Abstract
Allogeneic blood or marrow transplantation (BMT) is a curative therapy for chronic myeloid leukemia (CML). We have previously reported that the pharmacologic targeting of busulfan combined with cyclophosphamide (TBU/CY) can minimize regimen-related toxicity while preserving antileukemic effects. We report here on 131 consecutive chronic-phase CML patients treated with allogeneic related BMT using a TBU/CY preparative regimen, where the busulfan dose was targeted to achieve a steady-state plasma concentration of at least 900 ng/mL. The median age of the patients was 43 years (range, 14-66 years). Estimates of the probabilities of nonrelapse mortality, relapse, survival, and disease-free survival 3 years after transplantation were 14%, 8%, 86%, and 78%, respectively. Age had no statistically significant effect on survival. Although approximately 60% of the patients developed clinically extensive chronic graft-versus-host disease, the median Karnofsky score at last contact date among survivors was 95%. Of surviving patients, 11% were molecularly positive for the bcr-abl mRNA at last contact, with a median level of bcr-abl transcripts of 4.6 copies/μg RNA. These data suggest that TBU/CY is a very effective preparative regimen for CML in chronic phase, associated with an expected survival at 3 years of approximately 85%, with most patients being in molecular remission. (Blood. 2003;102:31-35)
Introduction
Several approaches are available for the treatment of chronic myeloid leukemia in chronic phase (CML-cp). α-Interferon, with or without cytarabine, can cause major cytogenetic responses in a subset of patients, and these patients appear to have a prolonged survival compared with those without a major cytogenetic response.1-3 The tyrosine kinase inhibitor imatinib mesylate has shown exciting promise in effecting cytogenetic responses in patients with interferon-resistant disease, and the efficacy of the drug in newly diagnosed CML cases is currently under study.4,5 The potential of imatinib to cause long-term responses, let alone cures, is unknown. Allogeneic transplantation remains the only therapy with a demonstrated ability to cure CML in a substantial proportion of patients, but its application is limited by the considerations of suitable donors, toxicity, and the constraints of patient age on outcome.6,7
Preparative regimens for CML transplantation have evolved considerably during the past decade. A randomized study that compared the preparative regimens of busulfan (BU) and cyclophosphamide (CY) versus total body radiation (TBI) and CY in CML-cp demonstrated similar survival and disease-free survival (DFS), but less toxicity in the BU/CY group.8 These data have recently been updated and continue to show similar outcomes in the 2 study arms.9 The availability of an assay for measurement of the concentration of busulfan in plasma has allowed us to correlate BU levels with posttransplantation survival and recurrent malignancy. These studies suggest that patients who had a steady-state busulfan concentration of 917 ng/L (the median busulfan level in the patient cohort) or more had a significantly lower relapse rate and better survival than patients with a level of less than 917 ng/L.10 Because of these data we began to adjust busulfan doses for all patients to ensure a “targeted” steady-state concentration (Css) of 900 mg/mL or more (TBU). In addition, the use of peripheral blood stem cells (PBSCs), compared with bone marrow (BM), has been reported to be associated with superior survival and a lower level of residual disease among patients with “high-risk” disease, as detected by sensitive molecular assays for the bcr-abl chimeric mRNA.11 Given these potential improvements in allogeneic transplantation and the development of new alternative treatment strategies using targeted therapies, we performed a retrospective analysis of our experience in 131 consecutive CML-cp patients with the TBU/CY preparative regimen, with bone marrow or PBSCs as the source of stem cell support.
Patients, materials, and methods
Patients
Between September 1995 and December 2000, 131 consecutive patients with a diagnosis of CML-cp received hematopoietic cell transplants from human leukocyte antigen (HLA)–identical (n = 130) or one antigen–mismatched (n = 1) relatives at the Fred Hutchinson Cancer Research Center. The diagnosis of CML-cp was established using standard criteria. All patients had cytogenetic evidence of the Philadelphia (Ph) chromosome. Informed consent was obtained using forms approved by the institutional review board of the Fred Hutchinson Cancer Research Center. All patients with a diagnosis of CML-cp who received targeted busulfan and cyclophosphamide were analyzed in this report. Some of the patients were eligible for a randomized trial of PBSC versus BM transplantation. If they refused randomization they were eligible for phase 2 studies using either PBSCs or BM. Thirty-nine of these patients were enrolled in the multicenter randomized study at the Fred Hutchinson Cancer Research Center, the City of Hope National Cancer Center, and the Stanford University Medical Center.12 The remaining 92 patients received either BM (n = 80) or PBSCs (n = 12).
Targeted busulfan-cyclophosphamide (TBU/CY) preparative regimen
The preparative regimen of TBU/CY consisted of BU, 1.0 mg/kg orally, 4 times daily on each of 4 successive days (total dose 16 mg/kg) followed by CY, 60 mg/kg intravenously, on each of 2 successive days.8 Busulfan targeting was performed as previously described.13 For oral busulfan, blood samples are obtained at 0, 30, 60, 90, 120, 180, 240, and 360 minutes after the first dose. For intravenous busulfan (one patient), samples were obtained at 120 (end of infusion), 135, 150, 180, 240, 300, and 360 minutes after the infusion was started. The estimate of busulfan clearance (dose/area under the curve [AUC]) was used to calculate the dose required to achieve the desired Css (clearance × Css × time between doses). Subsequent assessments of clearance are made for possible dose adjustment after doses 5 and 9, based on blood samples obtained 30, 60, 90, 120, 180, 300, and 360 minutes after the oral dose. Dose adjustments were capped at 30% after the first dose, with further adjustments made following the estimation of busulfan clearance after subsequent doses. Average busulfan exposure was calculated as mean dose/(mean clearance)(dosing interval). The mean clearance was calculated as the mean of the values measured after doses 1, 5, and 9.
Stem cells were infused on day 0. Stem cells were obtained either by bone marrow aspirations or by peripheral blood apheresis after granulocyte colony-stimulating factor (G-CSF) mobilization as previously described.12
Prophylaxis and treatment of GVHD
Methotrexate (MTX) was administered intravenously at a dose of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. Cyclosporine (CSP) was administered intravenously at a dose of 3 mg/kg per day in 2 divided doses starting on the day before the transplantation (day -1). Oral CSP, 6.25 mg/kg every 12 hours, was substituted for intravenous administration when tolerated. In the absence of graft-versus-host disease (GVHD), the CSP dose was tapered by 5% weekly starting on day 50, and administration was usually discontinued on day 180. Acute and chronic GVHD were diagnosed using established criteria.14,15 Acute GVHD was treated with prednisone, antithymocyte globulin, monoclonal antibodies, or other investigational agents. Chronic GVHD was treated with prednisone alone or with CSP.
HLA typing
Infection prophylaxis
Prophylaxis against cytomegalovirus (CMV) disease was accomplished by the use of CMV-negative blood products for CMV-seronegative patients with CMV-seronegative donors and with the administration of ganciclovir whenever CMV antigenemia was detected in all other patients.18,19 Pneumocystis carinii pneumonia (PCP) prophylaxis with trimethoprim-sulfamethoxazole began at the time of patient arrival, was held after transplantation until the granulocyte count reached 500, and then was continued until immunosuppressive treatment was stopped. Fluconazole was given as antifungal prophylaxis from the beginning of the preparative regimen until day 75 after transplantation.20
Disease monitoring after transplantation
Pathology, cytogenetics, and reverse transcriptase polymerase chain reaction (RT-PCR) monitoring were performed after transplantation on days 25 and 80, then at 6-month intervals for 2 years, and yearly thereafter. A 2-step, “nested” RT-PCR was used to amplify the chimeric bcr-abl mRNA, as previously described.21 Quantitative RT-PCR (Q-PCR) was performed using a 5′ exonuclease–based fluorogenic “real time” RT-PCR to detect bcr-abl and β-2 microglobulin (β2μ) mRNA. Assays were carried out in single tubes in an ABI PRISM 7700 sequence detector (PE Applied Biosystems, Foster City, CA), as previously described.22
Definitions of outcomes
The day of neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 500 or higher. Platelet engraftment was defined as the first of 7 consecutive days with platelet counts higher than 20 000 without transfusion support. The severity of acute GVHD was determined by a single investigator, according to published criteria.14,23 Relapse was defined by hematologic recurrence of disease, the presence of 5 or more Ph-positive metaphases on any occasion, or the presence of Ph-positive metaphases in 2 consecutive evaluations at least 3 months apart. The cause of death for patients with recurrent malignancy was categorized as leukemia. Deaths in the absence of persistent relapse were categorized as nonrelapse mortality (NRM).
Statistical analyses
Outcomes that were analyzed included GVHD, nonrelapse mortality, relapse, survival, and disease-free survival. Acute GVHD occurring during the first 100 days after transplantation was measured as grade 2 or higher or as grade 3 or 4. Estimates of survival were obtained by the method of Kaplan and Meier, where follow-up was censored at the date of last contact among survivors. The probabilities of relapse, nonrelapse mortality, and acute GVHD were estimated by cumulative incidence, where death without relapse was regarded as a competing risk for relapse, relapse was regarded as a competing risk for nonrelapse mortality, and death without acute GVHD was regarded as a competing risk for acute GVHD. Follow-up among disease-free survivors was censored on the date of last cytogenetic or molecular examination in evaluations of relapse, event-free survival, and nonrelapse mortality. In the evaluation of chronic GVHD, follow-up was censored at the date of last contact among surviving patients without GVHD.
Results
Patient characteristics
The median age for the entire cohort was 43 years (range, 14-66 years). The median time between diagnosis and BMT was 0.47 years (range, 0.2-7.7 years).
Busulfan concentrations
The target average exposure of BU Css of more than 900 ng/mL was achieved in 96% of the patients. Five of the 131 patients had an average exposure of less than 900 ng/mL, with a median of 2.4% below the lower limit of the target range of 900 ng/mL. Following dose 1, 48 (37%) of the 131 patients were below the target range of busulfan concentration and required upward dose adjustment (median 13% upward dose adjustment; range, 3%-36%) as described in “Patients, materials, and methods” to achieve Css more than 900 ng/mL. When all doses are considered, 76 patients (58%) required upward dose adjustment (median 16% upward dose adjustment; range, 3%-50%).
Engraftment
All patients achieved neutrophil engraftment, and the median time to engraftment was 20 days (range, 12-32 days). Of the 131 patients, 129 had a sustained platelet count of more than 20 000 at a median of 17 days (range, 10-66 days). One patient died at day 32 without platelet engraftment, and one patient did not achieve platelet engraftment by day 100 after transplantation.
GVHD
Acute GVHD (grades 2-4) occurred in 65% of patients, and 7% had grade 3 or 4 GVHD. The estimate of clinically extensive chronic GVHD was 60% at 1 year after transplantation among all patients.
Nonrelapse mortality
Sixteen patients died without recurrent malignancy, 4 before day 100 and a total of 12 before 1 year after bone marrow transplantation (BMT). The estimated probability of NRM was 10% (95% confidence interval [CI], 4%-15%) and 14% (95% CI, 7%-21%) at 1 and 3 years, respectively. Nonrelapse deaths occurred at a median of 197 days (range, 32-1111 days) from the date of transplantation. The causes of NRM are shown in Table 1. Pulmonary toxicity was the most common cause of NRM. Eight of the patients who died of NRM also had clinically extensive chronic GVHD at the time of death.
Outcomes among 131 CML patients after transplantation
Nonrelapse mortality | 16 |
| Pulmonary failure | 5 |
| Infection | 4 |
| Acute GVHD | 3 |
| Gastrointestinal bleed | 1 |
| Unknown* | 3 |
| Relapse death | 1 |
| Total deaths | 17 |
| Relapse | 7 |
| Survivors | 114 |
| Disease-free survivors | 107 |
Nonrelapse mortality | 16 |
| Pulmonary failure | 5 |
| Infection | 4 |
| Acute GVHD | 3 |
| Gastrointestinal bleed | 1 |
| Unknown* | 3 |
| Relapse death | 1 |
| Total deaths | 17 |
| Relapse | 7 |
| Survivors | 114 |
| Disease-free survivors | 107 |
These patients died at home and had either incomplete records or incomplete assessment of cause of death. All 3 were in remission at last contact.
Relapse
Seven patients had recurrent malignancy detected at a median of 346 days after transplantation (range, 76-1469 days). One of the 7 died of recurrent disease. The other 6 received various combinations of interferon, donor leukocyte infusions, and imatinib, and 3 achieved a cytogenetic remission. The cumulative incidence estimate of the probability of relapse is 3% (95% CI, 0%-7%) and 8% (95% CI, 1%-15%) at 1 and 3 years after transplantation, respectively. Nine patients were treated with interferon for one year after bcr-abl positivity was detected at 6 to 12 months after transplantation with no other evidence of persistent CML. None of these patients has relapsed. The median follow-up of patients without relapse is 459 days.
Of the 114 survivors, 111 have had posttransplantation PCR tests for bcr-abl. Twelve (11%) of the 111 were positive for bcr-abl at their last assay, at a median of 407 days after transplantation (range, 75-1731 days). The median level of bcr-abl transcripts in these patients was 4.6 copies/μg RNA (range, 0.4-87.0 copies/μg RNA).
Survival
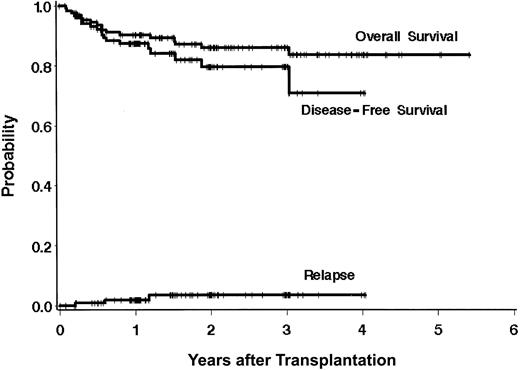
Overall survival is shown in Figure 1. Estimates of the probabilities of survival and relapse-free survival are 91% (95% CI, 86%-96%) and 87% (95% CI, 81%-93%) at 1 year after BMT, and 86% (95% CI, 80%-93%) and 78% (95% CI, 69%-88%) at 3 years after BMT, respectively. The median follow-up of the survivors is 771 days (range, 99-1980 days).
Outcomes after transplantation using a targeted BU/CY regimen. Estimates of survival, DFS, and relapse at 3 years after transplantation are 86%, 78%, and 8%, respectively.
Outcomes after transplantation using a targeted BU/CY regimen. Estimates of survival, DFS, and relapse at 3 years after transplantation are 86%, 78%, and 8%, respectively.
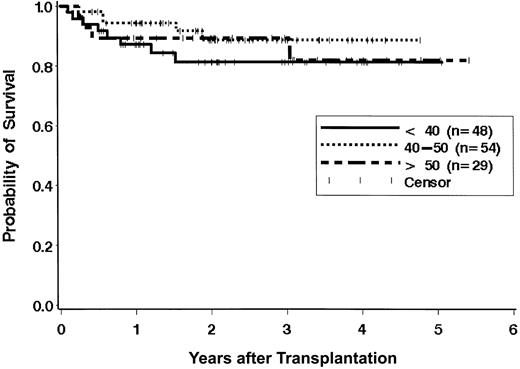
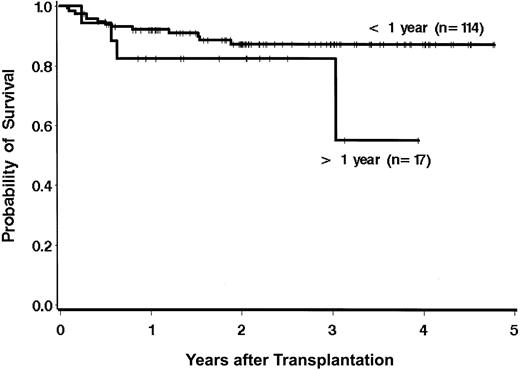
The survival results were evaluated to examine the effect of previously described risk factors on the hazard of mortality, recognizing that the ability to detect statistically significant differences is limited owing to the relatively low number of deaths (n = 17). There were no statistically significant differences in survival across the age groups as defined in Figure 2 (P = .55). Of the 29 patients aged 50 years or older, 14 were 55 years old or older (55 years, n = 4; 56 years, n = 1; 57 years, n = 2; 58 years, n = 2; 59 years, n = 2; 60 years, n = 1; 61 years, n = 1; 66 years, n = 1). Two (14%) of these 14 patents have died, while 2 of the 15 patients aged 50 to 55 years have died (Table 2). Of the 48 patients aged younger than 40 years, 12 were younger than 30 years, and 3 (25%) of these patients have died. Of the 36 patients aged 30 to 40 years, 5 (14%) have died. The time from diagnosis to transplantation showed a trend for association with the hazard of mortality when the analysis was performed with a dichotomous variable (≤ 1 year vs > 1 year). Only 17 patients received the transplant later than a year from diagnosis. The hazard ratio of mortality for patients receiving transplants more than 1 year from diagnosis was 2.2 times that of patients receiving transplants less than 1 year from diagnosis (95% CI, 0.7-6.8; P = .17). When time from diagnosis was modeled as a continuous variable, the trend for increased mortality associated with longer time from diagnosis to transplantation was evident, but it was not statistically significant (P = .10; Figure 3). There were no statistically significant differences in survival based on patient/donor gender combinations (data not shown; P = .41).
Effect of age on survival. There are no statistically significant differences in survival of patients aged younger than 40 years, 40 to 50 years, and older than 50 years (P = .55).
Effect of age on survival. There are no statistically significant differences in survival of patients aged younger than 40 years, 40 to 50 years, and older than 50 years (P = .55).
Risk variables and outcomes
Risk variables . | No. deaths (3-y survival, %) . | Relapse (3-y relapse, %) . |
|---|---|---|
| Patient age, y | ||
| Younger than 40, n = 48 | 8 (81) | 4 (11) |
| 40-50, n = 540 | 5 (89) | 2 (10) |
| Older than 50, n = 29 | 4 (82) | 1 (0) |
| Time from diagnosis to BMT | ||
| Less than 1 y, n = 114 | 13 (87) | 5 (7) |
| More than 1 y, n = 17 | 4 (82*) | 2 (13*) |
| Stem cell source | ||
| BM, n = 100 | 16 (81) | 6 (8) |
| PBSCs, n = 31 | 1 (96) | 1 (5) |
Risk variables . | No. deaths (3-y survival, %) . | Relapse (3-y relapse, %) . |
|---|---|---|
| Patient age, y | ||
| Younger than 40, n = 48 | 8 (81) | 4 (11) |
| 40-50, n = 540 | 5 (89) | 2 (10) |
| Older than 50, n = 29 | 4 (82) | 1 (0) |
| Time from diagnosis to BMT | ||
| Less than 1 y, n = 114 | 13 (87) | 5 (7) |
| More than 1 y, n = 17 | 4 (82*) | 2 (13*) |
| Stem cell source | ||
| BM, n = 100 | 16 (81) | 6 (8) |
| PBSCs, n = 31 | 1 (96) | 1 (5) |
Two-year Kaplan-Meier estimates, given lack of events at 3 years.
Outcomes and busulfan level
The effect of busulfan level on various outcomes was examined by modeling busulfan concentration as a continuous variable and by dichotomizing busulfan as more than or equal to 1000 ng/mL versus less than 1000 ng/mL. With busulfan modeled as a continuous (linear) variable, there was not a statistically significant association of busulfan with the hazard of mortality, although the hazard increased as concentration increased (P = .21). Since there was only one death that was preceded by relapse, the results for NRM are virtually the same as for overall mortality (positive association; P = .22). As busulfan concentration increased, the hazard of clinical extensive chronic GVHD actually decreased, but the association was not statistically significant (P = .24). The probability of grade 2 to 4 acute GVHD increased slightly as concentration increased, but the increase was not statistically significant (P = .35). When busulfan exposure was categorized as more than or equal to 1000 ng/mL versus less than 1000 ng/mL, the resulting groups showed similar hazards of mortality (hazard ratio [HR] = 1.0; 95% CI, 0.4-2.8; P = .96). The hazard of chronic GVHD was lower among patients who received 1000 ng/mL or more than among those who received less than 1000 ng/mL, but the difference was not statistically significant (HR = 0.8; 95% CI, 0.5-1.3; P = .40), and the probability of grade 2 to 4 acute GVHD was not statistically significantly different between the 2 groups (70% vs 60% for high versus low exposure, respectively; odds ratio = 1.5; 95% CI, 0.7-3.3; P = .28). If busulfan exposure was defined as 1100 ng/mL or more versus less than 1100 ng/mL, there were 4 deaths (21%) among 19 cases with higher exposure, compared with 13 deaths (12%) among 112 cases with lower exposure (HR = 1.9; 95% CI, 0.6-5.8; P = .27).
Long-term complications of transplantation
Seventy-eight of the 131 patients developed extensive chronic GVHD, for an estimate of 60% at 1 year after transplantation. Among the survivors who had Karnofsky scores (100 of 114 survivors), the median score was 95% (range, 30%-100%), with only 10% of survivors having a Karnofsky score of less than 80%.
Discussion
The TBU/CY preparative regimen, coupled with matched related allogeneic transplantation, was a safe and effective treatment in 131 consecutively treated CML-cp patients. Nonleukemic mortality was 14%, relapse was unusual (8%), and 3-year survival was high (86%). In these patients, age did not appear to influence outcome. We could not demonstrate a statistically significant effect of the time from diagnosis to transplantation and survival, although this analysis was difficult because so few patients had intervals of more than a year. The median Karnofsky score of survivors at last contact was 95%. In addition, only 11% of patients remained positive for bcr-abl at last contact, with very low quantitative levels (median, 4.6 bcr-abl copies/μg RNA; range, 0.4-87.0 copies/μg RNA). Our previous study22 showed a median bcr-abl level of more than 40 000 copies/μg at time of cytogenetic relapse, suggesting that these surviving patients have a residual disease burden approximately 4 logs less than expected at relapse. Taken as a whole, this study supports allogeneic transplantation as a very effective therapy for CML.
In contrast to our findings are the findings of a recent study of 36 patients receiving HLA-matched sibling transplants for CML. In that study, 1-year survival after allogeneic transplantation for CML following intravenous busulfan with 120 mg/kg CY was compromised by a busulfan AUC below 950 or above 1500 μMol/min after either oral or intravenous administration of busulfan.24 These AUC values correspond to Css values of 633 and 1000 ng/mL, respectively. Our results do not support an association of mortality and BU concentrations at these levels, although at concentrations at or above 1100 ng/mL the data are not as definitive in this regard. Thus, it would seem reasonable to suggest that busulfan levels be adjusted to achieve a level of 900 to 1100 ng/mL. It should also be noted that the interpatient coefficient of variation in clearance (the determinant of AUC and Css at a given dose) of intravenous busulfan is comparable to that of the oral drug formulation, while the intrapatient coefficient of variation is less in the intravenous formulation.25 Thus, the need for targeting with the 2 formulations is the same, but the intravenous formulation affords more reproducibility between doses in a given patient.
These data demonstrate the continued improvement in transplantation results for patients with CML-cp, beginning with the first series published, in 1986, in which survival was estimated near 50%.26 Apart from transplantation earlier in the course of the disease, it is difficult to identify the precise factors leading to the improved survival over the past 2 decades. However, the evolution from CY-TBI regimens through BU/CY and finally TBU/CY regimens has likely had a major part in the improved outcome over time. This can be especially demonstrated by the lack of an age effect on outcome, which was clearly a major factor in outcomes with TBI-based preparative regimens, and by the low relapse rate, which has fallen to less than 10% from a level of approximately 15% to 20% with TBI-CY transplants. The success of pharmacologic targeting of busulfan lays the groundwork for the potential targeting of other components of preparative regimens, such as cyclophosphamide.
The recent advent of reduced-conditioning or nonmyeloablative transplants in CML illustrates different strategies that may be similarly effective in controlling the disease.27-29 Thus, elimination of the malignant clone may occur after an optimal dose of a cytotoxic agent (targeted busulfan), causing the rapid elimination of CML cells, or after adoption of a new immune system, after the more gradual elimination of CML cells via the graft-versus-leukemia effect. As such, it is not surprising that the reduced-intensity approach appears more effective in chronic-phase CML, since the immune system has time to mount the attack, than in advanced CML, where the race to contain and eliminate the malignant clone is far more difficult.
The treatment of CML-cp has been radically altered by the introduction of the tyrosine kinase inhibitor imatinib.4,5 Recent data suggest that of patients with CML-cp who are resistant to interferon therapy, approximately 40% may achieve complete cytogenetic remissions with imatinib.5 The phase 3 randomized trial comparing imatinib and interferon/Ara-c is ongoing, but preliminary reports suggest that 70% of patients treated with imatinib will achieve a complete cytogenetic response, with less than 5% progression to advanced disease within the first year of therapy. These are extremely encouraging results, but there are still important questions that have not been answered. Is imatinib curative? If not, how long will responses last? When the disease recurs, does it return to chronic phase or to accelerated or blast phase? For those patients who fail to respond to imatinib, or who relapse in chronic phase, how much will the delay in transplantation affect outcome? Will treatment with imatinib predispose patients who subsequently undergo transplantation to more regimen-related toxicity?
Novel agents for the treatment of CML will continue to emerge, offering patients the opportunity for substantial benefit. Some of these agents may be curative or may prolong the course of the disease to a degree that is tantamount to cure. When considering currently available treatment options, however, patients and physicians must recognize that only transplantation has clearly been shown to cure CML.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-08-2619.
Supported in part by National Institutes of Health grant CA-18029.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal