Abstract
In 1995 and 1997 we proposed that gap junctions between stromal and hematopoietic cells formed by connexin43 (Cx43) determine hematopoiesis. If this were the case, are the critical gap junctions in this regard those between hematopoietic and stromal cells, or those between stromal cells alone? To test the first possibility, we compared hematopoietic repopulating capacity between fetal liver hematopoietic cells expressing the different mouse Cx43 genotypes, wild type (WT), hemizygous, or knock-out (KO) on WT host mice stroma. We deleted host glucose phosphate isomerase 1a (Gpi-1a) stems and then raced identifiable Cx43 WT host fetal liver against congenic donor Cx43 WT, hemizygous, or KO cells in sets, comparing their capacity to form 5 end cells. Hematopoietic capacity did not differ between the Cx43 WT and KO genotypes. The role of Cx43 gap junctions in hematopoiesis remains uncertain.
Introduction
Connexin43 (Cx43) forms the main gap junction in blood-forming tissue.1-12 Gap junctions have been comprehensively reviewed.13 In 1995 and 1997, we proposed1,14 that Cx43 gap junctions between stromal and hematopoietic cells determine hematopoiesis. If this were the case, was this effect because of coupling between hematopoietic and stromal cells or between stromal cells alone? This paper addresses the first possibility. To determine whether the Cx43 genotype altered the blood-forming capacity of stem cells we used hematopoietic cells of mice with the Cx43 deletion.15 Such KO mice die at birth of pulmonary atresia, but the 3 genotypes can readily be compared using fetal liver. We used competitive repopulation16 to race them.
Study design
Mice
Two congenic C57Bl/6J strains were used. The hosts were glucose phosphate isomerase 1a (Gpi-1a) and the donors of different Cx43 genotypes Gpi-1b.
Competitive repopulation
Cx43 KOs replace hematopoietic Cx43 with Cx45.17 We compared blood formation by WT and KO stems as published.1An intracellular injection of lucifer yellow18 into WT stroma spread over 4 to 5 cell diameters but did not pass from one KO cell to another. KO cells passed neurobiotin to the next cell but no further. The molecular mass of lucifer yellow is 457, its charge 2−, and maximum/minimum (max/min) dimensions 10.6/9.5, with an axial ratio of 1.1. Neurobiotin is smaller: molecular mass is 287; charge, 1+, maximum/minimum dimensions, 12.7/5.4, and axial ratio, 2.4. Mixed hematopoietic WT/KO junctions did not couple.
We grafted fetal liver cells19 from genotyped 15 dpc (days after coitus) embryos. Hosts' stems were deleted but they retained their WT stroma. Cx43 genotypes were raced in 10 Gpi-1a hosts grafted with an equal fetal liver dose of host WT and each Cx43 genotype separately (2 livers/mouse, 1.31 ± 0.75 × 107 viable cells/liver), ie, Gpi-1a Cx43 WT versus Gpi-1b WT, or Gpi-1a WT versus Gpi-1b hemizygous, or Gpi-1a WT versus Gpi-1b KO. In each mouse we compared Gpi-1a and Gpi-1b platelets (short lived), neutrophils, red cells, and B and T cells (long lived) formed by competing stems after separating Gpi-1a and Gpi-1b electrophoretically.19
Treatment with 5-fluorouracil (5-FU) or phenylhydrazine (PHZ)
5-FU (150 mg/kg) was injected intravenously into grafted animals as indicated in the figure legends. PHZ (60 mg/kg) was injected subcutaneously into grafted mice on the 2 days before blood collection.
Statistical significance
Prof Mervyn Stone (Statistics, University College London) studied our findings to assess if there were significant differences between the Cx43 genotypes. This subject will be discussed in detail elsewhere (M.R. and M. Stone, manuscript submitted).
Results and discussion
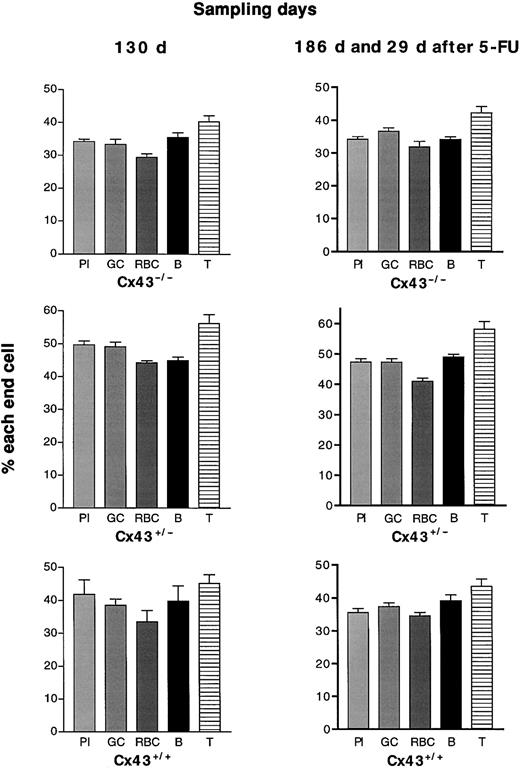
If Cx43 gap junctional communication between stem and stroma enabled resting stems to cycle, hematopoiesis in KOs, which replace Cx43 with Cx45 and cannot couple normally to host Cx43 stroma, would be disadvantaged. In 5 repopulation races (22-131 days after grafting), neither donor Cx43 WT nor KO genotype was consistently hematopoietically advantaged (Figure 1), although the proportions of end cells formed by fetal liver of the 3 genotypes did vary between one race and another. Nor did cyto-ablation of grafted mice by 5-FU (2 experiments 14 and 29 days after the drug) to reveal blood formation by more primitive stems20 alter the observation. The proportions of short-lived cells remained strikingly stable (Figure 2).
Percentage of all end cells formed by the 3 Cx43 genotypes of fetal liver and assayed at different intervals after grafting.
The 3 Cx43 genotypes are compared, each raced against the same dose of host fetal liver. Box-and-whiskers figures of the proportions of the pooled 5 end cells between 21 and 131 days after grafting are shown. No donor genotype is consistently advantaged. With the passage of time (out to 6 months, not shown here) Cx43+/−fetal liver formed more end cells than either WT or KO. This will be reported elsewhere (M.R. and M. Stone, manuscript submitted).
Percentage of all end cells formed by the 3 Cx43 genotypes of fetal liver and assayed at different intervals after grafting.
The 3 Cx43 genotypes are compared, each raced against the same dose of host fetal liver. Box-and-whiskers figures of the proportions of the pooled 5 end cells between 21 and 131 days after grafting are shown. No donor genotype is consistently advantaged. With the passage of time (out to 6 months, not shown here) Cx43+/−fetal liver formed more end cells than either WT or KO. This will be reported elsewhere (M.R. and M. Stone, manuscript submitted).
Undetectable effect of 5-FU on blood formation by stems of the 3 Cx43 genotypes.
Cell types derived from Gpi-1b stems were compared before and after 5-FU. Challenging grafted mice with 5-FU did not alter the proportions of their 5 end cells. Results shown are percentages ± SD.
Undetectable effect of 5-FU on blood formation by stems of the 3 Cx43 genotypes.
Cell types derived from Gpi-1b stems were compared before and after 5-FU. Challenging grafted mice with 5-FU did not alter the proportions of their 5 end cells. Results shown are percentages ± SD.
WT and KO fetal liver differed little, not in blood-forming capacity/liver, or in stem cell concentration, as shown in the spleen-colony assay (inasmuch as this is a stem cell assay), or the high-proliferation potential assay of colony-forming cells (HPP-CFC).21 This similarity was not confined to proportions of end cells. Eight and a half months after grafting, when WT liver formed 34.5% of red cells and KO of 31.8%, Gpi-1 phenotyping of 385 circulating HPP colonies showed similar proportions after PHZ, specifically WT of 23.8% and KO of 20.2%.
Although KO hematopoietic cells failed to form normal gap junctions with WT stroma, they displayed no hematopoietic disadvantage when compared with WT cells. If we were correct in proposing that Cx43 gap junctions are hematopoietic determinants, then either the Cx43 genotype of stem cells is not a determinant or normal homotypic junctions between them and stroma are not all important. Another report,22 based on WT and hemizygous animals, may suggest that gap junctions between stromal cells alone are determinant. But that raises even knottier problems. Does the connexin type of gap junction on stromal cells vary between WT and hemizygous? If so, how does it vary? What are the coupling consequences, and how does that affect blood formation? The role of hematopoietic Cx43 gap junctions is still uncertain.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-07-2028.
Supported by the Wellcome Trust and Royal Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin Rosendaal, Department of Anatomy and Developmental Biology, University College, Gower Street, London WC1E 6BT, United Kingdom; e-mail: m.rosendaal@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal