The Ikaros gene encodes a zinc finger transcription factor that is selectively expressed by all hematopoietic cells. Although Ikaros is required for lymphocyte differentiation, its role in the myeloid lineage is unclear. We show here that Ikaros expression is temporally regulated during neutrophil differentiation: Ikaros is primarily expressed at immature stages and significantly less so in mature neutrophils. Furthermore IkL/L mice, harboring a hypomorphic mutation at the Ikaros locus, exhibit several defects during neutrophil differentiation. (1) IkL/L fetal livers contain high numbers of neutrophil lineage cells, and this increase is reflected in the number of GM-CSF-dependent progenitor cells. (2) The migratory potential and survival of neutrophil progenitors is altered in vitro. (3) Expression of the Gr-1 marker is delayed and repressed. In contrast, neutrophil function appears normal. These data demonstrate that Ikaros regulates early neutrophil differentiation but is dispensable in mature neutrophils.
Introduction
Neutrophils are specialized effector cells critical for innate immunity. Mature neutrophils are distinguished by their characteristically segmented nuclear morphology, and contain specialized organelles responsible for phagocytosis, bacterial clearance, and inflammatory response. Neutrophils differentiate mainly in the adult bone marrow and can be classified into increasingly mature subsets on the basis of their morphology—promyelocytes, myelocytes, metamyelocytes, and band-segmented neutrophils. These subsets can also be neatly subtyped on the basis of their expression of the surface markers Mac-1 (CD11b) and Gr-1: Mac-1+Gr-1locells consist mostly of myelocytes and metamyelocytes, while Mac-1+Gr-1hi cells are composed mainly of mature band-segmented neutrophils.1
Neutrophil differentiation is driven primarily by the cytokines granulocyte-colony stimulating factor (G-CSF) and granulocyte macrophage-colony stimulating factor (GM-CSF).2,3 The expression of the G-CSF and GM-CSF receptors is regulated by key transcription factors, such as PU.1 and members of the CAAT/enhanced binding protein (C/EBP) family. PU.1 is not essential for neutrophil commitment,4 but PU.1-null neutrophils fail to fully differentiate, do not respond to G- or GM-CSF signaling, do not generate superoxide ions, and are defective at bacterial uptake and killing.5 Likewise, C/EBPα has been shown to be critical for early granulocyte (neutrophil and eosinophil) differentiation,6 prior to primary granule gene expression probably through an arrest of precursor proliferation by E2F repression,7 and acts as a positive switch to induce granulocyte commitment over monocyte maturation.8 In contrast, C/EBPε appears to regulate terminal neutrophil differentiation (metamyelocyte) at the time of secondary granule gene expression; C/EBPε-null mice are thus unable to clear bacterial infections resulting in secondary inflammatory responses.9,10 Finally, retinoic acid receptor α (RARα) has been shown to bidirectionally modulate neutrophil differentiation; RARα stimulates differentiation in response to exogenous retinoic acid but limits maturation in the absence of ligand.11
The Ikaros zinc finger transcription factor is expressed by all hematopoietic cells.12-14 Ikaros-null (Ik-C) mice as well as animals expressing a dominant-negative form of Ikaros (Ik-DN) exhibit profound defects in the lymphoid lineage. Homozygous Ik-DN mice lack B, T, natural killer (NK), and dendritic cells.15 Ik-C animals lack B and NK cells, and demonstrate specific T-lymphocyte defects.16 Ikaros appears to exert its effects by silencing target gene expression, as a large portion of Ikaros proteins colocalizes with heterochromatin and associates with the NURD complex in cycling lymphoid cells.12,17,18Conversely, Ikaros might also act as a transcriptional activator as some Ikaros proteins copurify with the SWI/SNF complex.19
There is growing evidence that Ikaros also plays an important role in the maturation and function of nonlymphoid hematopoietic cells. Long-term repopulating hematopoietic stem cells are greatly reduced or lacking in Ik-C and Ik-DN mice, respectively,20 with an age-dependent progressive reduction in spleen colony-forming unit (CFU-S) progenitors and erythroid-restricted precursors spleen colony-forming unit (BFU-E). Consistent with the defect in erythroid precursor populations, adult Ik-C mice are anemic and exhibit abnormal megakaryocytopoiesis as well as increased platelet formation.20,21 Moreover, embryonic to adult β-globin switching is delayed during fetal development.21 The influence of Ikaros on cells of the myeloid lineage is less clear. Normal numbers of precursors giving rise to multilineage and granulocyte- and/or monocyte-restricted colonies were detected in Ik-C bone marrow (BM) cells in response to interleukin-3 (IL-3), stem cell factor (SCF), and erythropoietin stimulation.20Yet Gr-1+ cells are lacking in the BM of Ik-C and Ik-DN animals, suggesting a potential defect in granulocyte differentiation.15,16 Finally, a dominant-negative isoform of Ikaros was detected in 7 of 10 patients with acute myeloid leukemia (AML), indicating the importance of Ikaros function in the myeloid lineage.22
We recently described a novel mouse line (IkL/L) in which the β-galactosidase (βgal) reporter was inserted into theIkaros locus.14 In the BM cells of these mice, very low levels of Ikaros proteins are produced that contain both the DNA-binding domain and the dimerization domain but lack exon 2-encoded sequences.14 The IkL/L mice exhibit essentially the same hematopoietic defects as those found in the Ik-C line, but in a milder form, suggesting that the mutation is hypomorphic. Interestingly, Gr-1 expression is weakened but not abolished in the BM cells of IkL/L mice; we exploited this observation to isolate and study the neutrophil lineage in these animals. In this report, we show that (1) Ikaros is dynamically regulated in the neutrophil lineage, and (2) Ikaros regulates early neutrophil differentiation by changing the migratory potential and survival of neutrophil precursors to G-CSF/SCF stimulation.
Materials and methods
Cell culture
IkL/L mice were described previously.14Samples of 5 × 104 fetal liver (FM) or BM cells (6- to 8-week-old mice) were cultured in methylcellulose media (Methocult 3234; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 2 ng/mL G-CSF plus 20 ng/mL SCF (R&D Systems, Minneapolis, MN), or 10 ng/mL GM-CSF (R&D Systems). Labeling with CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes, Eugene, OR) was performed by incubating cells for 10 minutes at 37°C in medium supplemented with 20 μg/mL CFSE, followed by 2 washes.
Antibodies and flow cytometry
The following antibodies were used: fluorescein isothiocyanate (FITC)-conjugated Gr-1 (Caltag, South San Francisco, CA); biotin-conjugated Mac-1 (BD Pharmingen, San Diego, CA); and streptavidin-phycoerythrin (PE; Jackson Immunoresearch Laboratories, West Grove, PA). Lineage-negative (Lin−) cells were purified as follows: BM cells were stained with antibodies (all rat IgGs) against CD4, CD8, CD3, B220, TER119, and F4/80 and depleted with sheep anti-rat IgG-coupled Dynabeads (Dynal, Oslo, Norway). Depleted cells were stained with PE-conjugated goat anti-rat IgG and PE-negative cells were sorted. Purity of the sorted cells was on average higher than 95%. Apoptosis was analyzed using FITC-conjugated anti-Annexin V (Caltag) and propidium iodide at a final concentration of 50 ng/μL. Samples were analyzed or sorted using a Coulter Epics Elite (Coulter Electronics, Hialeah, FL). βgal activity was detected using the substrate fluorescein digalactopyranoside (FDG) as previously described.23
Hematologic analysis
Leukocyte numbers from the blood were obtained using an STKS hematology flow cytometer (Coulter Electronics). The proportion of neutrophils within the leukocyte pool was then determined from a May-Grünwald/Giemsa-stained blood smear.
Immunofluorescence staining
Immunofluorescence staining was performed according to Wang et al.16 Sorted BM cells in phosphate-buffered saline (PBS) were cytospun for 5 minutes at 1200 rpm onto slides and fixed for 20 minutes in cold 4% paraformaldehyde/0.1% Tween/PBS. Slides were then washed 4 times in cold PBS. Samples were incubated with blocking buffer (Dulbecco modified Eagle medium [DMEM] supplemented with 3% heat-inactivated horse serum, 0.1% sodium azide, and 30 mM HEPES [4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid]) for 1 hour at 4°C and then with the primary antibody (polyclonal rabbit antiserum to the C-terminal part of Ikaros)24diluted 1:1000 in blocking buffer overnight at 4°C. After 4 washes in cold PBS, cells were incubated with the secondary antibody goat anti-rabbit IgG conjugated to cyanine 3 (Cy3; Jackson Immunoresearch Laboratories) diluted at 1:1000 in blocking buffer for 1 hour at room temperature (rt). After 4 washes in cold PBS, cells were incubated with Hoechst 33342 (Sigma, St Louis, MO) diluted at 1:200 in PBS for 2 minutes at rt. Samples were mounted in 5% propylgalate/80% glycerol/PBS and viewed at × 40 with a fluorescent microscope (Leica, Solms, Germany).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
RNA was isolated using the RNeasy Kit (Qiagen SA, Valencia, CA). Total RNA (1 μg) was reverse transcribed and one twentieth of the reaction was subjected to PCR using the following oligonucleotides: Ly-6G: 5′-GTCCCACCTGAGACTTCCTG-3′, 5′-GAGGAGTGGGGTGCCTATAC-3′; Gelatinase: 5′-GGCTCTGCTGTTCAGCAAGG-3′, 5′-GGCTCCCTAGGGATGCTCTC-3′; Lysozyme: 5′-CTGCAGGATGACATCACTGC-3′, 5′-TGCTGAGGCCTGTACTTAGAGG-3′; Lactoferrin: 5′-AAGCCAGGCTTGTCCTCTAG-3′, 5′-TCTCATCTCGTTCTGCCACC-3′; C/EBPε 5′-GCTACAATCCCCTGCAGTACC-3′, 5′-CACAAGGGCAAGGCA-3′; STAT3: 5′-GTTCTCGTCCACCACCAAGC-3′, 5′-CCAGACTCAGAGGTCTCTCC-3′; and β-actin: 5′-GTGACGAGGCCCAGAGCAAGAG-3′, 5′-AGGGGCCGGACTCATCGTACTC-3′. Quantification of the PCR products was obtained using a Typhoon 8600 image analyzer (Amersham, Piscataway, NJ) with ImageQuant software (Amersham).
G-CSF treatment
Recombinant human G-CSF (Amgen, Thousand Oaks, CA) was injected subcutaneously twice a day 12 hours apart for 5 days at a dose of 250 μg/kg body weight in 200 μL 0.1% bovine serum albumin (BSA)/PBS.25 Blood was collected 24 hours before the treatment and 2 hours after the last injection, at which time the mice were also killed and their BM and spleens analyzed. Control mice were injected with 0.1% BSA/PBS.
Neutrophil phagocytosis assay
Phagocytic activity was assessed with the Phagotest Kit (Orpegen Pharma, Heidelberg, Germany). Briefly, blood samples (100 μL) were cooled 10 minutes on ice before adding 40 μL of opsonized and FITC-conjugated Escherichia coli. Samples were then incubated for 1 hour at 37°C or 0°C (control). To distinguish between bacteria adhered to the cell surface and internalized bacteria, 100μL of neutralization solution was added to quench externally bound FITC bacteria. Cells were washed 2 times and erythrocytes were lysed with 2 mL of lysis solution for 20 minutes at rt. To exclude artefacts due to aggregated bacteria or cells, 200 μL of DNA staining solution was added for 10 minutes on ice. Samples were then analyzed by flow cytometry.
Oxidative burst assay
Respiratory burst was evaluated using Fc OxyBURST Assay Reagents (Molecular Probes). BM cells were prewarmed in KRP buffer (Sigma) for 15 minutes at 37°C. Immune complexes bound to dichlorodihydrofluorescein (H2DCF) were added at a concentration of 140 μg/mL and samples were incubated for 30 to 45 minutes at 37°C.26 Once internalized within the phagovacuole, H2DCF is oxidized and the fluorescent dichlorofluorescein (DCF) is released, which is then measured by flow cytometry.
Neutrophil chemotaxis
Chemotaxis was assessed in vitro using a 3-μm pore diameter polycarbonate membrane transwell apparatus (Costar, Cambridge, MA) as directed by the manufacturer. rIL-8 was added to the lower chamber at 0.3 μg/mL (R&D Systems). To the upper chamber, 106cells/mL of media were added and allowed to migrate for 90 minutes at 37°C. Negative control contained only Iscoves media (GIBCO-BRL, Grand Island, NJ) in the upper and lower compartments. Cell migration was assessed by counting the number of cells present in the bottom chamber after the incubation period. Chemotaxis was assessed in vivo by injecting intraperitoneally 5 mg/kg body weight of lipopolysaccharides (LPS; Sigma L-4391). At 20 hours after injection, the peritoneal cavity was washed with 5 mL of PBS. Mac-1+Gr-1+ cells were quantified by flow cytometry.
Electrophoretic mobility shift assay
Nuclear extracts were prepared as follows: 107 BM cells were resuspended in 500 μL hypotonic lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]) and iced for 10 minutes. Nuclei were pelleted and resuspended in 40 μL of the following buffer: 20 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA [ethylenediaminetetraacetic acid], 0.5 mM DTT, protease inhibitor cocktail. Lysates were iced for 20 minutes and vortexed thoroughly. After centrifugation, supernatants (nuclear extracts) were collected and quantified by the Bradford colorimetric assay. Nuclear extract (3 μg) was used for each sample. First, nuclear extracts were incubated on ice for 10 minutes with 2 μg polydeoxyisosinic-deoxycytidilic acid (poly dIdC) in 19 μL of binding buffer (10 mM Tris, pH 7.5, 100 mM KCl, 10% glycerol, and 1 mM DTT). Then, 5 × 104 cpm of end-labeled, double-stranded probe was added to each reaction and the mixture was incubated for 15 minutes at RT. Protein-DNA complexes were resolved on a 5% polyacrylamide gel and autoradiographed. Probes were the following: STAT3: 5′-CATTTCCCGTAAATCAT-3′; STAT3m: 5′-CATTGCACGTCAATCAT-3′, PU.1: 5′-GGGCTAAGCGGAAGTGGAGGTC-3′; and PU.1m: 5′-GGGCTAAGCTAGCGTGGAGGTC-3′.
Results
Ikaros expression during neutrophil differentiation
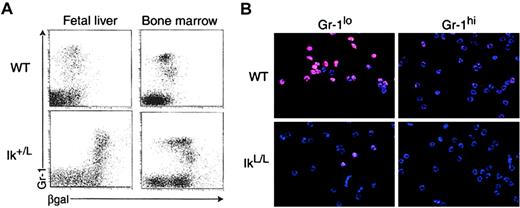
To investigate the potential role of Ikaros in neutrophils, we first evaluated its expression in this lineage. Gr-1+ cells from wild-type (WT) and heterozygous Ik+/L mice were analyzed by flow cytometry, using the β-galactosidase reporter as a measure of Ikaros promoter activity (Figure1A). Gr-1+ cells from both the FL and BM of Ik+/L mice showed βgal positivity above the background levels expressed by cells of WT animals, indicating that the Ikaros locus is active in neutrophil lineage cells. Interestingly, immature BM (but not FL) Ik+/L Gr-1lo cells (consisting mostly of myelocytes and metamyelocytes) expressed higher βgal levels than Gr-1hi cells (mainly neutrophils), suggesting that Ikaros expression is dynamically regulated during differentiation. Even within the Gr-1hi population, βgal was expressed differentially, ranging from high to background levels when compared with WT. To confirm this observation, WT BM cells were purified into immature Mac-1+Gr-1lo and mature Mac-1+Gr-1hi neutrophils and analyzed for their level of Ikaros protein by immunofluorescence staining, using a polyclonal antibody against the C-terminal end of Ikaros (Figure 1B, upper images). Both populations expressed Ikaros that localized predominantly in the cell nucleus (as verified by Hoechst staining of the same cells; data not shown) in a punctate fashion. However, Ikaros proteins were clearly more abundant in the immature Mac-1+Gr-1lo population.
Ikaros expression in WT and IkL/L neutrophils.
(A) Ikaros promoter activity was measured in heterozygous Ik+/L 16.5 dpc FL and adult BM cells using the Gr-1 marker to stain for granulocyte lineage cells and the substrate FDG to measure βgal activity. Note that all Ik+/L BM Gr-1locells express high levels of βgal, whereas Gr-1hi cells express βgal at lower levels. (B) Immunofluorescence detection of Ikaros proteins (red) and cell nuclei (Hoechst staining in blue) in WT and homozygous IkL/L Gr-1lo and Gr-1hi BM cells (see Figure 5A for sorting gates). One representative experiment is shown. Original magnification, × 400.
Ikaros expression in WT and IkL/L neutrophils.
(A) Ikaros promoter activity was measured in heterozygous Ik+/L 16.5 dpc FL and adult BM cells using the Gr-1 marker to stain for granulocyte lineage cells and the substrate FDG to measure βgal activity. Note that all Ik+/L BM Gr-1locells express high levels of βgal, whereas Gr-1hi cells express βgal at lower levels. (B) Immunofluorescence detection of Ikaros proteins (red) and cell nuclei (Hoechst staining in blue) in WT and homozygous IkL/L Gr-1lo and Gr-1hi BM cells (see Figure 5A for sorting gates). One representative experiment is shown. Original magnification, × 400.
In homozygous IkL/L neutrophils, the level of Ikaros proteins was greatly reduced on a per-cell basis when compared with WT cells (Figure 1B, bottom images). Both IkL/LMac-1+Gr-1lo and Mac-1+Gr-1hi cells stained only faintly with the anti-Ikaros antibody. There were a few brightly stained cells (between 10%-15%) in the immature Mac-1+Gr-1lo population, but these were also less positive than the WT cells. In fact, the majority of the immature Mac-1+Gr-1lo and all of the mature Mac-1+Gr-1hi, IkL/L cells contained less Ikaros than the weaker staining Mac-1+Gr-1hi subset from WT animals. Nevertheless, the Ikaros proteins in IkL/L and WT cells localized in the same punctate manner. The large reduction in Ikaros levels in the neutrophil lineage suggests that Ikaros function in these cells may be diminished, though not completely abolished, in IkL/L mice.
Neutrophil populations in IkL/L mice
Neutrophil differentiation in the FL, BM, and spleen of IkL/L mice was analyzed by flow cytometry using the markers Mac-1 and Gr-1 (Figure 2A). In the IkL/L FL, there was a striking increase in the proportion of Mac-1+Gr-1lo/+ cells during development, as tested over 3 time points (Figure 2A and not shown). Although B lymphocytes are absent in the IkL/L FL (and are present in WT FL cells from 14.5 days after coitus [dpc] on),14the observed increase appears to be independent of the change in lymphocyte numbers, as it preceded normal lymphocyte development and occurred with a concomitant reduction in erythroid cell numbers. At late gestation (16.5 to 18.5 dpc), Mac-1+ cells comprised the majority of IkL/L FL cells, in contrast to control (WT or Ik+/L) FL cells, which were predominantly composed of erythroid lineage cells (Figure 2A and not shown). Importantly, the cellularity of the FL was similar between WT and IkL/L mice at all stages analyzed (Figure 2B, left graph); therefore, the increased proportion of neutrophil lineage cells also translated into a large increase in absolute numbers of neutrophil lineage cells (Figure2B, right graph).
Neutrophil populations in IkL/L mice.
(A) Phenotypic analysis of WT and IkL/L FL and BM cells using the Gr-1 and Mac-1 markers. Numbers shown correspond to the percentage of cells in each gate. The experiment shown is representative of 3 experiments. (B) Absolute number of total (left) and Mac-1+ (right) FL cells corresponding to fetuses analyzed in Figure 2A. Note that because of the large variability in FL cellularity between fetuses from different litters of the same developmental stage, representative results from individual WT and IkL/L littermates are shown here. (C) Absolute numbers of total BM cells (left) and Mac-1+ cells (right) per femur in 6-week-old mice (n = 11 for WT; n = 13 for IkL/L). (D) Absolute numbers of neutrophil lineage cells in the blood of mice treated or not with G-CSF. (** indicates P < .01, n = 11; *, P < .05, n = 4).
Neutrophil populations in IkL/L mice.
(A) Phenotypic analysis of WT and IkL/L FL and BM cells using the Gr-1 and Mac-1 markers. Numbers shown correspond to the percentage of cells in each gate. The experiment shown is representative of 3 experiments. (B) Absolute number of total (left) and Mac-1+ (right) FL cells corresponding to fetuses analyzed in Figure 2A. Note that because of the large variability in FL cellularity between fetuses from different litters of the same developmental stage, representative results from individual WT and IkL/L littermates are shown here. (C) Absolute numbers of total BM cells (left) and Mac-1+ cells (right) per femur in 6-week-old mice (n = 11 for WT; n = 13 for IkL/L). (D) Absolute numbers of neutrophil lineage cells in the blood of mice treated or not with G-CSF. (** indicates P < .01, n = 11; *, P < .05, n = 4).
In adult mice, the proportion of Mac-1+ cells remained elevated in IkL/L BM (Figure 2A), but this increase was not reflected in an increase in absolute numbers (Figure 2C, right graph), as the cellularity of the IkL/L BM was 20% to 30% less than WT BM on average (per femur, WT: 20.8 ± 6.5 × 106, n = 11 vs IkL/L: 15.6 ± 3.9 × 106, n = 13) (Figure 2C, left graph). Furthermore, IkL/L neutrophils resembled WT ones (segmented or hyposegmented neutrophils, not shown). Interestingly, the neutrophil distribution in the periphery was markedly altered in IkL/Lmice. Blood neutrophil numbers were reduced approximately 2-fold (WT: 1.35 ± 0.38 × 103/mm3, n = 11 vs IkL/L: 0.72 ± 0.27 × 103/mm3, n = 11) (Figure 2D, left graph). Moreover, neutrophil blood numbers in the IkL/L animals remained depressed even after G-CSF treatment for 5 days, a procedure which normally provokes a massive influx of mature neutrophils from the BM into circulation.25 Even though the blood neutrophil counts in both IkL/L and WT mice increased in response to G-CSF, the levels in the blood of G-CSF-treated IkL/L mice were 3-fold less than those of G-CSF-treated control animals (Figure 2D, right graph).
Neutrophil differentiation in vitro
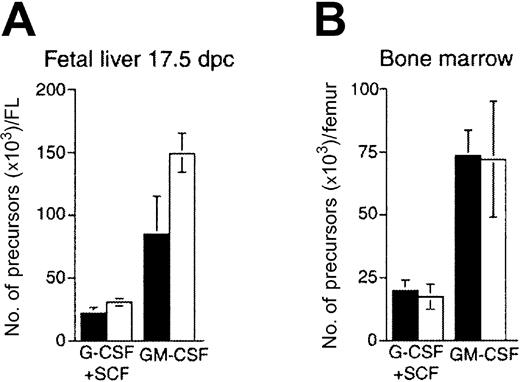
Since Ikaros is highly expressed in immature Mac-1+Gr-1lo cells, we evaluated neutrophil differentiation in vitro. At 17.5 dpc, FL and BM cells from IkL/L and WT mice were cultured for 7 days in G-CSF plus SCF, or GM-CSF alone, at which time the number of colonies per dish was quantified for each condition (Figure 3). In the FL, the absolute number of GM-CSF-dependent progenitors was increased by approximately 50% with respect to WT, while similar numbers of IkL/L and WT progenitors grew in response to G-CSF/SCF (Figure 3A). In contrast, similar numbers of progenitors from the BM were detected for both types of conditions (Figure 3B). Thus, an increase in GM-CSF-dependent progenitors might, at least in part, account for the increase in the neutrophil population of the IkL/L FL.
Myeloid precursors in IkL/Lhematopoietic organs.
Numbers of (A) FL-derived and (B) BM-derived precursors developing after a 7-day methylcellulose culture with G-CSF plus SCF, or GM-CSF. Graphs depict the means and standard deviation (SD) values of triplicate cultures from cells of one mouse. ▪ indicates WT; ■, IkL/L. One experiment representative of 3 is shown.
Myeloid precursors in IkL/Lhematopoietic organs.
Numbers of (A) FL-derived and (B) BM-derived precursors developing after a 7-day methylcellulose culture with G-CSF plus SCF, or GM-CSF. Graphs depict the means and standard deviation (SD) values of triplicate cultures from cells of one mouse. ▪ indicates WT; ■, IkL/L. One experiment representative of 3 is shown.
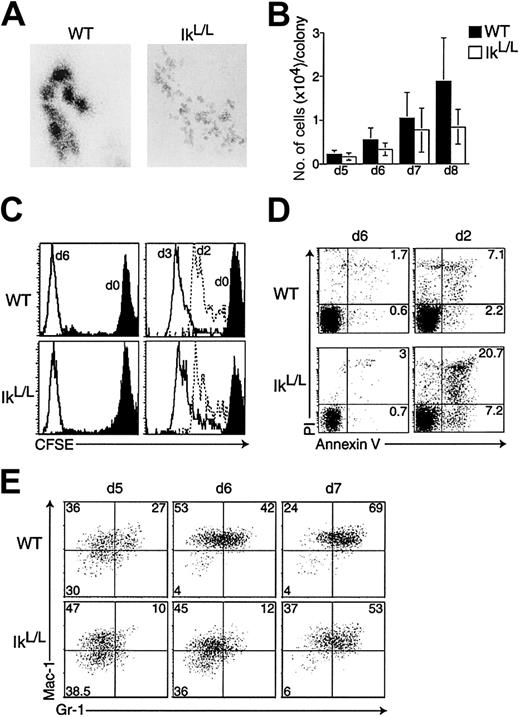
Although the numbers of G-CSF/SCF-dependent colonies derived from IkL/L BM or FL cells were similar after 7 days to those from WT cells, the morphology of the IkL/L colonies was very different (Figure 4A). Colonies generated from WT BM cells after 7 days of culture were typically composed of 5 to 10 large and densely packed clusters of cells. This morphology suggests that 2 successive events occur during differentiation in G-CSF/SCF: (1) the proliferating cells migrate away from their initial position, and (2) the cells lose their ability to migrate and can only proliferate in place to produce the clusters. In contrast, the colonies grown from IkL/L BM cells were composed of a larger number of small clusters (Figure 4A). In addition, the number of cells per IkL/L colony remained significantly lower over time in these cultures (Figure 4B).
Phenotypic analysis of G-CSF/SCF-dependent colonies.
WT and IkL/L BM cells were cultured in methylcellulose medium supplemented with G-CSF plus SCF. (A) Representative colonies (day 7) for each genotype. Original magnification, × 80. 6 independent experiments were performed. (B) BM cells were cultured as above for the indicated number of days. At each time point, the number of cells per colony was counted for the 10 largest colonies in each plate; bars represent the means ± SDs. (C) Similar proliferation kinetics between WT and IkL/L cells, as revealed by decrease of the CFSE label. Left: CFSE profile of BM cells at day 0 of culture (black histograms), and of representative WT and IkL/L colonies after 6 days of culture (white histograms). For each genotype, 6 colonies were analyzed, all of which showed similar CFSE levels. Right: Lin− BM cells were sorted, labeled with CFSE, and 105 cells were put in culture. Cells from entire plates were harvested after 1 to 3 days of culture and their CFSE levels analyzed. Days of harvest are indicated next to specific histograms. (D) Analysis of apoptosis by Annexin V/propidium iodide staining. Left: isolated colonies at day 6; 6 colonies were analyzed for each genotype and displayed similar phenotypes (with the exception of a single WT colony that had approximately 10% annexinV-positive cells, not shown). Right: 105 sorted Lin− cells were put in culture; cells from entire plates were harvested after 2 days and analyzed. (E) For each genotype, 10 colonies were pooled and analyzed for Gr-1 and Mac-1 expression after 5, 6, and 7 days of culture. Numbers indicate the percentage of cells in each quadrant.
Phenotypic analysis of G-CSF/SCF-dependent colonies.
WT and IkL/L BM cells were cultured in methylcellulose medium supplemented with G-CSF plus SCF. (A) Representative colonies (day 7) for each genotype. Original magnification, × 80. 6 independent experiments were performed. (B) BM cells were cultured as above for the indicated number of days. At each time point, the number of cells per colony was counted for the 10 largest colonies in each plate; bars represent the means ± SDs. (C) Similar proliferation kinetics between WT and IkL/L cells, as revealed by decrease of the CFSE label. Left: CFSE profile of BM cells at day 0 of culture (black histograms), and of representative WT and IkL/L colonies after 6 days of culture (white histograms). For each genotype, 6 colonies were analyzed, all of which showed similar CFSE levels. Right: Lin− BM cells were sorted, labeled with CFSE, and 105 cells were put in culture. Cells from entire plates were harvested after 1 to 3 days of culture and their CFSE levels analyzed. Days of harvest are indicated next to specific histograms. (D) Analysis of apoptosis by Annexin V/propidium iodide staining. Left: isolated colonies at day 6; 6 colonies were analyzed for each genotype and displayed similar phenotypes (with the exception of a single WT colony that had approximately 10% annexinV-positive cells, not shown). Right: 105 sorted Lin− cells were put in culture; cells from entire plates were harvested after 2 days and analyzed. (E) For each genotype, 10 colonies were pooled and analyzed for Gr-1 and Mac-1 expression after 5, 6, and 7 days of culture. Numbers indicate the percentage of cells in each quadrant.
To determine if the smaller size of the IkL/Lcolonies was due to reduced proliferation of the IkL/Lprogenitors in response to G-CSF and SCF, we evaluated, (in isolated colonies from day-6 cultures) the fluorescence intensity of BM cells labeled with the cytoplasmic dye CFSE (a compound that is linearly diluted upon cell division) at day 0 of culture.27Surprisingly, CFSE levels decreased to similar levels in cells from IkL/L and WT colonies (Figure 4C, left graphs), suggesting that both types of cells had undergone similar numbers of division. As the CFSE intensity of cells from 6-day colonies was extremely low and indistinguishable from background staining, we investigated similar cultures after 1 to 3 days of culture. Since isolated colonies contained very few cells at these earlier time points, CFSE levels were measured from all the cells of each culture dish at each time point. Furthermore, to analyze a more homogeneous population of progenitor cells, the latter cultures were initiated using purified lineage-negative (Lin−) cells. Again, no difference was observed between WT and IkL/L cultures (Figure 4C, right graphs). These observations strongly suggest that decreased proliferation does not account for the decreased cellularity of the IkL/L colonies.
We next evaluated cell death as an alternative mechanism. Dying cells were identified by staining for Annexin V and propidium iodide (PI) positivity; Annexin V is an early marker of apoptosis while dead cells are PI+. No significant cell death was observed in most WT and IkL/L colonies at day 6 of culture (Figure 4D, left graphs). In striking contrast, significantly higher numbers of cells were found to be Annexin V+ and/or PI+ after 2 days of culture (Figure 4D, right graphs). Similar results were obtained between 1 to 3 days of culture (not shown). Thus, a higher rate of apoptosis at early stages of G-CSF/SCF-induced maturation may account for the reduced size of the IkL/L colonies.
To follow the kinetics of differentiation in IkL/L cells, colonies grown in G-CSF plus SCF from IkL/L and WT BM cells were analyzed after 5, 6, and 7 days of culture by flow cytometry using antibodies specific for Gr-1 and Mac-1 (Figure 4E). For each time point, 10 colonies were pooled and analyzed. At each time point, cells from IkL/L colonies expressed less Mac-1 and Gr-1 than those from WT cells, suggesting that differentiation may be delayed. However, the same colonies studied after May-Grünwald/Giemsa staining revealed no obvious differences; the percentage of promyelocytes, myelocytes, metamyelocytes, and neutrophils were similar between WT and IkL/L colonies (not shown).
Gr-1 expression by differentiating neutrophils
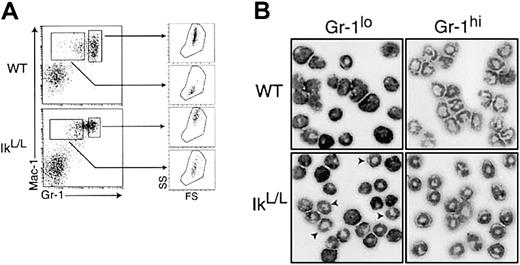
Our phenotypic analyses of the BM and the FL showed that a significant proportion of IkL/L neutrophils expressed low to intermediate levels of the Gr-1 antigen (Figure 2), suggesting an accumulation of immature cells. However, the in vitro experiments described above (Figures 4 and 5) indicated that differentiation is normal, but that Gr-1 expression may be delayed. To test this hypothesis, IkL/L and WT BM cells were stained with antibodies against Mac-1 and Gr-1 (Figure5A), and the Mac-1+Gr-1lo and Mac-1+Gr-1hi cells were assessed for their granular content by side-scatter analysis. Furthermore, these cells were purified and analyzed following May-Grünwald/Giemsa staining (Figure 5B). Mac-1+Gr-1hi cells from WT mice displayed high granular content, as expected of mature neutrophils. The neutrophil morphology of this population was also confirmed. Similarly, Mac-1+Gr-1hi BM cells from IkL/Lmice displayed high granular content and segmented nuclear morphology. Mac-1+Gr-1lo cells from WT animals consisted of immature neutrophils, exhibiting low granular content and metamyelocyte/myelocyte morphology (Figure 5A-B). In contrast, Mac-1+Gr-1lo IkL/L cells showed noticeably higher granular content and many segmented neutrophils were detected in this population. Thus, a significant proportion of terminally differentiated neutrophils in the IkL/L BM failed to express Gr-1 at high levels.
Gr-1 expression is reduced in IkL/Lneutrophils.
(A) Representative Mac-1/Gr-1 profiles of WT and IkL/L BM cells. Neutrophil lineage cells were gated into Gr-1lo and Gr-1hi cells. The graphs on the right show forward- and side-scatter profiles of the cells of each gate. IkL/LGr-1lo cells exhibit higher granular content than WT Gr-1lo cells. (B) Morphology of Gr-1lo and Gr-1hi cells purified according to the Gr-1 gates in panel A. Cells were cytospun and subjected to May-Grünwald/Giemsa staining. Arrows indicate cells displaying the doughnut-shaped nuclei characteristic of mature neutrophils.
Gr-1 expression is reduced in IkL/Lneutrophils.
(A) Representative Mac-1/Gr-1 profiles of WT and IkL/L BM cells. Neutrophil lineage cells were gated into Gr-1lo and Gr-1hi cells. The graphs on the right show forward- and side-scatter profiles of the cells of each gate. IkL/LGr-1lo cells exhibit higher granular content than WT Gr-1lo cells. (B) Morphology of Gr-1lo and Gr-1hi cells purified according to the Gr-1 gates in panel A. Cells were cytospun and subjected to May-Grünwald/Giemsa staining. Arrows indicate cells displaying the doughnut-shaped nuclei characteristic of mature neutrophils.
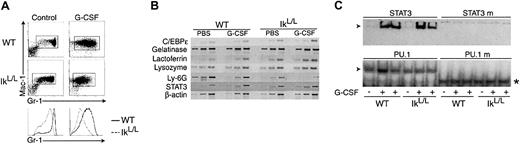
Similar results were obtained following G-CSF treatment in vivo, which allows one to follow granulopoiesis de novo in the BM as mature neutrophils are induced to exit. In WT mice treated with G-CSF for 5 days, Mac-1+ BM cells expressed intermediate to high levels of Gr-1 (Figure 6A). Strikingly, Mac-1+ IkL/L cells expressed very low levels of Gr-1, even though the majority of these cells were fully differentiated, as confirmed by May-Grünwald/Giemsa staining (not shown). The maturity of the Gr-1lo cells in G-CSF-treated IkL/L mice was confirmed by semiquantitative RT-PCR from RNA of WT and IkL/L BM cells. The expression of transcripts encoding secondary granule proteins (lactoferrin, gelatinase, and lysozyme) and C-EBPε (a marker of terminal neutrophil differentiation) were all present in IkL/L cells and at levels similar to WT (Figure 6B). Finally, we tested for STAT3 activity, as STAT3 is the main effector of G-CSF signaling.28 G-CSF treatment induced strong DNA binding activity to a STAT3 binding site in both WT and IkL/L BM cells (Figure 6C), suggesting that IkL/L granulocytes possess intact G-CSF-dependent signaling. In addition, similar binding activities were observed for a PU.1 binding site, also necessary for G-CSF-induced neutrophil differentiation.29 By all of these criteria, BM IkL/L neutrophils respond normally to G-CSF treatment. Their low level of Gr-1 expression therefore most likely reflects a failure to up-regulate this marker.
Analysis of BM neutrophils from mice injected with G-CSF.
(A) Phenotypic analysis of BM cells from WT and IkL/Lmice injected over 5 days with G-CSF or PBS (control). Cells were analyzed 2 hours after the last injection for their expression of Mac-1 and Gr-1 (top). Line graphs compare the intensity of the Gr-1 staining between WT and IkL/L cells for each condition. (B) Semiquantitative RT-PCR of transcripts encoding transcription factors (C/EBPε and STAT3), secondary granule markers (gelatinase, lactoferrin, and lysozyme), and Ly-6G in WT and IkL/L BM cells. Each panel represents ethidium bromide-stained products from PCR reactions corresponding to increasing numbers of cycles. The numbers of PCR cycles were 24, 27, 30 (C/EBPε, gelatinase, lysozyme, Ly-6G, STAT3); 27, 30, 33 (lactoferrin); and 18, 21, 24 (β-actin). (C) G-CSF induction of STAT3 binding. Electrophoretic mobility shift assay performed with 3 μg of nuclear extracts from BM cells derived from mice injected or not with G-CSF. Binding was performed using probes corresponding to WT or mutated (right lanes) STAT3- or PU.1-binding sites. The asterisk indicates a nonspecific band binding the mutated PU.1 site.
Analysis of BM neutrophils from mice injected with G-CSF.
(A) Phenotypic analysis of BM cells from WT and IkL/Lmice injected over 5 days with G-CSF or PBS (control). Cells were analyzed 2 hours after the last injection for their expression of Mac-1 and Gr-1 (top). Line graphs compare the intensity of the Gr-1 staining between WT and IkL/L cells for each condition. (B) Semiquantitative RT-PCR of transcripts encoding transcription factors (C/EBPε and STAT3), secondary granule markers (gelatinase, lactoferrin, and lysozyme), and Ly-6G in WT and IkL/L BM cells. Each panel represents ethidium bromide-stained products from PCR reactions corresponding to increasing numbers of cycles. The numbers of PCR cycles were 24, 27, 30 (C/EBPε, gelatinase, lysozyme, Ly-6G, STAT3); 27, 30, 33 (lactoferrin); and 18, 21, 24 (β-actin). (C) G-CSF induction of STAT3 binding. Electrophoretic mobility shift assay performed with 3 μg of nuclear extracts from BM cells derived from mice injected or not with G-CSF. Binding was performed using probes corresponding to WT or mutated (right lanes) STAT3- or PU.1-binding sites. The asterisk indicates a nonspecific band binding the mutated PU.1 site.
We analyzed the transcript levels of Ly-6G, the gene encoding Gr-1,30 by semiquantitative RT-PCR from RNA of WT and IkL/L BM cells (Figure 6B). Ly-6G mRNA levels were about 5-fold lower (5.3 for WT vs 1.0 for IkL/L in relative levels after normalization with β-actin) in IkL/L BM (despite the fact that the proportion of neutrophils was higher in IkL/L BM, Figure 2A). Transcript levels for Ly-6G were even further reduced when RNA extracted from BM cells of G-CSF-treated mice was analyzed (relative levels: 1.8 for WT and 0.2 for IkL/L, Figure 6B). Together, these data indicate that the reduction in Gr-1 expression is at least partially due to a reduction in the level of Ly-6G mRNA in IkL/L neutrophils.
Neutrophil function in IkL/L mice
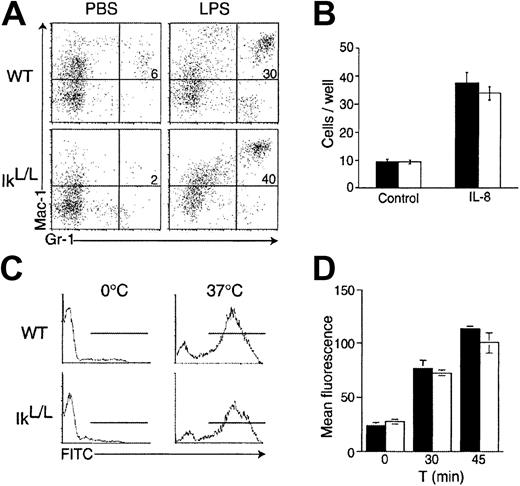
Finally we tested neutrophil function in IkL/Lmice in 4 assays. (1) Neutrophil response to chemotactic stimuli was tested in vivo by challenging WT and IkL/L animals with LPS-induced inflammation. LPS was injected into the peritoneal cavity of test mice, and neutrophil numbers were quantified in the peritoneal cavity 20 hours after challenge. Similar numbers of neutrophils were found in control and IkL/L mice (Figure7A), indicating that IkL/Lneutrophils can be efficiently recruited to a site of inflammation. (2) Neutrophil chemotaxis was measured in vitro in response to IL-8. WT and IkL/L BM cells were seeded in the top chamber of a transwell apparatus separated by a porous membrane from the bottom chamber to which IL-8 was added. The number of neutrophils that had migrated to the bottom chamber after 90 minutes at 37°C were quantified and the results are shown in Figure 7B; no differences were detected between control and IkL/L cells. (3) To measure phagocytic activity, blood cells from WT and IkL/L mice were incubated with fluorescein-labeled opsonized E coli. After one hour at 37°C, both IkL/L and WT cells had incorporated the bacteria with equal efficiency, as detected by flow cytometry (Figure 7C). (4) FcγRII/III-dependent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated respiratory burst was evaluated in IkL/L BM cells, after incubation of the cells with immune complexes bound to H2DCF. In this assay, the H2DCF molecule is internalized and oxidized within a phagocytic vacuole of the neutrophil, thereby releasing the DCF, which can then be visualized by flow cytometry. No differences were observed between WT and IkL/L neutrophils (Figure 7D). Thus, neutrophil function appears normal in IkL/L mice, as measured by these criteria.
Neutrophil function is not altered in IkL/Lmice.
(A) Appearance of Mac-1+Gr-1+ cells in the peritoneal cavity 20 hours after intraperitoneal LPS injection. Numbers represent the percentage of Mac-1+Gr-1+ cells. (B) Numbers of BM cells that had migrated from the upper chamber of a transwell apparatus to the lower chamber containing IL-8 after a 90-minute culture. Bars indicate the means ± SDs of triplicate cultures for each genotype of one representative experiment. (▪) indicates WT; (■) indicates IkL/L. (C) Phagocytic activity of WT and IkL/L blood cells as measured by the uptake of opsonized and FITC-conjugated E coli at 37°C for 1 hour. The proportion of internalized bacteria was subsequently measured by flow cytometry. (D) NADPH oxidase-mediated O burst was evaluated in BM cells after incubation with immune complexes bound to H2DCF for 30 and 45 minutes at 37°C. Following internalization of the H2DCF, O levels were assayed as a function of fluorescence emission by the reduced DCF by flow cytometry. Samples were run in triplicate and the results of a representative experiment are presented as means ± SDs. ▪ indicates WT; ■, IkL/L. One representative experiment is shown for each panel of Figure7.
Neutrophil function is not altered in IkL/Lmice.
(A) Appearance of Mac-1+Gr-1+ cells in the peritoneal cavity 20 hours after intraperitoneal LPS injection. Numbers represent the percentage of Mac-1+Gr-1+ cells. (B) Numbers of BM cells that had migrated from the upper chamber of a transwell apparatus to the lower chamber containing IL-8 after a 90-minute culture. Bars indicate the means ± SDs of triplicate cultures for each genotype of one representative experiment. (▪) indicates WT; (■) indicates IkL/L. (C) Phagocytic activity of WT and IkL/L blood cells as measured by the uptake of opsonized and FITC-conjugated E coli at 37°C for 1 hour. The proportion of internalized bacteria was subsequently measured by flow cytometry. (D) NADPH oxidase-mediated O burst was evaluated in BM cells after incubation with immune complexes bound to H2DCF for 30 and 45 minutes at 37°C. Following internalization of the H2DCF, O levels were assayed as a function of fluorescence emission by the reduced DCF by flow cytometry. Samples were run in triplicate and the results of a representative experiment are presented as means ± SDs. ▪ indicates WT; ■, IkL/L. One representative experiment is shown for each panel of Figure7.
Discussion
We have shown here that Ikaros is dynamically regulated in the neutrophil lineage. Ikaros is highly expressed in immature Mac-1+Gr-1lo BM neutrophils and significantly less so in mature Mac-1+Gr-1hi cells. In direct correlation, Ikaros appears to be most important in controlling early neutrophil differentiation. In the absence of normal levels of Ikaros (1) the number of myeloid lineage cells is strikingly increased in the FL of IkL/L embryos; (2) the migratory potential of neutrophil precursors from both the FL and BM is impaired; (3) G-CSF/SCF-responsive IkL/L neutrophil precursors exhibit increased cell death at early stages of maturation, but not at later stages; and (4) the induction of Ly-6G gene expression is impaired. Nonetheless, the function of mature neutrophils appears to be unaffected, as was demonstrated by in vitro and in vivo experiments. Interestingly, Ikaros transcripts were also found to be down-regulated in the human HL60 promyelocytic cell line treated with the differentiation-inducing agent 1,25(OH)2D3.31 Thus, our combined findings of a conserved regulation of Ikaros in the neutrophil lineage may indicate an evolutionally conserved function for this transcription factor in neutrophils.
The expansion in myeloid numbers in the IkL/L FL seems to result at least in part from an elevated number of precursors, as evidenced by the increase in GM-CSF-dependent colonies. This increase is unique to the FL, as the numbers of GM-CSF-dependent progenitors, and mature neutrophils, in the IkL/L BM remained unchanged vis-à-vis the WT BM. Furthermore, similar numbers of G-CSF/SCF-dependent progenitors were observed in both the FL and BM of WT and IkL/L animals, suggesting that Ikaros may regulate the expression of specific cytokine receptors on immature hematopoietic cells. The FL specificity seen here also suggests that signals or factors that control progenitor homeostasis in the myeloid lineage may differ between the FL and BM. Interestingly, differences in Ikaros activity or function between fetal and adult environments are also seen in the B- and T-cell lineages, which are selectively lacking in the liver and thymus of IkL/L fetuses, respectively.14
The increase in IkL/L FL myeloid cells at late gestation (16.5-18.5 dpc) occurs with a concomitant decrease in the number of erythroid cells. Adult IkL/L mice also have fewer erythroid cells in their BM and blood (P. Kastner, unpublished data, June 1999). One may therefore contend that the primary defect lies in the erythroid lineage, leading to an indirect increase of myeloid cells, possibly as a consequence of biased cell fate choice. This mechanism is unlikely, as (1) the increase in myeloid cell numbers is already seen at 14.5 dpc, when similar numbers of erythroid cells are present in the FL of WT and IkL/L mice, arguing in favor of a specific expansion of myeloid precursors; and (2) we have found that 17.5 dpc IkL/L and WT FLs harbor similar numbers of early committed erythroid progenitors (BFU-E) but greatly reduced numbers of late erythroid progenitors (CFU-E; A.D., unpublished data, October 1999). Thus, the decrease in erythroid cells at late fetal stages might result from a postcommitment defect of erythropoiesis, rather than from a biased choice of pluripotent progenitors toward the myeloid cell fate. Indeed, it has recently been shown that Ik-C mutants exhibit intrinsic defects during erythroid maturation.21 Interestingly, a large-scale gene expression analysis of RNA from 14.5 dpc FL of WT and Ik-C fetuses revealed enhanced transcript levels for many granulocyte-specific genes in the Ik-C FL.21 Although these authors suggested that the increases might reflect a role for Ikaros in the regulation of these genes, their finding is equally consistent with an expansion of the myeloid population in Ik-C FLs (which has thus far not been documented), similar to the one seen here.
The morphology of G-CSF/SCF-dependent neutrophil colonies was very different between WT and IkL/L mice: IkL/Lcolonies were smaller and contained numerous cellular clusters. These data show that Ikaros deficiency intrinsically affects the maturation of myeloid progenitors. Furthermore, our results are interesting from the viewpoint of cytokine signaling, as they provide evidence that G-CSF/SCF signaling can be separated into 2 independent events which are characterized first by migration and then by proliferation.
Blood neutrophils are severely reduced in IkL/L mice, and the phenotype is maintained even after G-CSF treatment in vivo, a procedure that normally stimulates the migration of BM neutrophils to the blood. Why this happens is unclear. One possibility is that IkL/L blood neutrophils have a shorter life span than their WT counterparts; this is unlikely as neutrophils in other organs appear normal, if not increased, in numbers. Furthermore, our preliminary experiments find that IkL/L blood neutrophils are not more apoptotic than WT cells (A.D., unpublished data, May 2000). Alternatively, IkL/L neutrophils may exhibit altered migratory or adhesive properties which can contribute to a change in anatomic distribution of these cells. For example, splenic neutrophil numbers are up 3 to 6 times in IkL/L animals (A.D., unpublished results, March 2000). However, as lymphocyte numbers are down in the spleen of these mice, the neutrophil effect may be due to compensation through homeostasis. Indeed, we detect similar increases in neutrophil numbers in the spleens of RAG-1−/− mice that lack mature lymphocytes (A.D., unpublished results, April 2000). Finally, IkL/L neutrophils respond normally to chemotactic signals such as IL-8. As Ikaros is crucial for the function of other hematopoietic cells, it is unclear whether the blood defect is intrinsic.
Gr-1 expression is reduced in IkL/L neutrophils and our data suggest that the Ly-6G gene is transcriptionally repressed in these cells. Interestingly, in the BM of Ik-C and Ik-DN mice, there is an accumulation of cells expressing Mac-1 but not Gr-1,15 16 demonstrating that Ikaros function is essential for Gr-1 expression. That Gr-1 is expressed by IkL/L BM cells reflects the residual Ikaros activity in these mice and the hypomorphic nature of the IkL/L mutation. Importantly, we show here that a decrease in Gr-1 expression does not correlate with a block in differentiation, as many neutrophils that express low Gr-1 levels are morphologically mature. Together, these data suggest thatLy-6G may be an Ikaros target gene. However, as the genomic sequence of the murine Ly-6G locus is not publicly available, it was not possible to determine whether this locus contains candidate binding sites for Ikaros.
Although the Ly-6G gene appears to be positively regulated by Ikaros, the 2 have patterns of expression that are sharply divergent in the neutrophil lineage. Cells that express the highest levels of Ly-6G also express the lowest levels of Ikaros, and vice versa. Ikaros may therefore act to initiate rather than to maintain Ly-6G expression, perhaps by recruiting other activators to the Ly-6G promoter. Such a scenario would be consistent with recent results from Koipally et al32 who showed, in the context of transfection experiments, that Ikaros binding to target promoters may be required to potentiate the activity of other positive factors. This type of activity would stand in contrast to the more conventional one, in which Ikaros is proposed to be involved in silencing gene expression, as supported by experimental work on the TdT, λ5, and VPAC-1 genes.33-35 Understanding how Ikaros participates in the regulation of the Ly-6G locus at the molecular level may therefore have broad implications for understanding how this factor contributes to gene activation.
In conclusion, our data show that Ikaros controls several aspects of neutrophil differentiation. Of importance is whether the effect observed with the IkL/L mutation reflects a decreased level of Ikaros activity or the activity of an Ikaros protein lacking exon 2-derived sequences, as exon 2 encodes a motif that may interact with the corepressor CtBP.36 This discrepancy is currently under investigation using in vivo systems. However, the apparent convergence between the IkL/L and Ik-C phenotypes, as well as the milder block of Gr-1 expression in IkL/L mice, suggests that the IkL/L mutation is hypomorphic due both to the low abundance and to their weakened repressive properties of the Ikaros proteins expressed in IkL/L mice. In future work, it will be important to understand how Ikaros functions in neutrophils at the molecular level.
We thank S. Smale for the generous gifts of Ikaros antibodies; C. Bronn and S. Duhautbois-Boine for excellent technical assistance; the IGBMC mouse facility for maintaining the IkL/L line; and C. Waltzinger, C. Ebel, and J. Barths for help with the flow cytometry.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-05-1336.
Supported by institute funds from the INSERM, CNRS, and the Hôpital Universitaire de Strasbourg, and by grants from the Ligue Nationale contre le Cancer (LNCC; P. Kirstetter). A.D. and P. Kirstetter received fellowships from the Ministère de la Recherche et de la Technologie and LNCC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Kastner and Susan Chan, IGBMC, BP10142, 67404 Illkirch Cedex, France; e-mail:scpk@igbmc.u-strasbg.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal