OX40 (CD134) is expressed on activated T cells; its ligand, OX40 ligand (OX40L) is expressed on dendritic cells, B cells, and activated endothelial cells. To determine how OX40-OX40L interaction affects graft-versus-host disease (GVHD), we used antagonistic anti-OX40L monoclonal antibody (mAb) or OX40−/−donor or OX40L−/− recipient mice. Similar degrees of GVHD reduction were observed with each approach. Despite the fact that OX40 is up-regulated on both CD4+ and CD8+ T cells isolated during GVHD, the major effects of OX40 ligation were on CD4+ and not CD8+ T-cell–mediated alloresponses as assessed in both GVHD and engraftment model systems. GVHD inhibition by blockade of the OX40/OX40L pathway did not require CD28 signaling. Some studies have indicated OX40 is essential for inducing T-helper type 2 (Th2) responses. However, in vivo blockade of OX40-OX40L interactions reduced GVHD mortality induced by either signal transducer and activator of transcription–6−/− (Stat-6−/−) (Th2-defective) or Stat-4−/− (Th1-defective) major histocompatibility complex (MHC)–disparate splenocytes, indicating that the GVHD-ameliorating effects did not require Stat-4 or Stat-6 signaling. Although OX40L has been reported to be expressed on activated T cells, no effects on GVHD were observed when OX40L−/− versus OX40L+/+ T cells were infused in different models. These data provide insights as to the mechanisms responsible for OX40/OX40L regulation of GVHD.
Introduction
T cells receiving signals via the antigen-specific T-cell receptor (TCR) require a second, costimulatory signal to stabilize cytokine mRNA and induce antiapoptotic proteins.1,2 Members of the immunoglobulin supergene and tumor necrosis factor (TNF)/TNF receptor families can function as costimulatory molecules. The latter include CD40 ligand (CD40L) (CD154); 4-1BB receptor (CD137); CD30; and OX40 (CD134).3OX40 is expressed on activated CD4+ and CD8+ T cells in mice and humans. OX40L (CD134L) is a type II membrane protein expressed on antigen-presenting cells (APCs), including dendritic cells (DCs), B cells, and macrophages, that have been activated by known inductive stimuli such as CD40 or proinflammatory mediators (eg, lipopolysaccharide [LPS]).4-7 In addition, OX40L expression has been reported on endothelial cells, microglial cells, and T cells.8-11 Signaling of OX40 receptor on antigen-specific CD4+ T cells results in production of T-helper type 1 (Th1) and Th2 cytokines, up-regulation of antiapoptotic proteins,12-16 clonal expansion, and development into memory cells.11,14 17-22
Because OX40 is up-regulated following T-cell activation, studies were performed to assess the role of OX40-OX40L interaction on graft-versus-host disease (GVHD), which is mediated by alloantigen-activated donor T cells. Tittle et al23 observed that increased numbers of alloreactive CD4+ T cells that coexpressed OX40 were present in the peripheral blood, lymphohematopoietic organs, and liver of nonirradiated F1 rat recipients of parental donor grafts that were experiencing a GVHD reaction. Chen et al13showed comparable allogeneic mixed leukocyte reaction (MLR) responses using OX40L−/− DCs as stimulators of CD4+ T-cell proliferation, and Pippig et al11 demonstrated that OX40−/− and OX40+/+ T cells have similar allogeneic MLR responses. Stuber et al24 have shown that blocking OX40-OX40L interactions with an OX40-immunoglobulin fusion protein can diminish the intestinal manifestations of acute GVHD using an analogous nonirradiation semiallogeneic system in mice as described by Tittle et al in rats.23 Tsukada et al25 extended these studies into lethally irradiated murine F1 recipients of parental donor grafts, representing the only published study to date examining the effects of blocking the OX40/OX40L pathway, using an OX40L monoclonal antibody (mAb), on GVHD-mediated lethality. Since OX40L has also been reported to be expressed on activated T cells, it is possible that the anti-OX40L mAb reduced GVHD lethality, at least in part, by the clearance or alteration of the biological function of donor T cells rather than simply blocking the engagement of OX40 receptor on donor T cells with OX40L on recipient cells.11
While the rodent studies described above provide evidence that OX40/OX40L blockade can reduce acute GVHD responses, the mechanisms responsible have not been fully elucidated.The present studies were undertaken to investigate the potency and mechanisms by which OX40-OX40L interactions regulate GVHD. Additionally, the potential role of this pathway on the engraftment of pan–T-cell–depleted (TCD) allogeneic bone marrow (BM) was examined. To strengthen our conclusions, we used complementary approaches designed to target the OX40/OX40L pathway: antagonistic and agonistic mAbs, OX40 receptor–deficient (OX40−/−) donors, and OX40L deletional mutant (OX40L−/−) recipients. Our data indicate that OX40-OX40L interactions preferentially regulate CD4+ T-cell–mediated alloresponses in vivo. The protective effects of OX40/OX40L blockade did not require signal transducer and activator of transcription–4 (Stat-4) or Stat-6 signaling. Despite data indicating that OX40L can be expressed on T cells, we have no evidence that such expression can significantly regulate GVHD. These data provide additional insights as to the mechanisms responsible for GVHD as influenced by OX40-OX40L interactions.
Materials and methods
Mice
B10.BR/SgSnJ (H2k), C57BL/6 (termed B6:H2b; CD45.2), CD28-deficient B6 (CD28−/−), C.H2bm1 (termed bm1; CD45.2), BALB/c severe combined immune deficient (BALB/c–SCID), BALB/c Stat-4 deficient (Stat-4−/−), BALB/c Stat-6 deficient (Stat-6−/−), and C.H2bm12 (termed bm12; CD45.2) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6-CD45.1 congenic mice were purchased from the National Institutes of Health (Bethesda, MD). OX40−/− mice were generated as described and backcrossed 9 generations with B6 mice.11 OX40L+/+ mice were generated as described and used as inbred 129/Sv mice or backcrossed 5 generations to BALB/c mice, as indicated.13 The 129/Sv OX40L−/− mice or littermate controls were used as donors in some experiments, while BALB/c OX40L−/− mice or littermates were used as recipients in other experiments. Mice were bred and housed in a specific pathogen-free facility in microisolator cages. Donors and recipients were used at 8 to 10 weeks of age.
mAb preparation
Agonistic anti-OX40 (clone M5, rat immunoglobulin G1 [IgG1], kindly provided by Dr Kenneth Mohler, Immunex, Seattle, WA) and antagonistic anti-OX40L (clone RM134L, rat IgG2b) were generated as ascites fluid and subsequently purified.15 26Control rat IgG was purchased from Rockland Laboratories (Gilbertsville, PA). Injections of mAb were given at a dose of 200 μg per injection intraperitoneally days −1 to +5 and then thrice weekly through day +28 unless otherwise indicated.
GVHD generation
Different GVHD systems were used to analyze the effects of the OX40/OX40L pathway on alloresponses in vivo. In the first type, recipients were heavily irradiated to simulate human transplantation conditions. B10.BR recipients were lethally irradiated with 8.0 Gy total body irradiation (TBI) by x-ray (39 cGy/min) on day −1 followed on day 0 by the intravenous infusion of pan-TCD BM (0.8 to 2 × 107), accomplished by treatment with anti-Thy1.2 (clone 30-H-12) plus rabbit complement.27 Donor BM cells were supplemented with splenocytes, purified lymph node (LN) T cells, or LN T-cell subpopulations from B6 wild-type, B6 OX40−/−, 129/Sv OX40L−/−, B6 CD28−/−, BALB/c Stat-4−/−, or BALB/c Stat-6−/− donors, as indicated. To measure CD4+ T-cell GVHD responses, bm12 recipients were lethally irradiated (8.0 Gy TBI) and then infused with TCD BM and splenocytes obtained from B6 donors.27 To determine the effects of OX40-OX40L interactions on GVHD and graft-versus-leukemia (GVL) induced by delayed lymphocyte infusion (DLI), B6 recipients were lethally irradiated (8.0 Gy TBI), reconstituted with TCD BM, and then given donor B10.BR splenocytes on day 21 after BM transplantation (BMT)28 along with either irrelevant or anti-OX40 mAb infusions as described. Some cohorts of mice were challenged with acute myeloid leukemia cells (C1498 derived from B6 mice) as previously described.27 To determine the effects of host OX40L expression on GVHD lethality, BALB/c OX40L−/− mice or littermate controls were lethally irradiated (6.0 Gy TBI) and reconstituted with B6 TCD BM along with purified LN T cells (0 or 1 × 106) obtained from B6 or B6 CD28−/− donors.
To more specifically determine the effect of OX40-OX40L interactions on the GVHD capacity of CD4+ or CD8+ T cells, we used a system in which purified T-cell subsets are given to major histocompatibility complex (MHC)–disparate, sublethally irradiated recipients.27 This system permits highly accurate quantification of the degree of GVHD responses as related to T-cell dose. MHC class II (bm12)– or class I (bm1)–disparate recipients were irradiated with 6.0 Gy TBI on day 0 from a137cesium source at a dose rate of 85 cGy/min. At 4 to 6 hours after TBI, purified LN CD4+ or CD8+ T cells from B6, B6 OX40−/−, 129/Sv OX40L−/−, or littermate control donors were infused. To complement studies in irradiated mice, one set of studies was performed in nonconditioned BALB/c–SCID recipients. Recipients were depleted of natural killer (NK) cells by antiasialo-GM1 antisera (25 μL on days −4 and −2) and infused with purified T cells obtained from 129/Sv OX40L−/− mice or littermate controls.
To purify LN cells, single cell suspensions of axillary, mesenteric, and inguinal LN cells were depleted of NK cells and enriched for CD4+ or CD8+ T cells by depletion with anti-CD8 (hybridoma 2.43, rat IgG2b, provided by Dr David Sachs, Charlestown, MA) or anti-CD4 (hybridoma GK1.5, rat IgG2b, provided by Dr Frank Fitch, Chicago IL), respectively. Rat mAb-coated T cells were passaged through a goat antimouse and goat antirat immunoglobulin-coated column (Biotex, Edmonton, AB, Canada). The final composition of T cells in the donor graft was determined by flow cytometry and was always found to be at least 94% T cells of the desired phenotype. Hematocrit values were obtained at periodic intervals as an indicator of the possible bone marrow–destructive effects of infused T cells.27 For all GVHD (and engraftment) systems, mice were monitored daily for survival and clinical appearance and weighed twice weekly.
Engraftment studies
Bm1 or bm12 mice were irradiated with 4.5 or 5.0 Gy TBI, as indicated, by x-ray on day −1 and given B6 CD45.2 TCD BM (0.7 to 1 × 107) cells on day 0.27 Recipients were given irrelevant anti-OX40 or anti-OX40L mAb (200 μg per dose) intraperitoneally daily from days −1 to +6, then twice weekly through day 14. Donor or host chimerism was monitored in peripheral blood at 6 weeks and 3 to 4 months after BMT by means of αCD45.2 (clone 104-2, rat IgG2a) and αCD45.1 (clone A20-1.7, rat IgG2a), both provided by Dr U. Hammerling (New York, NY).27 The T-cell, B-cell, and granulocyte/macrophage constituency of peripheral blood cells was measured with the use of mAb directed toward CD4 or CD8, CD19, and Mac1, respectively. All fluorochrome-labeled mAbs, unless otherwise indicated, were obtained from PharMingen (San Diego, CA). Cells were first incubated with 2.4G2 to block Fc receptors, and then incubated with an optimal concentration of fluorochrome-labeled mAb for 45 minutes at 4°C. Cells were washed 3 times and resuspended for analysis by 3-color flow cytometry by means of fluorescein isothiocyanate–, phycoerythrin-, or biotin (along with SA [streptavidin]–peridinin chlorophyll A protein [SA-PerCP])–conjugated mAb purchased from PharMingen or Becton Dickinson (Mountain View, CA). Irrelevant mAb control values were subtracted from values obtained with relevant mAbs. All results were obtained by means of a FACSCalibur (Becton Dickinson). Forward- and side-scatter settings were gated to exclude red cells and debris, and 1 × 104 cells were analyzed for each determination.
Statistical analyses
Group comparisons of continuous data were made by Studentt test. Survival data were analyzed by life-table methods by means of the Mantel-Peto-Cox summary of chi-square.29 Actuarial survival and relapse rates are shown. Probability (P) values of .05 or lower were considered significant.
Results
OX40-OX40L interaction regulates GVHD lethality
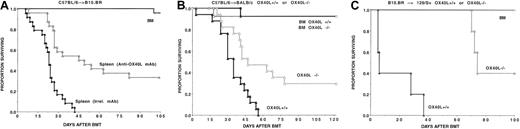
To determine the role of OX40-OX40L interactions in regulating GVHD responses, initial studies were undertaken to determine the magnitude of effects on OX40 receptor ligation in heavily irradiated B10.BR (H2k) recipients of B6 (H2b) donor BM and supplemental splenocytes (0, 5, or 15 × 106per recipient). The administration of an agonistic anti-OX40 mAb significantly increased GVHD severity, as evidenced by accelerated mortality, clinical appearance, and body weight loss (Figure 1A and not shown). On the basis of donor splenocyte dose titrations, anti-OX40 mAb infusion accelerated GVHD by at least 5-fold (Figure1A).
OX40 receptor regulation of GVHD in heavily irradiated MHC class I plus class II–disparate recipients.
Targeting of the OX40 receptor regulates GVHD in heavily irradiated MHC class I plus class II–disparate recipients. (A) B10.BR recipients (n = 8 per group) were lethally irradiated and reconstituted with B6 BM alone or containing supplemental splenocytes (S) from B6 donors. The splenocyte number × 106is shown. Recipients of splenocytes received either irrelevant or anti-OX40 mAb beginning on the day before splenocyte administration as described in “Materials and methods.” (B) B6 recipients (n = 10 per group) were lethally irradiated and reconstituted with B10.BR BM. On day 21 after BMT, recipients were given splenocytes (5 × 106) (DLI), followed 1 week later by challenge with acute myelogenous leukemia (AML) cells. Beginning on the day prior to DLI, cohorts of mice were given irrelevant or anti-OX40 mAb. Autopsies to detect gross evidence of AML cells were performed on mice that had received AML cells. (C) Mice (n = 8 per group per experiment) received transplants as described in panel A, except that splenocytes (15 × 106) were obtained from either OX40+/+(▴) or OX40−/− (▵) donors. Data from 2 replicate experiments with similar results are pooled. In each instance, targeting of the OX40 receptor had a significant impact on survival rates.
OX40 receptor regulation of GVHD in heavily irradiated MHC class I plus class II–disparate recipients.
Targeting of the OX40 receptor regulates GVHD in heavily irradiated MHC class I plus class II–disparate recipients. (A) B10.BR recipients (n = 8 per group) were lethally irradiated and reconstituted with B6 BM alone or containing supplemental splenocytes (S) from B6 donors. The splenocyte number × 106is shown. Recipients of splenocytes received either irrelevant or anti-OX40 mAb beginning on the day before splenocyte administration as described in “Materials and methods.” (B) B6 recipients (n = 10 per group) were lethally irradiated and reconstituted with B10.BR BM. On day 21 after BMT, recipients were given splenocytes (5 × 106) (DLI), followed 1 week later by challenge with acute myelogenous leukemia (AML) cells. Beginning on the day prior to DLI, cohorts of mice were given irrelevant or anti-OX40 mAb. Autopsies to detect gross evidence of AML cells were performed on mice that had received AML cells. (C) Mice (n = 8 per group per experiment) received transplants as described in panel A, except that splenocytes (15 × 106) were obtained from either OX40+/+(▴) or OX40−/− (▵) donors. Data from 2 replicate experiments with similar results are pooled. In each instance, targeting of the OX40 receptor had a significant impact on survival rates.
To determine whether the acceleration in GVHD lethality observed with anti-OX40 mAb was dependent upon the use of high-dose lethal irradiation, which induces proinflammatory cytokines, the effect of anti-OX40 mAb was investigated in a setting in DLI.30 DLI in the form of donor splenocyte infusion causes less GVHD mortality than when the same number is infused on the day of BMT.28 B6 recipients were lethally irradiated and reconstituted with B10.BR TCD BM. On day 21 after BMT, some cohorts of mice were given a low dose of donor splenocytes (5 × 106) and irrelevant or anti-OX40 mAb (beginning on day 20 after BMT).27 All mice surviving until day 28 were challenged with AML cells.27 Mice that received no DLI cells all succumbed to AML by day 50. Mice receiving DLI and irrelevant mAb experienced GVL but eventually died of AML. In contrast, recipients given both DLI and anti-OX40 mAb all died of GVHD by day 28, prior to AML cell infusion (Figure 1B). Thus, anti-OX40 mAb given later after BMT substantially increased GVHD mediated by low-dose DLI.
While administration of an agonistic anti-OX40 mAb clearly has a potent effect on GVHD acceleration, engagement of the OX40 receptor by mAb may overestimate the magnitude of effect that is physiologically conferred by the binding of OX40 to OX40L during the process of GVHD generation. Therefore, complementary studies were performed in which donor splenocytes were obtained from wild-type or OX40−/− mice to examine the effects of OX40 loss of function on GVHD (Figure 1C). Lethally irradiated B10.BR recipients were infused with B6 TCD BM and supplemental splenocytes (0 or 15 × 106). As compared with the uniform lethality of wild-type splenocytes, donor splenocytes deficient in OX40 had a significantly reduced GVHD capacity, with 30% of recipients surviving long term.
As further evidence that the OX40/OX40L pathway affects GVHD, 2 additional approaches were conducted that targeted the OX40L component of the pathway. In the first, lethally irradiated B10.BR recipients were infused with B6 TCD BM, supplemental splenocytes (0 or 25 × 106), and either irrelevant or anti-OX40L mAb (Figure 2A). Anti-OX40L mAb–treated recipients had 35% long-term survival, as compared with 0% in the controls. In the second approach, BALB/c OX40L−/− mice or wild-type littermate controls were lethally irradiated, reconstituted with B6 TCD BM, and given purified B6 T cells (Figure 2B). Whereas none of the wild-type recipients' T cells survived beyond 8 weeks after BMT, 32% of OX40L−/− recipients survived long term (4 months after BMT). Similar results were observed in a different MHC-disparate system in which B10.BR T cells (106) caused 100% GVHD lethality in 129/Sv (H2b) recipients within 40 days after BMT in contrast to 40% survival in 129/Sv OX40L−/− mice at 100 days (Figure 2C). Collectively, these data indicated that intact OX40-OX40L interactions are required for optimal GVHD generation in lethally irradiated recipients of fully allogeneic donor grafts.
Targeting of OX40L and GVHD lethality.
Targeting of OX40L regulates GVHD lethality. (A) B10.BR recipients (n = 8 per group per experiment) were lethally irradiated and BM reconstituted, and cohorts of mice were given supplemental splenocytes (25 × 106) as indicated. Splenocyte recipients were administered irrelevant (▴) or anti-OX40L (▵) mAb beginning 1 day prior to transplantation. Data from 2 replicate experiments with similar results are pooled. (B) BALB/c OX40L−/−mice (dotted lines) or OX40L+/+ (solid lines) littermate controls (n = 8 per group per experiment), as indicated, were lethally irradiated and BM reconstituted, and cohorts were given B6 purified T cells (106). Data from 2 replicate experiments with similar results are pooled. (C) The 129/Sv OX40L−/−mice or OX40L+/+ littermate controls (n = 5 per group), as indicated, were lethally irradiated and reconstituted with B10.BR BM, and cohorts were given purified B10.BR T cells (106). In each instance, targeting of OX40L had a significant impact on GVHD lethality.
Targeting of OX40L and GVHD lethality.
Targeting of OX40L regulates GVHD lethality. (A) B10.BR recipients (n = 8 per group per experiment) were lethally irradiated and BM reconstituted, and cohorts of mice were given supplemental splenocytes (25 × 106) as indicated. Splenocyte recipients were administered irrelevant (▴) or anti-OX40L (▵) mAb beginning 1 day prior to transplantation. Data from 2 replicate experiments with similar results are pooled. (B) BALB/c OX40L−/−mice (dotted lines) or OX40L+/+ (solid lines) littermate controls (n = 8 per group per experiment), as indicated, were lethally irradiated and BM reconstituted, and cohorts were given B6 purified T cells (106). Data from 2 replicate experiments with similar results are pooled. (C) The 129/Sv OX40L−/−mice or OX40L+/+ littermate controls (n = 5 per group), as indicated, were lethally irradiated and reconstituted with B10.BR BM, and cohorts were given purified B10.BR T cells (106). In each instance, targeting of OX40L had a significant impact on GVHD lethality.
OX40-OX40L interactions have a more pronounced effect on CD4+ T-cell– than on CD8+ T-cell–mediated alloresponses in both GVHD and alloengraftment
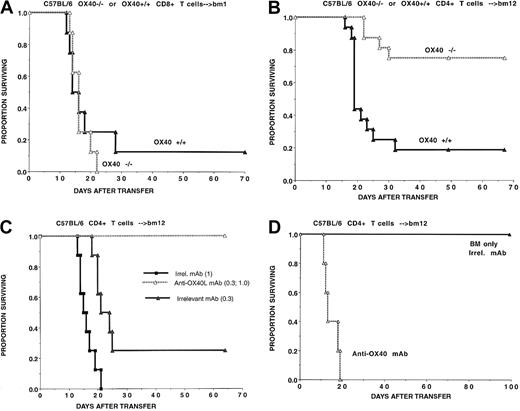
During GVHD induction in lethally irradiated B10.BR recipients of B6 BM and supplemental splenocytes (107), we have observed that the OX40 receptor is up-regulated on both CD4+ T cells (22% positive) and CD8+ T cells (20% positive) isolated from thoracic duct lymphatics on day 7 after BMT (data not shown). To determine whether precluding OX40-OX40L binding would have similar effects on CD4+ versus CD8+ T-cell–mediated GVHD, experiments were performed with the use of highly purified T-cell subsets infused into recipients with an isolated MHC class I or class II only disparity. Because residual host T cells remain in the sublethally irradiated recipients, the infusion of agonistic mAbs could affect GVHD responses by stimulating either donor antihost reactions, resulting in increased GVHD, or host antidonor responses, resulting in less GVHD lethality. To avoid this complication, these studies were performed with OX40−/− donor T cells.
To assess CD8+ T cells' responses, sublethally irradiated bm1 recipients were given B6 CD8+ T cells (0.3 × 106) obtained from OX40+/+ or OX40−/− donors. The infusion of B6 OX40−/−CD8+ T cells resulted in 0% survival as compared with 12% survival in recipients of wild-type CD8+ T cells, which was not of sufficient magnitude to be statistically significant (P = .06) (Figure 3A). Thus, OX40-OX40L interactions do not appear to be a major regulator of CD8+ T-cell–mediated GVHD lethality.
Comparison of OX40-OX40L interactions with and CD8+T-cell–mediated GVHD lethality.
OX40-OX40L interactions are more potent regulators of CD4+as compared with CD8+ T-cell–mediated GVHD lethality. (A) Sublethally irradiated bm1 recipients (n = 8 per group) were given highly purified CD8+ T cells (0.3 × 106) from OX40+/+ (▴) or OX40−/− (▵) donors as indicated. (B) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified CD4+ T cells (0.3 × 105) from B6 OX40+/+ (▴) or OX40−/− (▵) donors as indicated. Data from 2 replicate experiments with similar results were pooled. (C) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified B6 CD4+ T cells (0.3 or 1 × 105 as indicated in parentheses). Mice also were given either irrelevant (▴ or ■) or anti-OX40L (▵) mAb. (D) Lethally irradiated bm12 recipients (n = 5 per group) were given highly purified B6 CD4+ T cells (0.3 × 106). Mice also were given either irrelevant or anti-OX40 mAb.
Comparison of OX40-OX40L interactions with and CD8+T-cell–mediated GVHD lethality.
OX40-OX40L interactions are more potent regulators of CD4+as compared with CD8+ T-cell–mediated GVHD lethality. (A) Sublethally irradiated bm1 recipients (n = 8 per group) were given highly purified CD8+ T cells (0.3 × 106) from OX40+/+ (▴) or OX40−/− (▵) donors as indicated. (B) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified CD4+ T cells (0.3 × 105) from B6 OX40+/+ (▴) or OX40−/− (▵) donors as indicated. Data from 2 replicate experiments with similar results were pooled. (C) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified B6 CD4+ T cells (0.3 or 1 × 105 as indicated in parentheses). Mice also were given either irrelevant (▴ or ■) or anti-OX40L (▵) mAb. (D) Lethally irradiated bm12 recipients (n = 5 per group) were given highly purified B6 CD4+ T cells (0.3 × 106). Mice also were given either irrelevant or anti-OX40 mAb.
To ascertain the function of OX40/OX40L in a CD4+T-cell–mediated GVHD system, OX40+/+ or OX40−/− CD4+ T cells were infused into sublethally irradiated bm12 recipients. Recipients of OX40−/− CD4+ T cells (0.3 × 106) had a 75% survival rate versus 16% with wild-type cells (P = .0003) (Figure 3B). Anti-OX40L mAb treatment of bm12 recipients of wild-type B6 CD4+ T cells provided a high degree of GVHD lethality protection. Specifically, anti-OX40L mAb treatment rescued 100% versus 25% (P = .002) of recipients of low-dose (0.3 × 105) and 100% versus 0% (P = .0006) of recipients of high-dose (105) CD4+ T cells (Figure 3C). In lethally irradiated bm12 recipients, agonistic anti-OX40 mAb resulted in rapid mortality induced by a nonlethal dose of purified CD4+ T cells (P < .0001) (Figure 3D). Thus, the OX40/OX40L pathway is more critical in driving CD4+ than CD8+ T-cell alloresponses.
The data presented above clearly indicate that OX40-OX40L interactions preferentially accelerate GVHD induced by CD4+ donor T cells that encounter MHC class II antigens that are distributed throughout the host microenvironment. We next sought to determine whether OX40 receptor signaling regulates host CD4+ or CD8+ T-cell–mediated BM graft rejection, a situation in which BM cells alone serve as the source of alloantigen. Agonistic anti-OX40 mAb administration resulted in a marked reduction in engraftment in sublethally irradiated bm12 recipients of donor B6 TCD BM at 6 weeks (data not shown) or 4 months (Table 1) after BMT. In contrast, a blocking anti-OX40L mAb increased alloengraftment at 2 different TBI doses. Consistent with the lack of pronounced effects of OX40-OX40L interactions on modifying CD8+ T-cell–mediated GVHD, anti-OX40 mAb administration had only modest effects in influencing alloengraftment in a CD8+ T-cell–mediated rejection system (Table 1). Moreover, anti-OX40L mAb showed no evidence of engraftment promoting properties in this setting (Table 1). These data demonstrate a more pronounced role for the OX40/OX40L pathway in regulating CD4+ as compared with CD8+ T cells alloresponses in vivo.
The role of OX40/OX40L in the engraftment of MHC class I or II-disparate T-cell-depleted donor BM grafts
| Group . | TBI . | Day . | No. . | Donor, % (SD) . | Host, % (SD) . |
|---|---|---|---|---|---|
| B6-CD45.1→bm12 | |||||
| Irrelevant mAb | 5.0 | 115 | 15 | 88 (4) | 12 (4) |
| αOX40 mAb | 5.0 | 115 | 15 | 16 (2)* | 87 (2)* |
| αOX40L mAb | 5.0 | 115 | 15 | 97 (1)* | 6 (1) |
| Irrelevant mAb | 4.5 | 120 | 13 | 81 (7) | 20 (6) |
| αOX40L mAb | 4.5 | 120 | 14 | 94 (1)† | 10 (1)† |
| B6-CD45.1→bm1 | |||||
| Irrelevant mAb | 5.0 | 97 | 15 | 88 (6) | 12 (5) |
| αOX40 mAb | 5.0 | 97 | 15 | 68 (9)† | 33 (9)† |
| Irrelevant mAb | 4.5 | 100 | 15 | 79 (8) | 22 (7) |
| αOX40L mAb | 4.5 | 100 | 15 | 65 (10) | 36 (10) |
| Group . | TBI . | Day . | No. . | Donor, % (SD) . | Host, % (SD) . |
|---|---|---|---|---|---|
| B6-CD45.1→bm12 | |||||
| Irrelevant mAb | 5.0 | 115 | 15 | 88 (4) | 12 (4) |
| αOX40 mAb | 5.0 | 115 | 15 | 16 (2)* | 87 (2)* |
| αOX40L mAb | 5.0 | 115 | 15 | 97 (1)* | 6 (1) |
| Irrelevant mAb | 4.5 | 120 | 13 | 81 (7) | 20 (6) |
| αOX40L mAb | 4.5 | 120 | 14 | 94 (1)† | 10 (1)† |
| B6-CD45.1→bm1 | |||||
| Irrelevant mAb | 5.0 | 97 | 15 | 88 (6) | 12 (5) |
| αOX40 mAb | 5.0 | 97 | 15 | 68 (9)† | 33 (9)† |
| Irrelevant mAb | 4.5 | 100 | 15 | 79 (8) | 22 (7) |
| αOX40L mAb | 4.5 | 100 | 15 | 65 (10) | 36 (10) |
B6-CD45.1 recipients (n = 15 per group) were sublethally irradiated with TBI doses, as indicated, on day −1 and then given 0.7 to 1 × 107 TCD BM from bm1 or bm12 donors and either irrelevant IgG, anti-OX40, or anti-OX40L mAb as described in ‘Materials and methods.” On the indicated day after BMT, all surviving recipients were phenotyped to determine the donor or host origin of peripheral blood mononuclear cells. Values shown are mean percentages. The standard deviation of the mean is listed in parentheses.
P ≤ .05 versus irrelevant mAb controls.
.05 < P ≤ .10 versus irrelevant mAb controls.
OX40L expression on donor T cells does not have a major effect on GVHD lethality
Although OX40L expression has been reported on T cells,11 the function of OX40L on in vivo T-cell alloresponses has not been examined. CD4+ T cells (105) from 129/Sv OX40L−/− mice or wild-type littermate controls were given to sublethally irradiated bm12 recipients (Figure 4A). There was no significant difference in survival of recipients of OX40L−/− versus wild-type (WT) T cells (56% versus 38%; P = .24). This was reproduced at a higher T-cell dose (data not shown). These findings are in sharp contrast to the 100% long-term survival rates obtained with anti-OX40L mAb in the B6→bm12 system at 2 different CD4+ T-cell doses, indicating that the major effect of anti-OX40L mAb is not on blocking T-cell–expressed OX40L from binding with OX40 receptor–expressing cells. As might be anticipated from our findings on the relative lack of importance of the OX40/OX40L pathway on CD8+ T-cell alloresponses, there were no significant differences in median survival time in sublethally irradiated bm1 recipients of 129/Sv OX40L−/− versus WT CD8+ T cells (Figure 4B). Moreover, in nonconditioned, NK cell–depleted BALB/c–SCID recipients, CD4+ T cells obtained from OX40L−/− mice and WT littermate controls had a comparable efficacy in inducing GVHD lethality (Figure 4C). Under the conditions of these various GVHD models, we were unable to uncover a major role for OX40L expression on donor T cells in regulating in vivo GVHD.
OX40L expression on donor T cells and regulation of GVHD lethality.
OX40L expression on donor T cells is not a potent regulator of CD4+ T-cell–mediated GVHD lethality. (A) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified CD4+ T cells (105) from either 129/Sv OX40L−/− (○) mice or OX40L+/+(●) littermate controls, as indicated. Data from 2 replicate experiments with similar results are pooled. Groupwise comparisons revealed that P = .24. (B) Sublethally irradiated bm1 recipients (n = 5 per group) were given highly purified CD8+ T cells (1 or 3 × 106, as indicated in parentheses) from either 129/Sv OX40L−/− mice or OX40L+/+ littermate controls. No signficant differences were noted between relevant groups. (C) Antiasialo-GM1 antisera–pretreated BALB/c–SCID mice were given purified CD4+ T cells (106) from 129/Sv OX40L−/− (▵) mice or OX40L+/+ (▴) littermate controls (n = 5 per group). Survival was not improved in recipients receiving OX40L−/− versus OX40L+/+CD4+ T cells.
OX40L expression on donor T cells and regulation of GVHD lethality.
OX40L expression on donor T cells is not a potent regulator of CD4+ T-cell–mediated GVHD lethality. (A) Sublethally irradiated bm12 recipients (n = 8 per group per experiment) were given highly purified CD4+ T cells (105) from either 129/Sv OX40L−/− (○) mice or OX40L+/+(●) littermate controls, as indicated. Data from 2 replicate experiments with similar results are pooled. Groupwise comparisons revealed that P = .24. (B) Sublethally irradiated bm1 recipients (n = 5 per group) were given highly purified CD8+ T cells (1 or 3 × 106, as indicated in parentheses) from either 129/Sv OX40L−/− mice or OX40L+/+ littermate controls. No signficant differences were noted between relevant groups. (C) Antiasialo-GM1 antisera–pretreated BALB/c–SCID mice were given purified CD4+ T cells (106) from 129/Sv OX40L−/− (▵) mice or OX40L+/+ (▴) littermate controls (n = 5 per group). Survival was not improved in recipients receiving OX40L−/− versus OX40L+/+CD4+ T cells.
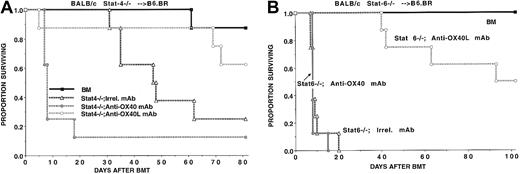
GVHD protection induced by OX40/OX40L blockade is not dependent upon Stat-4 or Stat-6 signaling
Some studies have proposed that OX40 receptor ligation preferentially supports Th2 differentiation, while others demonstrate that Th1 responses are also affected by the OX40 pathway.12-16,31,32 In some models, Stat signaling can regulate the propensity of T cells to differentiate into Th1 or Th2 cells. To determine whether the anti-GVHD effects of OX40/OX40L blockade were dependent upon Stat signaling, studies were performed with the use of splenocyte donors deficient in Stat-4 (typically Th1-defective) or Stat-6 (typically Th2-defective) signaling pathways.33 B6 recipients were lethally irradiated and reconstituted with BALB/c TCD BM and supplemental splenocytes (25 × 106) from Stat-4−/− or Stat-6−/− mice as indicated (Figure5). Cohorts of mice were given irrelevant, anti-OX40, or anti-OX40L mAb. Anti-OX40 mAb accelerated and anti-OX40L mAb inhibited GVHD lethality in recipients of Stat 4−/− splenocytes (Figure 5A). Anti-OX40L mAb also inhibited GVHD lethality induced by Stat-6−/− donor splenocytes (Figure 5B). Owing to the rapid lethality of Stat-6−/− splenocytes (88% lethality by day 7 after BMT), the effects of anti-OX40 mAb on accelerating GVHD lethality were not observed (Figure 5B). These data indicate that the inhibitory effect of OX40/OX40L blockade does not depend upon Stat-4 or Stat-6 signaling.
OX40/OX40L regulation of GVHD lethality and Stat-4 or Stat-6 signaling.
The regulation of GVHD lethality by the OX40/OX40L pathway does not depend upon Stat-4 or Stat-6 signaling in donor T cells. B6.BR recipients (n = 8 per group) were lethally irradiated, reconstituted with BALB/c BM, and given either no supplemental splenocytes or splenocytes (25 × 106) from BALB/c or BALB/c Stat-4−/− (panel A) or BALB/c Stat-6−/−(panel B) donors. Cohorts of mice that received splenocytes were given either irrelevant, anti-OX40, or anti-OX40L mAb, as indicated. Anti-OX40L mAb infusion significantly reduced GVHD lethality. Conversely, anti-OX40 mAb significantly increased GVHD lethality in recipients of Stat-4−/− splenocytes.
OX40/OX40L regulation of GVHD lethality and Stat-4 or Stat-6 signaling.
The regulation of GVHD lethality by the OX40/OX40L pathway does not depend upon Stat-4 or Stat-6 signaling in donor T cells. B6.BR recipients (n = 8 per group) were lethally irradiated, reconstituted with BALB/c BM, and given either no supplemental splenocytes or splenocytes (25 × 106) from BALB/c or BALB/c Stat-4−/− (panel A) or BALB/c Stat-6−/−(panel B) donors. Cohorts of mice that received splenocytes were given either irrelevant, anti-OX40, or anti-OX40L mAb, as indicated. Anti-OX40L mAb infusion significantly reduced GVHD lethality. Conversely, anti-OX40 mAb significantly increased GVHD lethality in recipients of Stat-4−/− splenocytes.
The effects of regulating OX40-OX40L interactions on GVHD lethality does not require CD28 signaling
Data from our group and others have shown that CD28/B7 interactions can modify GVHD responses.27,34 35 To determine if the anti-GVHD effects of OX40/OX40L blockade were redundant with CD28/B7 blockade, donor T cells were obtained from B6 CD28−/− mice and infused into lethally irradiated allogeneic recipients reconstituted with TCD B6 BM. B10.BR recipients received B6 CD28−/− splenocytes at 15 × 106 (Figure 6A) or 25 × 106 (Figure 6B), along with either irrelevant, anti-OX40, or anti-OX40L mAb. At both splenocyte doses, anti-OX40 mAb accelerated GVHD lethality while anti-OX40L mAb almost completely inhibited GVHD lethality. In other studies, the infusion of purified B6 CD28−/− T cells (3 × 106) into lethally irradiated, TCD BM-reconstituted BALB/c OX40L−/−recipients resulted in a significant survival advantage as compared with BALB/c OX40L+/+ littermate controls (Figure 6C). Taken together, these data indicate that the effects of the OX40/OX40L pathway on GVHD lethality in heavily irradiated recipients do not require CD28 signaling.
Effect of blockade of the OX40/OX40L pathway on GVHD lethality in settings in which CD28 signaling is precluded.
Blockade of the OX40/OX40L pathway is effective in inhibiting GVHD lethality in settings in which CD28 signaling is precluded. (A-B) B10.BR recipients (n = 8 per group) were lethally irradiated and reconstituted with B6 BM, and cohorts were given C28−/−splenocytes at 15 × 106 (panel A) or 25 × 106 (panel B). Recipients given splenocytes also received either irrelevant, anti-OX40, or anti-OX40L mAb. Survival was significantly different in groups receiving irrelevant mAb versus groups receiving either anti-OX40 or anti-OX40L mAb. (C) BALB/c OX40L−/− mice or OX40L+/+littermate controls (n = 5 per group) were lethally irradiated, reconstituted with B6 WT BM, and given either no T cells or B6 CD28−/− T cells (3 × 106). OX40L−/− recipients had a significantly higher survival rate than OX40L+/+ recipients.
Effect of blockade of the OX40/OX40L pathway on GVHD lethality in settings in which CD28 signaling is precluded.
Blockade of the OX40/OX40L pathway is effective in inhibiting GVHD lethality in settings in which CD28 signaling is precluded. (A-B) B10.BR recipients (n = 8 per group) were lethally irradiated and reconstituted with B6 BM, and cohorts were given C28−/−splenocytes at 15 × 106 (panel A) or 25 × 106 (panel B). Recipients given splenocytes also received either irrelevant, anti-OX40, or anti-OX40L mAb. Survival was significantly different in groups receiving irrelevant mAb versus groups receiving either anti-OX40 or anti-OX40L mAb. (C) BALB/c OX40L−/− mice or OX40L+/+littermate controls (n = 5 per group) were lethally irradiated, reconstituted with B6 WT BM, and given either no T cells or B6 CD28−/− T cells (3 × 106). OX40L−/− recipients had a significantly higher survival rate than OX40L+/+ recipients.
Discussion
The present study provides definitive data indicating that OX40-OX40L interactions are critical for CD4+ and far less critical for CD8+ T-cell alloresponses in vivo. The beneficial effects of blockade of the OX40/OX40L pathway did not require CD28 signaling. Although some studies have shown that the OX40/OX40L pathway is a more potent regulator of Th2 than Th1 responses, suggesting that this pathway may be preferentially affected by Stat-6 versus Stat-4 signaling, our studies using splenocytes obtained from Stat-4−/− or Stat-6−/− donors indicate that this is not the case.
We have conclusively demonstrated that the OX40/OX40L pathway is an important regulator of GVHD in a variety of GVHD models with different pathophysiological mechanisms. These data extend those of Tsukada et al,25 who used a single donor/recipient strain combination (C57BL/6→C57BL/6 × DBA/2)F1) and a single approach (anti-OX40L mAb administration) to reduce GVHD lethality. However, this strain combination typifies a Th1/Tc1-mediated GVHD response and may not be representative of other GVHD systems in which both Th1/Tc1 and Th2/Tc2 responses can contribute to lethality. Moreover, the separate contribution of CD4+ and CD8+ T cells to GVHD lethality in their GVHD was not examined, which is relevant since GVHD in mice and humans may be dominated by either CD4+ or CD8+ T cells. Additionally, anti-OX40L mAb may directly affect donor T cells since OX40L has been reported to be up-regulated on activated CD4+ and CD8+ T cells.
Our studies extend the literature by demonstrating the importance of the OX40/OX40L pathway in regulating GVHD by using 4 distinct and complementary approaches: agonistic and antagonistic mAbs and genetic deletion of either the OX40 receptor or OX40L. Three GVHD systems in which recipients were lethally irradiated and given MHC-disparate donor T cells were used to determine the effects of OX40/OX40L on GVHD induced early in the post-BMT period by both CD4+ and CD8+ T cells. Since the injury induced by heavy irradiation can lessen the requirement for T-cell costimulation early in the post-BMT period, additional studies using a DLI model were performed in which the OX40 receptor was purposefully engaged by agonistic mAb later in the post-BMT period, at a time when proinflammatory cytokine release and irradiation-induced tissue injury have subsided. In each experimental setting, the OX40/OX40L pathway had a major biological effect in regulating GVHD lethality.
We show in both GVHD and alloengraftment systems that the dominant effect of the OX40/OX40L pathway is on alloreactive CD4+ and not CD8+ T cells. Although the majority of the literature has focused upon CD4+ T-cell responses, several studies have shown that this pathway also regulates CD8+ T-cell responses. Chen et al13 observed decreased CD4+ and CD8+ T-cell proliferation in OX40L−/− mice challenged with oxazolone. Kjaergaard et al36 have reported that OX40 receptor is up-regulated on tumor-infiltrating CD8+ T cells. In addition, therapeutic antitumor effects due to CD8+ cells were observed in anti-OX40 mAb–treated recipients of adoptively transferred T-cell populations. De Smedt et al15 showed that anti-OX40 mAb administration enhanced the expansion and acquisition of cytotoxic T lymphocyte (CTL) activity by antigen-specific CD8+T cells that were exposed to antigen-pulsed DCs. In contrast, other studies have shown that CTL responses to viral challenge were intact in mice that lack either OX40 or its ligand.11,13 37 Even though our studies indicate a more modest direct effect of the OX40/OX40L pathway on in vivo CD8+ versus CD4+T-cell alloresponses, it is possible that CD8+ T cells may be more vigorously affected by this pathway under other circumstances. Also, it is important to note that although the OX40 pathway may have only a modest direct effect on CD8+ T cells, effects on CD4+ T-cell help may have critical indirect consequences on CD8+ T-cell expansion or the induction of CD8+CTLs. Therefore, blockade of the OX40 pathway may have significant impact on CD8+-dependent responses by the interference of CD4+ helper function to CTLs.
Our data indicate that the beneficial effects of blocking the OX40/OX40L pathway does not require CD28 signaling. It has been shown that these 2 costimulatory pathways can provide nonredundant functions as assessed in vitro since OX40 receptor expression can be induced on T cells from CD28−/− mice, and the stimulation of CD28−/− by activated B cells is substantially inhibited by anti-OX40L mAb.26 Walker et al38,39demonstrated that blockade of the OX40/OX40L pathway reduces the effects of anti-CD28 in restoring the defective germinal center formation in CTL antigen–4 (CTLA4)–immunoglobulin transgenic mice. Pippig et al have shown that the defective signaling of OX40−/− T cells could not be restored by anti-CD28 mAb, indicating that these pathways are nonredundant under these in vitro conditions,11 and Gramaglia et al17 have shown that B7-1 and OX40L act synergistically to stimulate proliferation and cytokine production by naive CD4+ T cells. In contrast, Ndhlovu and colleagues40 provide data indicating a dependency of the biological effects of the OX40/OX40L pathway on the CD28/B7 pathway in regulating experimental autoimmune encephalomyelitis (EAE) generation. Our current and previous studies, along with those of other investigators, have shown that blocking the CD28/B7 pathway alone by using CD28−/− donor T cells was insufficient to uniformly prevent lethal GVHD.27,34 35 Similarly, GVHD lethality is not uniformly prevented by OX40/OX40L blockade. The high survival rate of recipients given CD28−/− donor T cells and anti-OX40L mAb suggest that the coblockade of these 2 pathways may be particularly advantageous in inhibiting GVHD.
Compelling evidence exists that OX40 signaling can support CD4+ T cells to develop into Th2 cells.31,41,42 However, not all studies have shown the generation of Th2 responses to have an absolute dependence on the OX40/OX40L pathway.11 In our studies, GVHD lethality was lessened by the administration of anti-OX40L mAb to recipients of Th2- as well as Th1-defective donor splenocytes, indicating that the GVHD-preventive effect of anti-OX40L mAb was not strictly due to the preferential induction of Th2 responses, as has been suggested by other GVHD studies.28 Our studies indicate that the regulation of GVHD lethality by the OX40/OX40L pathway does not depend upon either Stat-4 (Th1) or Stat-6 (Th2) signaling.
Although OX40L expression has been reported on activated T cells, we did not observe differences in alloresponses in vitro as measured with the use of OX40L−/− T cells in primary MLR cultures.11,13 Consistent with those in vitro findings, donor OX40L−/− T cells were still capable of mediating lethal GVHD similarly to wild-type T cells. These data are in striking contrast to the in vivo infusion of anti-OX40L mAb, indicating that the major effects of this mAb are on recipient, and not donor, cells. Anti-OX40L mAb probably has its major effect by blocking the costimulation of donor T cells by recipient cells. Candidate recipient cells would include DCs, activated B cells, and activated endothelium. There are at least 2 main consequences of this blockade. The first would be to inhibit the initial expansion of alloreactive T cells and sustained expansion and long-term survival of antigen-activated CD4+ T cells. This could be accomplished by reducing the proportion of alloreactive CD4+ T cells that enter cell cycle upon encountering host alloantigens or by the induction of antiapoptotic genes, including bcl-xL and bcl-2.14,16-19 Our data also indicate that coblockade of the CD28/B7 pathway may serve to further inhibit initial expansion of alloreactive T cells. Conversely, strategies to augment OX40 signaling may be highly advantageous in generating antitumor cell responses by promoting initial expansion and the generation of a larger antitumor memory T-cell pool.21,36 Finally, an additional advantage of blocking the OX40/OX40L pathway may be to impede the homing and migration of activated donor T cells to GVHD target organs via binding to OX40L-expressing endothelial cells or cells within critical lymphohematopoietic organs that are essential for priming alloresponses.38 39
Although an agonistic anti-OX40 mAb was not well tolerated when given 3 weeks after BMT along with DLI, it is possible that anti-OX40 mAb could be used to induce antitumor effects after BMT when combined with either no DLI or lower DLI cell doses. Such an approach may be of particular benefit in an autologous setting because signaling via OX40 can break peripheral tolerance, which may be therapeutically helpful in patients with residual post-BMT hematological malignancy that may continue to be tolerized by tumor antigens.43
Our studies indicate that the OX40/OX40L pathway has a broad importance in GVHD induction. Several but not all studies in humans have suggested that OX40 up-regulation can precede acute GVHD generation and may be a marker of steroid-resistant acute GVHD or chronic GVHD.44-47 Regardless of whether the up-regulation of OX40 receptor is of prognostic significance, interruption of the OX40/OX40L pathway early in the post-BMT period warrants testing as an approach to prevent GVHD and allogeneic BM graft rejection.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-10-3048.
Supported in part by NIH grants ROI AI34495, ROI CA72669, ROI HL63452, and R37 HL56067.
B.R.B. and A.H.S. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, University of Minnesota Hospital, Box 109 Mayo Bldg, 420 SE Delaware St, Minneapolis, MN 55455; e-mail: blaza001@tc.umn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal