Abstract
In vitro studies suggest that activation of class IA phosphatidylinositol 3 (PI-3) kinase is necessary for normal erythroid cell development. However, when class IA PI-3 kinase–deficient mice were generated by a targeted deletion of the p85α regulatory subunit, fetal erythropoiesis was reportedly unaffected. Given the discrepancies between these studies, we performed a more detailed in vivo analysis of class IA PI-3 kinase–deficient embryos. Day-14.5 p85α-/- embryos are pale with a marked reduction of mature erythrocytes in their peripheral blood. Further, the absolute number and frequency of both early (erythroid burst-forming unit [BFU-E]) and late erythroid progenitors (erythroid colony-forming unit [CFU-E]) are reduced in p85α-/- fetal livers compared with wild-type controls, which is associated with reduced proliferation. Taken together, these data establish an important role for p85α and class IA PI-3 kinase in regulating the development of both early and late erythroid progenitors in fetal liver. (Blood. 2003;102:142-145)
Introduction
Erythropoiesis is a coordinated process regulated by specific signaling pathways. In vitro studies suggest that activation of class IA phosphatidylinositol 3 (PI-3) kinase following binding of erythropoietin (Epo) and kit-ligand (KitL) to their receptors is required for the proliferation, survival, and differentiation of erythroid progenitors.1-10 However, the physiologic role of class IA PI-3 kinase in regulating fetal and adult erythropoiesis is not known. A class IA PI-3 kinase knock-out mouse was generated by a targeted deletion of the p85α regulatory subunit of this kinase.11,12 Although p85α-/- mice die shortly after birth secondary to hepatic necrosis and chylous ascites,12 we used p85α-/- embryos to investigate the effect of p85α deficiency on fetal liver erythropoiesis in vivo.
Study design
Mice and fetal hematopoietic cell isolation
p85α+/- mice (129/SV × C57BL/6) were obtained from Dr Lewis Cantley at Harvard University (Boston, MA). Studies were conducted with a protocol approved by the Indiana University Animal Care and Use Committee. The p85α allele was genotyped by polymerase chain reaction (PCR) as previously described.11,12 p85α+/- mice were mated to produce day-14.5 p85α-/- and p85α+/+ embryos. Fetal liver cells were isolated as previously described.13 Single cell suspensions were prepared by pushing the hepatic tissues through a 23-gauge needle.
Peripheral blood analysis and fetal liver erythropoiesis
Embryonic blood was obtained from day-14.5 fetal hearts for peripheral smears, and fetal liver touch preps were performed as previously described14 and stained with Wright-Giemsa (Dade Behring, Newark, DE). Photomicrographs of peripheral smears and touch preps were taken with an Olympus DP11 microscope (Melville, NY).
Colony assays
Recombinant KitL and Epo were obtained from Peprotech (Rocky Hill, NJ) and Amgen (Thousand Oaks, CA), respectively. Erythroid burst-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) assays were performed exactly as previously described.15
c-kit+ cell isolation
Fetal liver cells were incubated with 1 μg phycoerythrin (PE)–conjugated c-kit monoclonal antibody (Pharmingen, San Diego, CA) per 106 cells, placed on ice for 20 minutes, pelleted, washed, and resuspended in phosphate-buffered saline (PBS). C-kit+ cells were purified by immunomagnetic bead enrichment as previously described.15
Apoptosis and proliferation assays
c-kit+ fetal liver cells were stained with fluorescein isothiocyanate (FITC)–annexin V (Pharmingen) and propidium iodide (Sigma, St Louis, MO) exactly per manufacturer's protocol followed by flow cytometric analysis as previously described.16 For thymidine incorporation assays, 10 000 c-kit+ fetal liver cells were plated in 96-well plates in 5% fetal calf serum (BioWhittaker, Walkersville, MD) with Epo and KitL or no growth factors. After 48 hours in culture, cells were pulsed with tritiated thymidine (New Life Sciences, Boston, MA) for 16 to 24 hours and harvested on glass fiber filters, and β emission was measured.
Results and discussion
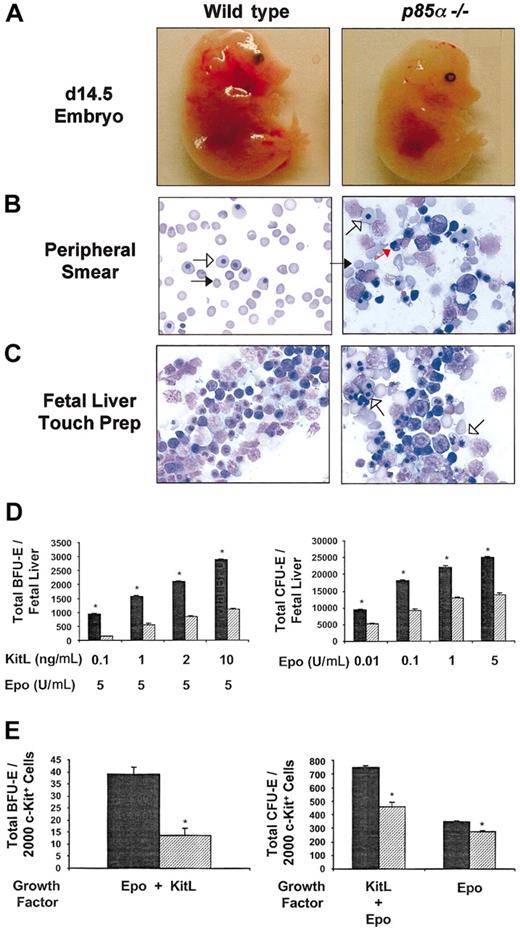
To determine the effect of p85α deficiency on fetal liver erythropoiesis, p85α+/- mice were intercrossed, and day-14.5 embryos were harvested for analysis. Day-14.5 p85α-/- embryos were pale compared with wild-type controls (Figure 1A) and demonstrated a marked reduction in the percentage of mature erythrocytes in their peripheral blood smears (Figure 1B). Most of the circulating cells in the p85α-/- embryos were nucleated megaloblasts or megalocytes (79% ± 5% versus 26% ± 8% for wild-type controls, n = 7, P < .05). In contrast, in wild-type peripheral blood, 80% to 90% of circulating cells were enucleated, mature red blood cells, or macrocytes, whereas the remaining cells were yolk sac–derived megaloblasts (Figure 1B). This pattern was also demonstrated in day-14.5 fetal liver touch preps. Although there was a continuum of erythropoiesis in wild-type fetal liver, a predominance of nucleated erythroid precursors was present in p85α-/- fetal livers (Figure 1C).
Effect of p85α deficiency on fetal liver erythropoiesis in day-14.5 embryos. (A) Phenotypic appearance of wild-type and p85α-/- embryos at day 14.5 of gestation. p85α-/- embryos are pale compared with wild-type controls. Original magnification, × 10. (B) Representative peripheral blood smears from day-14.5 wild-type and p85α-/- embryos. Blood smears were stained with Wright-Giemsa, and the total number of mature erythrocytes was quantified (“Results and discussion”). Mature erythrocytes (macrocytes) are indicated by black arrows, yolk sac–derived megaloblasts are indicated by short open arrows, and fetal liver-derived erythroid precursors are indicated by red arrows. p85α-/- peripheral blood contains fewer nonnucleated red cells and a large number of nucleated erythroblasts. Original magnification, × 40. (C) Representative fetal liver touch preps from wild-type and day-14.5 p85α-/- embryos. Touch preps were stained with Wright-Giemsa. Numerous erythropoietic cells in all stages of differentiation are observed in wild-type fetal livers. More megaloblasts are observed in the fetal livers of p85α-/- embryos (short open arrows). Original magnification, × 40. (D) Total number of BFU-Es and CFU-Es in day-14.5 wild-type (▦) and p85α-/- (▨) fetal livers. Cells were plated for growth of BFU-Es in methylcellulose medium containing various concentrations of KitL as indicated combined with a single concentration of Epo (5 U/mL). Cells were plated for growth of CFU-Es in methylcellulose medium containing various concentrations of Epo alone as indicated. CFU-Es and BFU-Es were counted by indirect microscopy after 2 and 7 days of culture, respectively. Results represent the mean number of colonies per fetal liver ± SEM of 5 independent experiments. *P < .05 by Student paired t test. (E) Frequency of erythroid progenitors in c-kit+–sorted fetal liver cells isolated from day-14.5 p85α-/- (▨) and wild-type (▦) fetal livers. c-kit+ cells were isolated by immunomagnetic bead enrichment and sorted as previously described.15 More than 90% of the cells were c-kit+ after sorting as tested by fluorescence cytometry (data not shown). c-kit+ cells/mL (2000) were plated in methylcellulose medium containing KitL (10 ng/mL) and Epo (5 U/mL), and the numbers of CFU-Es and BFU-Es were quantified after 2 and 7 days of culture, respectively. In addition, 2000 c-kit+ cells/mL were plated in methylcellulose medium containing Epo (5 U/mL) alone, and the number of CFU-Es was counted after 2 days of culture. Results represent the mean number of colonies per 2000 c-kit+ cells ± SEM of 3 independent experiments. *P < .05 by Student paired t test.
Effect of p85α deficiency on fetal liver erythropoiesis in day-14.5 embryos. (A) Phenotypic appearance of wild-type and p85α-/- embryos at day 14.5 of gestation. p85α-/- embryos are pale compared with wild-type controls. Original magnification, × 10. (B) Representative peripheral blood smears from day-14.5 wild-type and p85α-/- embryos. Blood smears were stained with Wright-Giemsa, and the total number of mature erythrocytes was quantified (“Results and discussion”). Mature erythrocytes (macrocytes) are indicated by black arrows, yolk sac–derived megaloblasts are indicated by short open arrows, and fetal liver-derived erythroid precursors are indicated by red arrows. p85α-/- peripheral blood contains fewer nonnucleated red cells and a large number of nucleated erythroblasts. Original magnification, × 40. (C) Representative fetal liver touch preps from wild-type and day-14.5 p85α-/- embryos. Touch preps were stained with Wright-Giemsa. Numerous erythropoietic cells in all stages of differentiation are observed in wild-type fetal livers. More megaloblasts are observed in the fetal livers of p85α-/- embryos (short open arrows). Original magnification, × 40. (D) Total number of BFU-Es and CFU-Es in day-14.5 wild-type (▦) and p85α-/- (▨) fetal livers. Cells were plated for growth of BFU-Es in methylcellulose medium containing various concentrations of KitL as indicated combined with a single concentration of Epo (5 U/mL). Cells were plated for growth of CFU-Es in methylcellulose medium containing various concentrations of Epo alone as indicated. CFU-Es and BFU-Es were counted by indirect microscopy after 2 and 7 days of culture, respectively. Results represent the mean number of colonies per fetal liver ± SEM of 5 independent experiments. *P < .05 by Student paired t test. (E) Frequency of erythroid progenitors in c-kit+–sorted fetal liver cells isolated from day-14.5 p85α-/- (▨) and wild-type (▦) fetal livers. c-kit+ cells were isolated by immunomagnetic bead enrichment and sorted as previously described.15 More than 90% of the cells were c-kit+ after sorting as tested by fluorescence cytometry (data not shown). c-kit+ cells/mL (2000) were plated in methylcellulose medium containing KitL (10 ng/mL) and Epo (5 U/mL), and the numbers of CFU-Es and BFU-Es were quantified after 2 and 7 days of culture, respectively. In addition, 2000 c-kit+ cells/mL were plated in methylcellulose medium containing Epo (5 U/mL) alone, and the number of CFU-Es was counted after 2 days of culture. Results represent the mean number of colonies per 2000 c-kit+ cells ± SEM of 3 independent experiments. *P < .05 by Student paired t test.
We next assayed for early (BFU-E) and late (CFU-E) erythroid progenitors in wild-type and p85α-/- fetal livers. A marked reduction in the total number of BFU-E and CFU-E progenitors was observed in p85α-/- fetal livers compared with wild-type controls (Figure 1D). The reduction in BFU-Es and CFU-Es in p85α-/- fetal livers could be secondary to p85α deficiency in hematopoietic progenitors or alternatively a defect in the p85α-/- microenvironment. To directly test the effect of p85α deficiency on erythroid colony formation independent of the effects of the microenvironment, we performed BFU-E and CFU-E assays with equal numbers of sorted wild-type and p85α-/- c-kit+ cells, which are enriched for hematopoietic progenitors. A marked reduction in the frequency of both BFU-Es and CFU-Es was observed in c-kit+ cells isolated from day-14.5 p85α-/- fetal livers compared with wild-type controls (Figure 1E). Interestingly, the reduction in CFU-E frequency observed in p85α-/- c-kit+ cells was significantly greater when cells were stimulated with both KitL and Epo in combination compared with Epo alone (Figure 1E). Collectively, these results argue that an intrinsic p85α-/- hematopoietic progenitor cell defect contributes in part to the reduction of erythroid progenitors in day-14.5 p85α-/- fetal livers in vivo and the lack of mature erythrocytes in the peripheral blood of these affected fetuses.
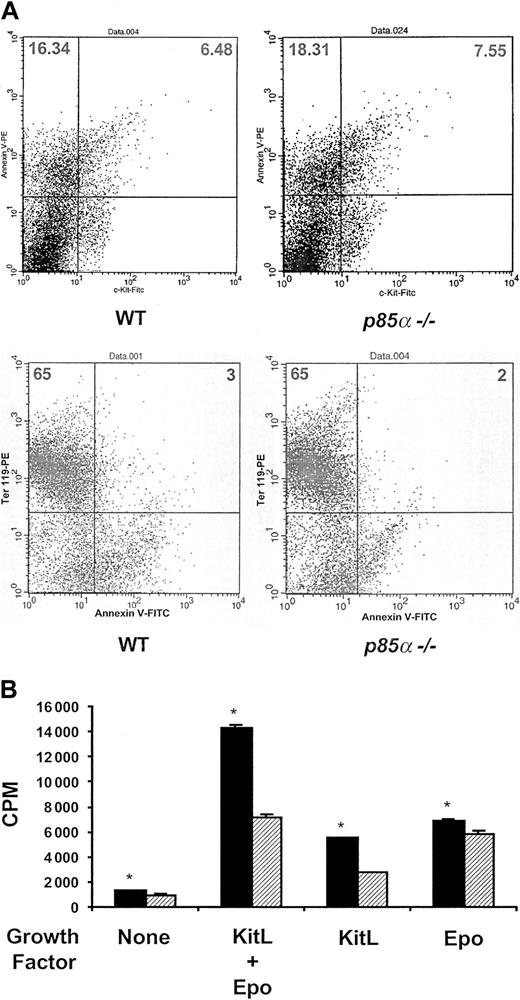
Because these data establish a previously unrecognized role for p85α in regulating fetal erythropoiesis in vivo, we next tested whether the p85α-/- erythroid phenotype could be linked to either a decrease in proliferation and/or survival of hematopoietic progenitors. Prior studies argue that activation of the PI-3 kinase/Akt pathway by either Epo or KitL promotes the survival of erythroid progenitors.1,6,8,17 To test this hypothesis in vivo, we compared the percentage of both freshly isolated c-kit+ cells (a marker for early erythroid progenitors) or ter119+ cells (a marker for late erythroid progenitors) undergoing apoptosis harvested from wild-type and p85α-/- day-14.5 fetal livers. Cells were stained with annexin to identify apoptotic cells. No differences in apoptosis in either c-kit+ or ter119+ cells isolated from the 2 experimental genotypes were observed (Figure 2A). These data are consistent with 2 recent reports which show that p85α-/- mast cells do not have an increase in apoptosis in response to KitL compared with wild-type controls.18,19
Apoptosis and proliferation of wild-type and p85α-/- c-kit+ fetal liver cells. (A) Apoptosis of wild-type and p85α-/- c-kit+ or ter119+ day-14.5 fetal liver cells. Freshly isolated fetal liver cells were stained with either annexin V–PE or c-kit–FITC antibodies to identify the percentage of c-kit+ cells (top row) or annexin V–FITC and ter119-PE antibodies (bottom row) to identify the percentage of ter119+ cells undergoing apoptosis by fluorescence cytometry in vivo. The numbers in each quadrant represent the percentage of total cells in that quadrant. A representative dot plot for each genotype is shown. Four other experiments showed similar results. (B) Proliferation of wild-type (▦) and p85α-/- (▨) c-kit+ day-14.5 fetal liver cells in response to no growth factors, Epo and KitL in combination, or alone. Freshly isolated c-kit+ fetal liver cells were plated in 96-well plates in replicates of 6 in the presence of 5% fetal calf serum with no additional growth factors or 10 ng/mL KitL and 5 U/mL Epo in combination or alone. After 48 hours of culture, cells were pulsed with tritiated thymidine and harvested 16 hours later for measurement of β emission. Results represent the mean thymidine incorporation ± SEM of 5 independent experiments. *P < .05 by Student paired t test.
Apoptosis and proliferation of wild-type and p85α-/- c-kit+ fetal liver cells. (A) Apoptosis of wild-type and p85α-/- c-kit+ or ter119+ day-14.5 fetal liver cells. Freshly isolated fetal liver cells were stained with either annexin V–PE or c-kit–FITC antibodies to identify the percentage of c-kit+ cells (top row) or annexin V–FITC and ter119-PE antibodies (bottom row) to identify the percentage of ter119+ cells undergoing apoptosis by fluorescence cytometry in vivo. The numbers in each quadrant represent the percentage of total cells in that quadrant. A representative dot plot for each genotype is shown. Four other experiments showed similar results. (B) Proliferation of wild-type (▦) and p85α-/- (▨) c-kit+ day-14.5 fetal liver cells in response to no growth factors, Epo and KitL in combination, or alone. Freshly isolated c-kit+ fetal liver cells were plated in 96-well plates in replicates of 6 in the presence of 5% fetal calf serum with no additional growth factors or 10 ng/mL KitL and 5 U/mL Epo in combination or alone. After 48 hours of culture, cells were pulsed with tritiated thymidine and harvested 16 hours later for measurement of β emission. Results represent the mean thymidine incorporation ± SEM of 5 independent experiments. *P < .05 by Student paired t test.
We next tested whether p85α deficiency would alter the proliferation of c-kit+ progenitor cells in response to either KitL or Epo alone or in combination. Remarkably, p85α-/- c-kit+ cells displayed a 40% to 50% reduction in proliferation in response to KitL alone or KitL in combination with Epo compared with wild-type cells (Figure 2B). Such a proliferative defect would account for the diminished number of erythroid progenitors measured in the day-14.5 p85α-/- embryos. This finding would explain why knock-in mice harboring a mutation in the p85α binding domain of the c-kit receptor are not anemic as adult animals,20 whereas we note an erythropoietic defect in p85α-/- fetal livers. It is well known that rates of hematopoietic progenitor proliferation are approximately 2-fold higher in fetal liver compared with adult bone marrow under normal homeostatic conditions.21 Thus, a defect in basal and stimulated progenitor proliferation would affect fetal liver more than adult hematopoiesis. Further, other hematopoietic growth factors or integrins may act in synergy with KitL to transmit signals via class IA PI-3 kinase to augment the proliferation of erythroid progenitors. This situation would result in a more anemic phenotype in p85α-/- animals compared with mice containing only a mutation in the p85α binding site of the c-kit receptor. Finally, p85α-/- c-kit+ cells stimulated with Epo alone demonstrated only a 10% reduction in proliferation compared with wild-type cells (Figure 2B). This finding is consistent with the observation that mutant mice harboring an Epo receptor, which lacks the p85α binding site, are viable with relatively normal adult erythropoiesis.22 This observation is also consistent with our clonogenic data, which showed that p85α-/- c-kit+ cells had only a minor decrease in CFU-E formation in response to Epo alone (Figure 1E).
Thus, these studies clearly establish and clarify a role for p85α and class IA PI-3 kinase in regulating normal fetal erythropoiesis in vivo at least in part by promoting the proliferation of hematopoietic progenitors via signaling through the KitL/c-kit pathway. Our studies did not find a role for p85α in controlling the survival of c-kit+ cells in vivo. However, Akt activation in p85α-/- c-kit+ cells in response to either Epo or KitL was reduced by only 30% to 40% compared with wild-type controls (data not shown), which is consistent with previous studies using other primary p85α-/- cell lines stimulated with different growth factors.11,12,18,19,23 The retention of a significant amount of Akt activity in our p85α-/- cells may explain the differences in our results and previous in vitro studies using pharmacologic inhibitors of PI-3 kinase. Future studies designed to further reduce class IA PI-3 kinase activity in vivo in genetically engineered animal models will clarify the role of this kinase in regulating both fetal and adult erythropoiesis.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-10-3245.
Supported by grants 1 KO8 CA096579-01 (D.A.I.) and R01HL63169 (M.C.Y.) from the National Institutes of Health and by grant P30 DK49218 (D.A.I.) from the National Institute of Diabetes and Digestive and Kidney Diseases. H.H. is a Howard Hughes Medical Institute Medical Student Research Training Fellow. D.A.I. is a recipient of a Basil O'Connor Award from the March of Dimes (5-FY02-254).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lewis Cantley for providing p85α+/- mice, Marsha Hippensteel for exceptional administrative support, and Drs L. Haneline and D. Clapp (Indiana University) for many valuable discussions and thoughtful review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal