (n = 6 mice minimum, each). Relevant P values are shown.
Hematopoietic progenitor assays. Bone marrow cells from wild-type (WT) or G-CSFR-/- (KO) mice were plated in methylcellulose-containing media supplemented with the appropriate cytokines for each progenitor type (“Materials and methods”). Hematopoietic colonies containing more than 30 cells were counted after 7 to 10 days and reported as the number of colonies per mouse femur. Data represent the mean ± SD (n = 6 mice minimum, each). Relevant P values are shown.
To assess the primitive multipotent progenitor population, an LTC-IC assay was performed. Evidence from several studies indicates that LTC-ICs are primitive multipotential progenitors that are closely related to hematopoietic stem cells (HSCs).12-16 In fact, a recent report showed that 50% of LTC-ICs demonstrate long-term repopulating ability.15 Interestingly, the frequency of LTC-ICs in the bone marrow of wild-type and G-CSFR-/- mice was similar: 1/16 700 (-1 SEM 1/19 400; +1 SEM 1/14 400) and 1/17 200 (-1 SEM 1/20 400; +1 SEM 1/14 600), respectively. Collectively, these data show that in addition to the defect in granulopoiesis, the loss of G-CSFR signals results in a perturbation of nongranulocytic lineages. Whereas the number of primitive multipotent progenitors is normal, progenitors in the common myeloid lineage are decreased and B-lymphoid progenitors are increased.
G-CSFR signals regulate granulopoiesis primarily through the production of myeloid-committed progenitors
The observed perturbations in hematopoiesis in G-CSFR-/- mice may be secondary to a cell-intrinsic mechanism caused by the loss of G-CSFR signals. Alternatively, the severe neutropenia present in these mice may induce compensatory changes that affect hematopoiesis. To distinguish between these possibilities, a competitive repopulation assay was performed. G-CSFR-/- (Ly5.2), also referred to as knockout (KO) cells, and wild-type (Ly5.1) bone marrow cells were mixed at ratios of 1:1, 3:1, and 9:1 (KO/WT) and transplanted into irradiated wild-type (Ly5.1) mice. As a transplantation control, a separate cohort of recipient (Ly5.1) mice received transplants of only wild-type (Ly5.2) cells. At 6 months after receiving transplants, mice were analyzed by flow cytometry to determine the contribution of G-CSFR-/- and wild-type cells to each hematopoietic lineage. Importantly, in the control transplants, more than 99% of blood leukocytes were of donor origin, confirming that the conditioning regimen resulted in complete ablation of endogenous hematopoiesis (data not shown). In the mixed chimeras, the contribution of G-CSFR-/- cells to the B- and T-lymphocyte lineages was near predicted levels (Table 1). However, the contribution of G-CSFR-/- cells to the granulocytic lineage was profoundly impaired, in such a way that in the 9:1 mixed chimeras, 92.0% of blood neutrophils were of wild-type origin. A significant skewing in the monocyte lineage toward wild-type cells was also observed. Collectively, these data show that under basal conditions, granulopoiesis is nearly completely dependent on G-CSFR signals.
Percentage of KO cells in G-CSFR KO/WT chimeras
Chimera, KO/WT* . | B-lymphocytes, % KO (KO/WT ratio)† . | Blood, % KO (KO/WT ratio)† . | . | . | Bone marrow neutrophils, % KO (KO/WT ratio)† . | ||
|---|---|---|---|---|---|---|---|
| . | . | T lymphocytes . | Neutrophils . | Monocytes . | . | ||
| 1:1 | 57 ± 11 (1.4:1) | 53 ± 7 (1.2:1) | 2 ± 1 (0.02:1) | ND | 4 ± 1 (0.04:1) | ||
| 3:1 | 76 ± 10 (3.0:1) | 68 ± 9 (2.4:1) | 4 ± 2 (0.04:1) | ND | 7 ± 2 (0.08:1) | ||
| 9:1 | 83 ± 4 (4.9:1) | 79 ± 6 (4.2:1) | 8 ± 5 (0.09:1) | 34 ± 12 (0.6:1) | 17 ± 8 (0.2:1) | ||
Chimera, KO/WT* . | B-lymphocytes, % KO (KO/WT ratio)† . | Blood, % KO (KO/WT ratio)† . | . | . | Bone marrow neutrophils, % KO (KO/WT ratio)† . | ||
|---|---|---|---|---|---|---|---|
| . | . | T lymphocytes . | Neutrophils . | Monocytes . | . | ||
| 1:1 | 57 ± 11 (1.4:1) | 53 ± 7 (1.2:1) | 2 ± 1 (0.02:1) | ND | 4 ± 1 (0.04:1) | ||
| 3:1 | 76 ± 10 (3.0:1) | 68 ± 9 (2.4:1) | 4 ± 2 (0.04:1) | ND | 7 ± 2 (0.08:1) | ||
| 9:1 | 83 ± 4 (4.9:1) | 79 ± 6 (4.2:1) | 8 ± 5 (0.09:1) | 34 ± 12 (0.6:1) | 17 ± 8 (0.2:1) | ||
ND indicates not determined.
The original transplant ratio.
The percentage of KO cells in each lineage at 6 months after transplantation (n = 6 minimum, each). Data represent the mean ± SD, followed by the calculated KO/WT ratio after transplantation.
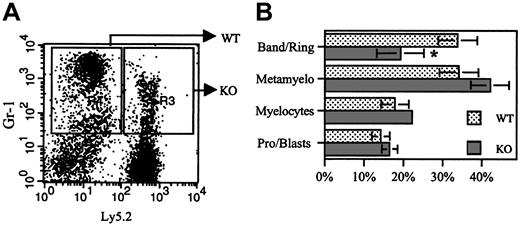
Previous studies showed that G-CSFR signals contribute to neutrophil release from the bone marrow17 and terminal granulocytic differentiation.18,19 To determine whether the loss of G-CSFR signals resulted in impaired neutrophil release, donor chimerism in bone marrow granulocytic cells was characterized. Relative to the blood, an approximately 2-fold increase in the number of G-CSFR-/- neutrophils in the bone marrow was observed in each group of chimeras (Table 1). These data, while consistent with a modest neutrophil-release defect, do not completely account for the decrease in G-CSFR-/- neutrophils in the blood. To determine whether the loss of G-CSFR signals resulted in a block in granulocytic differentiation, Gr-1+ (granulocytic) cells were sorted into G-CSFR-/- (Ly5.2+) and wild-type (Ly5.2-) fractions, and their morphology was examined (Figure 2). Compared with wild-type cells, a modest but significant decrease in band, ring, and segmented neutrophils was observed in the sorted G-CSFR-/- population. These data, though consistent with a modest defect in terminal granulocytic differentiation, also do not completely account for the defect in G-CSFR-/- granulopoiesis.
Morphologic examination of Gr-1+cells. (A) Gr-1+ (granulocytic) cells from a 9:1 (KO/WT) chimera were sorted into WT (Ly5.2-) and KO (Ly5.2+) fractions as shown. (B) Cytospins of each cell population were made and 500-count manual differentials performed. Data represent mean ± SD (n = 4 mice); *P < .05.
Morphologic examination of Gr-1+cells. (A) Gr-1+ (granulocytic) cells from a 9:1 (KO/WT) chimera were sorted into WT (Ly5.2-) and KO (Ly5.2+) fractions as shown. (B) Cytospins of each cell population were made and 500-count manual differentials performed. Data represent mean ± SD (n = 4 mice); *P < .05.
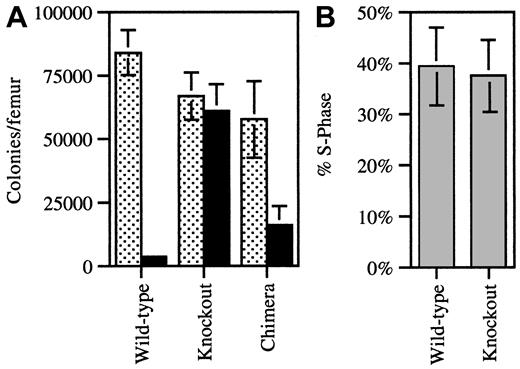
To further characterize the defect in G-CSFR-/- cell granulopoiesis, the number of CFU-GMs in the bone marrow was determined in the 9:1 (KO/WT) chimeras. The contribution of G-CSFR-/- versus wild-type progenitors was measured by determining the percentage of CFU-GMs that produced colonies in vitro in the presence of G418 (1 mg/mL); G-CSFR-/- progenitors contain the neomycin phosphotransferase gene and are therefore resistant to G418. In control experiments, in the presence of this dose of G418, only 4% of wild-type CFU-GMs grew, whereas nearly all of the G-CSFR-/- CFU-GMs grew (Figure 3A). Based on the analysis of G-CSFR-/- mice (Figure 1), it was anticipated that the great majority of CFU-GMs in the 9:1 chimeras would be of G-CSFR-/- origin. Instead, only a minority of CFU-GMs were of G-CSFR-/- origin (28% ± 8%), with a calculated KO/WT ratio of 0.4:1 (Figure 3A). To determine whether the loss of G-CSFR signals led to the impaired recruitment of CFU-GMs into the cell cycle in vivo, a tritiated thymidine suicide assay was performed. In the 9:1 chimeras, a similar percentage of WT and KO CFU-GMs were in S-phase (Figure 3B) (KO, 38% ± 7%; WT, 39% ± 8%). Thus, G-CSFR signals are not required in vivo to stimulate CFU-GM entry into the cell cycle. Collectively, these data suggest that the primary mechanism by which G-CSF regulates granulopoiesis is through the production and/or maintenance of committed granulocyte-macrophage progenitors (CFU-GMs).
Quantification of CFU-GMs and cell cycle analysis. (A) Bone marrow cells harvested from individual WT (n = 3), KO (n = 3), and 9:1 (KO/WT) chimeric (n = 8) mice were plated in methylcellulose with (solid bars) and without (dotted bars) G418 (1 mg/mL) and cultured in conditions supporting the growth of CFU-GMs. The number of colonies is reported per mouse femur. Cultures of wild-type and KO cells served as controls to confirm the ability of G418 to selectively inhibit the growth of wild-type colonies. (B) The number of CFU-GMs in S-phase from 9:1 KO/WT chimeric mice (n = 6) was determined by means of an H3-thymidine suicide assay. Selective growth in G418 was used to discriminate between WT and KO CFU-GMs. Data represent the mean ± SD.
Quantification of CFU-GMs and cell cycle analysis. (A) Bone marrow cells harvested from individual WT (n = 3), KO (n = 3), and 9:1 (KO/WT) chimeric (n = 8) mice were plated in methylcellulose with (solid bars) and without (dotted bars) G418 (1 mg/mL) and cultured in conditions supporting the growth of CFU-GMs. The number of colonies is reported per mouse femur. Cultures of wild-type and KO cells served as controls to confirm the ability of G418 to selectively inhibit the growth of wild-type colonies. (B) The number of CFU-GMs in S-phase from 9:1 KO/WT chimeric mice (n = 6) was determined by means of an H3-thymidine suicide assay. Selective growth in G418 was used to discriminate between WT and KO CFU-GMs. Data represent the mean ± SD.
G-CSFR signals are required for the normal production of progenitors in the common myeloid pathway
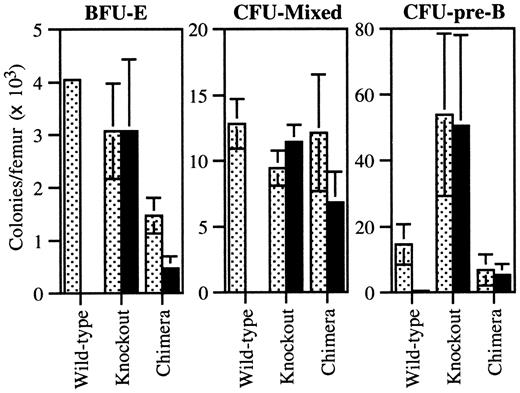
We next examined the contribution of G-CSFR-/- cells to other progenitor cell populations in the 9:1 chimeras by means of the G418 resistance assay (Figure 4). Surprisingly, the number of G-CSFR-/- BFU-Es was significantly reduced (31% ± 11%, with a KO/WT ratio of 0.5:1) compared with the original transplant ratio. Because there is little if any G-CSFR expression on erythroid progenitors,4,5,20 this result suggested a defect in a more primitive hematopoietic progenitor population. Indeed, the contribution of G-CSFR-/- cells to progenitors with mixed granulocyte-macrophage and erythroid potential (CFU-mixed) was also decreased (61% ± 22%, with a KO/WT ratio of 1.6:1).
Progenitor assays from chimeric mice. Bone marrow cells from individual WT (n = 9), KO (n = 9), and 9:1 (KO/WT) chimeric (n = 8) mice were plated in methylcellulose with (solid bars) and without (dotted bars) G418 (1 mg/mL) and cultured in conditions supporting the growth of BFU-E, CFU-mixed, or CFU-pre-B colonies. The number of colonies is reported per mouse femur. Cultures of wild-type and KO cells served as controls to confirm the ability of G418 to selectively inhibit the growth of wild-type colonies. Data represent the mean ± SD.
Progenitor assays from chimeric mice. Bone marrow cells from individual WT (n = 9), KO (n = 9), and 9:1 (KO/WT) chimeric (n = 8) mice were plated in methylcellulose with (solid bars) and without (dotted bars) G418 (1 mg/mL) and cultured in conditions supporting the growth of BFU-E, CFU-mixed, or CFU-pre-B colonies. The number of colonies is reported per mouse femur. Cultures of wild-type and KO cells served as controls to confirm the ability of G418 to selectively inhibit the growth of wild-type colonies. Data represent the mean ± SD.
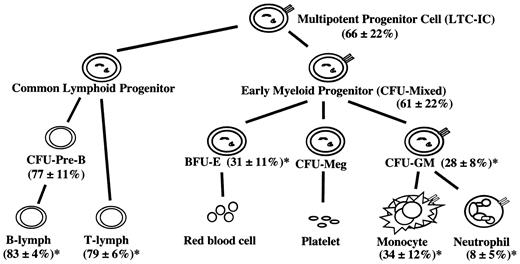
To indirectly assess the hematopoietic stem cell pool, individual LTC-IC clones in the bone marrow of seven 9:1 (KO/WT) chimeras were identified by limiting dilution. The G418 sensitivity of colony-forming cells derived from these LTC-IC clones was used to discriminate between G-CSFR-/- and wild-type LTC-ICs. On the basis of this assay, the contribution of G-CSFR-/- LTC-ICs was also reduced (66% ± 22%, with a KO/WT ratio of 1.9:1). In contrast, the contribution of G-CSFR-/- cells to CFU-pre-Bs was relatively preserved (77% ± 11%, with a KO/WT ratio of 3.3:1) compared with the original transplant ratio. A summary of the chimerism analyses is shown in Figure 5.
Summary of donor chimerism in 9:1 (KO/WT) chimeras. Shown is the summary of the data for the 9:1 (KO/WT) chimeric mice (n = 6-12). The mean percentage ± SD of the contribution of G-CSFR-/- cells in each cell population is shown. Note that, on the basis of the original transplant, a G-CSFR-/- percentage of 90% would be predicted in each lineage if no competitive advantage was present. *P < .05 when compared with the LTC-IC percentage.
Summary of donor chimerism in 9:1 (KO/WT) chimeras. Shown is the summary of the data for the 9:1 (KO/WT) chimeric mice (n = 6-12). The mean percentage ± SD of the contribution of G-CSFR-/- cells in each cell population is shown. Note that, on the basis of the original transplant, a G-CSFR-/- percentage of 90% would be predicted in each lineage if no competitive advantage was present. *P < .05 when compared with the LTC-IC percentage.
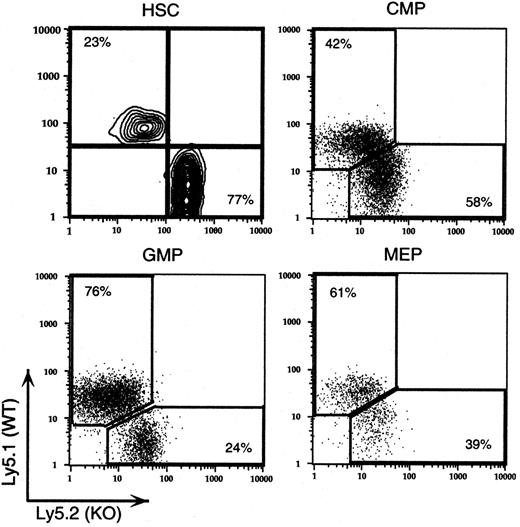
The recent development of multicolor flow cytometric techniques for the measurement of discrete progenitor populations provided an independent measure of the contribution of G-CSFR-/- cells to each progenitor cell population. A cohort of 9:1 (KO/WT) chimeras was analyzed to enumerate the number of stem and myeloid progenitor cells by multicolor flow cytometry (Figure 6). Previous studies have established that sorted cell populations based on antigenic markers contain progenitors that give rise to the appropriate progeny.8,21 The percentages of G-CSFR-/- cells from a representative 9:1 chimeric mouse in each progenitor cell population were as follows: HSCs, 77%, with a KO/WT ratio of 3.3:1; CMPs, 58%, with a KO/WT ratio of 1.4:1, GMPs, 24%, with a KO/WT ratio of 0.3:1; and MEPs, 39%, with a KO/WT ratio of 0.6:1. Unfortunately, for technical reasons, common lymphoid progenitors (CLPs) could not be analyzed. The percentage of G-CSFR-/- cells in each cell population was remarkably similar to that observed in the comparable functional progenitor assays.
Progenitor analysis by multicolor flow cytometry. Populations of hematopoietic stem cells (HSCs), common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) were identified by flow cytometry, as described in “Materials and methods.” Ly5.1 and Ly5.2 expression in each of the progenitor cell populations is shown for a single 9:1 (KO/WT) chimeric mouse. The percentage of KO and wild-type cells is shown in the corner of each individual plot. Data are representative of 4 mice analyzed. The KO/WT ratio for each population is reported in the text.
Progenitor analysis by multicolor flow cytometry. Populations of hematopoietic stem cells (HSCs), common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) were identified by flow cytometry, as described in “Materials and methods.” Ly5.1 and Ly5.2 expression in each of the progenitor cell populations is shown for a single 9:1 (KO/WT) chimeric mouse. The percentage of KO and wild-type cells is shown in the corner of each individual plot. Data are representative of 4 mice analyzed. The KO/WT ratio for each population is reported in the text.
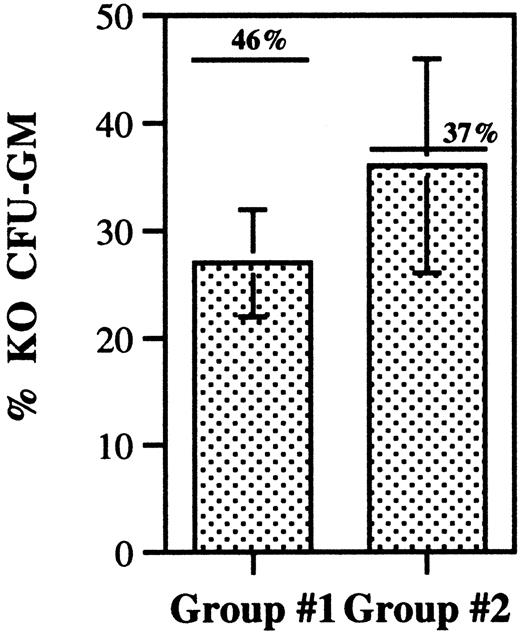
The decreased contribution of G-CSFR-/- cells to the HSC cell compartment in the mixed chimeras suggests a defect in HSC homing, engraftment, or self-renewal. HSC homing can be indirectly assessed by measuring the homing of CFU-GMs to the bone marrow.22-24 Asaumi et al25 showed that CFU-GMs are rapidly cleared from the blood and are detectable only in the bone marrow 24 hours after intravenous injection. We used this assay to compare the homing of wild-type and G-CSFR-/- CFU-GMs to the bone marrow. Bone marrow cells were harvested from wild-type and G-CSFR-/- mice, mixed in equal proportions, and transplanted into irradiated syngeneic mice. Prior to transplantation, an aliquot of this cell mixture was cultured to determine the number of wild-type and G-CSFR-/- CFU-GMs, by means of the G418 resistance assay (“Materials and methods”). At 24 hours after transplantation, the number of wild-type and G-CSFR-/- CFU-GMs in the femurs of recipient mice was also measured. Importantly, no CFU-GMs were recovered from control mice that were irradiated but had not received transplants of bone marrow cells (data not shown). Relative to the pretransplantation G-CSFR-/- percentage, only a modest and nonsignificant decrease in the percentage of G-CSFR-/- CFU-GM was observed in the bone marrow 24 hours after transplantation (Figure 7). The ratio of the percentage of G-CSFR-/- CFU-GMs measured after transplantation versus before transplantation was 0.8 ± 0.3. These data demonstrate that there is no significant defect in the homing of G-CSFR-/- CFU-GMs to the bone marrow.
CFU-GM homing assay. Wild-type and G-CSFR-/- (KO) bone marrow cells were mixed in a 1:1 ratio and injected intravenously into irradiated mice. Cells were recovered from the bone marrow 24 hours after transplantation, and the number of wild-type and KO CFU-GMs measured. The percentage of KO CFU-GMs is shown for 2 independent experiments (group 1, n = 4; group 2, n = 7). The pretransplantation percentage of KO CFU-GMs is shown above each column and is indicated by a single line. Data represent the mean ± SD.
CFU-GM homing assay. Wild-type and G-CSFR-/- (KO) bone marrow cells were mixed in a 1:1 ratio and injected intravenously into irradiated mice. Cells were recovered from the bone marrow 24 hours after transplantation, and the number of wild-type and KO CFU-GMs measured. The percentage of KO CFU-GMs is shown for 2 independent experiments (group 1, n = 4; group 2, n = 7). The pretransplantation percentage of KO CFU-GMs is shown above each column and is indicated by a single line. Data represent the mean ± SD.
Discussion
The role that external factors, in particular cytokines, play in the regulation of hematopoiesis is not fully defined. In the present study, as a model for cytokines in general, we characterized the contribution of G-CSFR signals to the regulation of hematopoiesis. Similarly to previous studies, we show that inbred G-CSFR-/- mice are neutropenic, with levels of circulating neutrophils approximately 16% those of strain-matched wild-type mice, confirming the importance of G-CSFR signals to granulopoiesis in vivo. However, the presence of morphologically mature neutrophils in these mice demonstrates that G-CSFR-independent mechanisms of granulopoiesis must also exist. In fact, previous studies suggest that both interleukin 6 (IL-6) and GM-CSF contribute to basal granulopoiesis in the absence of G-CSFR signals.26,27 The importance of these alternative pathways is not clear, since compensatory mechanisms may be induced by the chronic neutropenia in G-CSFR-/- mice. To address this possibility, a competitive repopulation assay was performed with G-CSFR-/- and wild-type hematopoietic cells. Even in mice reconstituted with a 9:1 ratio of G-CSFR-/- to wild-type cells, more than 80% of granulocytic cells in the bone marrow and blood were of wild-type origin. These data suggest that under basal conditions nearly all of granulopoiesis is driven by G-CSFR signals. It should be noted, however, that a previous report showed that the neutrophil response after infection with Candida albicans was normal in G-CSF-deficient mice, indicating that G-CSFR signals are not universally required during stress granulopoiesis responses.28
How do G-CSFR signals regulate granulopoiesis? Current and previous studies show that in G-CSFR- or G-CSF-deficient mice, only a modest reduction in CFU-GMs was observed, relative to the severe decrease in circulating neutrophils.7 This observation suggested that the primary role of G-CSFR signals in granulopoiesis is to stimulate the proliferation and differentiation of lineage-committed granulocytic precursor cells, downstream of CFU-GMs. However, our analyses of the KO/WT chimeras make it apparent that G-CSFR signals act in a cell-intrinsic fashion at multiple stages of granulocytic differentiation, including the regulation of the production and/or maintenance of CFU-GMs. In the mixed chimeras, the number of G-CSFR-/- CFU-GMs was markedly reduced from predicted levels. For example, in the 9:1 KO/WT chimeras, only 28% of the CFU-GMs were of G-CSFR-/- origin. Second, in agreement with previous studies reviewed,19 G-CSFR signals stimulate the proliferation of committed granulocytic progenitors downstream of CFU-GMs. Relative to CFU-GMs (KO/WT ratio, 0.4:1), the contribution of G-CSFR-/- cells to Gr-1+ (granulocytic) cells in the bone marrow was modestly reduced (0.2:1). Interestingly, G-CSFR signals do not appear to be required for entry of CFU-GMs into the cell cycle in vivo, since the percentage of G-CSFR-/- and wild-type CFU-GMs in S-phase was similar. Third, G-CSFR signals facilitate terminal granulocytic differentiation. Morphologic analysis of sorted populations of Gr-1+ (granulocytic) cells from the bone marrow of the mixed chimeras showed a significant decrease in the number of mature neutrophils in the G-CSFR-/- cell population. These data are consistent with our previous observation that G-CSFR signals contribute to, but are not required for, terminal granulocytic differentiation.7 Finally, consistent with previous studies,17 our data show that G-CSFR signals regulate the release of mature neutrophils from the bone marrow to blood. Note that the percentage of mature neutrophils of G-CSFR-/- origin in the blood was reduced 2-fold compared with the bone marrow.
In addition to being expressed on cells of the granulocytic lineage, the G-CSFR is expressed on certain populations of multipotential hematopoietic progenitors, including primitive Rhodamine-123lolineage-Sca+c-kit+ progenitors, but not on lymphoid-committed progenitors.4,5,20 The functional significance of G-CSFR expression on these progenitor populations has been assessed in vitro by means of hematopoietic progenitor assays. These studies showed that, in addition to stimulating committed myeloid progenitors, G-CSF acts synergistically with stem cell factor to induce the proliferation of multipotential progenitors, including LTC-ICs.29,30 In the present study, we show that in vivo G-CSFR signals play a significant role in the regulation of primitive multipotential progenitor cells. A reduction in the number of G-CSFR-/- HSCs (as measured by LTC-IC assays or flow cytometry to identify the c-KithiLin-Sca-1hi population) was observed in the 9:1 mixed chimeras (Figures 5-6). Since the frequency of LTC-ICs in the bone marrow of G-CSFR-/- mice is normal, these data suggest a defect in the homing, engraftment, or self-renewal of G-CSFR-/- HSCs. As there is no direct method for measuring the homing of HSCs, previous studies have used the homing of CFU-GMs to the bone marrow as a surrogate assay.22-25 Using this assay, we show that the homing of G-CSFR-/- CFU-GMs is comparable to that of wild-type CFU-GMs (Figure 7). Although these data suggest that a defect in the homing of G-CSFR-/- HSCs is not responsible for the defect in engraftment, definitive proof will require the direct measurement of HSC homing.
Perhaps most striking is the observation that the loss of G-CSFR signals results in a significant and near-uniform decrease in all measured progenitor populations in the myeloid lineage. These populations include progenitors identified by functional assays, CFU-GMs and BFU-Es, and progenitors identified by multicolor flow cytometry, GMPs and MEPs (Figures 4 and 6). Thus, these studies provide strong evidence that G-CSFR signals regulate the generation of multiple progenitors in the myeloid lineage. These data may provide an explanation for the observation that treatment with G-CSF can enhance the erythroid response to erythropoietin in certain clinical conditions. Specifically, treatment with G-CSF and erythropoietin approximately doubles the erythroid response compared with erythropoietin alone in patients with myelodysplastic syndrome.31,32
Current evidence supports a model in which the generation of CMPs and CLPs is the first and decisive lineage-commitment step in the differentiation of HSCs.8,21 The mechanism of how cytokines may regulate this initial step in differentiation is controversial. Two general models have been proposed. In the instructive model, cytokines transmit specific signals to multipotential hematopoietic cells, directing their lineage commitment and differentiation. Consistent with this model, Kondo et al33 showed that stimulation through exogenously expressed IL-2 or GM-CSF receptors redirected the differentiation of common lymphoid progenitors (CLPs) to the myeloid lineage. In the stochastic model, lineage commitment and terminal differentiation are intrinsically determined, with cytokines providing only growth and survival signals. Strong support for this model is provided by the observation that enforced expression of bcl-2 rescues the T-lymphocyte and monocyte defects in IL-7- or M-CSF-deficient mice, respectively.34-36 Although the mechanism by which G-CSF regulates the generation and/or expansion of multipotential myeloid progenitors remains to be established, the data presented are suggestive of an instructive mechanism. If G-CSFR signals were directing commitment to the common myeloid lineage, it might be predicted that the loss of G-CSFR signals would lead to an accumulation of cells of the lymphoid lineage. In fact, though the changes are only modest, the contribution of G-CSFR-/- cells to the lymphoid lineage in the mixed chimeras was increased relative to the myeloid lineage (Figure 5).
The current study raises the possibility that regulation of G-CSF levels may provide the organism with a mechanism to direct primitive multipotential hematopoietic progenitors into the common myeloid lineage in response to environmental stresses. Thus, the increased levels of G-CSF present after infectious or other stresses may result in the sustained production of cells of the myeloid lineage.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-02-0593.
Supported by the National Institutes of Health (NIH) Training Program and Developmental Hematology grant no. 5T32HD0749904 and by the Edward Mallinckrodt, Jr, Foundation (D.C.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Morgan McLemore and David Grenda for their thoughtful discussions as this work progressed. We thank Jill Woloszynek for her excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal