Abstract
Fibroblasts from patients with thiamine-responsive megaloblastic anemia (TRMA) syndrome with diabetes and deafness undergo apoptotic cell death in the absence of supplemental thiamine in their cultures. The basis of megaloblastosis in these patients has not been determined. Here we use the stable [1,2-13C2]glucose isotope-based dynamic metabolic profiling technique to demonstrate that defective high-affinity thiamine transport primarily affects the synthesis of nucleic acid ribose via the nonoxidative branch of the pentose cycle. RNA ribose isolated from TRMA fibroblasts in thiamine-depleted cultures shows a time-dependent decrease in the fraction of ribose derived via transketolase, a thiamine-dependent enzyme in the pentose cycle. The fractional rate of de novo ribose synthesis from glucose is decreased several fold 2 to 4 days after removal of thiamine from the culture medium. No such metabolic changes are observed in wild-type fibroblasts or in TRMA mutant cells in thiamine-containing medium. Fluxes through glycolysis are similar in TRMA versus control fibroblasts in the pentose and TCA cycles. We conclude that reduced nucleic acid production through impaired transketolase catalysis is the underlying biochemical disturbance that likely induces cell cycle arrest or apoptosis in bone marrow cells and leads to the TRMA syndrome in patients with defective high-affinity thiamine transport. (Blood. 2003;102: 3556-3561)
Introduction
The pentose cycle, or “pentose-monophosphate shunt,” allows de novo synthesis of ribose from glucose by either or both of 2 possible routes: a nonoxidative pathway via transaldolase and transketolase, and an oxidative pathway, via glucose-6-phosphate (G6P) and 6-phosphogluconate dehydrogenase (G6PDH). The oxidative pathway also generates nicotinamide adenine dinucleotide phosphate-positive (NADPH+) reducing equivalents. Whereas the oxidative branch of the pathway predominates in circulating red blood cells, in many tissues the nonoxidative branch is quantitatively more important for ribose synthesis destined for nucleic acids.1-3 Thiamine pyrophosphate is a cofactor for transketolase, a key rate-limiting enzyme in the nonoxidative pathway of the pentose cycle. Defective thiamine uptake in fibroblasts isolated from patients with thiamine-responsive megaloblastic anemia (TRMA) is associated with the mutation and loss of function of the high-affinity, low-capacity thiamine transporter.4 Fibroblasts isolated from patients with TRMA show cycle arrest and increased apoptosis in low-thiamine-containing media.5 Although thiamine deficiency has been known to cause apoptosis of cells in sensitive brain regions including the thalamus, mammillary, and medial geniculate nuclei,6 the biochemical disturbance resulting from defective high-affinity thiamine transport in human cells is yet to be elucidated.
Metabolic pathway flux changes appear to be critical factors in cell differentiation, in apoptosis, and in mediating the antiproliferative effect of imatinib mesylate in Bcr-Abl-positive myeloid leukemia cells in culture.7,8 Stable isotope-based dynamic metabolic profiling technology allows tracking of metabolic pathway substrate flow changes in response to growth modulating treatments, or changes in the nutrient environment (Figure 1).9-11
The stable isotope-based dynamic metabolic profile (SIDMAP) of mammalian cells is based on13C glucose carbon tracer rearrangements in metabolic intermediates. Schematic of pathways to ribose formation (1), glycolysis (2), and TCA cycle (3) from [1,2-13C2]glucose in cultured fibroblasts. This tracer broadly labels intermediary metabolites in cultured TRMA (-/-) cells that indicate substrate flow defects in comparison with wild-type (WT) (+/+) cells in low-thiamine medium. Using SIDMAP, the rate of glycolysis, pentose cycle ribose synthesis, TCA cycle anaplerotic flux, direct glucose oxidation, and de novo fatty acid synthesis are measured in one tracer experiment.
The stable isotope-based dynamic metabolic profile (SIDMAP) of mammalian cells is based on13C glucose carbon tracer rearrangements in metabolic intermediates. Schematic of pathways to ribose formation (1), glycolysis (2), and TCA cycle (3) from [1,2-13C2]glucose in cultured fibroblasts. This tracer broadly labels intermediary metabolites in cultured TRMA (-/-) cells that indicate substrate flow defects in comparison with wild-type (WT) (+/+) cells in low-thiamine medium. Using SIDMAP, the rate of glycolysis, pentose cycle ribose synthesis, TCA cycle anaplerotic flux, direct glucose oxidation, and de novo fatty acid synthesis are measured in one tracer experiment.
Here we demonstrate, using stable isotope-labeled glucose and its recovered intracellular metabolites, that the defective high-affinity thiamine transporter protein produced by the mutated SLC19A2 gene in TRMA impairs nucleic acid synthesis in the nonoxidative branch of the pentose cycle. The mutant protein also produces smaller, non-cell-specific effects on glycolysis, the oxidative pentose cycle, and distribution of glucose carbons for de novo fatty acid synthesis or glucose anabolic use in the tricarboxylic acid (TCA) cycle. These observations suggest a possible novel etiologic mechanism for megaloblastosis: namely, defective redistribution of glucose carbons among macromolecule biosynthesis pathways in affected precursor marrow cells of patients with TRMA.
Materials and methods
The tracer for this metabolic profiling study, stable isotope [1,2-13C2]-labeled d-glucose, was purchased with more than 99% purity and 99% isotope enrichment for each position from Isotec Laboratories, Maimisburg, OH. Approval was obtained from the Harbor-UCLA Research and Education Institute, Dana-Farber Cancer Institute, and the Harvard Medical School institutional review boards for these studies.
Cells and cell culture
Human fibroblasts were obtained from skin biopsies of patients and unaffected relatives of 2 previously reported TRMA kindreds, after obtaining informed consent from subjects.12,13 Cells were grown in minimum essential medium (MEM) in the presence of 15% fetal bovine serum (FBS), at 37°C in a 95% air-5% CO2. In order to compare glucose use rates, ribose synthesis, lactate production, fatty acid de novo synthesis, and anabolic glucose use in the TCA cycle, 75% confluent cultures of control and TRMA cell lines were incubated in [1,2-13C2]d-glucose-containing media (100 mg/dL total concentration = 5 mM; 50% isotope enrichment—ie, half unlabeled glucose, half labeled with the stable isotope 13C tracer). Cells were plated at a density of 106 per 100 mm2 dish. Medium depleted of thiamine (thi-) and dialyzed FBS was obtained from GIBCO/BRL (Grand Island, NY) as previously described.12 Medium glucose and lactate levels were measured using a Cobas Mira chemistry analyzer (Roche Diagnostics, Pleasanton, CA). Fibroblast metabolic profiles and macromolecule synthesis patterns were determined at days 2, 4, and 7 in order to reveal time dependence of thiamine depletion in cultured cells up to the time when apoptosis occurs in TRMA cells.
RNA ribose stable isotope studies
RNA ribose was isolated by acid hydrolysis of cellular RNA after Trizol purification of cell extracts. Total RNA amounts were assessed by spectrophotometric determination on triplicate cultures. Ribose was derivatized to its aldonitrile acetate form using hydroxylamine in pyridine with acetic anhydride (Supelco, Bellefonte, PA) before mass spectral analyses. We monitored the ion cluster around the m/z256 (carbons 1-5 of ribose) (chemical ionization, CI) and m/z217 (carbons 3-5 of ribose) and m/z242 (carbons 1-4 of ribose) (electron impact ionization, EI) to determine molar enrichment and the positional distribution of 13C in ribose. By convention, the base mass of 12C-compounds (with their derivatization agents) is given as m0 as measured by mass spectrometry. Ribose molecules labeled with a single 13C atom on the first carbon position (m1) recovered from RNA were used to gauge the ribose fraction produced by direct oxidation of glucose through the G6PD pathway (Figure 1). Ribose molecules labeled with 13C on the first 2 carbon positions (m2) were used to measure the fraction produced by transketolase. Doubly labeled ribose molecules (m2 and m4) on the fourth and fifth carbon positions were used to measure molar fraction produced by triose phosphate isomerase and transketolase.3,10,11
Lactate
Lactate from the cell culture media (0.2 mL) was extracted by ethylene chloride after acidification with hydrochloric acid (HCl). Lactate was derivatized to its propylamine-heptafluorobutyrate (HFB) form and the m/z328 (carbons 1-3 of lactate) (chemical ionization, CI) was monitored for the detection of m1 (recycled lactate through the pentose cycle) and m2 (lactate produced by the Embden-Meyerhoff-Parnas pathway) for the estimation of pentose cycle activity. Fragmental lactate analysis was not necessary because the [1,2-13C2]glucose tracer labels lactate on the third (m1) or the second and third (m2) carbon positions, which are clearly distinguished in the molecular ion.3 In this study we recorded the m1/m2 ratios in lactate produced and released by TRMA and control fibroblasts in order to determine pentose cycle activity versus glycolysis in response to thiamine depletion.
Glutamate
The TCA cycle metabolite alpha-ketoglutarate is in close equilibrium with glutamate, which is released by mammalian cells into the medium. Glutamate label distribution from glucose is suitable for determining glucose oxidation versus anabolic glucose use within the cycle, also known as anaplerotic flux.14 In order to obtain glutamate, tissue culture medium was first treated with 6% perchloric acid. The supernatant was passed through a 3-cm3 Dowex-50 (H+) column (Aldrich Chemical, Milwaukee, WI). Amino acids were eluted with 15 mL 2 N ammonium hydroxide. To further separate glutamate from glutamine, the amino acid mixture was passed through a 3-cm3 Dowex-1 (acetate) column then collected with 15 mL 0.5 N acetic acid. The dry glutamate fraction from tissue culture medium was converted to its trifluoroacetyl butyl ester (TAB).15 Under EI conditions, ionization of TAB-glutamate gives rise to 2 fragments, m/z198 and m/z152, corresponding to C2-C5 and C2-C4 of glutamate. Glutamate labeled on the 4 to 5 carbon positions indicates pyruvate dehydrogenase (PDH) activity, while glutamate labeled on the 2 to 3 carbon positions indicates pyruvate carboxylase activity for the entry of glucose carbons to the TCA cycle. TCA cycle anabolic glucose use is calculated based on the m1/m2 ratios of glutamate.16
Fatty acids
Palmitate, stearate, cholesterol, and oleate were extracted after saponification of cell pellets in 30% potassium hydroxide (KOH) and 100% ethanol using petroleum ether. Fatty acids were converted to their methylated derivative using 0.5 N methanolic-HCL. Palmitate, stearate, and oleate were monitored at m/z270, m/z 298, and m/z264, respectively, with the enrichment of 13C-labeled acetyl units, which reflect synthesis, elongation, and desaturation of the new lipid fraction in fibroblasts in response to thiamine depletion in the medium as determined by mass isotopomer distribution analysis (MIDA) of different isotopomers.17,18
Gas chromatography/mass spectrometry (GC/MS)
Mass spectral data were obtained on the HP5973 mass selective detector connected to an HP6890 gas chromatograph (Agilent Technologies, Palo Alto, CA). The settings were as follows: GC inlet, 250°C; transfer line, 280°C; MS source, 230°C; MS Quad, 150°C. An HP-5 capillary column (30-m length, 250-μm diameter, 0.25-μm film thickness; Sigma-Aldrich, St Louis, MO) was used for glucose, ribose, and lactate analyses. Because transketolase has the highest metabolic control coefficient in the nonoxidative branch of the pentose cycle and it is the only thiamine-dependent reaction associated with this cycle,19,20 we use the terms “nonoxidative pentose cycle” and “transketolase” interchangeably throughout the paper. It should be noted though that transketolase and transaldolase, besides other enzymes, are all participants of nonoxidative metabolism in human cells.
Data analysis and statistical methods
Each experiment was carried out at least twice for every condition, and triplicate cell cultures were used for each condition within each experiment. Mass spectroscopic analyses were carried out by 3 independent automatic injections of 1-μL samples by the automatic sampler and accepted only if the standard sample deviation was less than 1% of the normalized peak intensity. Statistical analysis was performed using the Student t test for unpaired samples. A 2-tailed significance at the 99% confidence interval (∞ ± 2.58σ), P value less than .01, indicated significant differences in glucose carbon metabolism in wild-type and TRMA cells in culture at various time points of thiamine depletion.
Results
The SIDMAP (stable isotope-based dynamic metabolic profiling) approach has been successful in determining pentose cycle activity and the contribution of the 2 branches of the pentose cycle to nucleic acid synthesis in HepG2 liver,18 MIA pancreatic adenocarcinoma,21 and K562 myeloid leukemia cells7,8 in previous studies. In this study, we incubated human wild-type and TRMA fibroblasts with [1,2-13C2]glucose in the presence or absence of thiamine in order to determine 13C label distribution in RNA ribose, lactate, and glutamate, and to determine the contribution of glucose carbons to de novo nucleic and fatty acid synthesis. Based on the number of 13C-labeled carbons present in the extracted small-molecular-weight intermediary metabolites, the percent of metabolite synthesis via each pathway was determined. Net de novo RNA synthesis was dramatically reduced in thiamine-depleted TRMA cells, compared with thiamine-replete mutant cells or to wild-type control cells (Figure 2).
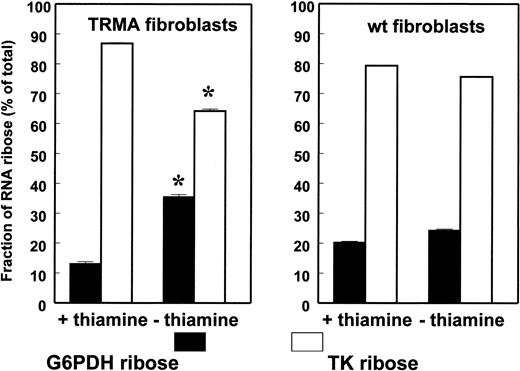
TRMA cells without thiamine exhibit decreased RNA ribose synthesis via transketolase. The fraction of RNA ribose derived from the oxidative (G6PD) and nonoxidative (TK) pathways is shown on the x-axis. The figure shows the significant rearrangement of oxidative/nonoxidative ribose synthesis pathways in TRMA cells, primarily shifting toward oxidative synthesis, while wild-type cells maintain metabolic activities and identical substrate flow in the pentose cycle after thiamine depletion. (Mean ± SD; n = 9) *P < .001.
TRMA cells without thiamine exhibit decreased RNA ribose synthesis via transketolase. The fraction of RNA ribose derived from the oxidative (G6PD) and nonoxidative (TK) pathways is shown on the x-axis. The figure shows the significant rearrangement of oxidative/nonoxidative ribose synthesis pathways in TRMA cells, primarily shifting toward oxidative synthesis, while wild-type cells maintain metabolic activities and identical substrate flow in the pentose cycle after thiamine depletion. (Mean ± SD; n = 9) *P < .001.
This analysis depends on the fact that net increases in 13C ribose can occur only by de novo ribose synthesis from [1,2-13C2]D-glucose. Change in the route of de novo ribose synthesis was evident after 2 days of culture. In the presence of thiamine, both the wild-type fibroblasts and the mutated cells synthesize approximately 80% of nucleic acid ribose directly through the nonoxidative branch and approximately 20% directly through the oxidative G6PDH reaction. Control normal fibroblasts showed no change in the contribution of transketolase and G6PD to nucleic acid ribose under thiamine-depleted conditions. TRMA fibroblasts, on the other hand, showed a significant 25% decrease in the contribution of transketolase and a corresponding 37% increase in the contribution of direct glucose oxidation through G6PD to nucleic acid ribose synthesis after 2 days of culture under thiamine-depleted conditions (Figure 2).
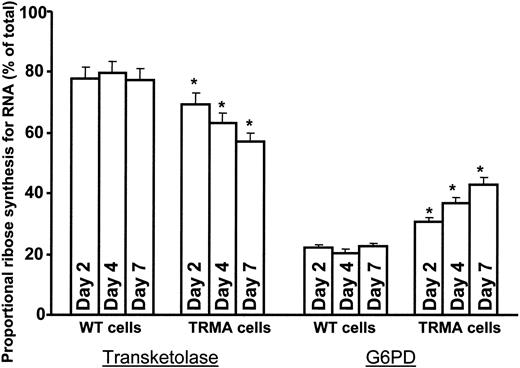
The impact of time on metabolic changes in TRMA cells in the absence of thiamine was also evident. A time-dependent decrease in the contribution of transketolase and a compensatory increase in the contribution of G6PDH to nucleic acid ribose synthesis was evident in TRMA fibroblasts incubated in thiamine-depleted medium with [1,2-13C2]glucose for 2, 4, and 7 days (Figure 3). Signs of apoptotic cell death accompanied the prominent decrease in the contribution of transketolase to nucleic acid ribose synthesis in TRMA cells at day 7 of thiamine depletion, similar to previous observations.12 This metabolic change was absent in thiamine-replete cultures where apoptosis did not occur, indicating that adequate thiamine supplementation is capable of maintaining nonoxidative ribose synthesis in TRMA cells and maintaining phenotype.
TRMA cells without thiamine exhibit a time-dependent decrease in RNA ribose synthesis via transketolase. There is a time-dependent decrease in de novo RNA ribose synthesis through the nonoxidative pathway in TRMA cells depleted of thiamine, and there is a compensatory increase in glucose oxidation and ribose synthesis though G6PDH. Over 7 days, the fraction of RNA ribose derived from the oxidative pathway under thiamine-depleted conditions is approximately twice that in thiamine-replete medium. WT cells did not exhibit significant change in pentose cycle carbon flow between glucose oxidation and nonoxidative ribose synthesis at any time point throughout our study. (Mean ± SD; n = 9) *P < .001.
TRMA cells without thiamine exhibit a time-dependent decrease in RNA ribose synthesis via transketolase. There is a time-dependent decrease in de novo RNA ribose synthesis through the nonoxidative pathway in TRMA cells depleted of thiamine, and there is a compensatory increase in glucose oxidation and ribose synthesis though G6PDH. Over 7 days, the fraction of RNA ribose derived from the oxidative pathway under thiamine-depleted conditions is approximately twice that in thiamine-replete medium. WT cells did not exhibit significant change in pentose cycle carbon flow between glucose oxidation and nonoxidative ribose synthesis at any time point throughout our study. (Mean ± SD; n = 9) *P < .001.
The sum of all labeled species (Σmn) found in RNA ribose is a measure of de novo synthesis from [1,2-13C2]D-glucose. Total ribose synthesis has also significantly declined in TRMA fibroblasts as shown in Figure 4. The rate of de novo incorporation was measured by the change (Δ) in 13C enrichment total RNA. There is a significant decrease of about 80% in TRMA cell total RNA synthesis from glucose after 2 days of thiamine depletion, a phenomenon that has not been observed in wild-type fibroblasts.
TRMA cells without thiamine exhibit a significant decrease in total RNA ribose synthesis after 2 days of thiamine depletion. There is a significant decrease of about 80% in TRMA cell total RNA synthesis from glucose after thiamine depletion, which has not been observed in wild-type fibroblasts. (Mean ± SD; n = 9) *P < .001.
TRMA cells without thiamine exhibit a significant decrease in total RNA ribose synthesis after 2 days of thiamine depletion. There is a significant decrease of about 80% in TRMA cell total RNA synthesis from glucose after thiamine depletion, which has not been observed in wild-type fibroblasts. (Mean ± SD; n = 9) *P < .001.
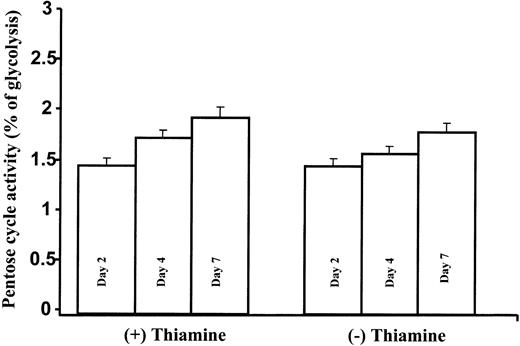
Pentose cycle activity relative to the Embden-Meyerhoff-Parnas pathway was calculated by the m2/m1 ratios measured in lactate using previously published methods and formulas.3 Pentose cycle activity showed a modest time-dependent increase in both thiamine-depleted and thiamine-replete TRMA cultures over time. This change did not differ with thiamine states: note that approximately only 2% of lactate is derived via the pentose cycle, while the remainder is derived from glycolysis in TRMA cells (Figure 5).
Slight time-dependent increase in pentose cycle activity relative to glycolysis in TRMA cells with or without thiamine treatment. Pentose cycle activity relative to the Embden-Meyerhoff-Parnas pathway is shown here by the m1/m2 ratios in lactate. Pentose cycle activity showed a modest time-dependent increase in both thiamine-depleted and thiamine-replete TRMA cultures. (Mean ± SD; n = 9).
Slight time-dependent increase in pentose cycle activity relative to glycolysis in TRMA cells with or without thiamine treatment. Pentose cycle activity relative to the Embden-Meyerhoff-Parnas pathway is shown here by the m1/m2 ratios in lactate. Pentose cycle activity showed a modest time-dependent increase in both thiamine-depleted and thiamine-replete TRMA cultures. (Mean ± SD; n = 9).
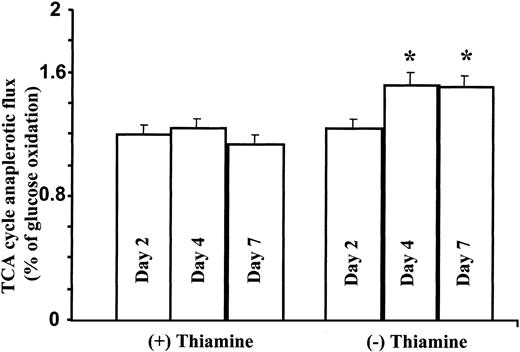
Total mass and fragmentation patterns in glutamate are unique and depend primarily on pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (α-KTGDH) activities as the thiamine-dependent steps in the TCA cycle. The position of the 13C label in glutamate (either C 2-3 or C 4-5) allows the estimation of TCA cycle anaplerotic flux relative to glucose oxidation. As shown in Figure 6, depletion of thiamine from the culture media of TRMA cells induces a significant increase in TCA cycle anaplerotic flux, indicating that mutation in the high-affinity thiamine transporter gene does not decrease TCA cycle anaplerotic flux and carbon flow.
TCA cycle anaplerotic flux increases in TRMA cells as measured by glutamate13C enrichment from glucose. As shown here, depletion of thiamine from the culture media of TRMA cells induces a significant increase in TCA cycle anaplerotic flux. (Mean ± SD; n = 9) *P < .001.
TCA cycle anaplerotic flux increases in TRMA cells as measured by glutamate13C enrichment from glucose. As shown here, depletion of thiamine from the culture media of TRMA cells induces a significant increase in TCA cycle anaplerotic flux. (Mean ± SD; n = 9) *P < .001.
Control wild-type cells showed no response in TCA cycle metabolism in response to thiamine depletion in the medium (not shown). Based on this finding, the only thiamine-dependent enzyme that also depends on the high-affinity transporter in TRMA cells is transketolase, an interrelationship demonstrated by the decrease in nonoxidative pentose cycle metabolism shown above (Figure 3).
Significant accumulation of 13C tracer from glucose did not occur in the fatty acids palmitate, stearate, and oleate, or in cholesterol, indicating that fatty acid de novo synthesis, chain elongation, and desaturation are not very active in control and TRMA cells. This confirms that the thiamine-pyrophosphate (PP) dependent pyruvate dehydrogenase enzyme complex is not affected by the defective high-affinity thiamine transporter in TRMA fibroblasts (data not shown).
Discussion
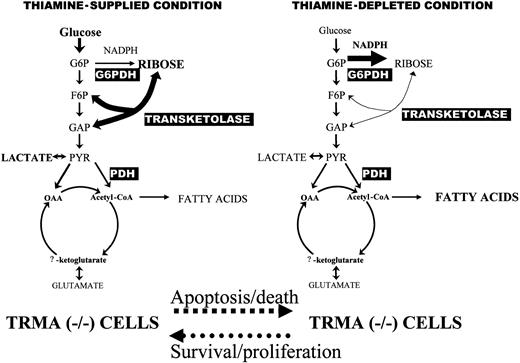
We have used stable isotope-based dynamic metabolic profiling to demonstrate defective de novo nucleic acid synthesis in TRMA fibroblasts subjected to thiamine deprivation. The fractional synthetic rate is reduced in thiamine-depleted TRMA cells. In addition, the fraction of ribose derived from the preferred nonoxidative (transketolase/transaldolase) portion of the pentose phosphate pathway is reduced in favor of the oxidative pentose pathway branch (via G6PDH). A comparison of SIDMAPs of TRMA (-/-) cells in thiamine-PP supplied versus depleted conditions is shown in Figure 7.
Metabolic profile changes associated with thiamine-PP depletion in TRMA (-/-) cultures exhibiting apoptosis. TRMA cells in the presence of thiamine use glucose as the major substrate for de novo nucleic acid synthesis through the nonoxidative steps of the pentose cycle (left). Nonoxidative ribose synthesis decreases while direct glucose oxidation increases in the pentose cycle (right). TCA cycle substrate flux showed no significant response to thiamine depletion indicating well-preserved pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase activities as other thiamin-dependent enzymes of metabolism. SIDMAP provides a virtual roadmap by which regulation of cell cycle, enzyme protein synthesis, as well as gene expression can be pursued for revealing the mechanism of TRMA cells' metabolic deficiencies.
Metabolic profile changes associated with thiamine-PP depletion in TRMA (-/-) cultures exhibiting apoptosis. TRMA cells in the presence of thiamine use glucose as the major substrate for de novo nucleic acid synthesis through the nonoxidative steps of the pentose cycle (left). Nonoxidative ribose synthesis decreases while direct glucose oxidation increases in the pentose cycle (right). TCA cycle substrate flux showed no significant response to thiamine depletion indicating well-preserved pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase activities as other thiamin-dependent enzymes of metabolism. SIDMAP provides a virtual roadmap by which regulation of cell cycle, enzyme protein synthesis, as well as gene expression can be pursued for revealing the mechanism of TRMA cells' metabolic deficiencies.
What are the likely consequences of this change? TRMA fibroblasts undergo apoptosis in thiamine-depleted conditions after about 2 weeks in culture.5 It is evident that the observed decreases in nucleic acid synthesis significantly contribute to this effect. In TRMA patients, the ambient thiamine concentration is not zero. However, hematopoietic cells in the marrow require very high nucleic acid synthesis rates to achieve division times, which are much greater than those of leisurely diploid fibroblasts in culture (doubling time < < one day vs several days). Drugs (eg, folate antagonists or purine analogs), metabolic disorders (orotic aciduria, Lesch-Nyhan syndrome), and nutritional states (B12 or folate deficiency), which reduce de novo nucleotide synthesis, lead to a “megaloblastic” appearance of the marrow, with nuclear-cytoplasmic dyssynchrony and delayed nuclear maturation. We postulate the same effect in TRMA erythropoiesis with the following notions. Since the initial report of TRMA in 1969 by Rogers et al,22 it has been clear that the megaloblastic changes in this disorder differ from other vitamin deficiency states. High-dose B12 and folate have no effect on the condition. Haworth et al performed deoxyuridine suppression tests on marrow from a Pakistani child with TRMA and found no defect.23 Deoxyuridine fails to inhibit incorporation of [3H]thymidine into DNA if it cannot be converted (via folate-dependent uridine methyltransferase) into thymidine monophosphate (TMP). This step is unaffected in TRMA fibroblasts. The high-affinity thiamine transporter defective in TRMA, designated SLC19A2, does not transport folate,24 though a report suggests that the homologue and reduced folate carrier, SLC19A1, could carry thiamine pyrophosphate in an experimental system.25 The SLC19A1 gene is not defective in TRMA, so that to the extent this observation is correct, it does not rescue TRMA fibroblasts from the biochemical disturbance noted here.
We acknowledge 2 potential limitations of this study. First, because fibroblasts grow very slowly and do not have the specialized requirement of erythropoietic precursors for rapid growth and hemoglobin synthesis, we cannot directly test the hypothesis about megaloblastic changes in these cells alone. Conditions to test thiamine metabolic effects in marrow are under development, but these studies are not straightforward. In any case, marrow from TRMA patients is not readily obtainable and patients are universally thiamine-treated. Second, the GC-MS analyses are highly accurate and reproducible with regard to molar fractions with varying label concentrations, but not well suited to accurate mass calculations without a tracer. Fortunately, as the labeled [1,2-13C2]glucose is initially incorporated into RNA, the change in molar enrichment directly reflects the de novo synthesis rate (as shown in Figure 1). We also experimented using 13C ribose tracer in TRMA fibroblast cultures, but these cells did not accumulate measurable tracer-ribose, probably due to the lack of ribose transport in human cells. Human cells primarily use glucose for ribose synthesis, and this makes glucose tracers superior for SIDMAP studies as presented herein.
Whereas hematologists are used to thinking of the pentose phosphate “shunt” as an erythrocyte pathway for generation of reducing equivalents, most mammalian cell types preferentially use the nonoxidative direction through the pentose phosphate pathway to generate net pentose synthesis.26 Reduced nicotinamide adenine dinucleotide (NADH) and NADPH can be generated by mitochondrial or lipolytic activities in most cell types, but not in erythrocytes. The observation that the transketolase (nonoxidative) pathway is preferred in cultured cells and organs is not new, having first been made more than 40 years ago using isotopic enrichment experiments similar in concept to those described here.1,2
Based on these data, the predominant metabolic disturbance in TRMA patients is decreased nucleic acid ribose synthesis through the nonoxidative branch of the pentose cycle (Figure 7). The prominent decrease observed in the contribution of transketolase to nucleic acid ribose synthesis indicates that thiamine-PP and nonoxidative ribose synthesis are required for human cell survival and proliferation. This observation may also identify a new enzymatic target site in the apoptotic pathway of mammalian cells, which is regulated primarily through high-affinity thiamine transport. Transaldolase, a nonoxidative pentose cycle enzyme, has been shown to regulate the metabolic flux between the oxidative and nonoxidative branches of the pentose cycle and to influence sensitivity to various cell death signals.27 Transketolase has a higher metabolic control coefficient in the pentose cycle, which makes it a close rate-limiting enzyme in nonoxidative ribose synthesis.19 Therefore it is not surprising that human cells are sensitive to thiamine depletion and reduced transketolase activity when their high-affinity thiamine transport fails. One interesting aspect of our study is that the data suggest that high-affinity low-capacity thiamine transport may be linked to transketolase-related metabolic activities, while TCA cycle metabolism, glycolysis, and fatty acid synthesis pathways are not affected. Therefore, it seems likely that high-affinity thiamine transport is tied to nucleic acid synthesis and cell replication as a primary and highly important mammalian cell function for survival and progression. Both the fractional rate of label incorporation and the route of label within the pentose cycle are abnormal in TRMA cells. This finding suggests that defective ribose synthesis for nucleic acids is the likely cause of megaloblastic changes in TRMA.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-05-1537.
Supported in part by RO1 HL66182-01A1, K24 HLK24HL004184, and March of Dimes grants to E.J.N.; PHS M01-RR0045 of the General Clinical Research Unit to W.-N.P.L.; and P01-CA42710 of the UCLA Clinical Nutrition Research Unit Stable Isotope Core through its preliminary/feasibility grant program to L.G.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the patients and their families for participating in this study.

![Figure 1. The stable isotope-based dynamic metabolic profile (SIDMAP) of mammalian cells is based on 13C glucose carbon tracer rearrangements in metabolic intermediates. Schematic of pathways to ribose formation (1), glycolysis (2), and TCA cycle (3) from [1,2-13C2]glucose in cultured fibroblasts. This tracer broadly labels intermediary metabolites in cultured TRMA (-/-) cells that indicate substrate flow defects in comparison with wild-type (WT) (+/+) cells in low-thiamine medium. Using SIDMAP, the rate of glycolysis, pentose cycle ribose synthesis, TCA cycle anaplerotic flux, direct glucose oxidation, and de novo fatty acid synthesis are measured in one tracer experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-05-1537/6/m_h82235251001.jpeg?Expires=1769158751&Signature=oj39v~YFb0Mcvs5Zoc0JWce1XibGe0p~yVOrm-bp~Q0MALbAqCrOEeTjp49v0fawKmfdnJylGbuJWsyxTohKCcX13hZZcsZA2kZiloOdSeWH4HUMY3HzaNDUQWTpHT83uV0zXkEyvfn8vZtVpbdHmQqyRK5WaXZg1sHdGA1xKHMNtZVJ8UX1ps7-2-Cy8UTs-Y6SQviBA97PcpFpTGqwVhLu~PbSn-uqiMIqqROoTzNNs-hKCdkOVFNR6CrzZryWp43pBKVHvR5P4toBJTaMOBUZdlR~KWx~3cefI769wsHn1KzNvhBvkCD0GB~ltiGoyKQcrhDRDRbRQSZDhNQ5eQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal