It was recently reported that crude bone marrow cells have the ability to differentiate into glomerular mesangial cells. However, the exact nature of the engrafting cells in the bone marrow was not known. We tested the hypothesis that hematopoietic stem cells are capable of reconstituting the mesangial cells by transplanting a clonal population of cells derived from a single stem cell. We cultured Lin−, Sca-1+, c-kit+, CD34− bone marrow cells from transgenic enhanced green fluorescent protein (EGFP) mice (C57BL/6-Ly-5.2 background) individually for 1 week in the presence of interleukin-11 and steel factor. We then transplanted viable clones individually into lethally irradiated C57BL/6-Ly-5.1 mice. Kidneys from 5 recipient mice showing high levels (60%-90%) of multilineage hematopoietic reconstitution were examined 2 to 6 months later, using differential interference contrast and epifluorescence microscopy. EGFP+ cells with a morphology characteristic of mesangial cells were evident within the glomeruli. Transplantation of 100 noncultured Lin−, Sca-1+, c-kit+, CD34− bone marrow cells also generated mesangial cells. Cultured EGFP+ glomerular cells from recipient mice contracted in response to angiotensin II. EGFP+ mesangial cells seen in male-to-male transplants revealed only one Y-chromosome. These data demonstrate that a single hematopoietic stem cell is capable of differentiating into glomerular mesangial cells and that the process does not involve cell fusion.
Introduction
Mesangial cells constitute a population of cells residing in the glomerular mesangium between the capillary endothelial cells and the basal membrane of the glomeruli. These irregularly shaped cells possess high levels of actin and myosin and contract upon exposure to a number of vasoactive agents such as angiotensin II.1 It is thought that one of the functions of mesangial cells is to regulate the blood flow through selected capillary loops. Mesangial cells also are capable of phagocytosis and are involved in a number of pathologic conditions affecting the kidney. Proliferation of mesangial cells is associated with IgA nephropathy, mesangial proliferative glomerulonephritis, and diabetic nephropathy. Despite the pathophysiologic implications, the exact nature and origin of mesangial cells has not been elucidated. Due to the contractile nature of these cells, it generally has been held that mesangial cells represent a specialized type of pericyte. Recently, Imasawa et al2reported that bone marrow (BM) cells can reconstitute mesangial cells in the kidney of lethally irradiated mice. However, since crude bone marrow contains both hematopoietic and nonhematopoietic cells (ie, stromal cells and capillary endothelial cells), the exact nature of the cells that engrafted the glomeruli is not known. To test the hypothesis that mesangial cells are derived from hematopoietic stem cells, we transplanted a clonal population of cells obtained from a single hematopoietic stem cell. The results unequivocally establish the hematopoietic origin of glomerular mesangial cells.
Materials and methods
Monoclonal antibodies and hybridomas
Phycoerythrin (PE)-conjugated D7 (anti-Ly-6A/E [anti–Sca-1]; rat immunoglobulin G2a [IgG2a]), allophycocyanin (APC)-conjugated 2B8 (anti-c-kit; rat IgG2b), biotin-conjugated RAM34 (anti-CD34; rat IgG2a), PE-conjugated A20 (anti-Ly-5.1; mouse IgG2a), PE-conjugated or purified RB6-8C5 (anti-Ly-6G [anti–Gr-1]; rat IgG2b), PE-conjugated RA3-6B2 (anti-CD45R/B220; rat IgG2a), PE-conjugated 30-H12 (anti–Thy-1.2; rat IgG2b), and purified TER-119 (anti-erythrocytes; rat IgG2b) were purchased from Pharmingen (San Diego, CA). PE-conjugated M1/70.15 (anti–Mac-1; rat IgG2b) was purchased from Caltag Laboratories (Burlingame, CA). Hybridoma 14.8 (anti-B220; rat IgG2b), M1/70.15.11.5 (anti–Mac-1; rat IgG2b), GK1.5 (anti-CD4; rat IgG2b), and 53.6.72 (anti-CD8; rat IgG2b) were purchased from American Type Culture Collection (Rockville, MD).
Mice
C57BL/6-Ly5.1 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Transgenic enhanced green fluorescent protein (EGFP)–mice (C57BL/6-Ly5.2 background)3 were kindly provided by M. Okabe (Osaka University, Japan). These 2 strains of mice were bred and maintained at the Animal Research Facility of the Veterans Affairs Medical Center, Charleston, SC. All aspects of the animal research have been conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee of the Department of Veterans Affairs Medical Center.
Cell preparation
Used as bone marrow donors were 10- to 12-week-old male or female EGFP mice. Used as irradiated recipients and as the source for radioprotective cells were 10- to 14-week-old male or female C57BL/6-Ly5.1 mice. Mice were humanely killed by CO2 inhalation, and BM cells were flushed from femurs and tibiae, pooled, and washed twice with Ca2+-, Mg2+-free phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY) containing 0.1% bovine serum albumin (BSA) (Sigma-Aldrich, St Louis, MO). Samples were made into single-cell suspensions by repeated pipetting and filtering through a 40-μm nylon mesh. BM cells with densities ranging from 1.063 to 1.077 g/mL were collected by gradient separation using Nycodenz (Accurate Chemical and Scientific, Westbury, NY). Lineage negative (Lin−) BM cells of EGFP mice were prepared by incubating cells with anti-Mac-1, anti-Gr-1, anti-B220, anti-CD4, anti-CD8, anti-TER-119, and by removing positive cells with immunomagnetic beads (Dynabeads M-450 coupled to sheep anti-rat IgG) (Dynal, Great Neck, NY). The resulting Lin− cells were stained with PE-conjugated anti-Sca-1, APC-conjugated anti-c-kit, and biotin-conjugated anti-CD34, followed by streptavidin-conjugated PharRed (Pharmingen). After addition of propidium iodide at a concentration of 1 μg/mL, the cells were washed twice, resuspended in PBS containing 0.1% BSA, and kept on ice for cell sorting. Five-color analysis and cell sorting were performed using a FACSVantage (Becton Dickinson, San Jose, CA) with appropriate isotype-matched controls. The Lin− cells were first enriched for the Sca-1+, c-kit+ population. Single Lin−, Sca-1+, c-kit+, CD34− cells were deposited into round-bottomed 96-well plates (Corning, Corning, NY) using CloneCyt system (Becton Dickinson).
Single-cell culture
To achieve a high efficiency of clonal hematopoietic engraftment, we combined single-cell deposition with short-term cell culture. The culture medium consisted of α-modification of Eagle medium (ICN Biomedicals, Aurora, OH), 20% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA), 1% deionized fraction V BSA, 1 × 10−4 M 2-mercaptoethanol (Sigma-Aldrich), recombinant rat steel factor (SF), and recombinant human interleukin-11 (IL-11). SF was provided by Amgen (Thousand Oaks, CA) and used at a concentration of 100 ng/mL. IL-11 (a gift from Genetics Institute, Cambridge, MA) was used at a concentration of 100 ng/mL. We have extensive experience with the use of this combination of cytokines in culture and have observed that it supports maintenance of stem cells for 1 week.4-6 Corning 96-well plates containing the above culture media and single cells were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. At 5 and 18 hours after single-cell deposition, we carefully inspected all wells of the plates using an inverted microscope. Eighteen hours after initiation of culture, single cells were present in approximately 70% of the wells. The remainders were partially degenerated cells, debris, or cell doublets. The wells containing single cells were marked and incubated for a total of 7 days.
Transplantation
Recipient Ly-5.1 mice were prepared with a single 950-cGy dose of total body irradiation using a 4 × 106 V linear accelerator. Only contents of the wells containing viable clusters of cells derived from single cells were injected into tail veins of the irradiated Ly-5.1 mice. Five hundred Lin−, Sca-1+, c-kit+, and CD34+ BM cells from Ly-5.1 mice, which contain only short-term repopulating cells, were also transplanted as radioprotective cells to prevent posttransplantation death.7 Mice also received 100 noncultured Lin−, Sca-1+, c-kit+CD34− BM cells from EGFP mice in order to exclude the effect of cell culture on mesangial cell engraftment.
Flow cytometric analysis of hematopoietic engraftment
Peripheral blood was obtained from the retro-orbital plexus of the recipient mice 2, 5, and 6 months after transplantation, using heparin-coated micropipettes (Drummond Scientific, Broomall, PA). Red blood cells were lysed with 0.15 M NH4Cl, and the samples were stained with PE-conjugated anti-Ly-5.1. The percentage of chimerism was calculated as (% EGFP+ cells) × 100/(% EGFP+ cells + % Ly-5.1+ cells). Donor-derived cells in T-cell, B-cell, granulocyte, and monocyte/macrophage lineages were analyzed by staining with PE-conjugated anti-Thy-1.2, anti-CD45R/B220, or a combination of anti-Gr-1 and anti-Mac-1, respectively.
Histologic analysis of engrafted EGFP+ cells
Kidneys from engrafted mice were excised, fixed in 3% paraformaldehyde/PBS solution for 2 hours (25°C), washed, and stored in PBS containing 0.01% sodium azide (PBSA) (4°C). The potential of engrafted EGFP+ cells to contribute to the adult kidney was evaluated by microscopic analysis of thick (vibratome) and thin (paraffin) sections. Vibratome sections (30-100 μm) were cut using an OTS-3000 vibratome (Electron Microscopy Sciences, Fort Washington, PA). Briefly, fixed tissue was gently dried with a paper towel, mounted onto a sectioning stage using instant-drying glue, submerged in PBSA, and sectioned. For paraffin sections, the fixed tissue was dehydrated through a graded series of ethanol/water solutions (30%, 50%, 80%, and 100%) for 10 minutes each. The tissue was infiltrated with 100% Histo-Clear (National Diagnostics, Atlanta, GA) for 2 hours and then processed through a graded series of Histo-Clear:paraffin solutions (3:1, 1:1, 1:3) for 15 minutes each. Finally, the tissue was infiltrated with 100% paraffin for 1 hour twice, embedded in fresh paraffin, cut into 7- to 10-μm sections using a Spencer microtome, floated onto glass slides, and allowed to dry over low heat. Sections were then cleared using Histo-Clear (3 × 10 minutes), rehydrated through a graded series of ethanol/water solutions (100%, 80%, 50%, and 30%) for 10 minutes each, and, finally, transferred into PBSA. Both vibratome and paraffin sections were mounted on glass slides under no. 0 cover slips (Fisher Scientific, Sewanee, GA) using an antiphotobleaching mounting medium. EGFP+ cells were imaged using a Leica DMR light microscope equipped with epifluorescence and differential interference contrast (DIC) optics (Vashaw Scientific, Norcross, GA). Images were processed using Adobe Photoshop 5.5 software (Adobe Systems, San Jose, CA).
Contractile response to angiotensin II in culture
To extend upon our histologic designation of the engrafted cells as mesangial cells, we next evaluated the ability of the cells to respond to a factor (angiotensin II) that is known to elicit a biologic response in mesangial cells.1 To conduct this experiment we established mesangial cell cultures. Briefly, the cultures were generated as follows. Kidneys from engrafted animals were surgically removed and the cortex separated from the medulla. Cortexes were placed in sterile RPMI-1640 medium (Mediatech, Herndon, VA) and cut into 1- to 2-mm3 pieces. The glomeruli were then separated from the cortex by sequential mechanical sieving as previously described.8 Isolated glomeruli were plated on chamber slides coated with collagen I (BIOCOAT culture slides; Falcon, Becton Dickinson Labware, Mountain View, CA) and cultured in RPMI-1640 medium containing 15% FBS, 100 μg/mL streptomycin, 100 units/mL penicillin, and 2 mM L-glutamine. Media were replaced every 48 hours.
After the cells reached a near-confluent state (about 2 weeks), they were washed once with serum-free RPMI-1640 and then imaged using a Leica DMR upright microscope equipped with DIC and epifluorescence optics. EGFP+ cells were selected and photographed prior to the administration of angiotensin II (Sigma-Aldrich) using both epifluorescence and DIC optics. The cells were then exposed to 10−6 M angiotensin II at room temperature and imaged at 1-minute intervals from 0-10 minutes using both epifluorescence and DIC optics.
Fluorescence in situ hybridization (FISH) analysis of Y-chromosome
In order to exclude the possibility that cell fusions caused the apparent plasticity of stem cells, we carried out male-to-male transplantation and performed FISH for Y-chromosome analysis of EGFP+ mesangial cells. Paraformaldehyde-fixed, paraffin-embedded kidney sections from the mouse that received a transplant were labeled with a Y-chromosome paint probe and Cy3-labeled GFP antibody as previously described.9 Briefly, paraffin-embedded sections were deparaffinized, hydrated to 70% ethanol, serially dehydrated, and reacted with biotinylated Y-chromosome paint probe (catalog No. BMPOY; Applied Genetics Laboratories, Melbourne, FL). The biotinylated paint probe was detected with fluorescein isothiocyanate (FITC)–conjugated streptavidin (Jackson Immunoresearch Laboratories, West Grove, PA). EGFP+ cells were identified by a primary antibody, AF594-rabbit anti-GFP (no. A-21312; Molecular Probes, Eugene, OR), followed by Cy3-labeled affinity-purified F(ab′)2 fragments of donkey anti-rabbit IgG (H+L) (Jackson Immunoresearch Laboratories). Nuclei were counterstained with bis-benzimide (Sigma-Aldrich). Sections were coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and viewed with a Zeiss Axioplan 2 microscope (Thornwood, NY). Images were captured with a Spot II CCD digital camera (Diagnostic Instruments, Sterling Heights, MI).
Results
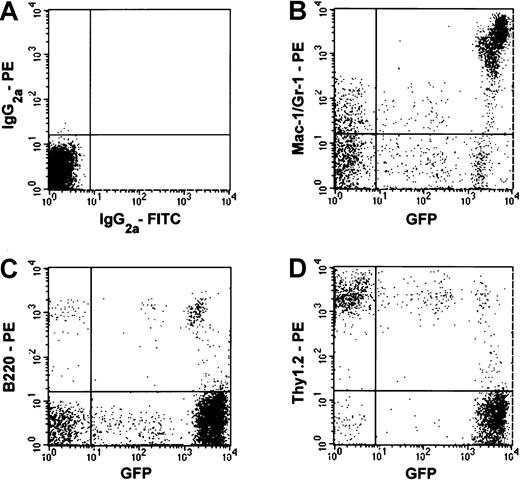
In order to efficiently generate mice that exhibit a high-level of hematopoietic reconstitution from a single EGFP+ stem cell, we have combined single-cell deposition on FACSVantage and cell culture. Clonal populations of cells derived from single hematopoietic stem cells were then transplanted into lethally irradiated recipient mice, Analysis of the nucleated blood cells from these mice 2 to 6 months later revealed variable levels of hematopoietic chimerism and lineage combinations. Presented in Figure1 is the flow cytometric analysis of the nucleated blood cells from a recipient mouse exhibiting a high level (about 90%) of multilineage engraftment 2 months after transplantation. Myeloid and lymphoid chimerism was seen, although macrophage/monocyte and granulocyte engraftment was predominant. The engrafting T cells appeared to consist of 2 populations as reported by Okabe et al.3
Multilineage hematopoietic reconstitution by cells derived from a single EGFP+ cell.
Nucleated peripheral blood cells of a recipient mouse were analyzed using flow cytometry 2 months after transplantation. (A) Blood cells from a control Ly-5.1 mouse that did not receive a transplant were stained with isotype-matched immunoglobulin. (B-D) Reconstitution by EGFP+ cells of Gr-1+ and/or Mac-1+cells, B220+ cells, and Thy-1.2+ cells are shown, respectively. The percentages of the engrafting cells in the granulocyte/macrophage, B-cell, and T-cell lineages were 79%, 77%, and 15%, respectively.
Multilineage hematopoietic reconstitution by cells derived from a single EGFP+ cell.
Nucleated peripheral blood cells of a recipient mouse were analyzed using flow cytometry 2 months after transplantation. (A) Blood cells from a control Ly-5.1 mouse that did not receive a transplant were stained with isotype-matched immunoglobulin. (B-D) Reconstitution by EGFP+ cells of Gr-1+ and/or Mac-1+cells, B220+ cells, and Thy-1.2+ cells are shown, respectively. The percentages of the engrafting cells in the granulocyte/macrophage, B-cell, and T-cell lineages were 79%, 77%, and 15%, respectively.
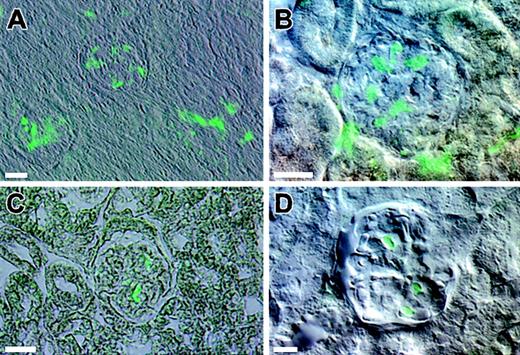
Five mice exhibiting high levels (60%-90%) of multilineage engraftment were killed and their kidneys examined microscopically. Analysis of vibratome and paraffin sections from each of the mice revealed an identical pattern of engraftment by EGFP+ cells into the kidney. Images depicted in Figure2 are composite DIC and epifluorescence images of kidney sections from engrafted animals. When vibratome sections were viewed at low magnification, a population of EGFP+ cells was consistently observed in the glomeruli (Figure 2A). Analysis of sections at higher magnification revealed that the EGFP+ cells had a morphology characteristic of mesangial cells (Figure 2B). The evidence that the EGFP+cells were indeed mesangial cells is supported by analysis of serial thin sections of engrafted kidneys. Figure 2C is a representative image from such a series. The cytoplasmic distribution of green fluorescent protein revealed an irregular shape and compact morphology of the cells lacking large cellular processes. This compact morphology clearly distinguishes these cells from podocytes, which have a highly branched morphology.
Composite DIC and epifluorescence images of kidney sections from engrafted animals.
The kidneys from mice with high levels of multilineage engraftment were examined microscopically. (A) A low-magnification image of a vibratome section depicts two glomeruli with associated EGFP+ cells. (B) A higher magnification image of a vibratome section depicts EGFP+ cells within the glomeruli. (C) A representative image is obtained from serial thin sections of an engrafted kidney. (D) A vibratome section of a kidney from a recipient of 100 Lin−, Sca-1+, c-kit+, and CD34− noncultured BM cells also shows similar EGFP+ cells. Bars equal 25 μm.
Composite DIC and epifluorescence images of kidney sections from engrafted animals.
The kidneys from mice with high levels of multilineage engraftment were examined microscopically. (A) A low-magnification image of a vibratome section depicts two glomeruli with associated EGFP+ cells. (B) A higher magnification image of a vibratome section depicts EGFP+ cells within the glomeruli. (C) A representative image is obtained from serial thin sections of an engrafted kidney. (D) A vibratome section of a kidney from a recipient of 100 Lin−, Sca-1+, c-kit+, and CD34− noncultured BM cells also shows similar EGFP+ cells. Bars equal 25 μm.
It was possible that the ability of the hematopoietic stem cells to generate mesangial cells was an artifact of culture and that hematopoietic stem cells do not possess the inherent capacity to differentiate into mesangial cells. In order to test this premise, we transplanted 100 noncultured Lin−, Sca-1+, c-kit+, and CD34− cells per mouse and examined kidneys 2 months after the transplantation. The kidneys revealed the presence of EGFP+ glomerular mesangial cells as shown in Figure 2D.
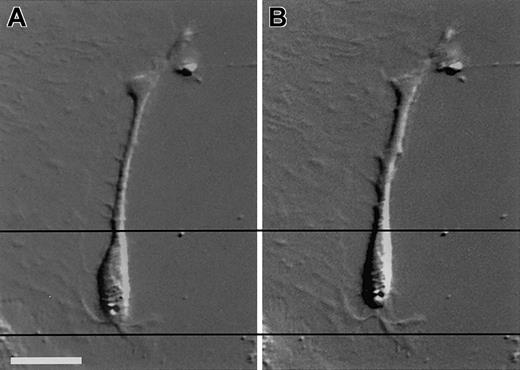
Although the location and the morphology of the cells strongly suggested that these cells were mesangial cells, we carried out cell culture experiments to evaluate the ability of these cells to respond to a known mesangial cell vasoactive agent (angiotensin II). Only mesangial cells, but not epithelial or endothelial cells, in the glomeruli contract upon exposure to angiotensin II.1Depicted in Figure 3 are composite DIC and epifluorescence images of a representative cultured EGFP+ cell taken prior to the administration of angiotensin II (Figure 3A) and 10 minutes after exposure to angiotensin II (Figure3B). The horizontal lines crossing the images are provided as reference points to illustrate the contractile response of the cells. Most of the EGFP+ cells contracted upon exposure to angiotensin II.
DIC images of an EGFP+ cell in a culture derived from the glomeruli of an engrafted kidney.
Cultured cells derived from the glomeruli of an engrafted kidney were exposed to 10−6 M angiotensin II at room temperature for 10 minutes. Photos show the cell prior to exposure to angiotensin II (A) and the cell 10 minutes after exposure to angiotensin II (B). Bars equal 25 μm.
DIC images of an EGFP+ cell in a culture derived from the glomeruli of an engrafted kidney.
Cultured cells derived from the glomeruli of an engrafted kidney were exposed to 10−6 M angiotensin II at room temperature for 10 minutes. Photos show the cell prior to exposure to angiotensin II (A) and the cell 10 minutes after exposure to angiotensin II (B). Bars equal 25 μm.
Discussion
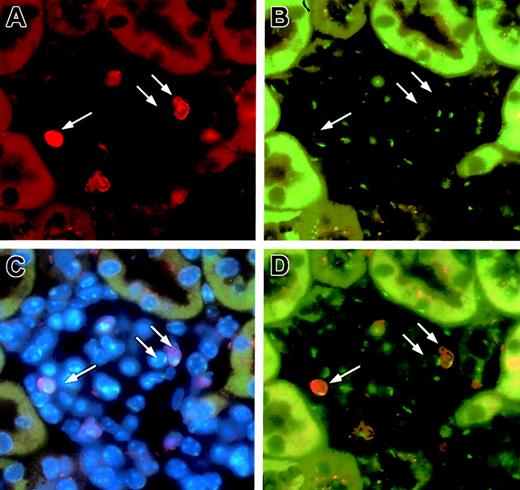
Recently, investigators in 2 laboratories observed spontaneous fusions between EGFP+ ES cells and somatic cells and together with authors of a number of commentaries, suggested that some of the data describing stem cell plasticity, including those based on transplantation, may be the result of spontaneous cell fusions.10 11 In order to exclude this possibility, we performed male-to-male transplantation and performed FISH for Y-chromosome analysis of EGFP+ mesangial cells by using a Y-chromosome paint probe and anti-GFP antibody (Figure4). Three independent reviewers analyzed 70-80 EGFP+ cells and found only a single Y-chromosome in each cell. This observation negated the possibility that the EGFP+ mesangial cells that we observed in the clonally engrafted mice are generated by spontaneous cell fusion.
FISH analysis of Y-chromosome in the glomerular mesangial cells in male-to-male transplantation.
A glomerulus was stained with anti-GFP (red; panel A) and an FITC-labeled Y-chromosome paint probe (green; panel B). Panel C shows triple labeling of the same glomerulus with a combination of anti-GFP, FITC-labeled Y-chromosome paint probe, and bis-benzimide (blue; nuclear label). Panel D is a superimposition of panels A and B. Two of 3 cells (arrows) are donor-derived mesangial cells with only one Y-chromosome. One cell shown has one Y-chromosome and is of recipient origin. Original magnification, × 63.
FISH analysis of Y-chromosome in the glomerular mesangial cells in male-to-male transplantation.
A glomerulus was stained with anti-GFP (red; panel A) and an FITC-labeled Y-chromosome paint probe (green; panel B). Panel C shows triple labeling of the same glomerulus with a combination of anti-GFP, FITC-labeled Y-chromosome paint probe, and bis-benzimide (blue; nuclear label). Panel D is a superimposition of panels A and B. Two of 3 cells (arrows) are donor-derived mesangial cells with only one Y-chromosome. One cell shown has one Y-chromosome and is of recipient origin. Original magnification, × 63.
Imasawa et al2 carried out time course analysis of the development of mesangial cells from bone marrow cells and noted the emergence of mesangial cells as early as 2 weeks after transplantation. We observed mesangial cells in all clonally engrafted mice at 2, 5, and 6 months after transplantation. Although the numbers and populations of bone marrow cells transplanted and the doses of radiation to the recipient mice differ between the 2 studies, the relative ease with which the mesangial cells were generated in both studies suggests rapid turnover of mesangial cells. In support of this is the estimate of 1% daily renewal made by Pabst and Sterzel.12 Although we observed mesangial cells in all 5 mice revealing clonal hematopoietic engraftment, these numbers are too small to answer the question of whether or not all hematopoietic stem cells are capable of generating mesangial cells. Analysis of kidneys from mice receiving transplants of paired progenies of stem cells is in progress to examine the mechanisms of stem cell commitment to the mesangial cell lineage.
Recently, Wagers et al13 examined organs of mice given transplants of single hematopoietic stem cells and did not report the presence of donor-origin glomerular mesangial cells. However, our methods of stem cell purification are slightly different. Of Lin−, Sca-1+, c-kit+ cells, they further selected a population for transplantation based on low level expression of Thy-1, whereas we chose CD34− cells. Wagers et al13 defined all CD45+ cells in the tissues as hematopoietic and most likely missed CD45+ glomerular mesangial cells.14
All circulating blood cells, including myeloid cells, lymphocytes, and platelets are derived from hematopoietic stem cells. In addition, hematopoietic stem cells are a source of tissue-residing cells such as mast cells, liver Kupffer cells, and osteoclasts. In this paper, we extended upon the recent observation of Imasawa et al2that suggested a bone marrow origin of glomerular mesangial cells and clearly established that hematopoietic stem cells give rise to glomerular mesangial cells. This concept may be important in the development of therapies for a number of kidney diseases that display mesangial cell hyperproliferation.
The authors thank Anne G. Livingston and Amanda C. LaRue for assistance in preparation of this manuscript, and the staff of the Radiation Oncology Department of the Medical University of South Carolina for assistance in the irradiation of mice.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-04-1076.
Supported by National Institutes of Health grants RO1-HL57375, RO1-HL52813, DAMD 17-00-1-0338, RO1-DK54197, PO1-CA78582; by American Heart Association, Southeast Affiliate; and by the Office of Research and Development, Medical Research Services, Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Makio Ogawa, Department of Veterans Affairs Medical Center, 109 Bee St, Charleston, SC 29401-5799; e-mail:ogawam@musc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal