Abstract
Rearrangements of the MLL locus, located on human chromosome 11q23, are frequent in both infant and therapy-related leukemias. Gene expression analysis of MLL-rearranged B-precursor acute lymphoblastic leukemias (MLL B-ALLs) has identified these cases as a unique subtype of leukemia, characterized by the expression of genes associated with both lymphoid and myeloid hematopoietic lineages. Here we show that MLL fusions also generate a distinct genetic subtype of T-lineage ALL (MLL T-ALL), in which leukemic cells are characterized by an early arrest in thymocyte differentiation, with suggestive evidence of commitment to the γδ lineage. Interestingly, multiple genes linked to cell proliferation (eg, PCNA, MYC, CDK2, and POLA) were down-regulated in MLL-fusion samples, relative to those transformed by other T-ALL oncogenes (P < .000 001, Fisher exact test). Overall, MLL T-ALL cases consistently demonstrated increased levels of expression of a subset of major HOX genes—HOXA9, HOXA10, and HOXC6—and the MEIS1 HOX coregulator (P < .008, one-sided Wilcoxon test), a pattern of gene expression that was reiterated in MLL B-ALLs. However, expression of myeloid lineage genes, previously reported in MLL B-ALLs, was not identified in T-lineage cases with this abnormality, suggesting that myeloid gene dysregulation is dispensable in leukemic transformation mediated by MLL fusion proteins. Our findings implicate dysregulation of HOX gene family members as a dominant mechanism of leukemic transformation induced by chimeric MLL oncogenes. (Blood. 2003;102:262-268)
Introduction
Translocations involving chromosome band 11q23 are found in a variety of hematologic malignancies, including both B-precursor and T-lineage acute lymphoblastic leukemias (B- and T-ALLs), acute myeloid leukemias (AMLs), and cases of myelodysplastic syndrome (MDS). Chromosome 11q23 rearrangements are present in most infant leukemias1-3 and in secondary myeloid leukemias occurring after chemotherapy with topoisomerase inhibitors.4-6 Invariably, such translocations generate a fusion gene encoding a chimeric protein in which the aminoterminal portion of MLL is fused to the carboxyterminal regions of more than 30 other proteins.7,8 MLL is a mammalian homologue of the Drosophila gene trithorax, and its product functions as a master transcriptional regulator of developmental patterning and cell fate decisions.9 The aminoterminal fragment of MLL in MLL fusion proteins uniformly includes 2 AT-hook domains and a region of homology with DNA methyltransferase,10,11 while the carboxyterminal fragments of the numerous fusion partners show little or no structural similarity to each other.
Armstrong et al12 recently analyzed the gene expression patterns of a series of B-precursor ALLs, demonstrating a characteristic, highly distinct profile for cases with a rearranged MLL gene (MLL B-ALLs). This finding was independently confirmed in a large group of patients studied at St Jude Children's Research Hospital.13 A major issue that remains to be addressed is the identification of the genes within the unique MLL B-ALL gene expression signature that serve as critical downstream targets during induction and maintenance of the leukemic phenotype. Among possible candidates, members of the HOX gene family are perhaps the most attractive.8 Trithorax, the Drosophila counterpart of MLL, is required for the proper maintenance of cell type—specific patterns of HOM-C gene expression during embryogenesis.14 In mice, heterozygous germ-line inactivation of Mll resulted in homeotic transformations of the axial skeleton associated with abnormalities in the maintenance of Hoxa7 and Hoxc9 gene expression.9 Retroviral insertional mutagenesis experiments in mice implicated Hoxa9 in collaboration with Meis1 in myeloid leukemogenesis,15 while subsequent transgenic studies showed that the aberrant expression of Hoxa9 and Hoxa10 in murine hematopoietic precursors induces leukemia in cooperation with Meis1.16,17 Finally, in a very recent report, MLL was shown to regulate HOX gene expression by binding directly to promoter sequences.18
In a previous study of human T-ALLs,19 we identified cases with the MLL-ENL fusion oncogene, but did not compare their gene expression profiles with those of T-ALLs harboring other oncogenic transcription factors or with those of MLL B-ALLs.19 Here we further analyze gene expression patterns in T-ALLs with MLL rearrangements and show that activation of HOXC6, HOXA9, and HOXA10, as well as the HOX gene cofactor MEIS1, defines a core gene transcriptional program shared by lymphoid leukemias expressing MLL fusion genes. Interestingly, in both the T and B lineages, MLL-associated leukemias also showed reduced expression levels of genes involved in cell proliferation. By contrast, aberrant myeloid gene expression and expression levels of FLT3 and other genes typically up-regulated in MLL B-ALL were not similarly up-regulated in MLL T-ALL, suggesting that these genes are not central to the MLL-dependent leukemogenic process. Thus, dysregulation of specific HOX genes appears to be a dominant mechanism of leukemic transformation initiated by MLL chimeric oncogenes.
Materials and methods
Leukemia samples
Briefly, the T-ALL cases consisted of 39 samples of cryopreserved lymphoblasts obtained, with informed consent, from children and young adults with T-ALL treated in Total Therapy Studies XI to XIII at St Jude Children's Research Hospital (Memphis, TN).19 B-ALL and MLL B-ALL samples were collected, after informed consent, from patients treated on Dana-Farber Cancer Institute protocols between 1980 and 2001 (n = 39), on the Interfant 99 protocol (n = 3), and at the Princess Margaret Hospital in Toronto (n = 2).12 Clinical and laboratory data from both series are shown in Tables S1 and S2 on the Blood website (see the Supplemental Material link at the top of the online article), and detailed information about the 2 series of patients analyzed in this study may be found in earlier publications.12,19
MLL-ENL detection and oncogene expression based on molecular subtype of T-ALL
Detailed methods and results of quantitative real-time reverse transcriptase—polymerase chain reaction (RT-PCR) to detect expression of the oncogenic transcription factors HOX11, HOX11L2, TAL1, and LYL1, as well as RT-PCR analysis of MLL-ENL fusion transcripts, were performed as previously described.19 These results have been previously reported elsewhere19 and are summarized in Table S1 of the supplemental material.
RNA purification labeling and analysis
T-ALL cases were analyzed with Affymetrix HU6800 arrays (Santa Clara, CA), while common B-ALL and MLL B-ALL cases were hybridized to U95 or U95Av2 arrays. RNA purification and labeling and microarray hybridization techniques have been described before12,19 ; detailed protocols are available from the Whitehead/MIT Genome Center Molecular Pattern Recognition website (http://www-genome.wi.mit.edu/mpr/publications/projects/Leukemia/protocol.html).
Data analysis and statistical methods
Biotinylated labeled RNA from 39 T-cell leukemia samples was hybridized to Affymetrix HU6800 arrays (6817 genes), and B-cell leukemia sample RNAs were analyzed using Affymetrix U95Av2 arrays (12 600 genes). Each gene in the array is analyzed using 40 oligonucleotide probes (20 perfectly matched probes and 20 mutated probes are used as controls for background and cross hybridization). An estimate (average difference) of the expression of each gene that takes into account the fluorescence intensity across the 20 perfectly matched probes and the 20 mutated probes was obtained using Affymetrix analysis software. Linear scaling of the data was performed using a typical sample (the one closer to the average fluorescence) as reference.
To perform an unsupervised hierarchic clustering analysis of T-ALL gene expression data, expression values from Affymetrix HU6800 arrays were first thresholded (floor, 100 units; ceiling, 16 000 units) and then filtered using fold-change (fold difference) and absolute variation (max-minute difference) to exclude genes with minimal variation across the samples using GeneCluster 2.0 software (Center for Genome Research, Whitehead Institute, Cambridge, MA). Using a filter consisting of a fold difference higher than 5 and a max-min difference more than 500 units, 1359 genes were selected. These genes were used to perform a hierarchic clustering analysis using GeneCluster and Treeview (Eisenlab, Stanford University, CA). GeneCluster and Treeview, together with detailed instructions for microarray data analysis, are available at http://www-genome.wi.mit.edu/cancer/software/software.html and http://rana.lbl.gov/EisenSoftware.htm, respectively.
Genes associated with a particular class distinction were identified by using the signal-to-noise statistics: Sx = ([μMLLleukemias - μnon-MLLleukemias]/[σMLLleukemias + σnon-MLLleukemias]), in which for each gene μ represents the mean value and σ represents the standard deviation within the group of samples described by the subscript in the formula (ie, MLL leukemias or non-MLL leukemias).20
Because of differences in platform structure (HU6800 arrays for T-ALL cases and U95Av2 arrays for B-ALL cases), as well as the hybridization procedures and scanning parameters, we performed our class distinction analysis in T-ALL and B-ALL samples separately. The results of this analysis for each series were compared to evaluate common findings and discrepancies. In order to facilitate this cross comparison, genes from both arrays were assigned a UniGene identifier.
The Wilcoxon rank-sum test was used to evaluate in T-ALL cases the expression levels of genes identified by Armstrong et al12 as being highly expressed in patients with MLL B-ALL. No correction for multiple comparisons was imposed, as the genes of interest were identified a priori. To assess whether genes associated with cell proliferation were preferentially down-regulated in the MLL T-ALL cases, we performed a nearest-neighbor analysis using the signal-to-noise statistic after preprocessing the data by thresholding (floor, 100; ceiling, 16 000) and applying a variation filtering (fold difference, > 5; max-min, > 500). Unsupervised hierarchic clustering (previous paragraphs) identified a cluster enriched for genes involved in DNA synthesis, nucleotide metabolism, and cell-cycle control. The frequency of such genes among the 100 nearest neighbors negatively associated with MLL T-ALL cases was compared with that in the remaining cases by Fisher exact test.
Results
MLL-rearranged cases constitute a distinct subtype of T-ALL
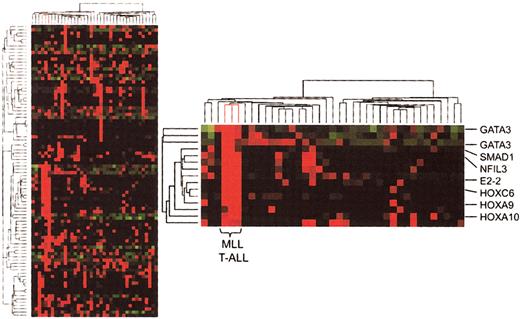
We first performed an unsupervised hierarchic clustering analysis of gene expression results in our 39 T-ALL cases, which included 3 cases expressing MLL-ENL fusion transcripts. In the resultant dendrogram (Figure 1), the 3 MLL-ENL samples cluster together as a result of high levels of expression of a series of genes that are generally not expressed or expressed at lower levels in other cases of T-ALL. These include numerous transcription factor genes involved in development and hematopoietic differentiation, such as GATA3, NFIL3, E2-2, BMI1, SMAD1, HOXA9, HOXC6, and HOXA10. Hence, this unsupervised analysis indicates that MLL T-ALL cases represent a distinct molecular subtype of T-ALL.19
Hierarchic clustering analysis (unsupervised) of gene expression in T-ALL. Partial view of the hierarchic tree of genes (rows) and samples (columns), with MLL-ENL+ T-ALL cases (lines highlighted in red). Gene expression levels are indicated by different shades of red (higher expression) and green (lower expression). The detailed view of the cluster of genes associated with MLL-ENL cases demonstrates the involvement of HOX genes and other transcription factors associated with hematopoietic differentiation.
Hierarchic clustering analysis (unsupervised) of gene expression in T-ALL. Partial view of the hierarchic tree of genes (rows) and samples (columns), with MLL-ENL+ T-ALL cases (lines highlighted in red). Gene expression levels are indicated by different shades of red (higher expression) and green (lower expression). The detailed view of the cluster of genes associated with MLL-ENL cases demonstrates the involvement of HOX genes and other transcription factors associated with hematopoietic differentiation.
Early differentiation arrest and γδ T-lineage commitment signatures in MLL T-ALL
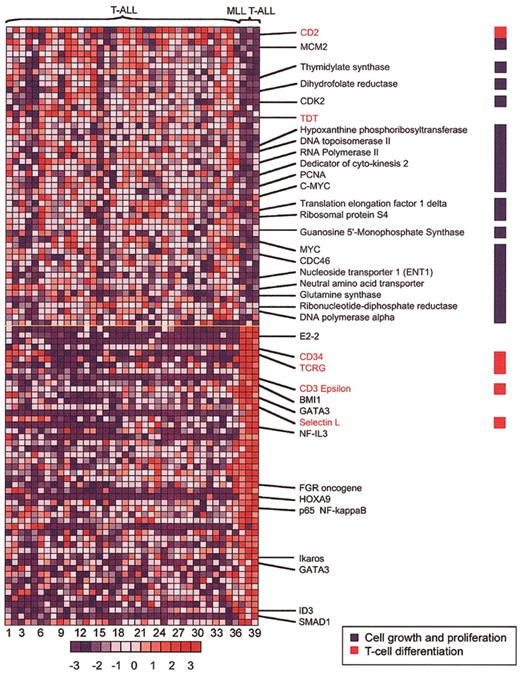
To better define the MLL-ENL gene expression signature in T-ALL, we compared patterns of gene up-regulation and down-regulation in MLL-ENL+ versus all other cases (Figures 2, 3). This analysis revealed a signature characterized by up-regulation of genes such as CD34, L-selectin, LSP1, LMO2, IL7R, and CD7, which are associated with early stages of thymocyte differentiation.21-27 A similarly increased expression of early T-cell differentiation genes was observed in LYL1+ samples19,28 (Figure 3). However, there was an apparent asynchrony of gene expression in MLL-ENL+ cases, in that genetic markers of more mature thymocytes, such as CD10, CD6, CD3E, LCK, and the TCRG and TCRD genes, were expressed in addition to the characteristic early T-cell signature (Figure 3). This gene expression profile is in agreement with the results from immunophenotype analysis, in which the 2 MLL T-ALL samples with available data showed the presence of both early and late T-cell surface markers (CD34+, CD10+, CD1-, and surface CD3+) (Table S1, supplemental material). This apparent asynchrony in T-cell development in association with the expression of the T-cell receptor (TCR) γ and δ genes (Figure 3) indicates a partial thymocyte developmental arrest in MLL T-ALL cases, associated with commitment to the γδ lineage. The up-regulation of ID3 expression (Figure 2) with γδ lineage commitment is consistent with data from retrovirally transduced murine thymocytes, showing that overexpression of ID3 inhibits αβ but not γδ T-cell development.29 High levels of ID3 could be mechanistically linked to T-cell transformation, because T-cell lymphomas rapidly and reproducibly arise in mice null for E2a or overexpressing Id1 early in thymocyte development.30
MLL-ENL—associated genes in T-ALL. The 100 genes most closely associated with MLL-ENL expression—either up-regulated (red) or down-regulated (blue)—are shown, based on a nearest-neighbor analysis. Each column represents a sample and each row, a gene. Numbers at the bottom indicate sample order (sample order is preserved in Figures 4, 5, and 6, and in Table S1 of the supplemental material). Normalized expression values are represented by colors (red, levels greater than the mean; blue, levels lower than the mean). The bar at the bottom shows the correlation between shifts in color and differences in gene expression, from - 3 to + 3 standard deviations. The full list of the top 100 genes positively and negatively associated with the MLL T-ALL category is included as Table S3 in the supplemental material.
MLL-ENL—associated genes in T-ALL. The 100 genes most closely associated with MLL-ENL expression—either up-regulated (red) or down-regulated (blue)—are shown, based on a nearest-neighbor analysis. Each column represents a sample and each row, a gene. Numbers at the bottom indicate sample order (sample order is preserved in Figures 4, 5, and 6, and in Table S1 of the supplemental material). Normalized expression values are represented by colors (red, levels greater than the mean; blue, levels lower than the mean). The bar at the bottom shows the correlation between shifts in color and differences in gene expression, from - 3 to + 3 standard deviations. The full list of the top 100 genes positively and negatively associated with the MLL T-ALL category is included as Table S3 in the supplemental material.
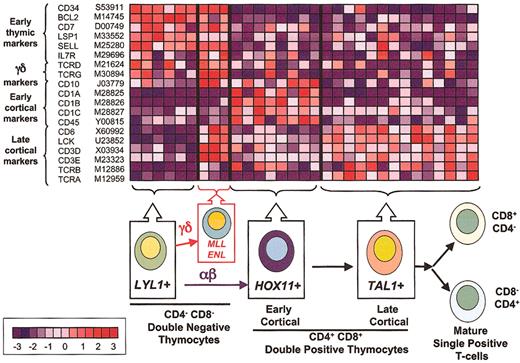
Correlation of gene expression profiles and thymocyte differentiation. The top panel illustrates the relative expression of genes (rows) differentially expressed during thymocyte cell differentiation in T-ALL samples (columns). Normalized expression values are indicated by red (levels greater than the mean) and blue (levels less than the mean). The bar at the bottom left shows the correlation between shift in color and differences in gene expression, from - 3 to + 3 standard deviations. LYL1+ samples are arrested at the earliest stages of T-cell differentiation, as demonstrated by expression of genes such as CD34, BCL2, and L-selectin. MLL-ENL+ cases combine the expression of this early developmental signature with the expression of genes associated with the TCR signaling machinery, such as LCK, CD3, and the TCRG and TCRD, suggesting commitment to the γδ lineage. By contrast, HOX11+ samples (characterized by the expression of CD10 and CD1 genes) show arrest at the early cortical stage of thymocyte development, while TAL1+ samples express the TCR signaling genes (eg, CD3 and LCK) together with TCRA and TCRB, demonstrating progression into the late cortical stage of thymocyte differentiation within the αβ lineage.
Correlation of gene expression profiles and thymocyte differentiation. The top panel illustrates the relative expression of genes (rows) differentially expressed during thymocyte cell differentiation in T-ALL samples (columns). Normalized expression values are indicated by red (levels greater than the mean) and blue (levels less than the mean). The bar at the bottom left shows the correlation between shift in color and differences in gene expression, from - 3 to + 3 standard deviations. LYL1+ samples are arrested at the earliest stages of T-cell differentiation, as demonstrated by expression of genes such as CD34, BCL2, and L-selectin. MLL-ENL+ cases combine the expression of this early developmental signature with the expression of genes associated with the TCR signaling machinery, such as LCK, CD3, and the TCRG and TCRD, suggesting commitment to the γδ lineage. By contrast, HOX11+ samples (characterized by the expression of CD10 and CD1 genes) show arrest at the early cortical stage of thymocyte development, while TAL1+ samples express the TCR signaling genes (eg, CD3 and LCK) together with TCRA and TCRB, demonstrating progression into the late cortical stage of thymocyte differentiation within the αβ lineage.
MLL-rearranged lymphoblastic leukemias down-regulate cell proliferation genes
Further analysis of the gene expression signature in MLL-ENL+ T-ALL cases revealed a significant down-regulation of genes associated with cell proliferation. A medium-stringency filter identified 1359 genes, which were subjected to nearest-neighbor analysis. Of these, 119 genes were identified in a cluster associated with cell proliferation. Among the 100 nearest neighbors negatively associated with the MLL T-ALL category were 52 genes from the cell proliferation cluster (52%); in contrast, the other 67 genes associated with cell proliferation constituted only 5% of the remaining 1259 genes under investigation (P < .000 0001 by Fisher exact test). The 52 cell proliferation genes down-regulated in MLL T-ALL cases included a broad range of genes involved in cell-cycle control and DNA synthesis, such as KI67, CDK2, PCNA, MYC, DNA polymerase A (POLA), dyhidrofolate reductase (DHFR), and thymidylate synthethase (TYMS) (Figure 2), suggesting that MLL-rearranged cases have a lower proliferative rate than do T-ALLs without this translocation. Relative down-regulation of numerous cell proliferation genes was also observed in most MLL B-ALL cases (Figure S1, supplemental material), suggesting that this could be a common feature of leukemias transformed by MLL translocations. Although this hypothesis will need to be confirmed by direct analysis of cell cycle in primary MLL tumor samples, our results are in agreement with previous in vitro studies showing a reduced proliferative capacity of MLL fusion gene—positive leukemic cells, whether primary samples or cell lines.31
HOX gene dysregulation is a general feature of MLL-rearranged leukemias
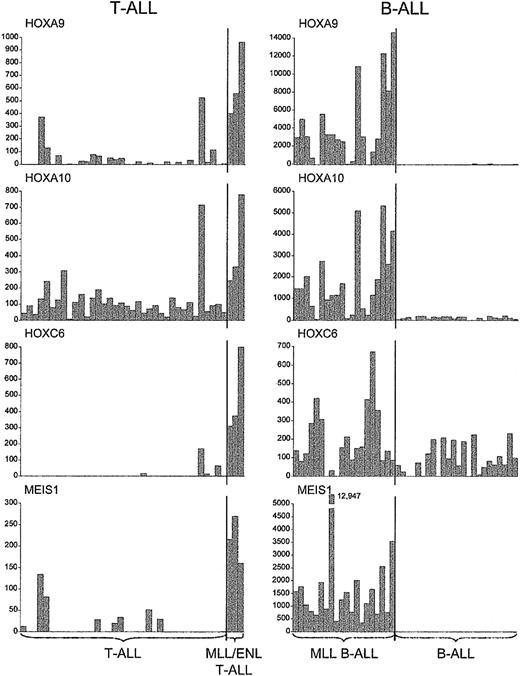
A further distinctive feature of MLL-ENL+ T-ALL cases was the significant up-regulation of specific homeotic genes, such as HOXA9, HOXA10, HOXC6, and the HOX cofactor MEIS1, as compared with results in all other T-ALL cases (P < .0008 for each comparison, one-sided Wilcoxon rank-sum test) (Figures 1, 2, 4). Importantly, these genes were also specifically up-regulated in MLL B-ALL cases.12 In light of recent data showing that MLL binds to specific HOX target loci18 together with the role of trithorax and MLL in the maintenance of HOM-C gene expression during embryonic development,10,11,32 these findings strongly suggest that MLL fusion oncoproteins directly regulate certain HOX genes in developing hematopoietic cells, to induce and maintain a leukemogenic program of gene expression.
Up-regulation ofHOXgenes inMLL-rearranged T- and B-lineage ALLs. Absolute fluorescence values for a subset of HOX genes and the HOX gene cofactor MEIS1, indicating consistent up-regulation in cases with MLL fusions, regardless of cell lineage.
Up-regulation ofHOXgenes inMLL-rearranged T- and B-lineage ALLs. Absolute fluorescence values for a subset of HOX genes and the HOX gene cofactor MEIS1, indicating consistent up-regulation in cases with MLL fusions, regardless of cell lineage.
Lineage-specific features of MLL-rearranged leukemias
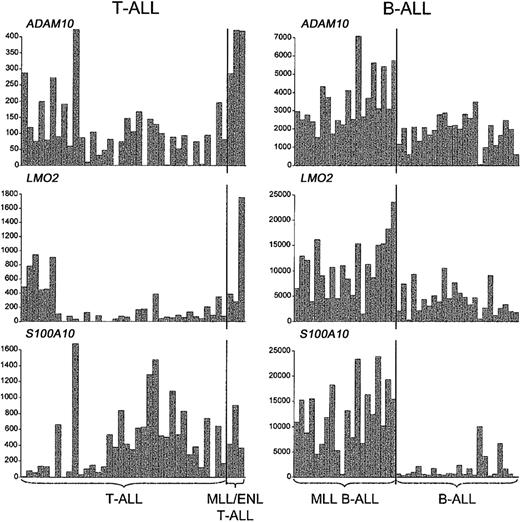
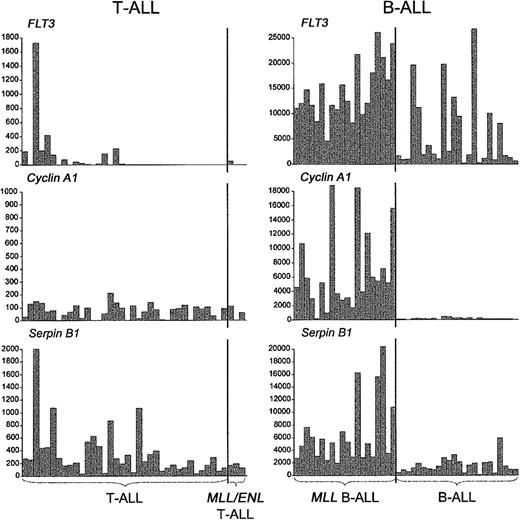
We also asked whether the distinctive activation of other genes in MLL B-ALL, including those related to the myeloid lineage, was recapitulated in MLL-ENL+ T-ALL.12,13 As shown in Figure 5, we detected the up-regulation of some of these genes, but in general their association with MLL-rearranged cases was not as specific as in MLL B-ALLs. For example, LMO2 activation was detected in both MLL-ENL+ and LYL1+ T-ALL cases, and S100A10 expression was found in both MLL-ENL+ and TAL1+ T-ALL cases, while ADAM10 was highly expressed not only in the MLL-ENL+ subgroup but also in a few cases of LYL1+ and HOX11+ T-ALL. More important, we could not establish an association between the MLL-ENL oncogene in T-ALL and the expression FLT3 and other myeloid genes, such as CCNA1 and SerpinB1/monocyte-neutrophil elastase inhibitor (ELANH2) (Figure 6), in contrast to findings in MLL B-ALL.12,13 Nearest-neighbor analysis of the 3 MLL B-ALL cases harboring the MLL-ENL fusion gene further indicated that these cases did not differ from other MLL B-ALL cases in their up-regulation of FLT3, CCNA1, or ELANH2 (Figure S2, supplemental material). Thus, the expression profile differences between MLL T- and B-ALLs highlight genes specifically up-regulated in these different types of lymphoid progenitor cells.
Up-regulation of representative non-HOXgenes inMLL-rearranged T- and B-lineage ALLs. A subset of genes other than HOX family members are up-regulated in both B- and T-ALLs with MLL translocations, as illustrated by the absolute fluorescence values of ADAM10, LMO2, and S100A10.
Up-regulation of representative non-HOXgenes inMLL-rearranged T- and B-lineage ALLs. A subset of genes other than HOX family members are up-regulated in both B- and T-ALLs with MLL translocations, as illustrated by the absolute fluorescence values of ADAM10, LMO2, and S100A10.
Expression ofFLT3and myeloid differentiation markers in T- and B-ALLs withMLLtranslocations. In contrast to the specific expression of FLT3 and other myeloid genes in MLL-rearranged B-ALLs, FLT3, Cyclin A1, and Serpin B1 were not up-regulated in MLL T-ALLs. A single, MLL-ENL—negative T-ALL case harboring an activating FLT3 internal tandem duplication expressed high levels of FLT3, Serpin B1, and other myeloid genes (Figure S3, supplemental material). Gene expression is reported in absolute fluorescence units.
Expression ofFLT3and myeloid differentiation markers in T- and B-ALLs withMLLtranslocations. In contrast to the specific expression of FLT3 and other myeloid genes in MLL-rearranged B-ALLs, FLT3, Cyclin A1, and Serpin B1 were not up-regulated in MLL T-ALLs. A single, MLL-ENL—negative T-ALL case harboring an activating FLT3 internal tandem duplication expressed high levels of FLT3, Serpin B1, and other myeloid genes (Figure S3, supplemental material). Gene expression is reported in absolute fluorescence units.
Discussion
Acute leukemias that express chimeric MLL oncogenes predominate in infants and in patients who have received chemotherapy with topoisomerase inhibitors. Despite the broad range of fusion partner genes that recombine with MLL and the involvement of MLL chimeras in hematologic cancers of different lineages, there are still no satisfactory mechanisms to explain the potent oncogenicity of these fusion proteins. An early favored hypothesis was that haploinsufficiency of the MLL locus combined with a dominant-negative effect of the oncogenic MLL fusions can lead to the loss of key MLL functions.33 However, identification of functional domains in MLL fusion partners that are required for transformation,34,35 together with rare cases in which MLL is fused to the CREB binding protein (CBP) transcriptional activator itself, suggests an active role of MLL partners in leukemogenesis.36 Our results, from comparative analysis of the gene expression profiles of T- and early B-lineage leukemias, implicate up-regulation of a subset of major HOX genes as the central mechanism of leukemic transformation by MLL oncoproteins.
The homeobox genes that were specifically up-regulated in both MLL B-ALL and MLL T-ALL include HOXA9, HOXA10, and HOXC6, together with the MEIS1 HOX cofactor. This observation may be of particular relevance because specific HOX genes are normally expressed in subsets of hematopoietic stem and progenitor cells.8,37 In addition, HOX gene dysregulation has been directly implicated in AMLs with translocation-induced fusion oncogenes: NUP98-HOXA9 [t(7;11)(p15;p15)], NUP98-HOXD11 [t(2;11)(q31; p15)], NUP98-HOXD13 [t(2;11)(q31;p15)], and less directly in pre—B-cell ALLs expressing the E2A-PBX1 [t(1;19)(q23;p13.3)] oncogene.38-42 Further studies in murine systems have shown that primary bone marrow cells can be transformed through enforced expression of Hoxa9 or Hoxa10 in collaboration with Meis1.16,17
The fundamental importance of HOX gene overexpression in B- and T-lineage leukemias with MLL translocations is indicated by the inconsistencies in gene expression profiles between our MLL T- and B-ALL cases.12,13 For example, S100A10 and LMO2 activation is highly predictive of the presence of MLL rearrangements in B-lineage leukemias, while in MLL T-ALL cases the effect is not specific, since a substantial number of TAL1+ or LYL1+ cases also express high levels of S100A10 and LMO2. Similarly, the myeloid gene component in MLL B-ALL is totally lacking in MLL T-ALL, an observation that has several plausible explanations. First, the myeloid lineage differentiation potential of MLL T-ALLs may be severely impaired in comparison with MLL B-ALLs, as this feature is limited to the earliest stages of T-cell development.43-45 The high frequency of myeloid surface markers on cells of the LYL1+ group of T-ALLs, which are blocked in the earliest stages of thymocyte differentiation,19 is in agreement with this hypothesis. It is also possible that the expression of myeloid features by MLL B-ALLs is associated with a secondary leukemogenic event specifically associated with the transformation of MLL-rearranged B-cell progenitors. An obvious candidate for this type of selective additional mutation is one resulting in FLT3 activation, which was found in a significant percentage of MLL B-ALL cases46 but in none of our MLL T-ALL cases.19 However, one LYL1+ T-ALL sample lacking MLL-ENL did show high levels of FLT3 expression in combination with an activating FLT3—internal tandem duplication (ITD) mutation.19 This case had strong myeloid gene expression, similar to the MLL B-ALL cases, except that high levels of granulocytic genes predominated in the FLT3-ITD T-ALL case, while monocytic genes predominated in MLL B-ALLs (Figure S3, supplemental material). Finally, FLT3 activation has been shown to induce myeloid differentiation of T-cell precursors47 and may contribute to the myeloid features of MLL B-ALLs as well.12 In any case, it is unlikely that myeloid genes are related to the oncogenic transcriptional program associated with MLL (ie, up-regulation of selected major HOX genes together with MEIS1) as they are found only in MLL B-ALLs but not in MLL T-ALLs.
Our results indicate that MLL-ENL+ T-ALLs are arrested at an early stage of thymocyte differentiation, after commitment to the γδ lineage. The mechanism of differentiation arrest may depend on the effect of MLL-ENL on other transcription factors involved in lymphoid differentiation and tumorigenesis, including p65NFKB, Ikaros, the helix-loop-helix factor E2-2 and the helix-loop-helix inhibitory factor ID3. The presence of high levels of ID3 expression may be of particular relevance in this regard, as the differentiation block observed in MLL-ENL+ T-ALLs is quite similar to that induced by the aberrant expression of ID3 in murine systems.29 We initially predicted that MLL-ENL+ cases with their characteristic up-regulation of HOX genes might resemble cases expressing HOX11 and HOX11L2, 2 orphan homeobox genes involved in the pathogenesis of T-ALL but located outside the clusters of the major HOXA, B, C, and D genes. Yet, the differences in stage-specific developmental arrest among these T-ALLs were striking. Similarly, cases harboring the helix-loop-helix factors TAL1 and LYL1, both of which are thought to act through inhibition of E12/E47 activity,48,49 are at opposite ends of the thymocyte developmental spectrum. These differences indicate that despite their structural and functional similarities, oncogenic transcription factors in T-ALL affect different developmental pathways that are relevant to the transformation of T-cell precursors.
A remarkable feature associated with the MLL-ENL fusion gene in T-ALL is the specific down-regulation of a broad range of genes involved in cell proliferation (Figure 2), a finding also observed in MLL B-ALL (Figure S1, supplemental material). Presumably, the lower proliferative capacity of leukemias harboring MLL fusion genes is offset by factors such as increased self-renewal of leukemic stem cells or inherent or acquired mutations that result in aberrantly increased cell survival within the leukemic clones.11 In contrast with the findings from gene expression analysis in other lymphoid tumors where the presence of a high proliferation gene expression signature is associated with a poorer prognosis,50 our data show that both MLL T-ALL (favorable prognosis) and most MLL B-ALL cases (adverse prognosis) have lower levels of expression of genes involved in cell proliferation.51,52 This suggests that the expression levels of genes associated with cell proliferation are not in themselves primary determinants of the different response to therapy between MLL T-ALL and MLL B-ALL cases.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-10-3221.
Supported in part by National Institutes of Health (NIH) grants CA68484, CA21765, and CA92551; a Leukemia and Lymphoma Society Fellowship Award (A.A.F.); and an American Society of Hematology Fellow Scholar Award (S.A.A.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Susana Raimondi for cytogenetic analysis, Fred Behm for providing cryopreserved samples from St Jude Children's Hospital Tissue Bank, Eric Smith for help with the figures, Patricia Ernst for critical review of the manuscript, John Gilbert for editorial assistance, and Doris Dodson for assistance with manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal