Abstract
Induction of immune tolerance on memory B cells specific to transplantation carbohydrate antigens was studied in the experimental animal model of α1,3galactosyltransferase knockout (KO) mice, which lack the α-gal epitope (Galα1-3Galβ1-4GlcNAc-R) and can produce the anti-Gal antibody against it. Memory anti-Gal B cells were generated by immunization of KO mice with pig kidney membranes (ie, xenogeneic cell membranes expressing an abundance of α-gal epitopes). Lymphocytes including memory anti-Gal B cells were administered into lethally irradiated KO mice, together with syngeneic wild-type (WT) lymphocytes expressing α-gal epitopes. Memory anti-Gal B cells were completely tolerized after being in vivo for 14 days with WT lymphocytes. This was indicated by the lack of anti-Gal immunoglobulin G (IgG) response following immunization with pig kidney membranes vs the extensive anti-Gal response in mice that did not receive WT lymphocytes. Tolerance induction was prevented if T cells were activated by alloantigens. This tolerance was highly specific to anti-Gal B cells and did not affect memory B cells with closely related specificity, such as B cells with anti–blood group A specificity. Tolerance induction on anti-Gal B cells was found to be time dependent and required more than 10 days of in vivo exposure of these B cells to WT lymphocytes. These observations suggest a novel method for induction of tolerance to transplantation carbohydrate antigens in humans, by in vitro transduction of autologous blood lymphocytes with an adenovirus containing the corresponding glycosyltransferase gene and administration of the transduced cells into the circulation after removal of natural antibodies to the antigen. (Blood. 2003;102:229-236)
Introduction
The study of immune tolerance to cell surface carbohydrate antigens, such as blood group ABO antigens, is of interest primarily in the area of transplantation of organs or tissues expressing incompatible carbohydrate antigens. Studying this type of tolerance has become feasible in recent years with the generation of α1,3galactosyltransferase (α1,3GT) knockout (KO) mice.1 These KO mice are syngeneic to the wild-type (WT) C57BL/6 strain, but they lack the α-gal epitope (Galα1-3Galβ1-4GlcNAc-R) because of the disruption of the α1,3GT gene. This gene encodes the glycosyltransferase that synthesizes α-gal epitopes. The α-gal epitope is absent in humans because of evolutionary inactivation of the α1,3GT gene.2,3 Nevertheless, this epitope constitutes part of the core structure of blood group B antigen, where a fucose is linked to the penultimate galactose (ie, Galα1-3(Fucα1-2)Galβ1-4GlcNAc-R) and of blood group A antigen, which is similar to blood group B antigen, but also has an N-acetyl group (NAc) linked to the terminal galactosyl (GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAc-R).4,5
The α-gal epitope is expressed on cells of nonprimate mammals (including mice) and in New World monkeys, all of which have active α1,3GT.2,3 Humans, apes, and Old World monkeys lack α-gal epitopes, and produce the natural anti-Gal antibody against it as approximately 1% of circulating immunoglobulins,6-8 because of continuous stimulation by gastrointestinal bacteria.9 Anti-Gal is of clinical significance, as the interaction between this antibody and α-gal epitopes on pig cells prevents xenotransplantation of pig organs into humans and monkeys.10-13 In addition, most of the elicited anti–blood group antibodies in patients that reject ABO-incompatible kidney allografts are in fact anti-Gal antibodies that bind to the core α-gal epitope within the incompatible blood group A or B antigens.14
In contrast to WT mice, which are immunotolerant to the α-gal epitope, KO mice have lost this tolerance and readily produce anti-Gal immunoglobulin G (IgG) in high titers following repeated immunizations with xenogeneic membranes expressing α-gal epitopes, such as pig kidney membranes (PKM).15,16 Chimerism studies with mouse bone marrow (BM) cells expressing α-gal epitopes have demonstrated the induction of tolerance to α-gal epitopes in KO mice.17-20 These studies led to the conclusion that BM cells expressing α-gal epitope can “educate” the immune system to “regard” this epitope as a self-antigen. Our objective has been to determine whether there are cells other than BM cells that can induce tolerance to the α-gal epitope. For this purpose we studied the ability of syngeneic WT lymphocytes (ie, lymphocytes with the same antigenic makeup as KO lymphocytes, but which also express approximately 1.5 × 105 α-gal epitopes per cell21 ) to induce such tolerance. In a recent study, we reported that as few as 2 × 106 syngeneic WT lymphocytes from C57BL/6 mice administered into naive KO mice tolerized them to the extent that the mice were incapable of producing anti-Gal following multiple PKM immunizations. This study suggested that exposure of naive B cells with an anticarbohydrate specificity to the cognate carbohydrate antigen on syngeneic lymphocytes results in tolerization of these B cells.22
The study of tolerance induction on naive anticarbohydrate B cells does not fully simulate anticarbohydrate immune response in humans. This is because human B cells capable of producing anti–blood group A or B or anti-Gal antibodies include a large proportion of memory B cells primed by carbohydrate epitopes on gastrointestinal bacteria.9,23,24 This raised the question of whether memory B cells capable of producing anti-Gal (memory anti-Gal B cells) can be tolerized by syngeneic lymphocytes expressing α-gal epitopes. Memory anti-Gal B cells can be generated by immunization with PKM. The many α-gal epitopes on PKM effectively activate anti-Gal B cells to proliferate, undergo isotype switch, and become either memory anti-Gal B cells or plasma cells secreting anti-Gal IgG in high titers.16 These elicited anti-Gal antibodies produced in PKM-immunized KO mice readily bind to α-gal epitopes on WT lymphocytes and induce their destruction. Therefore, the study of tolerance induction on memory anti-Gal B cells by WT lymphocytes had to be performed in the absence of circulating anti-Gal antibodies. This could be achieved by the adoptive transfer of memory anti-Gal B cells from PKM-immunized mice into lethally irradiated KO recipients. In these recipients, memory anti-Gal B cells were exposed to WT lymphocytes. We report here that in vivo exposure of memory anti-Gal B cells to syngeneic WT lymphocytes for 2 weeks results in specific tolerization of these B cells, and that this tolerance induction occurs only in the complete absence of T-cell activation during the tolerizing period.
Methods
Mice and immunization procedures
KO mice with disrupted α1,3GT gene on H-2b background were received as a gift from SangStat (Palo Alto, CA). Wild-type C57BL/6 mice on H-2b background (syngeneic WT mice) and WT C57BL/6 × Balb/c F1 mice (H-2bxd WT mice) were purchased from Jackson Laboratories (Bar Harbor, ME). Experiments were performed with both males and females and were found to yield similar results. The KO mice that served as donors for memory anti-Gal B cells were immunized intraperitoneally, 3 times at 1-week intervals, with a standard amount of 50-mg PKM homogenate in a volume of 0.2 mL, as previously described.16
Protocol for induction of tolerance on memory anti-Gal B cells
Lymphocytes that included memory anti-Gal B cells were obtained from PKM-immunized KO mice with documented anti-Gal IgG production. WT lymphocytes were obtained from syngeneic C57BL/6 mice. To study tolerance induction, memory anti-Gal B cells were transferred into same-sex lethally irradiated KO recipients. For this purpose, KO mice were lethally irradiated with 10.5 Gy and received on day 0, via the tail vein, 20 × 106 pooled lymphocytes from PKM-immunized KO mice and 20 × 106 pooled BM cells from same-sex naive KO mice. The latter cells were administered to provide blood cells for all nonlymphoid series. BM cells were obtained from femurs and tibias of naive KO mice. For tolerance induction, the KO recipients also received, on day 0, 0.2 × 106 to 20 × 106 same-sex WT syngeneic lymphocytes. In some of the studies KO recipients received WT lymphocytes from the F1 semiallogeneic C57BL/6 x Balb/c mice (H-2bxd). The presence of functional memory anti-Gal B cells was determined by anti-Gal response on day 28, after 2 PKM immunizations (50 mg) on days 14 and 21, as shown in Figure 1.
Timeline of the protocol for induction of tolerance on memory anti-Gal B cells.
Timeline of the protocol for induction of tolerance on memory anti-Gal B cells.
Measurement of anti-Gal IgG production
Anti-Gal IgG production was measured in serum samples obtained one week after the second immunization with PKM, as previously described.16 Anti-Gal activity was determined in sera placed into enzyme-linked immunosorbent assay (ELISA) wells at various dilutions in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), using synthetic α-gal epitopes linked to BSA (α-gal-BSA; Dextra, Reading, United Kingdom) as solid-phase antigen. Peroxidase-coupled goat antimouse IgG (Accurate Chemical, Westbury, NY) was used as secondary antibody.
Analysis of anti–blood group A antibody production
The production of anti–blood group A antibodies in mice immunized with human blood group A red-cell membranes was measured by ELISA with red cell membranes as solid-phase antigen, as previously described.14 Lysed blood group A red-cell membranes were washed, brought to a concentration of 2 mg/mL in carbonate buffer (pH 9.5), and dried overnight as 50-μL aliquots per well in ELISA plates (Falcon 3912), resulting in firm adherence to the wells. Wells were blocked with 1% BSA in PBS. Serum samples were adsorbed twice on equal volume of blood group O red-cell membranes to remove antihuman red-cell antibodies that were not anti-A. Sera were placed in the ELISA wells at 2-fold serial dilutions in PBS-BSA in 50-μL aliquots. After 2 hours, the plates were washed with PBS containing 0.05% Tween-20, then alkaline phosphatase–coupled goat antimouse IgG (diluted 1:1000; Sigma, St Louis, MO) was added as secondary antibody. The color reaction was developed with p-nitrophenylphosphate (Sigma) and absorbance measured at 405 nm.
ELISPOT analysis
The ELISPOT assay, used to measure the proportion of anti-Gal–secreting B cells, was performed as previously described.25 ELISPOT wells (Millipore, Bedford, MA) were coated with α-gal–BSA (5 μg/mL) or with BSA (5 μg/mL) for 20 hours at 4°C, then blocked for 1 hour with 0.4% BSA in RPMI 1640 medium. Lymphocytes were plated in the wells in 0.2-mL aliquots at various concentrations and incubated for 24 hours at 37°C. Subsequently, the wells were washed and incubated with peroxidase-coupled goat antimouse IgM combined with peroxidase-coupled goat antimouse IgG (Accurate Chemical). The spots representing anti-Gal antibodies secreted from individual plasma cells were identified with diaminobenzidine (DAB; Sigma). Wells containing BSA as solid-phase antigen served as background control.
Separation of lymphocyte subpopulations by magnetic beads
Spleen lymphocytes were incubated for 30 minutes with anti-IgG–or anti-CD3–coated biodegradable magnetic microbeads (Miltenyi Biotec, Auburn, CA). Subsequently, the lymphocytes binding the beads were retained within the magnetized metal bead column (Miltenyi Biotec) and eluted following removal of the magnetic field according to the manufacturer's specifications. Because the magnetic beads are biodegradable, the lymphocytes binding the beads can be studied for function by adoptive transfer.26 Staining of isolated populations with anti-IgG or anti-CD3 antibodies displayed more than 85% positive staining of cells with the corresponding antibodies, as assessed by flow cytometry (not shown).
Flow cytometry analysis of anti-Gal B cells and of WT lymphocytes
Anti-Gal B cells were identified by their ability to bind α-gal–BSA coupled to fluorescein isothiocyanate (FITC), according to a previously described method.16 The coupling with FITC (Pierce, Rockford, IL) was performed according to the manufacturer's instructions. Lymphocytes were incubated for 2 hours at 4°C with FITC–α-gal–BSA (1 μg/mL) in PBS containing 1% BSA and with phycoerythrin (PE)–conjugated antimouse Ig (Pharmingen, San Diego, CA) for double-color analysis of anti-Gal B cells. The stained cells were analyzed in a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
WT lymphocytes within KO recipients were identified by staining with the FITC-coupled Bandeiraea (Griffonia) simplicifolia IB4 lectin (BS lectin; Sigma). This lectin binds to α-gal epitopes on various cells.2,3 Lymphocytes (2 × 106/mL) were incubated with 10 μg/mL FITC-BS lectin for 30 minutes at 4°C. Subsequently the cells were washed and subjected to flow cytometry analysis.
Heart transplantation
WT hearts obtained from C57BL/6 mice were heterotopically transplanted in the abdominal cavity of KO mice by connecting the WT aorta to the KO aorta and the WT pulmonary artery to the KO inferior vena cava, as previously described.22 This transplantation was performed in tolerized KO mice and in control KO mice producing anti-Gal. The transplanted heart function was determined by daily palpation.
Results
Characterization of memory anti-Gal B cells
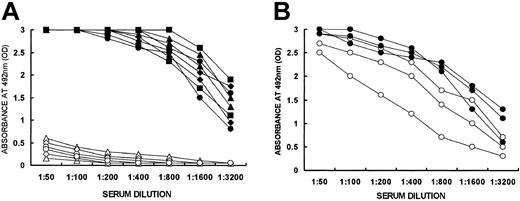
Memory anti-Gal B cells could be generated by PKM immunization of KO mice.16 However, such immunization also induces anti-Gal production.16 Circulating anti-Gal antibodies mask α-gal epitopes on WT lymphocytes and facilitate their elimination by complement-mediated lysis. Therefore, tolerization of memory anti-Gal B cells could be studied only after adoptive transfer of spleen cells from PKM-immunized mice into irradiated KO recipients, that is, in the absence of anti-Gal antibodies. The presence of memory anti-Gal B cells among the transferred spleen lymphocytes was demonstrated by a functional assay in which effective anti-Gal production was demonstrated 2 weeks to 3 months after adoptive transfer into lethally irradiated KO recipients. Lethally irradiated KO recipients (5 mice per group) received 20 × 106 lymphocytes from PKM-immunized KO mice. The recipients were immunized twice with PKM. The first immunization was given 2 weeks or 1, 2, or 3 months after adoptive transfer, and the second PKM immunization was given 1 week later. Anti-Gal IgG was produced in high titers following activation of memory anti-Gal B cells at all time points studied (Figure 2A). No significant spontaneous production of anti-Gal IgG was observed 14 days after adoptive transfer in the absence of PKM immunization (Figure 2A). These data imply that, similar to other memory B cells, memory anti-Gal B cells transferred from PKM-immunized mice can survive in irradiated KO recipients for prolonged periods. In contrast, irradiated KO mice that received naive KO spleen lymphocytes and that were immunized twice with PKM displayed no significant anti-Gal IgG response even when immunization was performed 3 months after adoptive transfer (Figure 2A). This suggests that the combined number of anti-Gal B cells transferred from naive KO mice and naive anti-Gal B cells generated in the recipient's bone marrow is too low to produce anti-Gal in measurable amounts following 2 PKM immunizations.
Production of anti-Gal by memory anti-Gal B cells. (A) Production of anti-Gal IgG in irradiated KO mice undergoing the following treatments: adoptive transfer of 20 × 106 spleen lymphocytes from PKM-immunized KO mice and 2 PKM immunizations, the first of which was given 14 days (•), 1 month (▴), 2 months (▪), or 3 months (♦) after adoptive transfer, or no PKM immunization and serum obtained on day 14 after adoptive transfer (▵); adoptive transfer of 20 × 106 spleen lymphocytes from naive KO mice and 2 PKM immunizations, the first of which was given 14 days (○), or 3 months (⋄) after adoptive transfer. Data are from 2 representative mice in each group of 5 mice. (B) Anti-Gal production in recipients of 5 × 106 B lymphocytes expressing cell-surface IgG from PKM-immunized mice and of 5 × 106 T cells from naive KO mice (○) or 5 × 106 primed T cells from KO mice immunized 3 times with PKM (•). Mice were immunized twice with PKM, starting on day 14 after adoptive transfer. Note that memory anti-Gal B cells expressing IgG can be activated by PKM in the presence of either naive or primed T cells.
Production of anti-Gal by memory anti-Gal B cells. (A) Production of anti-Gal IgG in irradiated KO mice undergoing the following treatments: adoptive transfer of 20 × 106 spleen lymphocytes from PKM-immunized KO mice and 2 PKM immunizations, the first of which was given 14 days (•), 1 month (▴), 2 months (▪), or 3 months (♦) after adoptive transfer, or no PKM immunization and serum obtained on day 14 after adoptive transfer (▵); adoptive transfer of 20 × 106 spleen lymphocytes from naive KO mice and 2 PKM immunizations, the first of which was given 14 days (○), or 3 months (⋄) after adoptive transfer. Data are from 2 representative mice in each group of 5 mice. (B) Anti-Gal production in recipients of 5 × 106 B lymphocytes expressing cell-surface IgG from PKM-immunized mice and of 5 × 106 T cells from naive KO mice (○) or 5 × 106 primed T cells from KO mice immunized 3 times with PKM (•). Mice were immunized twice with PKM, starting on day 14 after adoptive transfer. Note that memory anti-Gal B cells expressing IgG can be activated by PKM in the presence of either naive or primed T cells.
It should be stressed that anti-Gal response could be observed in recipients of memory anti-Gal B cells after one PKM immunization as well. However, anti-Gal response was 2- to 8-fold lower than that observed after 2 PKM immunizations. We chose to study tolerance induction after 2 PKM immunizations in order to demonstrate prevention of anti-Gal response in mice that are exposed for prolonged periods to xenoantigens. Repeated immunization with 50 mg PKM simulated the continuous presence of a xenograft with a size comparable to that of a mouse kidney, and which expresses α-gal epitopes.
Many of the memory anti-Gal B cells were characterized by expression of surface IgG following the isotype switch associated with the B-cell activation. IgG-expressing B cells were isolated from spleen lymphocytes of PKM-immunized mice by biodegradable magnetic microbeads26 that were coated with antimouse IgG. These cells were transferred into irradiated KO recipients (5 × 106 per mouse) together with 5 × 106 T cells isolated from naive KO mice by biodegradable magnetic microbeads coated with anti-CD3. T cells were needed because anti-Gal response is a T-dependent process, although T cells do not interact directly with the α-gal epitope.16,27 When immunized twice with PKM, starting 2 weeks after transfer, these recipients readily produced anti-Gal IgG (Figure 2B), implying that many of the transferred memory anti-Gal B cells express cell-surface IgG. Interestingly, when primed T cells from PKM-immunized mice were used instead of naive T cells, anti-Gal production by the transferred IgG-expressing memory anti-Gal B cells was only slightly higher than that observed in the presence of naive T cells (Figure 2B).
Tolerance induction on memory anti-Gal B cells by WT lymphocytes
The ability of α-gal epitopes on WT lymphocytes to tolerize memory anti-Gal B cells was studied by adoptive transfer of 20 × 106, 2 × 106, or 0.2 × 106 WT lymphocytes together with 20 × 106 lymphocytes from PKM-immunized mice. The mice were immunized with PKM on days 14 and 21 after adoptive transfer and anti-Gal IgG production was evaluated on day 28. When 20 × 106 or 2 × 106 WT lymphocytes were administered into the KO recipient of memory anti-Gal B cells, anti-Gal activity was marginal even at a serum dilution of 1:50 (Figure 3A). Comparison of anti-Gal response in presence and absence of WT lymphocytes could be performed by comparing the binding curves in ELISA. Although all mice received memory anti-Gal B cells from the same pool, production of anti-Gal was more than 50-fold higher in the absence of WT lymphocytes than in their presence. As shown in Figure 3A, the optical density (OD) observed at the lowest serum dilution of 1:50 in the tolerized mice was similar to that observed at the serum dilution of 1:3200 in mice that did not receive WT lymphocytes. Administration of 0.2 × 106 WT lymphocytes did not affect the anti-Gal response (not shown). These findings suggest that administration of as few as 2 × 106 WT lymphocytes results in effective tolerization of the memory anti-Gal B cells transferred from PKM-immunized mice.
Induction of tolerance on memory anti-Gal B cells by WT lymphocytes. (A) Anti-Gal response in KO recipients of 20 × 106 lymphocytes that include memory anti-Gal B cells from PKM-immunized mice and 20 × 106 syngeneic WT lymphocytes (▵); 2 × 106 syngeneic WT lymphocytes (○); or no WT lymphocytes (•). The mice were immunized with PKM on days 14 and 21 after adoptive transfer. Each data point shows the mean ± SE (n = 10). Statistical analysis by Student t test indicated significant differences (P < .0001) between the • group and the other 2 groups at all serum dilutions. (B) Repetition of the tolerance induction study of panel A with tolerizing WT lymphocytes depleted of stroma cells and macrophages (ie, adherent cells). Data are from 4 mice in each group receiving 20 × 106 lymphocytes from PKM-immunized KO mice and 20 × 106 syngeneic WT lymphocytes depleted of adherent cells (○) or no WT lymphocytes (•). (C) Repetition of the tolerance induction study of panel A in KO recipients with intact immune systems. Data are from 4 nonirradiated KO recipients in each group receiving 20 × 106 lymphocytes from PKM-immunized KO mice and 20 × 106 syngeneic WT lymphocytes (○) or no WT lymphocytes (•).
Induction of tolerance on memory anti-Gal B cells by WT lymphocytes. (A) Anti-Gal response in KO recipients of 20 × 106 lymphocytes that include memory anti-Gal B cells from PKM-immunized mice and 20 × 106 syngeneic WT lymphocytes (▵); 2 × 106 syngeneic WT lymphocytes (○); or no WT lymphocytes (•). The mice were immunized with PKM on days 14 and 21 after adoptive transfer. Each data point shows the mean ± SE (n = 10). Statistical analysis by Student t test indicated significant differences (P < .0001) between the • group and the other 2 groups at all serum dilutions. (B) Repetition of the tolerance induction study of panel A with tolerizing WT lymphocytes depleted of stroma cells and macrophages (ie, adherent cells). Data are from 4 mice in each group receiving 20 × 106 lymphocytes from PKM-immunized KO mice and 20 × 106 syngeneic WT lymphocytes depleted of adherent cells (○) or no WT lymphocytes (•). (C) Repetition of the tolerance induction study of panel A in KO recipients with intact immune systems. Data are from 4 nonirradiated KO recipients in each group receiving 20 × 106 lymphocytes from PKM-immunized KO mice and 20 × 106 syngeneic WT lymphocytes (○) or no WT lymphocytes (•).
The observed effective tolerance raised the question of whether it was induced by a specialized group of WT spleen cells, such as stroma cells, or whether it could be induced by purified WT lymphocytes. To address this question, tolerance induction was repeated with WT spleen lymphocytes that were depleted of adherent cells (ie, stroma cells and macrophages) by incubation for 90 minutes in tissue culture flasks. The proportion of lymphocytes among the nonadherent cells was found to be more than 98%, as determined by staining. Administration of these WT lymphocytes depleted of adherent cells, together with memory anti-Gal B cells, resulted in tolerization of the memory anti-Gal B cells as well as by the original WT spleen cells (Figure 3B). We have observed a similar tolerizing effect by transplantation of WT hearts into recipients of memory anti-Gal B cells (M.M.M. et al, submitted, February 2003). Taken together, these studies suggest that various syngeneic cells expressing α-gal epitopes may induce similar tolerance on anti-Gal B cells.
The tolerance induction described in Figure 3A-B was studied in lethally irradiated KO recipients. It was of interest to determine whether a similar tolerance could be achieved in recipients of memory anti-Gal B cells in which the lymphoid system was intact, that is, in recipients that were not irradiated. The tolerance-inducing study described in Figure 3A was repeated in naive KO mice (nonirradiated) receiving 20 × 106 lymphocytes, including memory anti-Gal B cells, from PKM-immunized mice and 20 × 106 WT lymphocytes. Anti-Gal production in the control mice that lacked WT lymphocytes was significantly lower than that observed in irradiated recipients (Figure 3C), possibly because many of the administered memory anti-Gal B cells failed to reach the sites of antibody production (eg, germinal centers) in secondary lymphoid organs. This is because such sites are occupied by the original lymphocytes of the recipient. Nevertheless, mice also receiving WT lymphocytes displayed tolerance, as they failed to produce anti-Gal in significant titers following 2 PKM immunizations (Figure 3C). These data imply that tolerance induction by WT lymphocytes can also be observed in recipients of memory anti-Gal B cells in which the immune system is intact.
Tolerance induction by WT lymphocytes was further supported by studies on transplantation of WT hearts. Five of the mice tolerized by 20 × 106 WT lymphocytes and immunized twice with PKM (Figure 3A) received transplants of WT hearts on day 28 after adoptive transfer. These WT hearts were not rejected and continued to function for at least 2 months. In contrast, all 5 control recipients that did not receive WT lymphocytes and that produced anti-Gal (Figure 3A) rejected the transplanted WT hearts within 1 hour, as in our previous study.22 These data support the observation on the lack of anti-Gal in the tolerized mice. Production of this antibody, even in low amounts, would have resulted in its binding to α-gal epitopes on the endothelial cells of the transplanted WT hearts and rejection of these grafts within days or a few weeks.
It should be stressed that mice receiving 20 × 106 WT lymphocytes and 20 × 106 KO lymphocytes separately, with a one-hour interval, displayed complete tolerance, as did mice receiving the 2 populations together (not shown). This implies that the lack of anti-Gal response is not associated with any unknown effect on memory anti-Gal B cells occurring during the brief in vitro mixing of WT and KO lymphocytes in the course of adoptive transfer into the irradiated KO recipients.
Kinetics of tolerance induction by WT lymphocytes
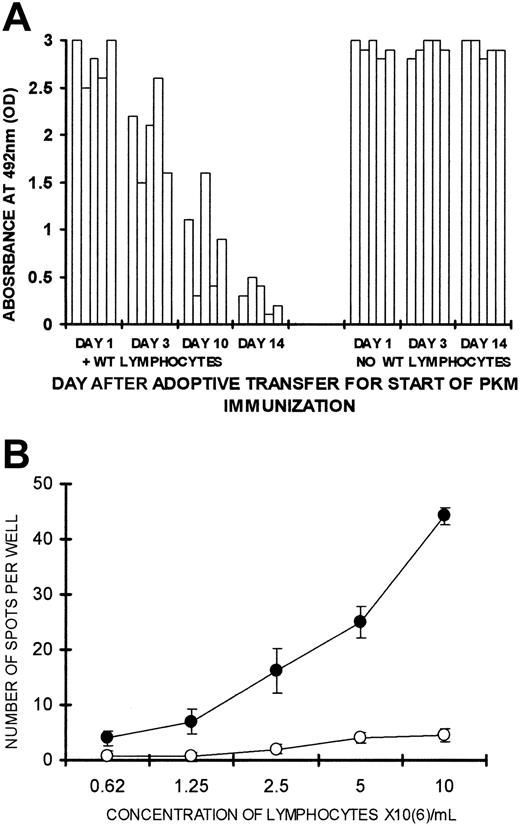
Tolerance induction by WT lymphocytes was found to be a time-dependent process. Lethally irradiated KO recipients of lymphocytes from PKM-immunized KO mice and 2 × 106 WT lymphocytes produced anti-Gal effectively when they were immunized twice with PKM, starting on day 1 or day 3 after adoptive transfer (Figure 4A). Three of 5 mice in which PKM immunization started on day 10 after adoptive transfer produced intermediate amounts of anti-Gal, and the remaining 2 mice failed to produce the antibody (< 0.5 OD). In contrast, all mice receiving the same lymphocytes and immunized with PKM starting on day 14 after adoptive transfer were tolerized and failed to display significant anti-Gal activity (< 0.5 OD). In the absence of WT lymphocytes, the mice displayed a similar effective anti-Gal IgG response if they were immunized by PKM starting 1, 3, or 14 days after adoptive transfer (Figure 4A). These findings further imply that the absence of anti-Gal production observed when PKM immunization starts on day 14 after adoptive transfer is not because of in situ adsorption of anti-Gal on α-gal epitopes of WT lymphocytes. Such adsorption, if it occurred, would also have prevented anti-Gal detection in mice immunized with PKM starting 1 or 3 days after adoptive transfer.
Kinetics of tolerance induction and ELISPOT in tolerized mice.(A) Production of anti-Gal in KO recipients of 20 × 106 lymphocytes that include memory anti-Gal B cells and 2 × 106 WT lymphocytes or no WT lymphocytes. The mice received the first of 2 PKM immunizations on days 1, 3, 10, or 14 after adoptive transfer. Anti-Gal was measured in serum samples diluted 1:100 in 5 mice in each group. (B) Analysis of lymphocytes secreting anti-Gal in tolerized or control KO mice, as measured by ELISPOT. Lymphocytes were obtained from KO recipients of 20 × 106 lymphocytes from PKM-immunized mice and 2 × 106 WT lymphocytes (○) or no WT lymphocytes (•). The mice were immunized with PKM on days 14 and 21 after adoptive transfer. ELISPOT assayed on day 28. Data shown are means ± SE (n = 5). Statistical analysis by Student t test indicated significant differences (P < .05) between the 2 groups at all lymphocyte concentrations.
Kinetics of tolerance induction and ELISPOT in tolerized mice.(A) Production of anti-Gal in KO recipients of 20 × 106 lymphocytes that include memory anti-Gal B cells and 2 × 106 WT lymphocytes or no WT lymphocytes. The mice received the first of 2 PKM immunizations on days 1, 3, 10, or 14 after adoptive transfer. Anti-Gal was measured in serum samples diluted 1:100 in 5 mice in each group. (B) Analysis of lymphocytes secreting anti-Gal in tolerized or control KO mice, as measured by ELISPOT. Lymphocytes were obtained from KO recipients of 20 × 106 lymphocytes from PKM-immunized mice and 2 × 106 WT lymphocytes (○) or no WT lymphocytes (•). The mice were immunized with PKM on days 14 and 21 after adoptive transfer. ELISPOT assayed on day 28. Data shown are means ± SE (n = 5). Statistical analysis by Student t test indicated significant differences (P < .05) between the 2 groups at all lymphocyte concentrations.
The absence of cells producing anti-Gal in the tolerized mice was further supported by ELISPOT assays for B cells secreting anti-Gal. Anti-Gal–secreting cells were readily detected as multiple spots in control PKM-immunized KO recipients (Figure 4B). However, almost no anti-Gal secreting cells were found in tolerized KO mice immunized with PKM (Figure 4B). These findings strongly suggest that tolerized KO mice that are repeatedly immunized with PKM lack anti-Gal–secreting cells, whereas such cells readily appear in control recipients following the repeated exposure of memory anti-Gal B cells to α-gal epitopes on the immunizing PKM.
WT lymphocytes in tolerized KO mice
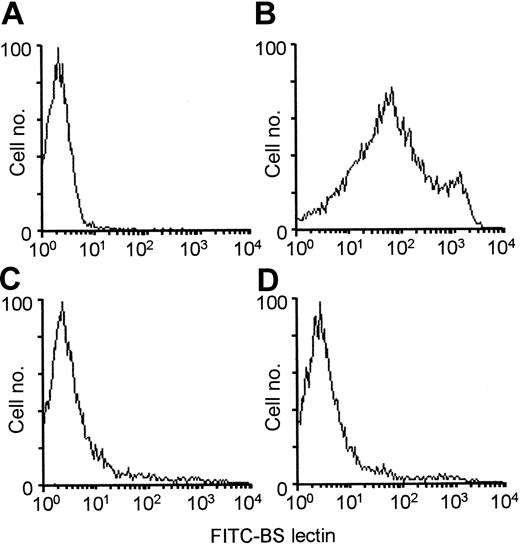
It could be argued that the observed lack of anti-Gal response in tolerized KO mice may be the result of preferential proliferation of WT lymphocytes following PKM immunizations. The proportion of WT lymphocytes in the spleens of these mice was determined by staining with BS lectin, which binds specifically to α-gal epitopes on cells.2,3 KO lymphocytes are not stained by BS lectin (Figure 5A), whereas this lectin does stain WT lymphocytes, as they all express α-gal epitopes (Figure 5B). In all KO recipients tolerized by WT lymphocytes and immunized twice with PKM, WT lymphocytes displayed fewer than 5% of spleen lymphocytes (Figure 5C-D). A similar low proportion of WT lymphocytes was observed in the mesenteric lymph nodes (not shown). Since each of the spleens in these mice contained approximately 1 × 108 lymphocytes, the number of WT lymphocytes did not exceed 5 × 106 per spleen. As the total number of lymphocytes in the peripheral lymphoid organs is less than 4 × 108, the number of WT lymphocytes within the tolerized mice is no more than 20 × 106 per mouse. As indicated in our previous study,22 in vitro adsorption of sera from control mice producing anti-Gal, on as many as 1 × 108 WT lymphocytes/mL, did not significantly affect this antibody activity. Taken together, these findings strongly suggest that the number of α-gal epitopes on WT lymphocytes in the tolerized mice is not sufficient for effective adsorption of anti-Gal.
Analysis ofBandeiraea simplicifolia IB4 (BS lectin) binding to α-gal epitopes on WT lymphocytes as measured by flow cytometry. BS lectin (10 μg/mL) binding to spleen lymphocytes from (A) KO mouse, (B) WT mouse, (C-D) 2 irradiated KO mice receiving 20 × 106 KO lymphocytes from PKM-immunized mice, 20 × 106 KO bone marrow cells, and 20 × 106 syngeneic WT lymphocytes; these 2 mice were immunized with PKM on days 14 and 21 and flow cytometry analysis was performed on day 28. Panels A and B show data from 1 representative mouse in each group of 5 mice; panels C and D show data from 2 representative mice in a group of 5.
Analysis ofBandeiraea simplicifolia IB4 (BS lectin) binding to α-gal epitopes on WT lymphocytes as measured by flow cytometry. BS lectin (10 μg/mL) binding to spleen lymphocytes from (A) KO mouse, (B) WT mouse, (C-D) 2 irradiated KO mice receiving 20 × 106 KO lymphocytes from PKM-immunized mice, 20 × 106 KO bone marrow cells, and 20 × 106 syngeneic WT lymphocytes; these 2 mice were immunized with PKM on days 14 and 21 and flow cytometry analysis was performed on day 28. Panels A and B show data from 1 representative mouse in each group of 5 mice; panels C and D show data from 2 representative mice in a group of 5.
Tolerance induction by WT lymphocytes is specific to memory anti-Gal B cells
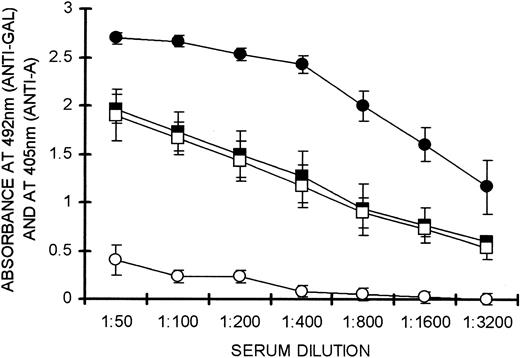
The specificity of tolerance induced by WT lymphocytes was demonstrated by analysis of anti–blood group A immune response. Blood group A differs from the α-gal epitope only in that an N-acetyl group is linked to the terminal galactosyl and a fucose is linked to the penultimate galactosyl unit. Mice were repeatedly immunized with 50 mg human blood group A red-cell membranes and 50 mg PKM. This resulted in the generation of both memory anti-A and memory anti-Gal B cells. Twenty million lymphocytes pooled from these mice, which included both types of memory B cells, were administered into irradiated KO recipients together with 20 × 106 WT lymphocytes. Two weeks after adoptive transfer, these recipients were immunized simultaneously with PKM and blood group A red-cell membranes. This immunization was repeated on day 21. Anti-Gal and anti-A production was measured on day 28 by the corresponding ELISAs. Memory anti-Gal B cells were tolerized in these mice, as indicated by the lack of anti-Gal response, whereas control mice that did not receive WT lymphocytes readily produced anti-Gal (open and solid circles, respectively, in Figure 6). In contrast, anti-A memory B cells were not affected by WT lymphocytes. This was indicated by the finding that production of anti-A IgG in mice receiving WT lymphocytes was similar to that observed in control mice that did not receive WT lymphocytes (open and solid squares, respectively, in Figure 6). These findings imply that tolerance induction by WT lymphocytes is specific to memory anti-Gal B cells and has no effect on memory B cells recognizing other structurally related carbohydrate antigens.
Specificity of tolerance induction as evaluated by production of anti–blood group A antibodies. Anti-A (□, ▪) and anti-Gal (○, •) IgG production in KO recipients of 20 × 106 KO lymphocytes that include memory anti-A and memory anti-Gal B cells and 20 × 106 WT lymphocytes (open symbols), or no WT lymphocytes (solid symbols). Anti-Gal activity was measured by absorbance at 492 nm and anti-A activity measured at 405 nm. Data shown are means ± SE (n = 5). Statistical analysis by Student t test indicated significant differences between groups • and ○ at all serum dilutions (P < .05). No significant difference was found between groups ▪ and □.
Specificity of tolerance induction as evaluated by production of anti–blood group A antibodies. Anti-A (□, ▪) and anti-Gal (○, •) IgG production in KO recipients of 20 × 106 KO lymphocytes that include memory anti-A and memory anti-Gal B cells and 20 × 106 WT lymphocytes (open symbols), or no WT lymphocytes (solid symbols). Anti-Gal activity was measured by absorbance at 492 nm and anti-A activity measured at 405 nm. Data shown are means ± SE (n = 5). Statistical analysis by Student t test indicated significant differences between groups • and ○ at all serum dilutions (P < .05). No significant difference was found between groups ▪ and □.
Analysis of anti-Gal B cells in tolerized mice by flow cytometry
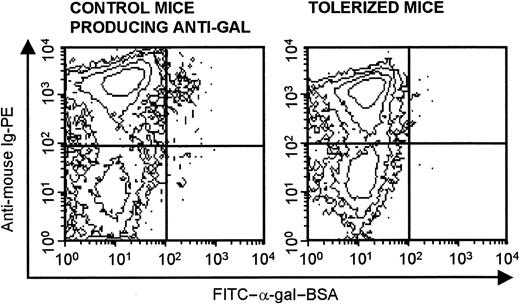
Previous studies on the mechanism of tolerance induction to a variety of protein antigens and DNA antigens were performed in mice that were transgenic for the antibody and also produced the antigen in question. These studies, in which the specific B cells constituted a large proportion of B lymphocytes, demonstrated the induction of tolerance by deletion,28,29 anergy,30 or receptor editing31,32 in the transgenic B cells. Because the B cells evaluated in the present study are physiologic memory B cells, their proportion is very small. Therefore, it is difficult to determine the mechanism of tolerance induction by flow cytometry. Specific staining of memory anti-Gal B cells by fluoresceinated α-gal BSA (ie, the cognate antigen) demonstrated labeling of approximately 1% of the B lymphocytes in PKM-immunized control KO recipients (Figure 7). Similar staining performed with lymphocytes from tolerized mice that were also PKM immunized revealed no cells binding α-gal BSA (Figure 7). This suggests that anti-Gal B cells were deleted in the tolerized mice. It should be stressed, however, that because of the small number of stained anti-Gal cells, this analysis is not sensitive enough to determine the actual fate of memory anti-Gal B cells within the tolerized mice. An appropriate anticarbohydrate transgenic animal model will enable reliable visual follow-up of antigen-specific B cells in the presence of tolerizing lymphocytes expressing the cognate antigen. Such a model will allow for accurate identification of the mechanism involved in the tolerization of anticarbohydrate B cells.
Identification of memory anti-Gal B cells by binding of FITC–α-gal–BSA. Flow cytometry of spleen lymphocytes identified as anti-Gal B cells by double staining with FITC–α-gal–BSA and PE-antimouse Ig. Staining of lymphocytes in control mice (ie, KO mice receiving 20 × 106 lymphocytes that include memory anti-Gal B cells) is shown in left panel; staining in mice that received the same lymphocytes but that were tolerized by WT lymphocytes is shown in right panel. Both groups were immunized with PKM on days 14 and 21 and staining of lymphocytes was performed on day 28 after adoptive transfer. Note that approximately 1% of B cells in control recipients of memory anti-Gal B cells bind FITC–α-gal–BSA, whereas no such cells are detected in the tolerized mice. Data are from 1 representative mouse out of 4 per group.
Identification of memory anti-Gal B cells by binding of FITC–α-gal–BSA. Flow cytometry of spleen lymphocytes identified as anti-Gal B cells by double staining with FITC–α-gal–BSA and PE-antimouse Ig. Staining of lymphocytes in control mice (ie, KO mice receiving 20 × 106 lymphocytes that include memory anti-Gal B cells) is shown in left panel; staining in mice that received the same lymphocytes but that were tolerized by WT lymphocytes is shown in right panel. Both groups were immunized with PKM on days 14 and 21 and staining of lymphocytes was performed on day 28 after adoptive transfer. Note that approximately 1% of B cells in control recipients of memory anti-Gal B cells bind FITC–α-gal–BSA, whereas no such cells are detected in the tolerized mice. Data are from 1 representative mouse out of 4 per group.
Prevention of tolerance induction by T-cell activation
The immune response to N-(asparagine)–linked carbohydrate antigens, such as ABO blood group antigens and the α-gal epitope, differs from the immune response to protein antigens in that carbohydrate antigens seem to be incapable of activating T cells. This is because epitopes on N-linked carbohydrate chains protrude too far from the surface of antigen-presenting cells (APCs) to allow for the accessory molecules of the T-cell receptor to engage the corresponding ligands of the surface of APCs.33 Accordingly, we have previously shown that T cells cannot be directly activated by α-gal epitopes.16 Since, with the exception of the α-gal epitope, all antigens on syngeneic WT lymphocytes are identical to those on KO lymphocytes, and since the α-gal epitope cannot activate T cells,16 WT lymphocytes cannot activate KO T cells. This raised the question of whether tolerization of memory anti-Gal B cells by WT lymphocytes can occur if KO T cells are activated. Such T-cell activation could be induced by the use of WT lymphocytes from C57BL/6 x Balb/c F1 mice (ie, spleen lymphocytes expressing both the autologous H-2b antigen and the allogeneic H-2d antigen) instead of the syngeneic WT C57BL/6 lymphocytes that express only the H-2b antigen, present also in KO mice. Fully allogeneic WT lymphocytes could not be used for this purpose because of the risk of a graft-versus-host (GVH) reaction.
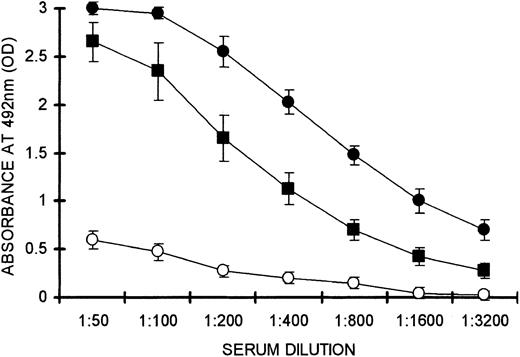
T-cell activation by an alloantigen was found to inhibit tolerance induction. KO mice received 2 × 106 WT lymphocytes on H-2bxd background together with 20 × 106 lymphocytes from PKM-immunized mice, then were immunized with PKM on days 14 and 21 after adoptive transfer. These mice were not tolerized and produced anti-Gal IgG (Figure 8). The activity of this antibody was somewhat lower than that in control mice receiving the same KO lymphocytes but no WT lymphocytes. In contrast, mice receiving the same memory anti-Gal B cells and 2 × 106 syngeneic WT C57BL/6 lymphocytes were tolerized, as indicated by the low anti-Gal activity (Figure 8). These findings strongly suggest that T cells activated by the H-2d alloantigen expressed on the semiallogeneic WT lymphocytes rescue memory anti-Gal B cells from being tolerized by α-gal epitopes on these WT lymphocytes. These rescued memory anti-Gal B cells were readily activated by α-gal epitopes on the immunizing PKM.
T-cell activation by an alloantigen prevents tolerance induction.Anti-Gal response to PKM immunization in KO mice after adoptive transfer of 20 × 106 KO lymphocytes that included memory anti-Gal B cells and 2 × 106 WT lymphocytes from semiallogeneic F1 C57BL/6 x BALB/c (ie, H-2bxd) mice (▪), 2 × 106 WT lymphocyte from syngeneic C57BL/6 (ie H-2b) mice (○), or no WT lymphocytes (•). Note that the immune response to the alloantigen prevents tolerance induction by WT lymphocytes. Data shown are means ± SE (n = 4). Statistical analysis by Student t test indicated significant differences (P < .001) between groups (•) and (○) at all serum dilutions. Significant differences (P < .05) were found between groups (•) and (▪) at serum dilutions of 1:400 to 1:1600, but not at serum dilutions of 1:50 to 1:200.
T-cell activation by an alloantigen prevents tolerance induction.Anti-Gal response to PKM immunization in KO mice after adoptive transfer of 20 × 106 KO lymphocytes that included memory anti-Gal B cells and 2 × 106 WT lymphocytes from semiallogeneic F1 C57BL/6 x BALB/c (ie, H-2bxd) mice (▪), 2 × 106 WT lymphocyte from syngeneic C57BL/6 (ie H-2b) mice (○), or no WT lymphocytes (•). Note that the immune response to the alloantigen prevents tolerance induction by WT lymphocytes. Data shown are means ± SE (n = 4). Statistical analysis by Student t test indicated significant differences (P < .001) between groups (•) and (○) at all serum dilutions. Significant differences (P < .05) were found between groups (•) and (▪) at serum dilutions of 1:400 to 1:1600, but not at serum dilutions of 1:50 to 1:200.
Discussion
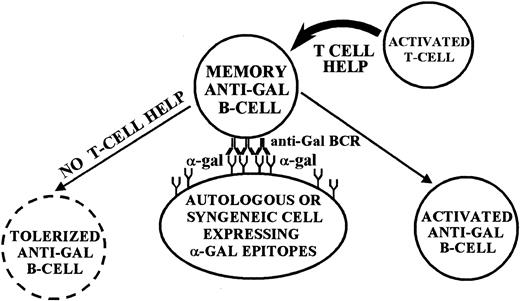
The present study demonstrates in vivo tolerization of memory anti-Gal B cells by syngeneic WT lymphocytes expressing the α-gal epitope. In a previous study we demonstrated a similar tolerance induction on naive anti-Gal B cells.22 Taken together, these 2 studies suggest that both naive and memory anticarbohydrate B cells can be tolerized when their B-cell receptors (BCRs) engage the cognate carbohydrate antigen on syngeneic lymphocytes (Figure 9). This phenomenon of tolerance induction by syngeneic lymphocytes expressing the tolerizing carbohydrate antigen is novel. Previous studies in KO mice demonstrated tolerance induction on naive anti-Gal B cells by chimerism with BM cells expressing α-gal epitopes.17-20 The present study and our previous report22 are the first to indicate that such tolerance induction is not an exclusive characteristic of BM cells, as it can also be induced by lymphocytes expressing the cognate carbohydrate antigen. Moreover, the tolerance can be induced on both naive and memory anti-Gal B cells. This tolerance is highly specific, as demonstrated by the effective activation of memory B cells with anti-Aspecificity in mice tolerized for memory anti-Gal B cells (Figure 6).
Proposed model for tolerance induction on memory anti-Gal B cells by α-gal epitopes. Anti-Gal BCR molecules engaging α-gal epitopes on autologous or syngeneic cells deliver a tolerizing signal to the anti-Gal B cell in the complete absence of T-cell help. If, however, helper T cells are activated, their help rescues the anti-Gal B cell from being tolerized and induces activation of the B cell.
Proposed model for tolerance induction on memory anti-Gal B cells by α-gal epitopes. Anti-Gal BCR molecules engaging α-gal epitopes on autologous or syngeneic cells deliver a tolerizing signal to the anti-Gal B cell in the complete absence of T-cell help. If, however, helper T cells are activated, their help rescues the anti-Gal B cell from being tolerized and induces activation of the B cell.
The mechanism by which naive and memory anti-Gal B cells are tolerized is not clear at present. Staining with the cognate antigen suggested the deletion of the tolerized cells. However, a reliable identification of the tolerizing mechanism will require the use of an experimental animal model that is transgenic for the anticarbohydrate antibody. In such a model, the anticarbohydrate B cells will constitute a large proportion of the total B cell population. Deletion, anergy, or receptor editing in the tolerized B-cell population will be possible to track in anticarbohydrate transgenic mice by the use of a genetic marker or by the binding of the cognate antigen and analysis by flow cytometry.
The inability to induce tolerance in mice in which T cells are activated by alloantigen (Figure 8) strongly suggests that anticarbohydrate B cells engaging the cognate antigen on lymphocytes may escape tolerance induction if T-cell help is provided. Our previous studies indicated that in order for anti-Gal B cells to be activated, these cells require T-cell help.16 In the case of PKM immunization, this help is provided by T cells activated by the many processed and presented immunogenic xenopeptides originating in the immunizing pig membranes.16 The present study suggests that T-cell help is also important in rescuing anti-Gal B cells from being tolerized (Figure 9). It may be that the presented tolerance reflects a more general phenomenon associated with the previously described antigen-specific B-cell suppression by multihaptenated polymers.34 In that study Dintzis and Dintzis reported that multihaptenated FITC-dextran down-regulates anti-FITC production in mice immunized with FITC-ovalbumin.34 It remains to be determined whether dextran-bearing multiple α-gal epitopes can induce a similar tolerizing effect in the presence of xenogeneic membranes expressing α-gal epitopes and whether the immune complexes between the natural anti-Gal and the multihaptenated polymer have no detrimental effects on recipients of such polymers.
Tolerance induction on memory anti-Gal B cells was found to be a time-dependent process. At least 10 days were required to induce tolerance by WT lymphocytes within the KO recipients (Figure 4A). As suggested in the model presented in Figure 9, tolerance induction requires memory anti-Gal B cells and WT lymphocytes to find each other in order to establish physical contact between BCRs on the B cell and α-gal epitopes on the WT lymphocyte. In the absence of T-cell help, this interaction elicits a tolerizing signal to the B cell. Prevention of tolerance induction by activated helper T cells is likely to be the reason for the lack of tolerance in KO recipients that were immunized by PKM on days 1 or 3 after adoptive transfer (Figure 4A). Immunization with PKM prior to the tolerization of memory anti-Gal B cells results in the activation of a large number of helper T cells by the numerous immunogenic pig xenopeptides.16 As in the case of lack of tolerance with semiallogeneic WT lymphocytes (Figure 8), it is possible that these activated T cells rescue memory anti-Gal B cells, which otherwise would later be tolerized by α-gal epitopes on WT lymphocytes.
Our observations on tolerization of anticarbohydrate B cells may be of clinical significance, since they suggest a novel approach for inducing a similar tolerance to ABO-incompatible blood group antigens on allografts or to α-gal epitopes on xenografts. One could hypothesize that autologous human blood lymphocytes could be manipulated in vitro to express these carbohydrate antigens. This might be achieved by in vitro transduction of lymphocytes with an adenovirus vector containing the corresponding glycosyltransferase gene. Subsequent administration of the transduced lymphocytes back into the individual that was depleted of natural anticarbohydrate antibodies might result in tolerization of B cells with specificity for the carbohydrate antigen expressed on the transduced lymphocytes. Depletion of anti–blood group antibodies in humans or of anti-Gal in monkeys was shown to be feasible with affinity columns expressing the corresponding carbohydrate epitope.35,36 In addition, we have recently shown that expression of α-gal epitopes on human cells can be achieved by transduction with a proliferation-defective adenovirus vector containing the α1,3GT gene.37 Tolerance induction by KO lymphocytes expressing α-gal epitopes, after being transduced in vitro with this adenovirus vector, is currently under study.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-11-3515.
Supported by NIH grant AI45849 and by a grant from the American Heart Association.
M.M.M. and H.O. contributed equally to this study.
This study is a continuation of a previous report.22
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal