Abstract
This study investigates whether genetically modified orally administered Lactococcus lactis (L lactis) could be used as an HIV vaccine. L lactis is immunogenic and extremely safe when delivered orally. We created a recombinant L lactis vector expressing the envelope protein of HIV on its cell surface. Oral immunization with this vector induced high levels of HIV-specific serum IgG and fecal IgA antibodies. Cell-mediated immune responses also were generated in both the regional lymph nodes and the spleen. Dendritic cells are readily infected by L lactis and appear to play a potential role in mediating the development of these immune responses. The protective efficacy of this vaccine strategy was demonstrated by challenging mice intraperitoneally with an HIV Env–expressing vaccinia virus. Their viral loads were 350-fold lower than those of control mice. These findings support the further development of L lactis–based HIV vaccines. (Blood. 2003; 102:223-228)
Introduction
A safe, effective, and inexpensive vaccine capable of inducing both mucosal and systemic immunity may be required to limit the spread of HIV. This study examines whether Lactococcus lactis (L lactis) engineered to express the Env gene of HIV might serve as such a vaccine.
In the past decade, multiple strategies to produce an immunogenic HIV vaccine have been explored. This included production of HIV subunit peptide vaccines,1 DNA vaccines,2 recombinant virus-vector vaccines (including modified vaccinia virus,3 adenovirus,4 rabies virus,5 flavivirus,6 friend murine leukemia virus,7 Venezuelan equine encephalitis virus,8 and adeno-associated virus9,10 ), and bacterial vector-vaccines (Bacille Calmette-Guerin11 ). Each of these strategies showed some promise in animal models, either alone or in combination. Yet there is still a great need for a safe and highly effective HIV vaccine.
In this context, we became interested in using L lactis as a vaccine vector, based on its extraordinary safety profile. L lactis is a noninvasive, nonpathogenic, gram-positive bacterium and has a long history of use in the production of fermented milk products. L lactis lacks the ability to multiply in vivo, except in gnotobiotic mice.12 When live L lactis were fed to animals and human volunteers, they passed rapidly through the gastrointestinal tract without colonization.12,13 Yet genetically modified L lactis have been effective in delivering antigen to the mucosal immune system and inducing a local immune response.14
L lactis have been engineered to express several bacterial and viral antigens.15,16 Mice fed these vectors generate antigen-specific immune responses.14 However, the use of L lactis to present HIV antigens never has been attempted. In this study, L lactis was modified to express the V2-V4 loop of HIV Env. Surface expression of Env was achieved by fusing the secreting signal and cell wall anchor to the N- and C-terminal ends of the V2-V4 loop. Mice orally immunized with this recombinant vector mounted an effective immune response against an HIV Env–expressing vaccinia virus.
Materials and methods
Plasmid constructs
A 900-bp fragment containing the V2-V4 loop from HIV-1IIIB was amplified by polymerase chain reaction (PCR) using the following primer pairs, with EcoRI or SalI sites underlined (5′-AGAATTCGTAACTCAGTCATTAC-3′, 5′-AGTCGACTCTTTTTTCTCTCTGCACCAC-3′). The resulting EcoRI/SalI fragment was fused to the surface display vector pSGANC332 under transcriptional control of an Em promoter (the vector was kindly supplied by Yakult, Tokyo, Japan) (Figure 1A). In brief, pSGANC33217 has a signal sequence of staphylokinase and a cell-wall anchor sequence of the C-terminal 332 amino acid residues of cell-wall–associated 763 proteinase of L lactis NCDO763. The anchor sequence contains sorting signals such as an LPXTG motif, hydrophobic domain, and a positively charged tail, which are commonly observed in gram-positive bacterial cell-wall–associated proteins. L lactis (IL1403 strain) was cultured in M17 broth medium (Difco Laboratories, Detroit, MI) containing 0.5% glucose (GM17) at 32°C overnight. The shuttle vector was transformed into IL1403 by electroporation using a Gene Pulser (BioRad, Hercules, CA) at 25 μF, 2000 V, and 200 Ω with 0.1-cm electrode cuvette (Bio-Rad) to generate an HIV Env–expressing IL1403 (IL1403-pHIV). As a negative control, IL1403 was transformed with an empty shuttle vector to generate IL1403-pEmpty.
Shuttle vector construct and expression of IL1403-pHIV. A 900-bp fragment containing HIV env V2-V4 loop was fused with the surface-secreting signal and the anchor gene at the N- and C-terminus, respectively, to produce the shuttle vector (A); the HIV protein expression of the IL1403-pHIV was confirmed by Western blot analysis (B); and the expression on the surface of IL1403 was examined with flow cytometry analysis after surface staining with FITC-anti-Env Ab (C). The IL1403-pcyHIV–expressing HIV protein in cytoplasma was used as a negative control to rule out the penetration of the Ab. pAMb1 represents the replication region and the erythromycin resistance gene of pAMβ1 derived from Enterococcus faecalis. The replication region functions in L lactis, and the erythromycin resistance gene functions both in L lactis and in E coli. pUC19 represents the replication region of pUC19 functioning in E coli.
Shuttle vector construct and expression of IL1403-pHIV. A 900-bp fragment containing HIV env V2-V4 loop was fused with the surface-secreting signal and the anchor gene at the N- and C-terminus, respectively, to produce the shuttle vector (A); the HIV protein expression of the IL1403-pHIV was confirmed by Western blot analysis (B); and the expression on the surface of IL1403 was examined with flow cytometry analysis after surface staining with FITC-anti-Env Ab (C). The IL1403-pcyHIV–expressing HIV protein in cytoplasma was used as a negative control to rule out the penetration of the Ab. pAMb1 represents the replication region and the erythromycin resistance gene of pAMβ1 derived from Enterococcus faecalis. The replication region functions in L lactis, and the erythromycin resistance gene functions both in L lactis and in E coli. pUC19 represents the replication region of pUC19 functioning in E coli.
HIV protein expression of IL1403-pHIV
To confirm the expression of the HIV Env insert, IL1403-pHIV and IL1403-pEmpty were cultured in GM17 medium supplemented at 32°C overnight. The culture was centrifuged at 5000 × g for 10 minutes at 4°C. Pellets were washed twice with wash buffer (0.5 M sucrose, 10% glycerol in phosphate-buffered saline [PBS]), and bacteria were suspended in equal volumes of 2 × sodium dodecyl sulfate (SDS) buffer (125 mM Tris[tris(hydroxymethyl)aminomethane]-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.01% bromophenol blue, and 10% β-mercaptoethanol). After boiling for 10 minutes, the cell lysates were electrophoresed on a 4%-12% gradient polyacrylamide gel and then transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, England). Protein was detected using polyclonal human anti-HIV antibody followed by affinity-purified horseradish peroxidase (HRP)–conjugated anti-human IgG (Jackson ImmunoResearch Lab, West Grove, PA). The membrane was radiographed on X-film using the ECL Western Blotting Detection System (Amersham Pharmacia Biotech).
To investigate whether HIV Env was expressed on the surface of IL1403, IL1403-pHIV was centrifuged at 5000 × g for 10 minutes at 4°C. The pellet was washed twice and the bacteria were then incubated with polyclonal human anti-HIV antibody at 4°C for 30 minutes followed by fluorescein isothiocyanate (FITC)–conjugated anti–human IgG. Binding of Ab to the surface of the bacteria was assessed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ). The IL1403-pcyHIV (expression of HIV Env in cytoplasma) was used as a control.
Animals and animal immunizations
Eight-week-old BALB/c female mice were purchased from Japan SLC, Shizuoka, Japan. The mice were housed in the Animal Center of Yokohama City University, where they were kept on a 12-hour day–night cycle. After overnight fasting, mice were immunized orally with 100 μL 108 CFU (colony-formation units) of IL1403-pHIV or IL1403-pEmpty plus 5 μg cholera toxin (Sigma, St Louis, MO) using a 21-gauge feeding tube (Poper & Sonslne, New Hyde Park, NY). Immunizations were given at week 0, 2, 4, 6, and 8.
Sample collection and ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed as described elsewhere.10 Samples of immune sera (collected by retro-orbital puncture) and feces were collected 2 weeks after final immunization. Sera were stored at -20°C until use. Fecal pellets (100 mg) were suspended in 0.5 mL PBS. After centrifugation at 15 000 × g for 5 minutes, the supernatants were collected and tested for Ig by ELISA. Briefly, 96-well microtiter plates were coated with 10 μg/mL HIV V3 region peptides (NNTRKRQRGPGRAFVTIGKIGN-multiantigen peptide, HIV V3-MAP peptide) overnight at 4°C. The wells were blocked with PBS-1% bovine serum albumin (BSA) and incubated for 2 hours at room temperature. Serially diluted serum or fecal suspension (100 μL) was added for 1 hour at 37°C. Bound Ab was detected using HRP-conjugated anti–mouse IgG (Sigma) or HRP-conjugated goat anti–mouse IgA (Zymed Laboratories, S. San Francisco, CA). The mean Ab titer was expressed as the reciprocal of the serial serum dilution that reached the cutoff value plus 2 SD.
IFN-γ ELISpot assay
IFN-γ–producing cells were quantified one week after the final immunization using an ELISpot kit for mouse IFN-γ as recommended by the manufacturer (Cat. 3321-2A-2, MabTech, Nacka, Sweden). Briefly, a MultiScreen-IP plate (Millipore, Bedford, MA) was treated with 70% ethanol and washed with PBS. The plate was coated with 10 μg/mL anti–mouse IFN-γ mAb (AN18) in PBS overnight at 4°C. The plate was washed and then blocked with RPMI 1640 plus 10% fetal calf serum (FCS) for 2 hours at room temperature. Lymphocytes (1-10 × 105) isolated from spleen or intestinal lymph nodes were added to each well. The lymphocytes were stimulated with 10 μg/mL of the HIV V3 peptide (NNTRKRIQRGPGRAFVTIGKIGN) for 24 hours at 37°C. Control wells contained unstimulated cells. After incubation, the plates were washed, incubated with 1 μg/mL biotinylated anti–mouse IFN-γ antibody (R4-6A2), streptavidin-alkaline phosphatase, and then 50 μL/well BCIP/NBT phosphatase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The numbers of spots were automatically determined by a computer-assisted video image analysis (KS Elispot, Carl Zeiss, Tokyo, Japan). All assays were performed in triplicate.
Intracellular cytokine staining (ICCS) assay
CD8+ T cells containing interferon (IFN)–γ were detected by the protocol recommended by the manufacturer's instructions (Cytofix/CytoPerm Plus kit, PharMingen, San Diego, CA). Briefly, lymphocytes were isolated from mouse spleen or intestinal lymph nodes one week after the final immunization. A single cell suspension was incubated with the HIV V3 peptide (10 μg/mL) for 24 hours at 37°C, and 1 μg/mL GolgiPlug was added 2 hours before the end of incubation. The cells were washed with staining buffer (3% FCS, 0.1% NaN3 in PBS), blocked with 4% normal mouse sera, and stained with phycoerythrin (PE)–conjugated anti–mouse CD8 antibody (Ly-2, PharMingen). Then the cells were suspended in 250 μL Cytofix/Cytoperm solution at 4°C for 20 minutes, washed with Perm/Wash solution, stained with FITC-conjugated anti–mouse IFN-γ antibody (PharMingen) at 4°C for 30 minutes, followed by flow cytometric analysis.
Tetramer assay
Tetramer assays were conducted one week after the final immunization. The H-2Dd/p18 tetramer (RGPGRAFVTI)18 conjugated to PE was prepared by the AIDS Research and Reference Reagent Program, National Institutes of Health (NIH), Bethesda, MD. The tetramer assay was performed as previously described.10 Briefly, isolated lymphocytes were incubated for 30 minutes at 4°C with 4% normal mouse serum in PBS. Cells were stained with anti–mouse CD8-FITC antibody (Ly-2, PharMingen) at 0.5 μg/106 cells for 30 minutes at 4°C. After being washed twice with the staining buffer (3% FCS, 0.1% NaN3 in PBS), the cells were incubated with the tetramer reagent for 15 minutes at 37°C, followed by analysis of flow cytometry (Becton Dickinson).
Recombinant vaccinia virus used for challenge study
The virus challenge experiment was performed as described previously.10,19 Recombinant vaccinia virus vPE16 expressing the HIV-1 env gene (AIDS Reagent Program, NIH, MD; Cat. No. 362) was used. One week after the last immunization, mice were challenged intraperitoneally with 108 plaque-forming units (PFUs) of vPE16 vaccinia virus. The mice were killed 6 days after challenge, and their ovaries were sonicated and assayed for vPE16 titer by serial 10-fold dilution on a plate of CV1 cells. Infected cells were detected by staining with crystal violet, and plaques were counted at each dilution.
Analysis of IL1403-pHIV–infected DCs
Dendritic cells (DCs) were isolated from BALB/c mouse bone marrow as previously described.20 Briefly, bone marrow (BM) was removed from the tibia and femur of BALB/c mice. 5 × 105 cells/mL were cultured in RPMI 1640 containing 10% FCS plus 1 ng/mL rGM-CSF (Kirin Beer, Tokyo, Japan) and rIL-4 for 6 days. CD11c+ DCs were purified using CD11c (N418) microbeads (Miltenyi Biotech, Auburn, CA) as recommended by the manufacturer's instructions. CD11c+ DCs were infected with L lactis at 37°C for 2 hours at 100 bacteria/cell. The infected cells were washed with PBS, smeared onto a slide glass, dried, and fixed with acetone for 10 minutes. After being washed with PBS, the cells were stained with anti–L lactis sera derived from L lactis–immunized mice at 37°C for 1 hour followed by staining with FITC-conjugated anti–mouse IgG (MBL, Nagoya, Japan) at 4°C for 30 minutes. The L lactis was visualized by fluorescence microscopy.
To examine whether L lactis can induce the maturation of BM-derived DCs, each CD11c+ DC was infected with 100 L lactis in RPMI 1640 containing 10% FCS and 1 ng/mL lipopolysaccharide (LPS) for 2 days at 37°C in a 5% CO2 in air incubator. The cells were then stained with FITC-conjugated major histocompatibility class II (MHC class II), CD86, or CD40 mAb (PharMingen) followed by fluorescence-activated cell-sorter scanner (FACS) analysis (Becton Dickinson). Cells incubated in 1 ng/mL LPS were used as positive controls.
To investigate whether the IL1403-pHIV–infected DCs could present HIV peptide to T cells, CD11c+ DCs were infected with IL1403-pHIV at 100 bacteria/cell at 37°C for 2 hours. Then the cells were washed, and 5 × 106 cells were transferred intravenously into recipient BALB/c mice. One week after administration, the number of HIV-specific IFN-γ–secreting splenocytes was measured by ICCS assay as described above.
Data analysis
All values are expressed as means ± standard error (SE). Statistical analysis of the experimental and control data were conducted by one-way factorial analysis of variance. Significance was defined as a P value less than .05.
Results
HIV expression by IL1403-pHIV
The IL1403-pHIV vector was engineered to express the V2-V4 loops of HIV Env on its cell surface. To evaluate whether the encoded protein was actively produced by IL1403-pHIV bacteria, cell lysates were analyzed by Western blotting. As seen in Figure 1B, a band of the size corresponding to HIV Env plus the anchor residues (57 kDa) was detected by anti-HIV Ab in lysates of IL1403-pHIV but not IL1403-pEmpty. To determine whether this HIV Env protein was expressed on the surface of the L lactis via the secreting signal and cell wall anchor, the cells were washed, stained with anti–Env Ab, and analyzed by flow cytometry. Results show that 98.3% of the IL1403-pHIV cells stained positive for Env (Figure 1C).
Oral immunization with IL1403-pHIV induces both mucosal and humoral immune responses
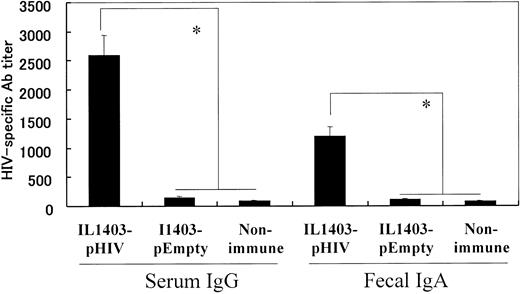
108 colony-formation units (CFUs) of IL1403-pHIV were administered orally to BALB/c mice 5 times over an 8-week period. The effect of vaccination on the production of HIV-specific serum IgG and fecal IgA antibodies was examined 2 weeks after the final dose of IL1403-pHIV. Results indicate that immunized mice produced more than 300-fold more HIV-specific serum IgG and 100-fold more fecal IgA than did control animals “vaccinated” with IL1403-pEmpty (P < .05, Figure 2).
HIV-1–specific antibody detected by ELISA. Mice were orally immunized with 108 CFUs of IL1403-pHIV or IL1403-pEmpty on weeks 0, 2, 4, 6, and 8. Ab titers were monitored 2 week after the last immunization. *Mean values significantly different between the groups.
HIV-1–specific antibody detected by ELISA. Mice were orally immunized with 108 CFUs of IL1403-pHIV or IL1403-pEmpty on weeks 0, 2, 4, 6, and 8. Ab titers were monitored 2 week after the last immunization. *Mean values significantly different between the groups.
IL1403-pHIV immunization stimulates a protective cellular immune response
Three assays (ELISpot, ICCS, and tetramer staining) were used to detect HIV-specific cell-mediated immunity. These assays were performed on cells from the spleen and peritoneal lymph nodes of mice immunized 5 times by the oral route. The number of HIV-specific IFN-γ–secreting cells in IL1403-pHIV–immunized mice was significantly greater than in controls (P < .05, Figure 3A). The absolute number of HIV-specific IFN-γ–secreting lymphocytes was even higher in the intestinal lymph nodes than spleen (456 vs 186, P < .05), suggesting that oral immunization was particularly effective at inducing mucosal immunity. Consistent with this conclusion, the number of IFN-γ–producing HIV-specific CD8+ T cells also was greater in the intestinal lymph nodes than spleen of IL1403-pHIV–immunized mice (0.26% vs 0.12%, P < .05, Figure 3B).
Antigen-specific cell-mediated immune responses. HIV-specific IFN-γ–secreting lymphocytes were detected by ELISpot assay (A) and ICCS assay (B). The number of CD8+ lymphocytes expressing T-cell receptor (TCR) that recognized the MHC class I–restricted p18 tetramer from HIV Env was examined by tetramer assay (C). The assays were performed one week after the final immunization. The figures represented one mouse of each group, and the data represent the average of 4-6 mice/group. LN indicates intestinal lymph node; * and **, mean values significantly different between the groups. Error bars indicate the standard error deviation from the mean of 4-6 mice (A). The data represent the average and SEM of 4-6 mice per group in panels B-C.
Antigen-specific cell-mediated immune responses. HIV-specific IFN-γ–secreting lymphocytes were detected by ELISpot assay (A) and ICCS assay (B). The number of CD8+ lymphocytes expressing T-cell receptor (TCR) that recognized the MHC class I–restricted p18 tetramer from HIV Env was examined by tetramer assay (C). The assays were performed one week after the final immunization. The figures represented one mouse of each group, and the data represent the average of 4-6 mice/group. LN indicates intestinal lymph node; * and **, mean values significantly different between the groups. Error bars indicate the standard error deviation from the mean of 4-6 mice (A). The data represent the average and SEM of 4-6 mice per group in panels B-C.
A tetramer binding assay was used to identify MHC class I–restricted HIV-specific T cells in immunized animals.21 Nearly 8-fold more CD8+ splenocytes from IL1403-pHIV–immunized mice bound the p18 HIV peptide when compared with control mice (0.15% vs 0.02%, P < .05, Figure 3C). An even higher percentage of lymph node cells showed this specificity (0.21% vs 0.02%, P < .05).
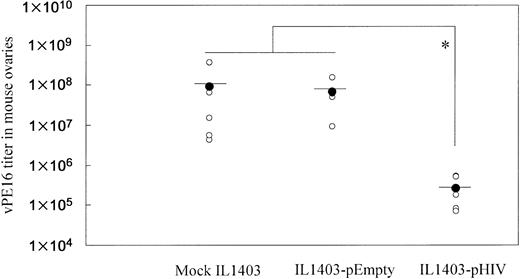
To examine whether oral administration with IL1403-pHIV induced a protective immune response, vaccinated mice were intraperitoneally challenged with vaccinia virus vPE16, which expresses the HIV env gene. As shown in Figure 4, oral administration of IL1403-pHIV vaccine reduced viral load by 350-fold (P < .05).
Resistance of immunized mice to infection by vaccinia virus (vPE16) expressing the HIV env gene. Mice (5 mice/group) were challenged intraperitoneally with 108 PFUs of vPE16 virus one week after the final immunization. Vaccinia virus titers in the mouse ovaries were measured 6 days after virus challenge. *Mean values significantly different between the groups.
Resistance of immunized mice to infection by vaccinia virus (vPE16) expressing the HIV env gene. Mice (5 mice/group) were challenged intraperitoneally with 108 PFUs of vPE16 virus one week after the final immunization. Vaccinia virus titers in the mouse ovaries were measured 6 days after virus challenge. *Mean values significantly different between the groups.
DCs exposed to IL1403-pHIV present HIV peptide to T cells
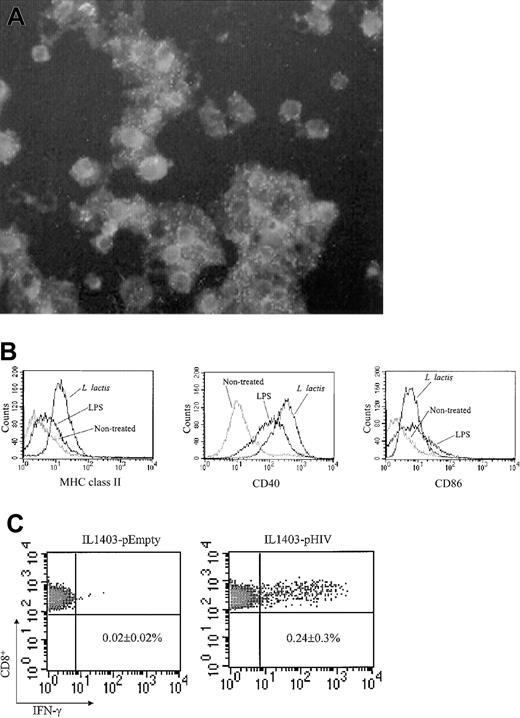
The role of DCs during IL1403-pHIV–induced immune responses was examined. DCs were purified from the bone marrow (BM) of unimmunized mice. These CD11c+ DCs were successfully infected when exposed to L lacti (Figure 5A). Infection by L lacti significantly up-regulated the expression of CD40, CD86, and MHC class II molecules by these DCs (Figure 5B). DCs then were treated with IL1403-pHIV and injected intravenously into normal mice. The frequency of HIV-specific IFN-γ–secreting splenocytes was monitored 7 days after DC administration using the ICCS assay. Recipients of IL1403-pHIV–infected DCs had 12-fold more HIV-specific IFN-γ–secreting CD8+ T cells than did recipients of DCs infected with IL1403-pEmpty (P < .05, Figure 5C). These data demonstrate that IL1403-pHIV–infected DCs can induce HIV-specific cytotoxic T lymphocyte (CTL) responses in vivo, consistent with such cells playing an important role in IL1403-pHIV vaccine-induced immune responses.
Infection of BM-DC by L lactis (A) CD11c+ DCs were infected with L lactis. The infected cells were stained with polyclonal mouse anti–L lactis Ab and FITC-conjugated anti-mouse IgG. Original magnification, × 400. (B) Maturation of BM-DCs induced by L lactis. The L lactis–infected CD11c+ DCs were stained with FITC-conjugated MHC class II, CD86, or CD40 mAb followed by analysis of flow cytometry. Lipopolysaccharide (LPS) was used as a positive control. (C) Induction of HIV-specific CTL response in vivo by transfer of IL1403-pHIV–transfected DCs. The L lactis–infected CD11c+ DCs were washed and injected intravenously into naive recipient BALB/c mice. One week later, splenocytes were isolated and stimulated with the V3 peptide for 24 hours. IFN-γ–secreting CD8+ splenocytes were detected using the ICCS assay. Similar results were obtained from 2 additional experiments. The data represent the average and SEM of 3 mice.
Infection of BM-DC by L lactis (A) CD11c+ DCs were infected with L lactis. The infected cells were stained with polyclonal mouse anti–L lactis Ab and FITC-conjugated anti-mouse IgG. Original magnification, × 400. (B) Maturation of BM-DCs induced by L lactis. The L lactis–infected CD11c+ DCs were stained with FITC-conjugated MHC class II, CD86, or CD40 mAb followed by analysis of flow cytometry. Lipopolysaccharide (LPS) was used as a positive control. (C) Induction of HIV-specific CTL response in vivo by transfer of IL1403-pHIV–transfected DCs. The L lactis–infected CD11c+ DCs were washed and injected intravenously into naive recipient BALB/c mice. One week later, splenocytes were isolated and stimulated with the V3 peptide for 24 hours. IFN-γ–secreting CD8+ splenocytes were detected using the ICCS assay. Similar results were obtained from 2 additional experiments. The data represent the average and SEM of 3 mice.
Discussion
This study demonstrates that oral administration of recombinant L lactis encoding the V2-V4 loop of the HIV env gene can induce HIV-specific mucosal and systemic immunity. This humoral and cell-mediated immunity is of sufficient magnitude to confer protective immunity against an HIV Env–expressing vaccinia virus challenge in mice.
Several bacteria have been used as vaccine vectors, including Bacillus Galmette-Guerin,11 Listeria monocytogenes,22 Salmonella,23 Shigella,24 and L lactis.14 Our vaccine showed similar levels of immunogenicity with Shigella,24 Salmonella,25 and attenuated Listeria26 vector vaccines. However, unlike other recombinant bacterial vaccines, orally administered L lactis is extremely non-pathogenic in humans, having been used in the production of fermented milk products for decades. Previous studies established that L lactis could be used as a vector to express heterologous genes.15
To explore whether an anti-HIV immune response could be induced by oral administration of recombinant L lactis, we constructed a recombinant vector in which the HIV Env protein was expressed on the surface of the bacterium (IL1403-pHIV). Mice were immunized orally every 2 weeks with this vector. After the fifth immunization, a significant increase in serum IgG and fecal IgA anti-HIV Abs was detected (Figure 2). Furthermore, vaccination induced strong HIV-specific mucosal and systemic cell-mediated immunity (Figure 3). The potential efficacy of this vaccine was established by challenging immunized mice with an Env-expressing vaccinia virus. Results show that viral load was reduced 350-fold (Figure 4). While the protective efficacy of IL1403-pHIV was a little weaker than Shigella DNA vaccine24 and optimal HIV DNA vaccine27 after intraperitoneal challenge. In this study, cholera toxin was used as an adjuvant of L lactis vaccine for oral administration. The IL1403-pHIV vaccine alone generated about 30% levels of immune responses of the L lactis vaccine with cholera toxin (data not shown). The cholera toxin not only prevents mucosal tolerance, but also elicits specific T- and B-cell–mediated immunity.28-30 However, cholera toxin induces diarrhea in healthy volunteers.31 Mutant cholera toxin molecules have been generated that retain adjuvant activity in the absence of toxicity,32 and these have provided a useful standard for the characterization of additional safe and effective mucosal immunization regiments.
We also examined the size of protein that could be expressed in L lactis. Preliminary studies showed that gene segments of 1 kb or less were effectively translated into proteins that could be stably expressed on the bacterial cell surface. The V2-V4 loop was of the appropriate length and encoded the most immunogenic epitopes from HIV Env in a native configuration.33-35 Immunogenic bacterial vectors that can be administered orally are promising candidates for use as HIV vaccines. The L lactis vector used in this report was relatively inexpensive to manufacture and well suited to large-scale administration in developing countries. Our findings indicate that oral administration of these vectors can induce a strong mucosal immune response, which may be critical for the prevention of HIV transmission.
Immature DCs are prevalent at mucosal surfaces, where they sample the environment for foreign pathogens. Contact with nonself stimulates the DCs to mature and migrate to central lymphoid organs, where they efficiently present the foreign antigen to naive T cells in the context of MHC class I and II molecules.36 The quality of the immune response initiated by DCs, particularly their ability to induce CTL, impacts host resistance to infection.37 Our study reveals that L lactis can infect immature DCs. Once infected, these cells mature, express costimulatory molecules, and effectively present the encoded HIV peptides to T cells in vivo (Figure 5). These data suggest that L lactis provides a powerful activation stimulus to DCs, resulting in up-regulation of costimulatory and MHC molecule production. These findings indicate a potential role for DCs in mediating the potent cellular immune responses to L lactis–derived antigens.
In summary, we constructed a recombinant L lactis that expressed the V2-V4 loop of HIV Env on their surface. Oral administration of this recombinant vaccine induced strong HIV-specific humoral and cell-mediated immune responses that significantly reduced viral load following challenge with an HIV-Env–expressing vaccinia virus. These findings make recombinant L lactis a promising candidate for HIV vaccine development.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2003-01-0110.
Partially supported by a Grant-in-Aid from the Ministry of Education, Science, Sports, Culture of Japan; The Ministry of Health and Welfare of Japan; and The Japan Health Sciences Foundation (K-1027, SA24713).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Ms M. Kawano, T. Takeishi, and A. Ohishi for their technical assistance and A. De La Fuente for her secretarial assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal