Abstract
The unknown biochemical basis for neurologic dysfunction in cobalamin deficiency and the frequent divergence between neurologic and hematologic manifestations led us to study homocysteine metabolism in 22 patients with pernicious anemia. Serum levels of total homocysteine (tHcy), methionine, S-adenosylmethionine (AdoMet), cysteine, cysteinylglycine (cys-gly), and glutathione (GSH) were measured. Only levels of tHcy and cysteine were increased and only GSH was decreased in cobalamin deficiency as a whole, compared with 17 control subjects. AdoMet correlated only with methionine levels (P = .015) and cysteine only with cys-gly (P = .007) in healthy subjects, but in cobalamin-deficient patients AdoMet correlated instead with cysteine, cys-gly, and folate levels only (P = .008,P = .03, and P = .03, respectively). Significant differences appeared in clinically subgrouped cobalamin-deficient patients. The 11 patients with neurologic defects had higher mean levels of folate (27.9 versus 15.4 nM), AdoMet (117.2 versus 78.6 nM), cysteine (462 versus 325 μM), and cys-gly (85.0 versus 54.7 μM) than the 11 neurologically unaffected patients. Cobalamin therapy restored all metabolic changes to normal. The results indicate that changes in several metabolic pathways differ in patients with and without neurologic dysfunction. Cysteine levels were the most significant predictors of neurologic dysfunction, but it is unclear if they are direct or indirect indicators of neurotoxicity. The higher AdoMet levels in neurologically affected patients may result from inhibition of glycine N-methyltransferase by those patients' higher folate levels. The origin of the folate differences is unclear and possibly varied. Low AdoMet and GSH levels were independent predictors of anemia.
Introduction
The biochemical basis for the neurologic dysfunction and the reason some patients develop it whereas others do not remain important unresolved questions in cobalamin deficiency. Most evidence favors the impairment of the methionine synthase reaction, which requires both cobalamin and 5-methyltetrahydrofolate (methylTHF), as the biochemical point of origin for both the hematologic and neurologic defects.1-3However, it appears plausible that the metabolic consequences that follow methionine synthase impairment differ between patients who develop neurologic problems and those who develop hematologic problems. Those unknown metabolic differences must also account for several clinical paradoxes. One paradox is that, despite some limited clinical similarities, the neurologic findings in cobalamin and folate deficiencies, which both affect methionine synthase activity, differ substantially,4 whereas the anemias are identical. The other paradox is that neurologic and hematologic manifestations not only diverge in cobalamin-deficient patients,5 but their occurrence and severity tend to be inversely related to each other.6,7 Furthermore, whichever clinical manifestation predominates also tends to recur if the deficiency relapses.7 8
Therefore, we studied patients with pernicious anemia, a disease in which deficiency is limited largely to cobalamin. The goal was to compare serum metabolites relevant to homocysteine metabolism, both its methylation to methionine and its catabolism via the transsulfuration and related thiol pathways, in patients with and without neurologic abnormalities. Some combinations of these metabolites in cobalamin deficiency have been compared with those present in healthy people,9-12 but clinical subsets, especially of neurologically impaired patients, have not been identified or compared with each other.
Patients, materials, and methods
Patients and blood samples
Patients with pernicious anemia were studied as the purest available models of cobalamin deficiency. Selection was retrospective, except for 2 patients identified after the study was begun. All patients met the following criteria: evidence of cobalamin deficiency based on a low serum cobalamin level, abnormal findings in serum total homocysteine (tHcy) as well as methylmalonic acid and/or deoxyuridine suppression testing, and in most cases evidence of clinical response to cobalamin therapy; proof of pernicious anemia by Schilling test and/or intrinsic factor antibody and serum gastrin testing; availability of blood samples before cobalamin therapy was given; and a serum creatinine level of 115 μM (1.3 mg/dL) or lower. Known use of folate supplements excluded patients from the study. Dietary intake andS-adenosylmethionine (AdoMet) supplement use were not assessed; however, most patients were seen before food fortification with folate was introduced and, being mostly older inner city dwellers, who rarely use supplements, few if any patients could afford the expensive AdoMet supplements.
Blood count results and blood smears were available in all but 3 patients, whose results were lost but for whom notes indicated the presence of megaloblastic anemia. Mean hemoglobin level was 85 ± 42 g/L (8.5 ± 4.2 g/dL), and mean corpuscular volume (MCV) was 109 ± 16 fL. Neurologic dysfunction was attributed to cobalamin deficiency if the manifestations were consistent with this and the patient had no coexisting disorders, such as diabetes or chronic alcoholism, that cause similar manifestations. The 11 patients with neurological abnormalities had the following findings: 3 presented with difficulty walking (and had positive Romberg signs, extensive vibratory sense loss, reflex changes, and numbness and tingling in the legs; 1 of these patients also had mental changes); 2 others presented with poor memory and/or disorientation (in 1 of them accompanied by myelopathic findings); 5 other patients noted numbness of the feet (extending to the hips and/or hands in several cases, and accompanied by typical findings on examination); and the last patient had no neurologic symptoms, but vibratory sense, proprioception, and deep tendon reflexes were diminished. Follow-up was available in all but 3 cases and showed neurologic improvement after cobalamin therapy. None of the 11 neurologically intact patients had neurologic symptoms or abnormal physical findings.
In some patients, additional serum samples were obtained after parenteral cobalamin therapy was begun. All pretreatment and posttreatment samples were centrifuged within 4 hours of venipuncture, usually within 2 hours. After centrifugation, all aliquots, except those from the 2 new, prospectively diagnosed cases, were stored for 1 to 8 years at −20°C. To control for the effects of sample processing and storage, identically processed specimens stored for 1 to 8 years were selected from 17 control subjects with documented normal cobalamin status.
Laboratory methods
Methionine, AdoMet, total cysteine, total cysteinylglycine (cys-gly), and total glutathione (GSH) were measured by reversed-phase high-performance liquid chromatography (HPLC) with coulometric electrochemical detection.13 14 We measured tHcy by fluorescence-polarization immunoassay (IMx; Abbott Diagnostics, Abbott Park, IL) according to the manufacturer's instructions. Serum cobalamin and folate levels had been measured by radioisotopic dilution assay at the time of each patient's diagnosis, by means of the Dualcount-boil kit (Diagnostic Products, Los Angeles, CA).
Because homocysteine is released from blood cells at room temperature when separation is delayed, comparisons were made to ascertain if similar in vitro changes apply to other metabolites. First, we compared results of 29 serum and simultaneously obtained and processed plasma samples from control subjects and cobalamin-deficient patients. Pairedt test analyses showed virtually identical values in serum and plasma, with the exception of methionine levels. Methionine levels, although significantly correlated in the matched samples for each subject, were lower in serum than in plasma (16.9 ± 9.0 versus 24.6 ± 9.3 μM). The effect of time elapsed to centrifugation was also assessed in 4 healthy volunteers; aliquots of their fresh blood samples were incubated for 1 and 5 hours after venipuncture. AdoMet and S-adenosylhomocysteine (AdoHcy) levels were not significantly different at 5 hours than at 1 hour; manipulation of cell concentrations in aliquots showed that the hematocrit of the sample had no effect (data not shown), suggesting that anemic samples do not behave differently from nonanemic ones.
To address the effect of prolonged storage of frozen samples, we also assayed 17 control sera stored for 1 to 8 years. The results, shown in Table 1, were consistent with those in fresh control samples established in our laboratory14 and with data from other laboratories,9,12,15-18 including studies that used specimens stored for several years.10 19The sole problem occurred with AdoHcy; our control sera produced abnormally high mean values and wide variations among samples. Therefore, AdoHcy results were deemed unreliable in the stored samples and are not reported here.
Results in all 22 untreated patients with cobalamin deficiency (pernicious anemia)
| . | Cobalamin-deficient patients, n = 22 . | Control subjects, n = 17 . |
|---|---|---|
| Total homocysteine level, μM | 98.1 ± 54.4* | 11.1 ± 2.0 |
| S-adenosylmethionine level, nM | 97.9 ± 31.9 | 87.6 ± 37.3 |
| Methionine level, μM | 22.3 ± 10.0 | 17.9 ± 10.8 |
| Cysteine level, μM | 390 ± 106* | 242 ± 49 |
| Cysteinylglycine level, μM | 69.1 ± 24.4 | 59.0 ± 15.9 |
| Glutathione level, μM | 2.1 ± 1.3† | 4.9 ± 2.8 |
| Cobalamin level, pM | 33 ± 34* | 291 ± 115 |
| Folate level, nM | 21.5 ± 11.8 | 32.0 ± 17.6 |
| . | Cobalamin-deficient patients, n = 22 . | Control subjects, n = 17 . |
|---|---|---|
| Total homocysteine level, μM | 98.1 ± 54.4* | 11.1 ± 2.0 |
| S-adenosylmethionine level, nM | 97.9 ± 31.9 | 87.6 ± 37.3 |
| Methionine level, μM | 22.3 ± 10.0 | 17.9 ± 10.8 |
| Cysteine level, μM | 390 ± 106* | 242 ± 49 |
| Cysteinylglycine level, μM | 69.1 ± 24.4 | 59.0 ± 15.9 |
| Glutathione level, μM | 2.1 ± 1.3† | 4.9 ± 2.8 |
| Cobalamin level, pM | 33 ± 34* | 291 ± 115 |
| Folate level, nM | 21.5 ± 11.8 | 32.0 ± 17.6 |
Significantly different from control values (P< .0001).
Significantly different from control values (P = .01).
Statistical analysis
When continuous variables in the study were positively skewed, log scales were applied to them in tests of significance that require assumptions of normal distribution. Between-group comparisons were tested by means of one-way analysis of variance, 2-group ttests, or Fisher exact test. Correlations between continuous variables were evaluated by means of Pearson correlations. Following univariatet test analyses, stepwise logistic regression analyses were performed to sort out variables as independent predictors of the dependent variable. Stepwise analysis of covariance was used to test discontinuous variables, such as neurologic or hematologic status, as well as continuous metabolic variables, to predict metabolic findings. All statistical analyses were done by means of SAS computer software, release 6.12 (SAS Institute, Cary, NC).
Results
The 22 patients with pernicious anemia showed that cobalamin deficiency was associated not only with significantly higher tHcy levels but also with higher cysteine and lower GSH levels than in control subjects (Table 1). The control subjects showed significant correlations only between AdoMet and methionine levels (r = .61, P = .015) and between cysteine and cys-gly levels (r = .86, P = .007) among all the metabolite results. However, the metabolic associations were quite different in cobalamin deficiency. In the 22 cobalamin-deficient patients, AdoMet did not correlate with methionine but with cysteine (r = .56, P = .008), cys-gly (r = .47, P = .03), and folate levels (r = .47, P = .03) instead; the last 3 levels also correlated with each other (cysteine and cys-gly,r = .57, P = .01; cysteine and folate,r = .54, P = .02; and cys-gly and folate,r = .59, P = .007).
Following these preliminary analyses, attention focused on comparing neurologically affected and unaffected patients. The presence or absence of megaloblastic anemia was also subjected to metabolic analyses.
Metabolic profiles associated with neurologic status
Table 2 compares data in the 11 cobalamin-deficient patients who had neurologic abnormalities and the 11 who did not. The patients with neurologic abnormalities had significantly higher AdoMet, cysteine, cys-gly, and folate levels than the patients without neurologic deficits (Table 2). Stepwise logistic regression analysis showed that cysteine (P = .003) was the most significant predictor of neurologic status.
Comparison of results in cobalamin-deficient patients with and without neurologic abnormalities
| . | With neurologic abnormalities, n = 11 . | Without neurologic abnormalities, n = 11 . |
|---|---|---|
| Total homocysteine level, μM | 97.6 ± 60.8 (104.3) | 98.5 ± 50.2 (88.9) |
| S-adenosylmethionine level, nM | 117.2 ± 24.7* (126.5) | 78.6 ± 26.6* (80.5) |
| Methionine level, μM | 20.7 ± 10.4 (18.3) | 23.9 ± 9.8 (20.4) |
| Cysteine level, μM | 462 ± 90† (449) | 325 ± 73†(297) |
| Cysteinylglycine level, μM | 85.0 ± 24.5‡(88.0) | 54.7 ± 13.0‡ (52.5) |
| Glutathione level, μM | 2.3 ± 1.4 (1.9) | 1.9 ± 1.2 (2.0) |
| Cobalamin level, pM | 32 ± 29 (31) | 35 ± 39 (37) |
| Folate level, nM | 27.9 ± 12.52-153 (29.0) | 15.4 ± 7.52-153 (17.9) |
| Hemoglobin level, g/L | 108 ± 452-153(118) | 63 ± 312-153 (52) |
| MCV, fL | 109 ± 19 (111) | 110 ± 14 (115) |
| . | With neurologic abnormalities, n = 11 . | Without neurologic abnormalities, n = 11 . |
|---|---|---|
| Total homocysteine level, μM | 97.6 ± 60.8 (104.3) | 98.5 ± 50.2 (88.9) |
| S-adenosylmethionine level, nM | 117.2 ± 24.7* (126.5) | 78.6 ± 26.6* (80.5) |
| Methionine level, μM | 20.7 ± 10.4 (18.3) | 23.9 ± 9.8 (20.4) |
| Cysteine level, μM | 462 ± 90† (449) | 325 ± 73†(297) |
| Cysteinylglycine level, μM | 85.0 ± 24.5‡(88.0) | 54.7 ± 13.0‡ (52.5) |
| Glutathione level, μM | 2.3 ± 1.4 (1.9) | 1.9 ± 1.2 (2.0) |
| Cobalamin level, pM | 32 ± 29 (31) | 35 ± 39 (37) |
| Folate level, nM | 27.9 ± 12.52-153 (29.0) | 15.4 ± 7.52-153 (17.9) |
| Hemoglobin level, g/L | 108 ± 452-153(118) | 63 ± 312-153 (52) |
| MCV, fL | 109 ± 19 (111) | 110 ± 14 (115) |
Values are shown as mean ± SD and, in parentheses, median. Significant differences are highlighted in boldface type.
Significant difference between the 2 groups' values,P = .002.
Significant difference between the 2 groups' values,P = .001.
Significant difference between the 2 groups' values,P = .004.
Significant difference between the 2 groups' values,P = .02.
Hemoglobin levels, adjusted for sex, were higher in patients with neurologic dysfunction than in those without (P = .02; Table 2). Because of the inverse relationship between neurologic and hematologic abnormalities, analyses examined whether the metabolic differences were related to the presence or absence of neurologic abnormality or to the presence or absence of megaloblastic anemia. The cobalamin-deficient patients were redivided into 3 groups: 1 group with neurologic dysfunction but with no or only minimal anemia (ie, having a sex-adjusted hemoglobin level exceeding 130 g/L [13 g/dL]); 1 with anemia (adjusted hemoglobin level 32 to 127 g/L [3.2 to 12.7 g/dL]) but no neurologic dysfunction; and 1 with both manifestations together (Table3). Cysteine, AdoMet, cys-gly, GSH, and folate levels differed significantly among the 3 subgroups, but methionine and cobalamin levels did not. Higher AdoMet, folate, cysteine, and cys-gly values tended to segregate in neurologically impaired patients, whether or not the patients had anemia. GSH levels, on the other hand, were lower in anemic patients with and without neurologic dysfunction than in patients with neurologic dysfunction alone. The tHcy pattern differed from the other substances in that patients with combined neurologic and hematologic abnormalities had higher tHcy levels than patients with either manifestation alone.
Comparison of results in cobalamin-deficient (pernicious anemia) patients with neurologic abnormalities only, hematologic abnormalities only, or both
| . | Manifestations of cobalamin deficiency . | P3-150 . | ||
|---|---|---|---|---|
| . | Neurologic only, n = 5 . | Anemia only, n = 11 . | Both, n = 6 . | |
| tHcy level, μM | 45.8 ± 34.9 | 98.5 ± 50.1 | 140.8 ± 38.5 | .008 |
| AdoMet level, nM | 126.0 ± 12.3 | 78.6 ± 26.6 | 109.9 ± 30.9 | .006 |
| Methionine level, μM | 24.0 ± 13.6 | 23.8 ± 9.7 | 17.9 ± 7.1 | NS |
| Cysteine level, μM | 503 ± 83 | 325 ± 73 | 421 ± 84 | .002 |
| Cys-gly level, μM | 82.6 ± 9.7 | 54.7 ± 13.0 | 87.4 ± 35.3 | .009 |
| GSH level, μM | 3.3 ± 1.2 | 1.9 ± 1.2 | 1.3 ± 0.8 | .03 |
| Cobalamin level, pM | 39 ± 43 | 35 ± 39 | 27 ± 13 | NS |
| Folate level, nM | 30.1 ± 13.1 | 15.4 ± 7.5 | 26.3 ± 12.9 | .048 |
| Hemoglobin level, g/L | 148 ± 10 | 63 ± 31 | 81 ± 38 | .001 |
| MCV, fL | 97 ± 9 | 109 ± 14 | 117 ± 19 | NS |
| . | Manifestations of cobalamin deficiency . | P3-150 . | ||
|---|---|---|---|---|
| . | Neurologic only, n = 5 . | Anemia only, n = 11 . | Both, n = 6 . | |
| tHcy level, μM | 45.8 ± 34.9 | 98.5 ± 50.1 | 140.8 ± 38.5 | .008 |
| AdoMet level, nM | 126.0 ± 12.3 | 78.6 ± 26.6 | 109.9 ± 30.9 | .006 |
| Methionine level, μM | 24.0 ± 13.6 | 23.8 ± 9.7 | 17.9 ± 7.1 | NS |
| Cysteine level, μM | 503 ± 83 | 325 ± 73 | 421 ± 84 | .002 |
| Cys-gly level, μM | 82.6 ± 9.7 | 54.7 ± 13.0 | 87.4 ± 35.3 | .009 |
| GSH level, μM | 3.3 ± 1.2 | 1.9 ± 1.2 | 1.3 ± 0.8 | .03 |
| Cobalamin level, pM | 39 ± 43 | 35 ± 39 | 27 ± 13 | NS |
| Folate level, nM | 30.1 ± 13.1 | 15.4 ± 7.5 | 26.3 ± 12.9 | .048 |
| Hemoglobin level, g/L | 148 ± 10 | 63 ± 31 | 81 ± 38 | .001 |
| MCV, fL | 97 ± 9 | 109 ± 14 | 117 ± 19 | NS |
tHcy indicates total homocysteine; AdoMet,S-adenosylmethionine; cys-gly, cysteinylglycine; GSH, glutathione; and NS, not significant.
All levels for which P is given are significant.
Stepwise analyses of covariance in the entire patient cohort supported the likelihood that the metabolic changes reflected neurologic status more than hematologic status. Univariate analyses had shown that neurologic status (P = .002), hematologic status (P = .02), cysteine levels (P = .01), cys-gly levels (P = .03), and folate levels (P = .03) were significantly associated with AdoMet levels. Only neurologic status remained a significant predictor for AdoMet levels (P = .02) after the analyses of covariance. Neurologic status also remained the sole significant predictor of cysteine (P = .001) and cys-gly levels (P = .002) in analyses of covariance.
Patients with and without anemia (regardless of neurologic status) differed significantly in their AdoMet (P = .001), GSH (P = .02), and cysteine levels (P = .03). Stepwise logistic regression analysis showed that AdoMet (P = .01) and GSH (P = .02) levels were the sole independent predictors of anemia. Four anemic patients had GSH levels below 1.0 μM, and 2 others had levels of 1.0 and 1.2 μM; nonanemic patients had low GSH levels too, but none below 2.2 μM.
Effect of cobalamin therapy
Serum specimens after initiation of cobalamin therapy were available in 7 patients with neurologic abnormalities (Table4) and 3 without neurologic abnormalities (Table 5). In all cases, tHcy levels decreased with therapy, from 119.6 ± 44.6 to 13.6 ± 3.5 μM (P < .0001).
Changes in serum total homocysteine,S-adenosylmethionine, cysteine, and glutathione levels after therapy in 7 cobalamin-deficient patients with neurologic abnormalities
| Patient . | Abnormality . | Day of Cbl therapy . | tHcy level, μM . | AdoMet level, nM . | Cysteine level, μM . | Cys-gly level, μM . | GSH level, μM . | |
|---|---|---|---|---|---|---|---|---|
| CNS . | Anemia . | |||||||
| 1 | Yes | Yes | Pretherapy | 163.3 | 132.1 | 393 | 121.7 | 0.7 |
| > 90 days | 13.5 | 117.3 | 245 | 98.6 | 2.9 | |||
| 2 | Yes | Yes | Pretherapy | 130.3 | 133.2 | 398 | 68.0 | 1.9 |
| 2 days | 27.4 | 113.9 | NA | NA | NA | |||
| 12 days | 15.3 | 139.8 | 181 | 69.1 | 8.0 | |||
| 30 days | 12.9 | 76.1 | 198 | 88.5 | 7.6 | |||
| 3 | Yes | Yes | Pretherapy | 181.5 | 125.6 | 540 | 98.3 | 2.4 |
| > 90 days | 15.3 | 74.5 | 175 | 68.6 | 4.0 | |||
| 4 | Yes | Yes | Pretherapy | 136.2 | 126.5 | NA | NA | NA |
| > 90 days | 9.0 | 95.1 | 165 | 82.3 | 9.2 | |||
| 5 | Yes | No | Pretherapy | 37.2 | 135.2 | 595 | 74.6 | 2.2 |
| Folate; 60 days4-150 | 33.44-150 | 96.64-150 | 1894-150 | 74.94-150 | 10.34-150 | |||
| 30 days4-151 | 9.44-151 | 56.64-151 | 1894-151 | 74.44-151 | 10.44-151 | |||
| 6 | Yes | Yes | Pretherapy | 72.2 | 80.2 | 315 | 35.9 | 0.8 |
| 2 days | 25.1 | 113.2 | NA | NA | NA | |||
| 22 days | 19.9 | 136.5 | 195 | 64.1 | 2.4 | |||
| 7 | Yes | No | Pretherapy | 104.3 | 109.6 | 436 | 92.2 | 4.4 |
| > 90 days | 16.3 | 122.5 | 190 | 73.7 | 5.3 | |||
| Patient . | Abnormality . | Day of Cbl therapy . | tHcy level, μM . | AdoMet level, nM . | Cysteine level, μM . | Cys-gly level, μM . | GSH level, μM . | |
|---|---|---|---|---|---|---|---|---|
| CNS . | Anemia . | |||||||
| 1 | Yes | Yes | Pretherapy | 163.3 | 132.1 | 393 | 121.7 | 0.7 |
| > 90 days | 13.5 | 117.3 | 245 | 98.6 | 2.9 | |||
| 2 | Yes | Yes | Pretherapy | 130.3 | 133.2 | 398 | 68.0 | 1.9 |
| 2 days | 27.4 | 113.9 | NA | NA | NA | |||
| 12 days | 15.3 | 139.8 | 181 | 69.1 | 8.0 | |||
| 30 days | 12.9 | 76.1 | 198 | 88.5 | 7.6 | |||
| 3 | Yes | Yes | Pretherapy | 181.5 | 125.6 | 540 | 98.3 | 2.4 |
| > 90 days | 15.3 | 74.5 | 175 | 68.6 | 4.0 | |||
| 4 | Yes | Yes | Pretherapy | 136.2 | 126.5 | NA | NA | NA |
| > 90 days | 9.0 | 95.1 | 165 | 82.3 | 9.2 | |||
| 5 | Yes | No | Pretherapy | 37.2 | 135.2 | 595 | 74.6 | 2.2 |
| Folate; 60 days4-150 | 33.44-150 | 96.64-150 | 1894-150 | 74.94-150 | 10.34-150 | |||
| 30 days4-151 | 9.44-151 | 56.64-151 | 1894-151 | 74.44-151 | 10.44-151 | |||
| 6 | Yes | Yes | Pretherapy | 72.2 | 80.2 | 315 | 35.9 | 0.8 |
| 2 days | 25.1 | 113.2 | NA | NA | NA | |||
| 22 days | 19.9 | 136.5 | 195 | 64.1 | 2.4 | |||
| 7 | Yes | No | Pretherapy | 104.3 | 109.6 | 436 | 92.2 | 4.4 |
| > 90 days | 16.3 | 122.5 | 190 | 73.7 | 5.3 | |||
Methionine values did not change significantly or consistently with therapy and are not shown.
CNS indicates central nervous system; Cbl, cobalamin; and NA, not available. See Table 3 for other abbreviations.
The patient was incorrectly treated for 60 days with folic acid instead of cobalamin.
Results 30 days after folate was discontinued and parenteral cobalamin was begun.
Changes in serum total homocysteine,S-adenosylmethionine, cysteine, and glutathione levels after therapy in 3 cobalamin-deficient patients without neurological abnormalities
| Patient . | Abnormality . | Day of Cbl therapy . | tHcy level, μM . | AdoMet level, nM . | Cysteine level, μM . | Cys-gly level, μM . | GSH level, μM . | |
|---|---|---|---|---|---|---|---|---|
| CNS . | Anemia . | |||||||
| A | No | Yes | Pretherapy | 159.2 | 54.4 | 335 | 67.2 | 0.5 |
| 14 days | 17.0 | 79.1 | 181 | 64.1 | 2.4 | |||
| B | No | Yes | Pretherapy | 88.9 | 50.0 | 316 | 52.5 | 1.0 |
| 30 days | 11.3 | 128.3 | 189 | 70.9 | 6.2 | |||
| C | No | Yes | Pretherapy | 122.0 | 81.8 | 472 | 47.7 | 0.9 |
| > 90 days | 10.9 | 110.4 | 174 | 81.1 | 6.6 | |||
| Patient . | Abnormality . | Day of Cbl therapy . | tHcy level, μM . | AdoMet level, nM . | Cysteine level, μM . | Cys-gly level, μM . | GSH level, μM . | |
|---|---|---|---|---|---|---|---|---|
| CNS . | Anemia . | |||||||
| A | No | Yes | Pretherapy | 159.2 | 54.4 | 335 | 67.2 | 0.5 |
| 14 days | 17.0 | 79.1 | 181 | 64.1 | 2.4 | |||
| B | No | Yes | Pretherapy | 88.9 | 50.0 | 316 | 52.5 | 1.0 |
| 30 days | 11.3 | 128.3 | 189 | 70.9 | 6.2 | |||
| C | No | Yes | Pretherapy | 122.0 | 81.8 | 472 | 47.7 | 0.9 |
| > 90 days | 10.9 | 110.4 | 174 | 81.1 | 6.6 | |||
Methionine cycle.
Among the 7 retested neurologically affected patients, patients 1 through 5 (Table 4) had high-normal pretreatment levels of AdoMet (exceeding 125 nM). Their AdoMet levels all decreased after cobalamin therapy (from 130.5 ± 4.2 to 83.9 ± 23.1 nM;P = .002). AdoMet levels rose from 75.2 ± 24.1 to 115.4 ± 22.4 nM (P = .03) with therapy in all 3 patients without neurologic abnormalities (Table 5) and in the 2 neurologically affected patients who had lower pretreatment levels (patients 6 and 7; Table 4). Interestingly, the rise in the lower AdoMet levels began within 2 days of starting therapy (patient 6), but the decrease of high-normal AdoMet values with therapy was delayed until longer than 12 days elapsed (patient 2) even though tHcy levels improved within 2 days in both cases.
Of incidental note, mistaken folate therapy given instead of cobalamin induced partial improvement in AdoMet levels, even though tHcy levels remained unchanged after 60 days (Table 4; patient 5). After appropriate cobalamin therapy was instituted, tHcy levels responded and AdoMet levels improved further.
No consistent changes occurred in methionine levels; levels were 23.5 ± 11.1 μM before and 21.9 ± 10.0 μM after cobalamin therapy (P = .74).
Transsulfuration and related thiol pathways.
Serum total cysteine levels, elevated in all the patients, fell consistently with cobalamin therapy, from 422 ± 99 to 193 ± 21 μM (P < .0001; Tables 4-5). GSH levels, low in all untreated patients, rose consistently with cobalamin therapy, from 1.6 ± 1.2 to 5.3 ± 2.7 μM (P = .002). The response of cys-gly to therapy was variable (Tables 4-5); the higher cys-gly levels tended to fall and the lower levels tended to rise, but with marginal or no statistical significance.
Discussion
The fundamental observation in this study is that metabolic changes are not uniform in cobalamin deficiency. Significant differences between patients with and without neurologic dysfunction characterized all pathways of homocysteine metabolism. Differences in serum folate levels were also apparent. An intriguing example of the metabolic differences was AdoMet, the agent for many important methylation reactions and a regulator and coordinator of homocysteine and methionine metabolism. The AdoMet findings, although made in serum, suggest that existing views of cellular AdoMet status in cobalamin deficiency may need modification. Because many biochemical differences were found and interrelations within homocysteine-methionine metabolism are complex, the observations will be discussed in groups as well as individually.
Folate
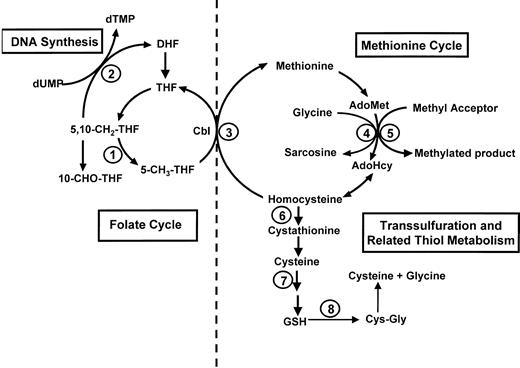
Serum folate levels rise in 20% to 30% of patients with cobalamin deficiency while red-cell folate levels tend to fall,5 largely as a consequence of the trapping of methylTHF following impairment of methionine synthase activity (Figure1, reaction 3). Significantly higher serum folate levels were found in neurologically affected patients than in unaffected ones, as previously noted by others.6 20 The lower levels in neurologically unaffected patients were not, with one possible borderline exception, in the range that indicates folate deficiency.
Homocysteine and methionine metabolism (right) and folate metabolism (left).
AdoHcy indicates S-adenosylhomocysteine; AdoMet,S-adenosylmethionine; Cbl, cobalamin; 5,10-CH2-THF, 5,10-methylenetetrahydrofolate; 5-CH3-THF, 5-methyltetrahydrofolate; 10-CHO-THF, 10-formyltetrahydrofolate; Cys-Gly, cysteinylglycine; DHF, dihydrofolate; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; GSH, glutathione; THF, tetrahydrofolate. Mediating enzymes for reactions are as follows: (1) 5,10-methylenetetrahydrofolate reductase; (2) thymidylate synthase; (3) methionine synthase (requires cobalamin and 5-methyltetrahydrofolate); (4) glycine N-methyltransferase; (5) a variety of cellular methyltransferases; (6) cystathionine β-synthase; (7) γ-glutamylcysteine synthetase, followed by a reaction mediated by glutathione synthetase; (8) γ-glutamyl transpeptidase (occurs extracellularly).
Homocysteine and methionine metabolism (right) and folate metabolism (left).
AdoHcy indicates S-adenosylhomocysteine; AdoMet,S-adenosylmethionine; Cbl, cobalamin; 5,10-CH2-THF, 5,10-methylenetetrahydrofolate; 5-CH3-THF, 5-methyltetrahydrofolate; 10-CHO-THF, 10-formyltetrahydrofolate; Cys-Gly, cysteinylglycine; DHF, dihydrofolate; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; GSH, glutathione; THF, tetrahydrofolate. Mediating enzymes for reactions are as follows: (1) 5,10-methylenetetrahydrofolate reductase; (2) thymidylate synthase; (3) methionine synthase (requires cobalamin and 5-methyltetrahydrofolate); (4) glycine N-methyltransferase; (5) a variety of cellular methyltransferases; (6) cystathionine β-synthase; (7) γ-glutamylcysteine synthetase, followed by a reaction mediated by glutathione synthetase; (8) γ-glutamyl transpeptidase (occurs extracellularly).
This folate difference between subgroups may have influenced some of the metabolic differences in our study, but its origin is unclear. Supplement usage could not be implicated, and most of the patients received diagnoses before food fortification with folate began in the United States. Although dietary intake differences cannot be ruled out, variations in metabolic handling of folate, particularly methylTHF, the predominant folate in plasma, must also be considered. Genetic polymorphisms, such as those involving methylenetetrahydrofolate reductase, provide one such possibility. However, neither studies of DNA from 8 of our patients (data not shown) nor a larger survey21 found any obvious associations of the 677C>T and 1298A>C mutations with clinical manifestations in pernicious anemia. Prospective studies will need to address the origin of the folate differences, which could have more than one explanation.
Methionine cycle
Although impaired methionine synthase activity in cobalamin deficiency would predict low methionine levels, many studies,9,10 12 including ours, found serum methionine to be normal. It is not clear how the normal levels are maintained. We found no methionine differences between neurologically affected and unaffected patients and no significant or consistent changes with therapy.
The disappearance of the normal correlation of AdoMet and methionine levels suggests that influences other than methionine availability determine AdoMet levels when cobalamin deficiency develops. AdoMet levels were normal rather than low in cobalamin deficiency as a whole, as also described by others.12 However, the normal mean level masked significant subgroup differences. Serum AdoMet tended to rise in neurologically affected patients and decline in unaffected ones. Although the levels were neither drastically high nor drastically low in either subgroup, 7 of the 11 neurologically impaired patients had AdoMet levels exceeding 125 nM and none had levels lower than 60 nM, whereas none of the 11 neurologically intact patients had levels exceeding 125 nM and 3 had levels lower than 60 nM. Consistent with this finding, a patient with the cblG mutation and severe myelopathy22 also had a high-normal AdoMet level of 124.9 nM (data not shown).
The AdoMet levels, whether high or low, responded to cobalamin therapy. The fall of the higher levels in patients with neurologic dysfunction lagged behind the response of tHcy levels and appeared more delayed than the rise within 2 days in the lower AdoMet levels. The latter rise, which paralleled the rapidity of tHcy response, presumably reflects the rapid recovery of methionine synthase activity after cobalamin therapy, whereas the slower fall of high AdoMet levels may require wider normalization of the metabolic cycle. Suggesting an important influence of folate, the high AdoMet level improved partially even when patient 5 was mistakenly treated with folate instead of cobalamin, whereas tHcy did not respond (Table 4).
The significantly higher serum AdoMet levels accompanying neurologic dysfunction and the finding that low AdoMet levels were an independent predictor of anemia do not support the hypothesis that AdoMet deficiency causes the neurologic dysfunction in cobalamin deficiency. That hypothesis had been suggested by studies done largely in animals,2-4,23-29 but data on AdoMet levels have been inconsistent.2,25,27,28,30 Human data are limited to a preliminary report of low cerebrospinal fluid AdoMet levels in 4 cobalamin-deficient patients with myelopathy.31 Reports of low cerebrospinal fluid AdoMet in neurologic diseases unrelated to cobalamin deficiency32-34 and in inborn errors of folate and methionine metabolism35 are of interest but limited applicability. The relationships between serum and central nervous system AdoMet levels have not been defined yet.
We propose folate-mediated inhibition of glycineN-methyltransferase (GNMT) as a likely explanation for the higher serum AdoMet levels in neurologically affected patients. GNMT metabolizes AdoMet in the apparently nonessential methylation of glycine to sarcosine (Figure 1, reaction 4) and is relatively resistant to end-product inhibition by AdoHcy.36 The enzyme thus modulates the intracellular availability of methyl groups. GNMT is inactivated by the binding of methylTHF.37 Increases in methylTHF levels are associated with increases in AdoMet levels, and decreases in methylTHF levels with decreases in AdoMet levels. Although we did not measure methylTHF or GNMT, levels of serum folate, which consists primarily of methylTHF, especially in cobalamin deficiency, were higher in the neurologically affected patients, who had high AdoMet levels. It is noteworthy that folate deficiency, with its low methylTHF levels, has been associated with low AdoMet levels in animal studies,38 39 unlike cobalamin deficiency.
The lower folate (and presumably methylTHF) and AdoMet levels in our neurologically intact cobalamin-deficient patients thus may fit within a continuum that extends from the very low methylTHF and AdoMet levels and no or minor neurologic deficits in folate deficiency to the higher folate and AdoMet levels in neurologically impaired cobalamin-deficient patients. If this is true, this spectrum of metabolic circumstances may help explain why some cobalamin-deficient patients develop often very severe neurologic dysfunction while others exhibit anemia only. It also suggests various metabolic consequences of folate supplementation to cobalamin-deficient patients beyond supplying 5,10-methylenetetrahydrofolate for thymidylate synthesis to mask anemia. Patient 6 had full correction of cysteine and GSH abnormalities but only partial correction of AdoMet levels and no correction of tHcy levels after prolonged folate supplementation (Table 4).
A role in neurologic dysfunction has also been suggested for increased AdoHcy, which inhibits methylation by AdoMet.3 AdoHcy is elevated and the AdoMet-AdoHcy ratio is decreased in cobalamin deficiency as a whole.12 However, without valid AdoHcy results, we cannot say whether the AdoHcy or the AdoMet-AdoHcy ratio differs in neurologically affected and unaffected patients.
Transsulfuration and related thiol metabolism
Plasma cysteine derives largely from hepatic release of GSH. Despite constant export from the liver, plasma GSH levels are kept relatively low by immediate degradation to cys-gly by the cell surface enzyme, γ-glutamyl transpeptidase (Figure 1, reaction 8), and then to cysteine.40 This extracellular activity provides the major mechanism for transport of cysteine and cys-gly to tissues, such as the central nervous system which lacks the complete transsulfuration pathway and must depend on these plasma-derived precursors for in situ synthesis of GSH.41 In this way, hepatic thiol status in cobalamin deficiency is directly related to antioxidant defense maintenance in the central nervous system by GSH.
The regulatory effects of AdoMet on homocysteine metabolism include its stimulation of cystathionine β-synthase activity (Figure1).42 Cystathionine levels rise in cobalamin deficiency,43 but were not measured in our study. It is unclear whether the quantitative changes in this pathway derived from the differences in our patients' AdoMet status. The correlation of AdoMet with cysteine and cys-gly levels in cobalamin-deficient patients but not in control subjects favors such an influence. The significantly greater cysteine elevation in neurologically affected patients could also be due to the greater stimulation of transsulfuration by the higher AdoMet levels in these patients than in anemic patients. However, the cysteine levels appeared to normalize more rapidly than AdoMet levels after cobalamin therapy. Of possible relevance, the rise in serum GSH after cobalamin therapy coincided with the fall in cysteine levels and preceded the fall of elevated AdoMet levels.
The parallel increase in cysteine and cys-gly levels in cobalamin deficiency is consistent with elevated hepatic transport of GSH and elevated γ-glutamyl transpeptidase activity but is inconsistent with active uptake by peripheral tissues, such as neural tissue, for increased GSH synthesis. Interference with cellular transport mechanisms offers a possible explanation. Almost 90% of cysteine transport into brain cells occurs by a low-affinity mechanism mediated by the X(AG) family of glutamate transporters.44 Excess glutamate is a noncompetitive inhibitor of cysteine uptake via the glutamate transporter and blocks cysteine entry into the cell.41 Insufficient cysteine uptake depletes intracellular GSH, resulting in neuronal cytotoxicity.44It is not known if glutamate is increased in cobalamin deficiency, but methylcobalamin protects against glutamate neurotoxicity in vitro.45 An alternative explanation for elevated serum cysteine is reduced activity of γ-glutamylcysteine synthetase (GCS), the rate-limiting enzyme for GSH synthesis (Figure 1, reaction 7). Both S-nitrosocysteine andS-nitrosocysteinylglycine are potent inhibitors of GCS mediated by nitric oxide release and are increased under conditions of GSH depletion and nitric oxide excess.40 The markedly elevated cysteine in our neurologically impaired patients may also be converted to cysteine sulfinic acid, an established excitatory and neurotoxic metabolite.
Previous reports of normal, increased, and decreased cysteine levels in cobalamin deficiency9-11,46 are hard to interpret because they often involved patients with either unclear cobalamin status or only mild deficiency; moreover, neurologic status was not analyzed. Long-term storage of serum does not account for our high results, because normal levels were demonstrated after therapy and in control sera. Moreover, a previous report of normal cysteine levels was based on samples left unprocessed for 4 hours and also stored for many years.10
Decreased GSH is additionally relevant as an independent predictor of anemia, in the presence of which the serum GSH level was often lower than 1 μM. This suggests the possibility that oxidative damage by GSH depletion accounts for the unexplained hemolytic process in severe megaloblastic anemia,5,47 much as hereditary disorders of GSH metabolism cause hemolytic anemia.48
The study results provide the first details of extensive metabolic differences associated with variations in clinical manifestations of cobalamin deficiency. It is necessary to note the limitations as well. One is the retrospective nature of the study and the resulting limitations of specimen collection and availability. The difficulty of studying adequate numbers of patients with the relatively uncommon disease of pernicious anemia, especially the minority who have neurologic dysfunction, is exacerbated by the need to test them before they are treated and by the many exclusion criteria necessary for study of the issues they raise. Our resultant dependence on less than ideal specimens was partially addressed in several ways: (1) Results in control sera that were processed and stored like the test samples closely matched our13 and others' reference ranges9,12 15-18 in freshly obtained samples; the sole exception was AdoHcy, which was therefore not included. (2) Experiments on sample-processing variations identified no relevant artifacts (see “Patients, materials, and methods”). (3) Results in the 2 new patients identified during the study were consistent with those in the earlier patients. (4) The normal posttherapy results in identically processed and stored sera strongly suggest that the abnormalities were not sample artifacts.
Another difficulty is that serum levels may not reflect intracellular metabolic events accurately, especially those occurring within the central nervous system. This is a common dilemma in human studies, which rarely have adequate access to neural tissue. Studies of cerebrospinal fluid may not be free of shortcomings either but will be of obvious interest.
The findings raise new questions in addition to providing new information that may help explain how neurologic dysfunction arises in cobalamin deficiency. Future studies should clarify the sequence and hierarchy of the significant metabolic differences that we have identified with the presence or absence of neurologic dysfunction, including the still unknown role and origins of variations in folate status. It will also be important to determine which of the findings represent metabolic events directly responsible for the neurologic defect or, conversely, its absence; which ones are effects; and which ones represent constitutional, genetic, or acquired metabolic characteristics that “program” a patient to respond to cobalamin deficiency with neurologic or hematologic sequelae.
We thank Conrad Wagner, PhD, for critical reading of the manuscript; Dajun Qian, PhD, for statistical analyses; and Anne Flateau, PhD, and James Parker for expert technical help.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-09-2746.
Supported by grant DK32640 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralph Carmel, NY Methodist Hospital, 506 Sixth St, Brooklyn, NY 11215; e-mail:rac9001@nyp.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal