Myeloid lineage–derived dendritic cells (DCs) are considered the professional antigen-presenting cell type responsible for eliciting T-cell–mediated immune responses. Acute myelogenous leukemia (AML) is a disease in which tumor antigens are expressed by the malignant clone that also has the potential to differentiate into DC-like cells (leukemic DCs) with antigen-presenting capacity. This study investigated whether the constitutive expression of the cytokine interleukin-7 (IL-7) in primary AML cells during their differentiation toward leukemic DCs results in superior antigen-presenting cells. A bicistronic retroviral vector encoding the IL-7cytokine and the surface immunoselectable low-affinity nerve growth factor receptor (LNGFr) gene was constructed and used for transduction experiments. A serum-free system was used to transduce and differentiate leukemic cells toward leukemic DCs. The study included 8 patients with AML. The transduction efficiency with the cytokine vector varied among patients, ranging from 5% to 30% as judged by LNGFr expression. The leukemic origin of the transduced cells was confirmed in a patient with a chromosomal translocation t(9:11) by fluorescence in situ hybridization analysis. Cytokine modified-cells consistently secreted IL-7 (mean, 415 pg ± 190/106 cells/48 hours; n = 5). We demonstrate thatIL-7–transduced cells are included in the differentiated leukemic DC subset, and, as shown in a particular case, that about half of the mature CD80+ and CD83+ populations coexpress the LNGFr transgene. In addition, IL-7–modified leukemic cells induce stronger allo-T-cell stimulation and higher amounts of IL-2 production in T cells compared with control groups. Finally, cytokine-transduced leukemic DCs can effectively prime and generate cytotoxic T lymphocytes against autologous leukemic blasts.
Introduction
Genetic modification of malignant cells with cytokine and/or costimulatory molecule genes yields potent tumor vaccines in several animal models, including leukemia models.1-7 This approach also leads to promising results in humans (reviewed in Pardoll8).
Dendritic cells (DCs) act as natural adjuvants and are thus of special importance for immunotherapy of cancer.9 The central role of DCs in the priming of T-cell-mediated immune responses has prompted studies to further potentiate DC function by gene transfer. Besides genes coding for tumor antigens, DCs have been engineered to express immunomodulatory cytokines, such as interleukin-7 (IL-7), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-12, and α interferon (αIFN), resulting in an enhancement of T-cell priming and antitumor immunity.10-13
Primary acute myelogenous leukemia (AML) cells are the malignant counterpart to myeloid lineage-committed hemopoietic progenitor cells. The recent demonstration that a proportion of primary AML cells can be differentiated in vitro into a DC-like cell type14-17 has provided the rationale for another form of immunotherapy: the use of in vitro–generated DC-like AML (hereafter referred to as leukemic DCs), naturally expressing tumor antigens, to induce antigen-specific T cells leading to the eradication of unmodified cancer cells.
Herein, we investigated whether cytokine gene transfer could be applied to these leukemias during their differentiation toward leukemic DCs, thus further potentiating their function. IL-7 was selected because of its known effects on survival, activation, and development of T cells,18,19 as well as its regulatory effects on T-cell cytokine production.20 21 Our results demonstrate the feasibility of introducing the IL-7 cytokine gene in these DC-like leukemia cells. Furthermore, IL-7–transduced leukemic DCs cells proved to be superior antigen-presenting cells enhancing T-cell responses.
Patients, materials, and methods
Patient samples
Peripheral blood samples were collected with informed consent from patients with AML at presentation or relapse. All selected patients had more than 80% blast cells in the peripheral blood. Peripheral blood mononuclear cells (PBMCs) containing a homogenous blast population were isolated by discontinuous Ficoll/Hypaque (Pharmacia, Upsala, Sweden) density gradient centrifugation. Cells were cryopreserved in RPMI + 20% Octaplas (Octapharma, Vienna, Austria) and 10% dimethyl sulfoxide (Sigma Aldrich, Vienna, Austria) until use. Patients' details are given in Table 1.
Patient characteristics and transduction efficiency
| Patient no. . | FAB type . | Cytogenetics . | %LNGFr+ . | |
|---|---|---|---|---|
| LXLN . | IL-7LN . | |||
| 1 | M1 | inv.3 | 26 | 16 |
| 2 | M5a | t(9:11) | 17 | 10 |
| 3 | M5b | 46XX | 20 | 16 |
| 4 | M5b | 46XY | 35 | 30 |
| 5 | M5b | 46XY | 26 | 21 |
| 6 | M2 | 46XY | 12 | 9 |
| 7 | M2 | ND | 16 | 7 |
| 8 | M4 | 46XY | 6 | 5 |
| Patient no. . | FAB type . | Cytogenetics . | %LNGFr+ . | |
|---|---|---|---|---|
| LXLN . | IL-7LN . | |||
| 1 | M1 | inv.3 | 26 | 16 |
| 2 | M5a | t(9:11) | 17 | 10 |
| 3 | M5b | 46XX | 20 | 16 |
| 4 | M5b | 46XY | 35 | 30 |
| 5 | M5b | 46XY | 26 | 21 |
| 6 | M2 | 46XY | 12 | 9 |
| 7 | M2 | ND | 16 | 7 |
| 8 | M4 | 46XY | 6 | 5 |
Leukemic blast were cultured in serum-free medium with SCF, GM-CSF, FL, IL-3, and TNFalpha for 48 hours before 3 rounds of infection with viral supernatants containing either the IL-7 cytokine (IL-7LN) or the reporter gene alone (LXLN). Transduction efficiency was monitored by flow cytometry using specific LNGFr mAb, 48 to 72 hours after the last transduction (n = 8, mean ± SD). Mean % LNGFr+ values (± SDs) were 19.8 ± 9 for LXLN and 12.7 ± 8.3 for IL-7LN.
FAB indicates French-American-British; ND, not done.
Generation of bicistronic retroviral vector and packaging cell lines
A bicistronic retroviral vector encoding the IL-7 cytokinegene and the truncated form (not expressed in hematopoietic cells) of the low-affinity nerve growth factor receptor (LNGFr) reporter gene, as well as a control vector encoding the reporter gene alone, were constructed as follows. A 1529–base pair (bp) (EcoR1-Xho) LNGF fragment from plasmid pLNSN (generous gift of Dr Tiefenthaler, Boehringer Mannheim, Penzberg, Germany) was inserted into the Moloney leukemia virus retroviral vector pLXSN (Invitrogen, Groningen, The Netherlands), in which the neomycin gene cassette has been deleted. This was followed by the insertion of an internal ribosomal entry site and a multiple cloning site to facilitate accommodation of further gene inserts. The generated pLXMILN plasmid was used as control vector (hereafter referred to as LXLN). The IL-7 cDNA was excised from plasmid pGEMBL (kindly provided by Immunex, Seattle, WA) and subcloned into the Bam-Nsi site of the multiple cloning site of pLXMILN, generating the final bicistronic vector pLXMILN-IL-7 (hereafter referred to as IL-7LN). All constructs were verified by sequencing.
These plasmid constructs were transfected into the Phoenix packaging cell line (kindly provided by Dr Nolan, Stanford, CA) using a calcium phosphate system (Stratagene, Amsterdam, The Netherlands). Cell-sorting selection was used to generate stable producer cell lines strongly expressing the LNGFr gene. Virus supernatants were prepared in serum-free medium (X-VIVO 15; Bio Whittaker, Walkersville, MD) supplemented with l-glutamine at 32°C and 5% CO2, collected between 36 to 48 hours and followed by cryopreservation. The producer cell line expressing IL-7LN secreted up to 23 ng/106 cells of IL-7 in 48 hours, measured by standard enzyme-linked immunosorbent assay (ELISA).
The generated retroviral vectors were tested for their ability to infect different cell types (CD34+ progenitor cells, leukemia cell lines, PBMCs isolated from healthy volunteers, and monocyte-derived DCs) under optimal culture conditions. As it could be expected, PBMCs and monocyte-derived DCs were not susceptible to viral infection since they are not cycling cells (unpublished observations, C.B.-F., April 1999).
Generation of leukemic DCs and retroviral infection
Cryopreserved leukemic cells were thawed, washed, and resuspended in serum-free medium (X-VIVO 15) supplemented withl-glutamine (2.5 mM), penicillin (125 TU/mL), and streptomycin (125 mg/mL). A total of 0.5 to 1 × 106blast cells/mL were plated in fibronectin-coated (RetroNectin 10 μg/cm2; Takara, Shiga, Japan) 6-well plates (Costar, Vienna, Austria) and cultured in serum-free medium in the presence of the following proliferative cytokine cocktail: stem cell factor (SCF; 20 ng/mL, Peprotech, London, United Kingdom), granulocyte-macrophage colony stimulating factor (GM-CSF; 100 ng/mL, Novartis Research Institute, Vienna, Austria), flt3 ligand (FL; 100 ng/mL kindly provided by Immunex), tumor necrosis factor α (TNFα; 50 U/mL, Bender, Vienna, Austria) and IL-3 (100 ng/mL, Novartis Research Institute). About one third of samples showed a viability less than 70% (trypan blue exclusion) after 3 to 4 days in culture and were excluded. After 48 to 72 hours of stimulation, leukemia samples were infected in 3 consecutive rounds with viral supernatants in the presence of 4 μg/mL polybrene (Sigma) and cytokines as previously described.22 The transduction efficiency was monitored by immunofluorescence staining using a LNGFr monoclonal antibody, 48 to 72 hours after the last infection.22 Comparative studies were initially performed to define a proper cytokine cocktail allowing viability and differentiation of transduced leukemic cells. A serum-free differentiation cytokine cocktail containing lower concentrations of SCF and FL (10 ng/mL and 50 ng/mL, respectively) plus GM-CSF (100 ng/mL), IL-4 (200 U/mL, Novartis), CD40 ligand (500 ng/mL, Immunex), and TNFα (50 U/mL) was selected. Transduced cells were further differentiated for 7 to 10 days prior to being tested in functional assays.
Immunophenotype and cell-sorting analysis
Unmanipulated and transduced AML samples were stained with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mouse monoclonal antibodies (mAbs) against CD86, human leukocyte antigen (HLA)–DR, CD54, CD58, CD4, CD8 (Pharmingen, Heidelberg, Germany), CD80, HLA-DQ (Becton Dickinson, Erembodegem, Belgium), CD83 (Immunotech, Marseille, France) CD1a (clone VIT 6, produced in our institute), and LNGFr (mAb 20.4 purchased from American Type Culture Collection [ATCC]), or with appropriate isotype control mAb. Cells were incubated at 4°C for 20 minutes, washed, and analyzed in a FACScan flow cytometer with CellQuest version 3.3 and analysis software (Becton Dickinson). Forward- and side-scatter gates or propidium iodide exclusion (Sigma) was used to exclude cell debris.
Cell sorting for LNGFr-positive and -negative subsets was performed 48 to 72 hours after transduction. To this end, AML cells were stained with the LNGFr mAb, sorted based on LNGFr+ surface expression (FACS Vantage flow cytometer [Becton Dickinson]), and replated in a differentiation cytokine cocktail for further studies.
Fluorescence in situ hybridization analysis (FISH)
Dual-color FISH was performed on cytospin preparations of naive leukemia blasts and sorted leukemia-derived DCs using the 3′ (labeled with Cy3, red) and 5′ (labeled with FITC, green) probes that flank the breakpoint of the MLL gene (kindly provided by Ed Schuuring, the Netherlands) as described.23 Slides were mounted in Vectashield with the fluorescence dye 4,6-diamino-2-phenyl indole (DAPI). Cells were visualized using a fluorescence microscope (Axioplan; Zeiss, Goettiengen, Germany). In each sample, 100 cells were analyzed.
Allogeneic mixed leukocyte reaction (MLR)
Responder cells for the allogeneic MLR were highly purified T cells isolated from the PBMCs of healthy donors as previously described.22 Freshly isolated or 10- to 14-day cultured/transduced leukemia cells were irradiated with 30 Gy and used as stimulators. A constant number of 5 × 104 T cells were cultured in triplicates and incubated with graded number of irradiated leukemia cells. After 5 days in culture, stimulation of responding T cells was monitored by measuring methyl-3H-thymidine incorporation (Amersham, Buckinghamshire, United Kingdom) for an additional 18 hours. Radioactivity was determined using a 1450 microbeta Wallac-Trilux instrument (Life Science, Vienna, Austria).
Measurement of cytokine production
Virus supernatants as well as supernatants from fresh leukemia cells, cytokine-modified leukemia cells, or cells modified with control vector were analyzed for IL-7 secretion using the specific IL-7 capture and detection mAbs (Pharmingen), following the manufacturer's instructions. The IL-7 concentration was extrapolated to 106 cells in 48 hours.
The cytokine production by T cells was determined as follows: 4 to 5 × 104 of fresh leukemias, cytokine-modified cells, cells transduced with control vector, or untransduced cells were cultured (in RPMI medium supplemented with 10% fetal calf serum [FCS]) alone or in the presence of 1 × 105allogeneic T cells in 96-well plates (Costar). After 48 to 72 hours of culture, supernatants were harvested and measured for IL-2, IL-4, and γIFN secretion using specific ELISA, as previously described.24
Induction of autologous CTL
AML blasts transduced either with the IL-7LN or control LXLN vector were sorted, differentiated to leukemic DCs, irradiated (30 Gy), and used to stimulate autologous PBMCs obtained after complete remission. About 106 nonadherent PBMCs were cocultured with 2.5 × 105 irradiated leukemic DCs in 1.5 mL Yssel medium (Gibco Lofer, Austria) containing 8% FCS,l-glutamine, and penicillin/streptomycin. Recombinant IL-2 (rIL-2; 20 ng/mL, Novartis) was added after 48 hours and then every 2 to 3 days. On day 7, a second restimulation with autologous irradiated IL-7LN leukemic DCs or LXLN leukemic DCs was performed in medium containing rIL-2. CTL activity was measured 5 to 7 days after the second stimulation.
T-cell cytotoxicity assay
The described nonradioactive Europium (Eu3+)-release assay25 was used to monitor the cytotoxic potential of the in vitro–expanded peripheral blood lymphocytes, used as effector cells. Thawed leukemia cells were cultured for 3 to 5 days in X-VIVO 15 medium containing rGM-CSF (50 ng/mL), allowing a more efficient labeling with Eu3+, and used as target cells. Briefly, 5 × 106 leukemic cells were treated with the Eu3+-DTPA-containing buffer (following the manufacturer's instructions; Wallac Oy, Turku, Finland), washed, incubated for one hour in RPMI phenol red–free medium at 37°C, and used in the assay. Labeled target cells (5 × 103) and serial dilutions of effector cells were set up in triplicates in 96-well plates in RPMI phenol red–free medium containing 10% FCS and incubated for 4 to 7 hours at 37°C and 5% CO2. Finally, 25 μL of supernatant and 200 μL of enhancement solution (Wallac) were mixed in 96-well flat-bottom plates and measured in the 1234 DELFIA Research fluorometer (Pharmacia). The percentage of cytotoxicity was calculated as follows: Percent specific lysis = [(Experimental Eu3+ release − Spontaneous Eu3+ release)/(Maximum Eu3+ release − Spontaneous Eu3+ release)] × 100.
Spontaneous Eu3+ release was less than 12% of the maximun release obtained by 2% Triton X-100 (Sigma) lysis.
Statistics
Data are presented as mean values ± SDs. For comparison of 2 groups, the paired Student t test was used. Pvalues of less than .05 were considered statistically significant.
Results
Feasibility of using bicistronic retroviral vectors to transduce primary AML cells during their differentiation toward leukemic DCs
We and others have previously described that retroviral vectors are suitable vehicles to transfer genes into DCs' progenitors, and per se do not influence the functional properties of these cells.22 26-28 This observation led us to investigate whether primary AMLs, on their differentiation to leukemic DCs, could be transduced with retroviral vectors encoding cytokine genes. IL-7 was selected based on its ability to enhance T-cell properties.
We initially performed a set of experiments to define the optimal culture conditions of these leukemia cells for retroviral infection, since these vectors only stably integrate into dividing cells. A serum-free system containing SCF, GM-CSF, Flt3 ligand, TNFα, and IL-3 proved to be the most potent cocktail that reliably induced proliferation in most cases (data not shown).
A bicistronic retroviral vector encoding the IL-7 and theLNGFr reporter gene and a control vector encoding the reporter gene alone were generated in our laboratory and used to infect primary AML cells (“Patients, materials, and methods”). The proportion of transduced leukemic cells expressing the LNGFr was determined by flow cytometry 48 to 72 hours after the last infection. The transduction efficiency obtained with both cytokine and control vectors in 8 patients, as well as patients' details are displayed in Table 1. Transduction rates with the cytokine vector, as judged by LNGFr expression, ranged from 5% (patient no. 8) to 30% (patient no. 4) (mean, 12.7% ± 8.3% SD; n = 8), whereas with the control vector gene transfer efficiencies ranged from 6% to 35% (mean, 19.8% ± 9% SD; n = 8). Higher transduction efficiency with the control vector versus the bicistronic vector was consistently observed in most cases, as it has been described.29 These results demonstrate that primary AMLs can be efficiently infected with retroviral vectors encoding cytokines and a selectable marker.
Cytokine-transduced cells are included in the leukemic DC population
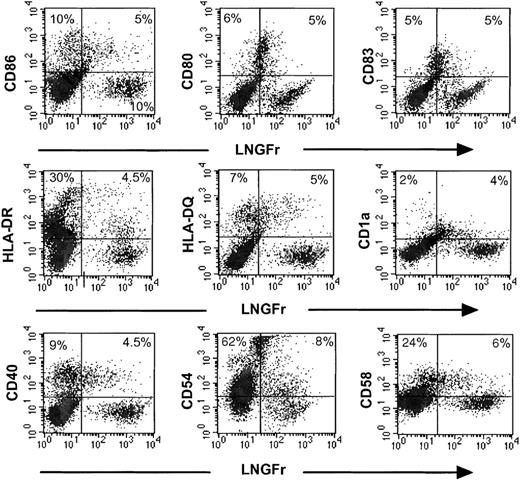
It is generally accepted that a proportion of AMLs can be differentiated in vitro to leukemic DCs.14-17 We defined leukemic DC phenotype as those culture samples with at least one de novo expression of the CD80, CD86, CD83, or CD1a molecules and up-regulation of 2 or more of the following molecules: HLA-DR, HLA-DQ, CD54, or CD58. Of 8 patients tested, 6 fulfilled these criteria (patient nos. 1 to 6) and were selected for further studies. Transduced AML cells were cultured for 7 to 10 days in the serum-free differentiation cytokine medium (described in “Patients, materials, and methods”) and subsequently double stained using the LNGFr antibody, which only detects retroviral infected cells, and a DC marker. Figure 1 shows the results from a patient with AML subtype M5b (patient no. 5). In this case, about one third of the IL-7LN+-transduced cells have a DC phenotype coexpressing CD86, CD80, and CD83 DC markers. This implies that in the mature leukemic DC population expressing CD83+ (10%) and CD80+ (11%), about 50% of these cells also contain the LNGFr transgene. Likewise, the transduced LNGFr+ population also coexpresses different levels of MHC class II, DQ, CD40, CD54, CD58, and CD1a (Figure 1). These results demonstrate that bicistronic retroviral vectors can target leukemic DCs during their in vitro generation. In addition, the proportion of leukemic DCs generated in the cytokine-transduced versus control population was not significantly different (data not shown).
Immunophenotype of IL-7LN-transduced leukemic DCs.
Leukemic cells from patient no. 5 were cultured in a cytokine containing serum-free medium (see “Patients, materials, and methods”) for 48 hours prior to 3 rounds of retroviral transduction with the IL-7LN vector. On day 7, cells were replated in a differentiation cytokine cocktail (see “Patients, materials, and methods”) for an additional 8 days. Double stainings with the LNGFr and CD86, CD80, CD83, HLA-DR, HLA-DQ, CD1a, CD40, CD54, and CD58 mAbs were performed at indicated time point. Viable cells were gated based on forward scatter and side scatter (FSC-SSC) features. Markers were set according to an isotype-matched control mAb. Fluorescence intensities are displayed in logarithmic scale. The numbers represent the percentages of positive cells.
Immunophenotype of IL-7LN-transduced leukemic DCs.
Leukemic cells from patient no. 5 were cultured in a cytokine containing serum-free medium (see “Patients, materials, and methods”) for 48 hours prior to 3 rounds of retroviral transduction with the IL-7LN vector. On day 7, cells were replated in a differentiation cytokine cocktail (see “Patients, materials, and methods”) for an additional 8 days. Double stainings with the LNGFr and CD86, CD80, CD83, HLA-DR, HLA-DQ, CD1a, CD40, CD54, and CD58 mAbs were performed at indicated time point. Viable cells were gated based on forward scatter and side scatter (FSC-SSC) features. Markers were set according to an isotype-matched control mAb. Fluorescence intensities are displayed in logarithmic scale. The numbers represent the percentages of positive cells.
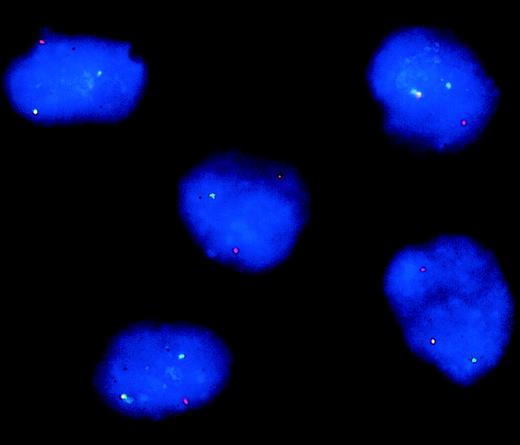
To further prove that the IL-7 retroviral vector targets predominantly leukemic cells and not residual normal cells contained in peripheral blood samples (blast cells source), we performed dual-color FISH analysis in a patient (no. 2) whose leukemic cells harbored a translocation t(9:11) (p22;q23) and the respective MLL/AF9 gene fusion (“Patients, materials, and methods). The separation of the 3′ and 5′ probes in one allele confirmed the presence of a translocation involving the MLL gene in 98% of the IL-7LN+ sorted population (Figure 2), in 95% of the LNGFr− cells (untransduced), and in 92% of the naive leukemic blasts (data not shown). These data indicate that almost all cytokine-modified cells are of leukemic origin and therefore, also the transduced leukemic DCs. Normal mononuclear cells that could be present in the peripheral blood of these patients and that could eventually generate DCs, are not susceptible to retroviral infection using our type of vectors (“Patients, materials, and methods”) as it is also described.30 Thus, by analyzing retroviral-transduced cells, the possibility of including nonleukemic DCs in our study is very unlikely.
FISH analysis of IL-7LN+ cells from patient no. 2 with a translocation t(9:11) and the respective MLL/AF9 gene fusion.
IL-7LN-transduced and sorted cells were hybridized with probes that flank the MLL breakpoint and were counterstained with DAPI. The MLL5′ probe is labeled with FITC (green signal) and the MLL3′ probe with CY3 (red signal). The colocalized signals define the normal allele, whereas the separated red and green signals represent the translocated allele.
FISH analysis of IL-7LN+ cells from patient no. 2 with a translocation t(9:11) and the respective MLL/AF9 gene fusion.
IL-7LN-transduced and sorted cells were hybridized with probes that flank the MLL breakpoint and were counterstained with DAPI. The MLL5′ probe is labeled with FITC (green signal) and the MLL3′ probe with CY3 (red signal). The colocalized signals define the normal allele, whereas the separated red and green signals represent the translocated allele.
Cytokine-modified leukemia cells secrete IL-7
After 48 to 72 hours of retroviral infection with control and cytokine vectors, cells were sorted based on the surface expression of the LNGFr reporter gene. Supernatants from sorted IL-7LN+and LXLN+ cells as well as from untransduced cells or fresh leukemias were tested for IL-7 secretion using specific ELISA. Table2 shows the results obtained in 5 different patients. An average of 415.5 pg ± 190 of IL-7 is secreted by 106 transduced leukemia cells in 48 hours. The cytokine was never detected in supernatants from fresh leukemia cells, unmodified cells, or cells transduced with the control vector.
IL-7 secretion in pg by 106 transduced leukemia cells per 48 hours, determined by ELISA
| Patient no. . | IL-7LN transduced . |
|---|---|
| 1 | 450 |
| 2 | 567 |
| 5 | 617 |
| 7 | 276 |
| 8 | 168 |
| Patient no. . | IL-7LN transduced . |
|---|---|
| 1 | 450 |
| 2 | 567 |
| 5 | 617 |
| 7 | 276 |
| 8 | 168 |
IL-7 production in LXLN-transduced cells was undetectable in all patients. Mean secretion was 415.5 ± 190 (SD) pg by 106 transduced leukemia cells per 48 hours.
IL-7-transduced leukemic DCs are superior stimulators in allogeneic MLRs
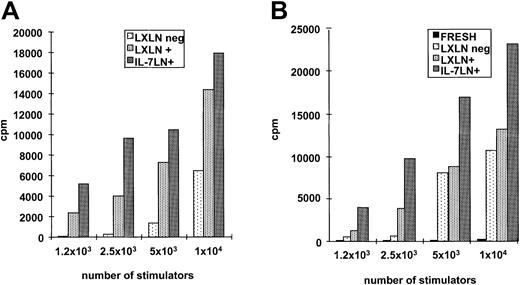
Primary AMLs often have low immunogenicity due in part to lack of costimulatory molecules, whereas differentiated leukemic DCs acquire the ability to activate T cells.14-17 We investigated whether the constitutive expression of IL-7 into leukemic DCs could improve their T-cell stimulatory capacity. Primary AML cells transduced with either control or IL-7 vector were replated in the described differentiation cytokine cocktail (“Patients, materials, and methods”) for an additional 7 to 10 days and used at several stimulator-responder ratios in a classical allogeneic MLR. Figure3A shows the results of 6 patients at the ratio 2 × 104 stimulators: 5 × 104 T cells. Although there was variability with overall stimulatory capacity among different patients, the same pattern was observed in all cases. Cytokine-modified leukemia cells induced consistently higher (up to 2.3-fold) stimulation of T cells compared with cells transduced only with the control vector. Interestingly, a patient with low (< 10%) IL-7LN transduction efficiency (patient no. 6) displayed the lowest T-cell stimulatory capacity compared with cells transduced with the control vector. Differences between control andIL-7–transduced samples showed a significance ofP = .048 (Student t test). In titration experiments, the strongest T-cell proliferation capacity (2.3-fold) was observed at a higher number of stimulators (patient no. 4: transduction efficiency 35% LXLN and 29% with the IL-7LN vector) as depicted in Figure 3B. In all 6 cases, fresh leukemia cells did not induce T-cell activation (data not shown).
Proliferation of allogeneic T cells in response to IL-7LN– or control vector (LXLN)–transduced leukemic DCs.
Allo-MLR was performed with 5 × 104 T cells as responders. Leukemia cells were transduced with both IL-7LN and control vector and differentiated in a serum-free cytokine system. On day 14, cells transduced with control (LXLN) or cytokine vector (IL-7LN) were used as stimulators at different ratios. Proliferation was measured 5 days after stimulation by 3H-thymidine incorporation (cpm). Panel A shows the results obtained in 6 patients with AML using 2 × 104 cells. Panel B shows a titration experiment performed with cells from patient no. 4.
Proliferation of allogeneic T cells in response to IL-7LN– or control vector (LXLN)–transduced leukemic DCs.
Allo-MLR was performed with 5 × 104 T cells as responders. Leukemia cells were transduced with both IL-7LN and control vector and differentiated in a serum-free cytokine system. On day 14, cells transduced with control (LXLN) or cytokine vector (IL-7LN) were used as stimulators at different ratios. Proliferation was measured 5 days after stimulation by 3H-thymidine incorporation (cpm). Panel A shows the results obtained in 6 patients with AML using 2 × 104 cells. Panel B shows a titration experiment performed with cells from patient no. 4.
To further prove that the effects observed were mainly due to theIL-7 transgene, we performed sorting experiments based on LNGFr expression. The IL-7LN+ and LXLN+ subsets as well as the untransduced (LXLN−) population were sorted, differentiated and used as stimulators in a similar manner as with the unsorted population described in “Patients, materials, and methods.” Results of 2 patients (patient nos. 5 and 3), of 4 tested, are shown in Figure 4. AgainIL-7–transduced leukemia cells from patient no. 5 (Figure4B) have the ability to induce up to 2-fold higher T-cell proliferation compared with cells modified with control vector (LXLN+), whereas fresh leukemia cells are not stimulators at all. This effect is less pronounced but consistently present in patient no. 3, especially at a lower number of stimulators, whereas IL-7LN+ cells also induced about a 2.2-fold increase in T-cell proliferation (Figure 4A).
Proliferation of allogeneic T cells in response to transduced and sorted populations.
Leukemia cells from patient no. 3 (A) and patient no. 5 (B) were transduced with both IL-7LN and control vector (LXLN). Cell-sorting selection was based on LNGFr expression using a specific mAb. Sorting subsets IL-7LN+, LXLN+, and LXLN−were replated in a serum-free differentiation cytokine cocktail for an additional 7 days and used as stimulators at different stimulator-responder ratios. Panel B also includes fresh leukemia cells as stimulators. Allo-MLR was performed with 5 × 104 T cells as responders. Proliferation was measured 5 days after stimulation by 3H-thymidine incorporation (cpm). Indicated values represent the mean of triplicates.
Proliferation of allogeneic T cells in response to transduced and sorted populations.
Leukemia cells from patient no. 3 (A) and patient no. 5 (B) were transduced with both IL-7LN and control vector (LXLN). Cell-sorting selection was based on LNGFr expression using a specific mAb. Sorting subsets IL-7LN+, LXLN+, and LXLN−were replated in a serum-free differentiation cytokine cocktail for an additional 7 days and used as stimulators at different stimulator-responder ratios. Panel B also includes fresh leukemia cells as stimulators. Allo-MLR was performed with 5 × 104 T cells as responders. Proliferation was measured 5 days after stimulation by 3H-thymidine incorporation (cpm). Indicated values represent the mean of triplicates.
The stimulatory capacity of the LXLN− subset was lower than (patient no. 3, Figure 4A) or similar to that of the control LXLN+ population (patient no. 5, Figure 4B), a phenomenon probably related to the differentiation stage of these cells. In fact, the phenotypic features of patient no. 5 show that half of the mature CD80+ and CD83+ cells are in the LXLN− fraction (Figure 1), and their stimulatory capacity is similar to the control vector.
Finally, addition of recombinant IL-7 to the proliferation and/or differentiation cytokine cocktail prior to MLR assays did not significantly increase the stimulatory capacity of unmodified leukemic DCs. Furthermore, the phenotypic features of these cells were, by contrast to previous observations,31 not significantly affected (not shown).
IL-7-transduced leukemic DCs induce higher levels of IL-2 in T cells
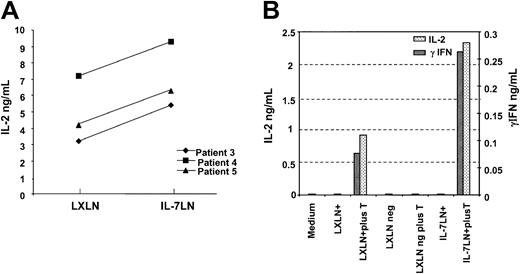
We next studied whether the constitutive expression of the IL-7 cytokine gene into leukemic DCs influences the cytokine secretion profile in T cells. Unsorted and sorted leukemic cells transduced either with control or cytokine vector were cultured alone or with allogeneic T cells for 48 to 72 hours. Supernatants were harvested and tested for IL-2, IL-4, and γIFN production using specific ELISA assays. Figure 5A shows the results with the unsorted population in 3 different patients (patient nos. 3, 4, and 5). IL-7 modified-leukemia cells induced consistently higher levels of IL-2 secretion (mean, 7 ng/mL ± 2 SDs; n = 3) compared with the control LXLN group (mean, 4.8 ng/mL ± 2 SDs; n = 3) with a significance of P = .002 (paired t test). No IL-4 or γIFN production was detected in the unsorted population (not shown). In sorted cells of patient no. 2, cytokine-modified cells induced up to 3.5-fold higher IL-2 secretion than cells transduced with vector control or untransduced cells. In addition γIFN production was 2.5-fold higher in IL-7–transduced leukemic cells versus control vector (280 pg/mL vs 110 pg/mL, respectively; Figure 5B), and again no IL-4 was measured in any of the analyzed subsets. Fresh leukemia cells do not produce any of these mentioned cytokines (not shown). These findings further confirm the ability of IL-7–modified leukemic DCs to enhance T-cell response and potentiate the secretion of cytokines considered to be of Th1 prototype and involved in tumor immunity.
Cytokine-transduced leukemia cells increase IL-2 and γIFN production in T cells.
Leukemia cells transduced with the IL-7LN or control (LXLN) vector were differentiated in a serum-free cytokine cocktail for one week. Subsequently, 4 to 5 × 104 unsorted or sorted cells were cultured alone or with 105 T cells. After 48 to 72 hours supernatants were harvested and measured for IL-2 and γIFN production using specific ELISA. Panel A shows IL-2 production in 3 different patients using unsorted IL-7LN and LXLN populations. Panel B shows (in 2 different scales) IL-2 and γIFN production by sorted IL-7LN and LXLN-transduced cells from patient no. 2. Controls included in the study are indicated.
Cytokine-transduced leukemia cells increase IL-2 and γIFN production in T cells.
Leukemia cells transduced with the IL-7LN or control (LXLN) vector were differentiated in a serum-free cytokine cocktail for one week. Subsequently, 4 to 5 × 104 unsorted or sorted cells were cultured alone or with 105 T cells. After 48 to 72 hours supernatants were harvested and measured for IL-2 and γIFN production using specific ELISA. Panel A shows IL-2 production in 3 different patients using unsorted IL-7LN and LXLN populations. Panel B shows (in 2 different scales) IL-2 and γIFN production by sorted IL-7LN and LXLN-transduced cells from patient no. 2. Controls included in the study are indicated.
Generation of autologous antileukemic activity by IL-7LN–transduced leukemic DCs
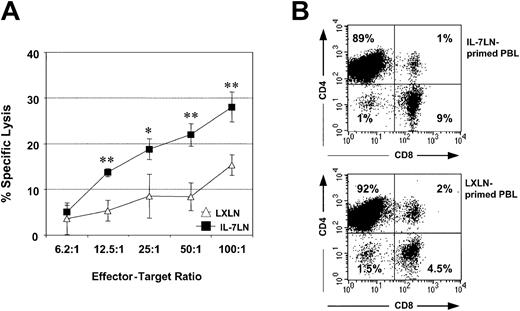
It has been reported that leukemic DCs stimulate autologous lymphocytes that can destroy unmodified leukemia cells.15 17 We investigated whether the constitutive expression of IL-7 in leukemic DCs results in the generation of superior CTL. In Figure 6A, the antileukemic cytotoxicity of autologous T cells primed with irradiated leukemic DCs transduced either with IL-7LN or with the control LXLN vector obtained in patient no. 2 is displayed. The percentage of lysis (means of triplicates ± SDs) was determined by measuring the Eu3+ release after 7 hours of coculture (“Patients, materials, and methods”). T cells stimulated with cytokine-modified leukemic DCs are consistently able to generate, at different effector-target ratios, superior antileukemic cytotoxicity compared with T cells stimulated with the control vector. This functional effect might be due, in part, to differences in the expanded T-cell population. Although most of the T cells are CD4+ (Figure6B), the proportion of CD8+ T cells generated with primed IL-7LN leukemic DCs was 2-fold higher (9%) compared with the percentage of CD8+ T cells obtained with control LXLN leukemic DCs (4.5%) (Figure 6B). No quantitative difference in T-cell expansion was observed between IL-7LN and LXLN groups (not shown).
Cytotoxicity of autologous T cells primed with IL-7LN-transduced leukemic DCs or with control LXLN-transduced DCs against autologous AML blasts.
Effector cells were autologous T cells cultured with irradiated IL-7-modified leukemic DCs or control LXLN leukemic DCs for 14 days. Autologous AML blasts were used as target cells. Panel A represents the cytotoxicity of triplicate cultures (means ± SDs) obtained at a different effector-target ratio in patient no. 2. The significance between the IL-7LN (•) and control group (▵, LXLN) at different E/T ratio is indicated as: *P < .03 and **P < .005. Panel B shows the phenotype of the effector T cells used in the cytotoxic assay. Cells were stained with CD8-FITC and CD4-PE mAbs and analyzed by flow cytometry. Makers were set according to an isotype-control mAb. The number in each box represents the percent of positive cells.
Cytotoxicity of autologous T cells primed with IL-7LN-transduced leukemic DCs or with control LXLN-transduced DCs against autologous AML blasts.
Effector cells were autologous T cells cultured with irradiated IL-7-modified leukemic DCs or control LXLN leukemic DCs for 14 days. Autologous AML blasts were used as target cells. Panel A represents the cytotoxicity of triplicate cultures (means ± SDs) obtained at a different effector-target ratio in patient no. 2. The significance between the IL-7LN (•) and control group (▵, LXLN) at different E/T ratio is indicated as: *P < .03 and **P < .005. Panel B shows the phenotype of the effector T cells used in the cytotoxic assay. Cells were stained with CD8-FITC and CD4-PE mAbs and analyzed by flow cytometry. Makers were set according to an isotype-control mAb. The number in each box represents the percent of positive cells.
Discussion
This study shows that bicistronic retroviral vectors can be used to introduce genes, like the IL-7 cytokine gene, into primary myeloid leukemic cells during their differentiation toward leukemic DCs. We also demonstrate that IL-7 increases the T-cell stimulatory capacity of these cells, enhances the Th1 cytokine profile in T cells, and potentiates the generation in vitro of autologous antileukemic CTL.
Retroviral vectors can transduce a wide range of hematopoietic cells including DCs' progenitors of myeloid origin22,27,28 and hematopoietic malignancies.32,33 AML is a malignant disease of myeloid precursors that can be driven to a DC-type;14-17 thus, we postulated that retroviral vectors might also deliver genes into these leukemic DCs. Because these vectors infect only cycling cells, a serum-free system used to target DC progenitors22 was further optimized for primary AMLs, which are known to have low proliferation rates in many cases. In general the combination of 5 cytokines, namely SCF, GM-CSF, IL-3, FL, and TNFα gave us an optimal proliferation, and up to 30% of the leukemic cells could be transduced with the IL-7 vector. Transduction efficiency displayed great variability among patients and did not correlate with proliferation stage (not shown) as previously described.33 Gene transfer in AMLs may be further improved by using vectors like lentiviral vectors since they do not need cycling cells,29 although the clinical applications of lentiviruses require higher biosafety conditions. Adenoviral vectors can effectively target DCs and do not need dividing cells,34 however the standard adenoviral vectors have poor infectivity in primary AML, requiring further manipulation.35 Furthermore, immunogenicity of adenoviruses may be of concern, in particular when repeat applications are envisaged.
In preclinical models, transfection with costimulatory molecules of undifferentiated leukemic cells (which per se are not expressed in these cells) and/or cytokine genes resulted in good therapeutic effects.5,7,36-38 Herein, we demonstrate, that following culture and transduction with the IL-7 vector in the serum-free medium described, leukemic DCs display CD86, CD80, and CD83 DCs markers as well as IL-7, as judged by the identification of the immunoselectable LNGFr transgene and the detection of the IL-7 protein only in the supernatants of cytokine-modified cells. The proportion of leukemic DCs transduced varied considerably among patients. For example, a great majority (> 70%) of the IL-7-modified cells of patient no. 1 displayed phenotypic DC features, whereas in patient no. 6 only a low proportion (<20%) of the LNGFr+ cells showed DC markers (not shown); and in patient no. 5 half of the transduced cells coexpress CD80+ and CD83+ (Figure 1). In addition, the percentage of leukemic cells expressing CD80 and CD83 DC markers was in most cases less than 25%, in the lower range compared with published data.16,17 This may be explained by the known heterogeneity of AMLs.17
The main advantage of cytokine-modified DCs relies on their functional properties. DCs migrate and home to T-cell areas in lymphatic tissues where they encounter and prime naive T cells. The local and continuous secretion of the cytokine in this environment probably could not be achieved by its systemic in vivo administration. In addition, the side effects often observed under systemic cytokine treatment would be avoided. The transgene expression of IL-7 in leukemic DCs could be of particular relevance since IL-7 induces proliferation of naive T cells,39regulates T-cell survival, enhances cytolytic T-cell function,40 and contributes to the generation of memory T cells.41 Thus, it is tempting to speculate that the local secretion of IL-7 during the leukemic DCs T-cell interaction occurring in secondary lymphoid organs might contribute to the generation of a more efficient T-cell response, and it could also influence humoral immunity by acting on the B cells present in these areas. Indeed, Westermann et al10 reported the in vitro modulation of DC function by IL-7 using monocyte-derived DCs. They observed up to a 2.7-fold increase in T-cell activation, findings that are in line with our leukemic DCs study (Figures 3-4). We also noticed stronger T-cell stimulation with increasing number of cytokine-modified cells. In addition, IL-7–modified leukemic cells induced consistently higher levels of the Th1 prototype cytokines IL-2 and γIFN, whereas no IL-4 secretion, the Th2 prototype cytokine, was observed under any condition tested. These observations are in line with previous work showing the regulatory effect of IL-7 on IL-2 and γIFN gene expression,20,21 and they support the role of lL-7 as immune adjuvant in antitumor immunity in which a cell-mediated Th1 response is generally required. Furthermore, in vitro T-cell priming with IL-7 leukemic DCs resulted in more efficient killing of autologous leukemic blast, due in part, to higher expansion of CD8+ T cells (Figure 6). The mechanisms responsible for IL-7-induced cytolytic activity of CD8+ CTLs are not entirely understood but likely involve the induction of pore-forming proteins and granules as well as the up-regulation of cytotoxic molecules such as γ-IFN.21 42
Any immunotherapeutic approach to leukemia/cancer treatment will be most effective at the stage of minimal residual disease. The immunosupppression related to the disease or derived from the treatment might limit its success. Recent studies described IL-7 as a regulator of naive CD8+ and CD4+ T-cell expansion in lymphopenic hosts, restoring immunity;41 43 whether this effect could also take place in the context of cancer vaccines remains to be explored.
To our knowledge this is the first study that shows the possibility of introducing genes into primary leukemic DCs. The transgene expression of IL-7 in these cells results in superior antigen-presenting cells. Cytokine transduction of leukemic DCs may thus further be exploited as a potentially powerful form of immunotherapy of AML.
We thank Manfred Berger for his excellent technical advice. We are grateful to Dr Ornella Parolini for the critical reading of the manuscript. We also thank Drs Andreas Szekeres and Karel Drbal for the useful discussions and suggestions.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-02-0378.
Supported by the ICP program of the Austrian Federal Ministry for Education, Science and Culture.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Concha Bello-Fernández, Institute of Immunology, Vienna International Research Cooperation Center (VIRCC), Brunnerstrasse 59, A-1235 Vienna, Austria; e-mail: concha@kabsi.at orconcha.bello@univie.ac.at.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal