Immunodeficiency following autologous CD34+-purified peripheral blood stem cell (PBSC) transplantation could be related to T-cell depletion of the graft or impaired T-cell reconstitution due to thymus irradiation. Aiming to assess the role of irradiated thymus in T-cell repopulation, we studied 32 adults with multiple myeloma, randomly assigned to receive high-dose therapy including total body irradiation (TBI) followed by autologous transplantation with either unselected or CD34+-selected PBSCs. The median number of reinfused CD3+ cells was lower in the selected group (0.03 versus 14 × 106/kg; P = .002). Lymphocyte subset counts were evaluated from month 3 to 24 after grafting. Naive CD4+ T cells were characterized both by phenotype and by quantification of T-cell receptor rearrangement excision circles (TRECs). The reconstitution of CD3+ and CD4+ T cells was significantly delayed in the CD34+-selected group, but eventually led to counts similar to those found in the unselected group after month 12. Mechanism of reconstitution differed, however, between both groups. Indeed, a marked increase in the naive CD62L+CD45RA+CD4+subset was observed in the selected group, but not in the unselected group in which half of the CD45RA+CD4+ T cells appear to be CD62L−. Age was identified as an independent adverse factor for CD4+ and CD62L+CD45RA+CD4+ T-cell reconstitution. Our results provide evidence that infusing PBSCs depleted of T cells after TBI in adults delays T-cell reconstitution but accelerates thymic regeneration.
Introduction
Advances in the treatment of hematologic malignancies have been reported with the use of high-dose myeloablative therapy with or without body irradiation and followed by hematopoietic stem cell transplantation (HSCT). Immunodeficiency is an important clinical problem in patients treated with such intensive approaches.1,2 The incidence of severe infectious adverse events and second cancers may be high,3-5 responsible for a significant treatment-related mortality.
Of all hematopoietic lineages, T-lineage lymphocytes (T cells) undergo the most extensive maturation process after leaving the marrow. Maturation of thymic T-cell precursors includes T-cell–receptor (TCR) gene rearrangement followed by negative and positive selection, T cells entering then the peripheral pool as naive T cells. Numerous studies of T-cell regeneration after myeloablative therapy followed by HSCT have shown that T cells may be regenerated through 2 different pathways.6 One is thymus dependent and might be considered as a recapitulation of ontogeny. In addition, when the activity of the thymus is low, the T-cell pool can be repopulated through peripheral expansion of reinfused mature T cells. Some studies suggest that the thymus-dependent pathway is age dependent and becomes limited after childhood, presumably due to thymic involution.7 8
In an autologous setting, ex vivo treatment of hematopoietic stem cells has been proposed as a means of reducing contamination of the graft by residual tumoral cells.9 The positive selection of peripheral blood stem cells (PBSCs) expressing the CD34 antigen has been used with this aim.10-12 The enrichment of the graft in CD34+ cells is accompanied by a profound T-cell depletion that may compromise the peripheral expansion of reinfused T cells following HSCT.
The combination of high-dose therapy (HDT) including a thymic irradiation with the reinfusion of CD34+-enriched autologous cells might thus be potentially responsible for a profound induced immunodeficiency, because both T-cell regeneration pathways might be altered in these patients. As a matter of fact, recent works provide evidence that adult thymus may contribute substantially to immune reconstitution in some cases.13 14 However, the effect of thymic irradiation on thymic function in the autologous setting has not yet been evaluated.
In the present study, we report an evaluation of T-cell repopulation kinetics and mechanisms in patients with multiple myeloma treated with HDT including total body irradiation (TBI) followed by reinfusion of autologous stem cells, using either unselected or CD34+-selected PBSCs. The primary objective of the study was to assess the capacity of irradiated adult thymus in T-cell repopulation in such a setting and factors influencing this function. To measure thymic output and to characterize naive T cells, we used both phenotype and molecular criteria, namely, CD45RA+CD62L+ phenotype and quantification of excisional DNA products of TCR gene rearrangement.15
Patients, materials, and methods
Patients and treatments
All 32 patients included in the present study (17 men and 15 women; median age, 50 years; range, 36-56 years) were treated in a multicenter prospective trial where patients younger than 56 years with newly diagnosed symptomatic multiple myeloma were randomly assigned up-front to receive either a single HDT (HDT1 arm) or 2 sequential HDTs (HDT2 arm). In addition, all patients were independently randomized to receive transplants with CD34+-selected PBSCs (CD34+-selected group) or unselected PBSCs (unselected group).
All patients first received 1 or 2 courses of high-dose steroid-containing regimens and PBSCs were thereafter mobilized by cyclophosphamide (4 g/m2) and lenograstim granulocyte colony-stimulating factor (10 μg/kg/d). When appropriate (CD34+-selected group), part of the collected PBSCs were CD34+ selected using the Isolex 300i system (Baxter, Irvine, CA). The selection procedure resulted in a median purity of 95% (65%-100%) and in a significant tumor cell depletion. In the HDT1 arm, HDT was preceded by 3 monthly courses of a vincristine, adriamycine, and dexamethasone (VAD)–like regimen and combined a multidrug regimen (carmustine, etoposide, melphalan 140 mg/m2 and cyclophosphamide 60 mg/kg) with a TBI (12 Gy in 6 fractions). Patients treated in the HDT2 arm received melphalan 140 mg/m2 alone, always supported by unmanipulated PBSCs, followed 2 to 3 months later by a second course of melphalan 140 mg/m2 combined with etoposide (30 mg/kg) and 12 Gy TBI. In both HDT groups, TBI-containing HDTs were supported with unselected or CD34+-selected PBSCs, according to the randomization procedure. No maintenance therapy was planned after TBI-containing HDT. After a pneumonia related to Pneumocystis carinii infection occurred 4 months after HDT in patient 9, all patients received oral cotrimoxazole as prophylactic treatment after HDT. All patients gave informed consent and the trial was approved by the Hôpital Saint-Louis (Paris, France) ethics committee.
Blood samples for immunologic studies
Heparinized blood samples were collected at month 1, 3, 6, 9, 12, 15, 18, and 24 after the last PBSC reinfusion. In addition, blood samples from diagnosis were available for 22 patients (12 from the CD34+-selected group and 10 from the unselected group). Peripheral blood mononuclear cells (MNCs) were separated from blood on Ficoll-Hypaque density gradients (Pharmacia, Uppsala, Sweden). MNCs were washed twice with RPMI 1640 (Gibco, Paisley, United Kingdom).
Flow cytometry
Lymphocyte subset analysis.
Absolute CD4+ and CD8+ cell counts were performed on fresh blood samples by 4-color flow cytometry analysis of cells positive for CD45, CD3, CD4, or CD8, using fluorescent beads as an internal standard (Coulter, Margency, France). The flow cytometry analysis of lymphocyte subpopulations was performed on the same whole blood specimen in 3-color immunofluorescence, using a panel of monoclonal antibodies to the following cell surface proteins: CD3 (peridinin chlorophyll protein [PerCP]), CD4 (fluorescein isothiocyanate [FITC], phycoerythrin [PE], or PerCP), CD8 (PE), CD45 (FITC)/CD14 (PE; Becton Dickinson, Pont de Clay, France) and CD8 (phyco-cyanine 5), CD45RA (PE), CD45RO (FITC), CD62L (FITC), CD16 (FITC)/CD56 (PE; Immunotech, Marseille-Luminy, France). Irrelevant antibodies of respective isotype were used to ascertain background staining. All antibodies were incubated with 100 μL whole blood for 20 minutes at 20°C before red cell lysis. After washings with phosphate-buffered saline (PBS), 5000 lymphocytes defined by forward/side scatter (FSC/SSC) gate combined to CD14−CD45+ staining were acquired into the FACScan cytometer and analyzed according to Cell Quest (Becton Dickinson, San Jose, CA). The mean values ± SEM in healthy people are 900 ± 185/mm3 for CD4+ cells, 482 ± 70/mm3 for CD45RO+CD4+cells, 420 ± 150/mm3 for CD62L+CD45RA+CD4+ cells, 649 ± 202/mm3 for CD8+CD3+cells, and 243 ± 95/mm3 for CD3−CD16+CD56+ cells.
Ki67 staining.
This antibody recognizes an unknown nuclear antigen present on proliferating cells. This antigen is specifically expressed during the end of G1, S, G2, and M phases of the cell cycle. Surface staining was performed on whole blood, as described (see “Lymphocyte subset analysis”). After red cell lysis, cells were washed with PBS, 0.5% bovine serum albumin (BSA; Sigma, St Quentin, France) and then fixed with PBS, 4% paraformaldehyde (PFA; Sigma) for 10 minutes at room temperature. Cells were permeabilized in a PBS, 0.1% saponin solution (Sigma) and stained with an anti-Ki67-FITC monoclonal antibody for 20 minutes. Fifty thousand lymphocytes were acquired into the FACScan cytometer and analyzed according Cell Quest. The mean value of the expression of the Ki67 antigen within the CD4+ and CD8+ T-lymphocyte population established in 10 healthy people is 1.6% ± 0.04%. After Ki67 intracellular staining, cells were washed and incubated with 10 mg/mL Topro-3-iodide (Molecular Probes, Leiden, The Netherlands) and 5 mg/mL RNase (Sigma) for 45 minutes at 4°C. Topro-3-iodide is excited at 633 nm and is emitted at 675 nm. A total of 150 000 lymphocytes defined by FSC/SSC gate are acquired into a FACScalibur cytometer fitted with a double helium-neon (633 nm)/argon (488 nm) laser.
TCR rearrangement excisional circles
Quantification of TCR rearrangement excisional circles (TRECs) was performed at month 24 of follow-up on purified cell subsets, when sufficient amounts of CD45RA+CD4+ T cells were reached. CD45RA+CD4+ and CD45RA−CD4+ T cells were purified from fresh PBMCs by magnetic separation over columns, using the MiniMACS multisort kit according to the manufacturer's instructions (Miltenyi Biotec, Sunnyvale, CA). Briefly, after 15 minutes' incubation with 20 μL CD4-conjugated magnetic beads per 107 cells, CD4+ T cells were isolated from PBMCs by positive selection over MiniMACS separation columns. Magnetic beads were then released and cells were incubated with CD45RA-conjugated magnetic beads and passed over columns. With this technique, at least 90% purity of the fractions was achieved. Truly naive T cells are better defined by the coexpression of CD45RA and CD62L antigens. However, due to practical reasons, TREC analysis was performed in purified CD45RA+CD4+ T cells as well as in the CD45RA−CD4+ T-cell counterpart. DNA was purified using the DNAzol technique (Life Technologies, Cergy Pontoise, France). Real-time polymerase chain reaction (PCR) assay (Taqman, Perkin Elmer, Les Ulis, France) for signal joint (Sj) TRECs was performed in 50 mL containing 100 to 400 ng DNA, 25 mL Universal Master Mix (Perkin Elmer), 400 nM forward (5′-CACATCCCTTTCAACCATGCTGACA-3′) and reverse primers (5′-AGAACGGTGAATGAAGAGCGACA-3′) and 200 nM specific probe (5′-TGCCCACTCCTGTGCACGGGTG-3′) under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, followed by 45 cycles of amplification (95°C for 15 seconds, 60°C for 1 minute).16 A nonrepeated region of the GAPDHgene was amplified in every sample test as an internal control measurement for imput DNA (forward primer 5′-CTCCCCACACACATGCACTTAC-3′, reverse primer 5′-CCTAGTCCCAGGGCTTTGATT-3′, and the probe 5′-AAAAGAGCTAGGAAGGGAAAGGACAGGCAACTTGGC-3′). For each sample the number of Sj TREC copies was determined using a dilution series of the pGTH310 clone containing the Sj fragment in the SacI site of a pGEM 4 vector.
Statistical methods
The Fisher exact test was used for binary variable comparison. The Mann-Whitney test was used for continuous variable comparison, such as T-cell subset counts (median comparison). In addition, the kinetics of T-cell subsets recovery per unit time were evaluated through the slope of the line fitted to the data over time (simple linear regression). For that, we assumed that the rate of disappearance of cells is negligible at the time scale of the study. Correlation between kinetics of recovery and age at the moment of transplantation was tested by the correlation t test. Multivariate comparisons were performed using logistic regression and tested by the likelihood ratio test. P < .05 was considered to indicate statistical significance. All calculations were performed using the STATA software, version 7.0 (Stata, College Station, TX).
Results
Patient population
The distribution of the 32 patients included in the present study within the 4 randomization groups was as follows: HDT1-unmanipulated arm, 10 patients; HDT1-CD34 arm, 8 patients; HDT2-unmanipulated arm, 6 patients; HDT2-CD34 arm, 8 patients. The impact of the CD34+ selection was assessed on the comparison of the group of 16 patients receiving CD34+-selected PBSCs to the group of 16 patients receiving unselected PBSCs after the last course of HDT. The median age was similar in both groups (49 years, range, 36-56 years in the selected group; 51 years, range, 38-56 years in the unselected group; P = .89). There was no difference in the single versus 2 courses of HDT distribution between the patient groups. As indicated in Table 1, the median number of reinfused CD34+ cells was similar in both groups. Conversely, the median number of reinfused CD3+ cells was significantly lower in the CD34+-selected PBSC group, representing a significant depletion in T lymphocytes compared with the unselected PBSC group. Myeloid recovery after transplantation was similar in the 2 groups (data not shown).
Characteristics of reinfused cells
| . | Unselected PBSC group . | CD34+-selected PBSC group . | P . |
|---|---|---|---|
| MNC (× 109) | 16 (6-42) | 0.4 (0.05-1.1) | <.0001 |
| MNC/kg (× 109) | 0.2 (0.07-0.7) | 0.005 (0.0007-0.013) | <.0001 |
| CD34+ cells (× 106) | 209 (41-1176) | 421 (44-1193) | .19 |
| CD34+ cells/kg (× 106) | 3.5 (0.4-17) | 4.9 (0.7-13) | .56 |
| CD3+ cells (× 106) | 720 (120-4320) | 2.4 (0.06-12) | .0009 |
| CD3+ cells/kg (× 106) | 14 (2-80) | 0.03 (0.0009-0.12) | .0019 |
| . | Unselected PBSC group . | CD34+-selected PBSC group . | P . |
|---|---|---|---|
| MNC (× 109) | 16 (6-42) | 0.4 (0.05-1.1) | <.0001 |
| MNC/kg (× 109) | 0.2 (0.07-0.7) | 0.005 (0.0007-0.013) | <.0001 |
| CD34+ cells (× 106) | 209 (41-1176) | 421 (44-1193) | .19 |
| CD34+ cells/kg (× 106) | 3.5 (0.4-17) | 4.9 (0.7-13) | .56 |
| CD3+ cells (× 106) | 720 (120-4320) | 2.4 (0.06-12) | .0009 |
| CD3+ cells/kg (× 106) | 14 (2-80) | 0.03 (0.0009-0.12) | .0019 |
Both groups included 16 patients; mean values are given, with ranges in parentheses.
Consequences of CD34+ selection
T-cell subset reconstitution.
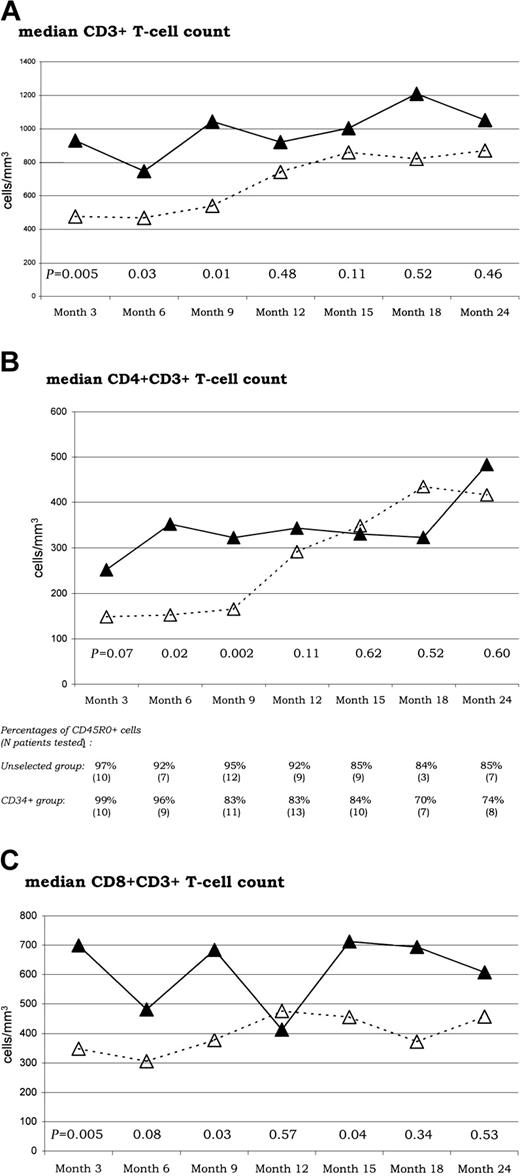
The CD3+ depletion in the reinfused cells associated with the CD34+ selection led to a significant delay in T-cell reconstitution (Figure 1). The median number of CD3+ (Figure 1A), CD4+CD3+ (Figure 1B), and CD8+CD3+ (Figure 1C) cells detected in the peripheral blood was generally lower in patients grafted after ex vivo CD34+ selection than in those grafted without ex vivo manipulation, especially during the early follow-up. For the CD3+ and CD4+CD3+ T-cell subsets, median comparisons showed statistically significant differences until month 9. The mean number of CD4+CD3+ T cells remained below 200 cells/mm3 until month 9 in the selected group, but not in the unselected group. The CD34+ selection had, however, no influence on long-term T-cell reconstitution after 1 year of follow-up because similar levels were reached in both groups. Baseline T-cell subset evaluation (CD3+, CD4+CD3+, and CD8+CD3+subsets) could be performed at diagnosis in 22 of the 32 patients (12 from the CD34+-selected group and 10 from the unselected group). These cell subset counts did not differ significantly from healthy controls. In addition, there were no differences between patients from selected and unselected groups (data not shown).
Recovery of CD3+, CD4+CD3+, and CD8+CD3+T cells.
Sequential assessments of median T-cell counts (at months 3, 6, 9, 12, 15, 18, and 24) are shown for the whole CD3+(A), the CD4+CD3+ (B), and the CD8+CD3+ (C) T-cell subsets, respectively. P values for median comparisons between the unselected (▵) and CD34+ selected (▴) groups are given at each time of follow-up. The table included in the CD4+CD3+ section sequentially indicates the percentages of CDR5RO+ cells within the CD4+CD3+ T-cell subset in tested patients from both unselected and CD34+-selected groups.
Recovery of CD3+, CD4+CD3+, and CD8+CD3+T cells.
Sequential assessments of median T-cell counts (at months 3, 6, 9, 12, 15, 18, and 24) are shown for the whole CD3+(A), the CD4+CD3+ (B), and the CD8+CD3+ (C) T-cell subsets, respectively. P values for median comparisons between the unselected (▵) and CD34+ selected (▴) groups are given at each time of follow-up. The table included in the CD4+CD3+ section sequentially indicates the percentages of CDR5RO+ cells within the CD4+CD3+ T-cell subset in tested patients from both unselected and CD34+-selected groups.
We also studied the pattern of expression of the CD45 isoforms in the CD4+ T-cell population. As indicated in Figure 1B, CD45RO+ cells contribute almost exclusively to CD4+ T-cell reconstitution for the first year in the unselected group but for the first 6 months only in the selected group. In the 2 groups, early CD4+ T-cell reconstitution was accompanied by increased Ki67 staining. We confirmed that the Ki67+CD4+ T cells were CD45RO+(data not shown). At month 1, 19% of the CD4+ and 24% of the CD8+ T cells exhibit the Ki67 antigen. These percentages decreased as soon as the third month after grafting and remained at values slightly above normal range (2%-5%) until month 15 without any difference between the CD4+ and CD8+ subsets between the selected and unselected groups. The distribution of Ki67+CD4+ T cells throughout the cell cycle was determined by analyzing DNA content with Topro-3-iodide staining. The Ki67 antigen was distributed through all cell cycle phases, that is, G0/G1 as well as G2 plus M phases as in healthy individuals.
Naive CD4+ T-cell reconstitution.
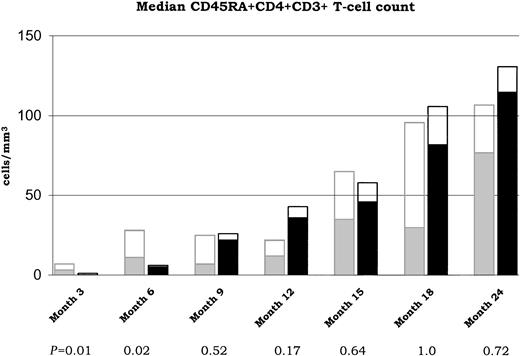
CD45RA+ cells steadily increased during the follow-up after transplantation and were detectable as soon as month 6 in the unselected group but only at month 9 in the selected group. The median number of CD45RA+CD4+ T cells was lower in the selected group compared with the unselected group during the short-term follow-up only (P = .01 and .02 at month 3 and 6, respectively; Figure 2). No differences were observed at month 9 and after. As indicated in Figure 2, almost half of the CD45RA+ cells did not exhibit the CD62L in the unselected group, at any time of the follow-up. Conversely, most of the CD4+CD45RA+ cells coexpress the CD62L in the selected group. Accordingly, a higher median number of CD62L+CD45RA+CD4+ T cells was observed in the selected group compared with the unselected from month 9. This difference reached the statistically significant level at months 9 and 12 (P = .02 and .02, respectively).
Recovery of naive CD4+ T cells.
Sequential assessments of median CD45RA+CD4+CD3+ T-cell counts (at months 3, 6, 9, 12, 15, 18, and 24) are shown for both unselected ( ) and CD34+ selected (■) groups. P values for median comparisons between groups are given at each time of follow-up. Fractions of CD62L+ T cells are represented in □.
) and CD34+ selected (■) groups. P values for median comparisons between groups are given at each time of follow-up. Fractions of CD62L+ T cells are represented in □.
Recovery of naive CD4+ T cells.
Sequential assessments of median CD45RA+CD4+CD3+ T-cell counts (at months 3, 6, 9, 12, 15, 18, and 24) are shown for both unselected ( ) and CD34+ selected (■) groups. P values for median comparisons between groups are given at each time of follow-up. Fractions of CD62L+ T cells are represented in □.
) and CD34+ selected (■) groups. P values for median comparisons between groups are given at each time of follow-up. Fractions of CD62L+ T cells are represented in □.
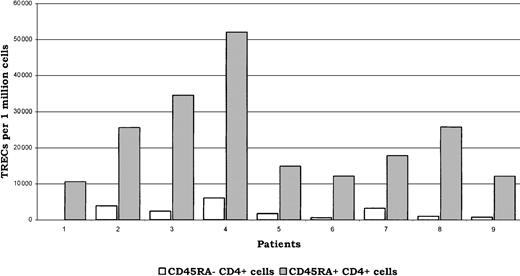
To confirm that cells expressing CD45RA+CD4+phenotype were enriched in naive CD4 T cells, we measured the number of TRECs in the selected CD45RA+CD4+ and CD45RA−CD4+ subpopulations at month 24 in 9 patients (5 patients from the CD34+-selected and 4 patients from the unselected group; Figure3). We found that the number of TRECs in these phenotypically designed naive CD45RA+ cells was 10-fold higher than in the CD45RA− counterpart (median TREC number, 18 versus 1.8 × 103/106 cells;P < .001), without marked differences between patients from the selected or unselected group. In both CD45RA− and CD45RA+ subpopulations, results obtained in healthy controls were in similar ranges (not shown). At month 24, CD45RA+CD62L+ cells accounted for at least 75% of the whole CD45RA+ T-cell population in each individual patient studied for TRECs. In addition, there was a positive correlation between the number of TRECs measured and the percentage of CD62L+ cells within the CD45RA+CD4+T-cell subset tested (R = 0.91; P = .001).
TRECs.
Numbers of TRECs in both CD45RA−CD4+ and CD45RA+CD4+ T-cell subsets are indicated in white and gray, respectively, for the 9 tested patients. Patients nos.1 to 5 belonged to the CD34+-selected group and patients nos. 6 to 9 belonged to the unselected group. The median number of TRECs was 10-fold higher in the CD45RA+ compared with the CD45RA− subset in patients receiving CD34+-selected graft, as well as in those receiving unselected graft (18 versus 1.8 × 103/106cells in the whole population of patients tested;P < .001).
TRECs.
Numbers of TRECs in both CD45RA−CD4+ and CD45RA+CD4+ T-cell subsets are indicated in white and gray, respectively, for the 9 tested patients. Patients nos.1 to 5 belonged to the CD34+-selected group and patients nos. 6 to 9 belonged to the unselected group. The median number of TRECs was 10-fold higher in the CD45RA+ compared with the CD45RA− subset in patients receiving CD34+-selected graft, as well as in those receiving unselected graft (18 versus 1.8 × 103/106cells in the whole population of patients tested;P < .001).
Non–T-cell subset reconstitution.
Reconstitution of CD45+CD14+ and CD3−CD16+CD56+ cell subsets has been followed-up until month 15. During this period, CD45+CD14+ cell counts remained in the normal ranges in all patients from the unselected as well as from the CD34+-selected group. Concerning the natural killer (NK) subset, a significant difference between both patient groups was observed at month 3 only. At that time, the mean number of CD3−CD16+CD56+ cells (± SEM) was higher in patients from the CD34+-selected group compared with those from the unselected group (229 ± 57 versus 113 ± 22/mm3; P = .004). This difference was no longer observed after this early period, as CD3−CD16+CD56+ cell counts were within the normal ranges in both patient groups.
Role of age
T-cell subset reconstitution.
Age had no impact on T-cell reconstitution until month 15, but a negative impact on long-term CD3+ and CD4+CD3+ cell reconstitution was observed. At month 18 and month 24, the mean number of CD3+ cells (± SEM) was 1310 ± 222 and 1250 ± 128 /mm3 in the 16 patients younger than 50 years versus 729 ± 208 and 893 ± 133 /mm3 in the 16 patients older than 50 years (P = .05 and .04, respectively). At month 18 and month 24, the mean number of CD4+CD3+ cells was 508 ± 184 and 558 ± 66/mm3 in the 16 patients younger than 50 years versus 259 ± 128 and 357 ± 59/mm3 in the 16 patients aged older than 50 years (P = .01 and .02, respectively). These differences remained significant after adjustment of the CD34+ selection criteria. Even when considered as a continuous variable in a logistic regression analysis including the CD34+ selection criteria, age was still an independent predictive factor of bad reconstitution at month 24 (P = .045 and .04 for CD3+ and CD4+CD3+ T cells, respectively).
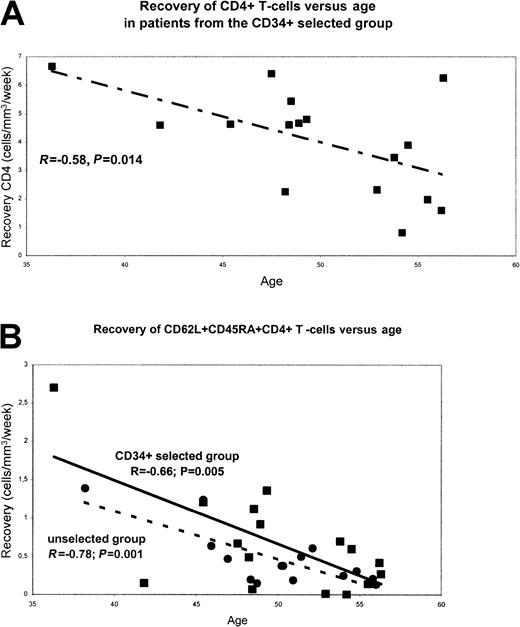
When kinetics of CD4+ T-cell recovery were correlated with age using a correlation t test, we found a negative correlation between advanced age and recovery of the whole CD4+ population in patients from the CD34-selected group (R = −0.58; P = .014; Figure4A) but not in those from the unselected group (not shown). Age had no significant influence on CD8+T-cell long-term reconstitution.
Naive CD4+ T-cell reconstitution.
As indicated in Figure 4B, we also found a negative correlation between age and CD62L+CD45RA+CD4+ T-cell recovery in patients from the CD34-selected group and in those from the unselected group (R = −0.66 and −0.78; P = .005 and .001, respectively). At month 18 and month 24, the mean number of these naive CD62L+CD45RA+CD4+ cells (± SEM) was 98 ± 21 and 163 ± 40/mm3 in the 16 patients younger than 50 years versus 23 ± 8 and 38 ± 9/mm3 in the 16 patients older than 50 years. Of note, the kinetics of recovery of the whole CD45RA+CD4+ T-cell population was significantly affected by age in patients from the CD34-selected group only (R = −0.8; P = .0001).
Role of previous treatment
The role of the 2 treatment arms (HDT1 versus HDT2) on immune reconstitution was analyzed in the whole patient population as well as in CD34+-selected and unselected patients separately. No significant difference was found with the exception of a late transient deficiency in CD3+ and CD8+CD3+cells observed at month 18 in patients who received 2 HDT compared with those who received a single HDT (P = .02 and .02 for the CD3+ and CD8+CD3+ subset, respectively, by the Mann-Whitney test). Of note, this transient deficiency in CD8+CD3+ cells associated with the HDT2 arm appeared to be mainly observed in patients from the unselected PBSC group. This lack of marked difference in immune reconstitution between both treatment arms and the fact that both selected and unselected patient groups were well balanced for these 2 treatment arms allow us to eliminate a potential bias introduced by a significant impact of previous therapy.
Role of intercurrent events
Opportunistic infections.
Because the immune reconstitution may have been influenced by the occurrence and treatment of intercurrent infections in some patients, we retrospectively registered all opportunistic infections that occurred during the 2-year follow-up in the 32 patients studied. We did not observe any significant difference in incidence of opportunistic infection between the patient groups in this limited population of patients (6 events in 5 patients from the CD34+-selected group compared with 3 events in 3 patients from the unselected group). As indicated in Table 2, the 3 patients from the unselected group who experienced opportunistic infections had quite normal CD4+CD3+ cell levels at the time of the infection. In the CD34+-selected group, patients who experienced opportunistic infections had low CD4+CD3+ cell levels at the time of infection. However, these levels were not significantly lower in these patients compared with those who had no infection at the corresponding times (P = .43, .64, and .28 at month 3, 9, and 24, respectively).
Occurrence of opportunistic infections
| . | Type of infection . | Mo of onset . | CD4+CD3+ cells,*cells/mm3 . | Treatment . |
|---|---|---|---|---|
| CD34+-selected group | ||||
| Patient 4 | VZV pancreatitis | 24 | 234 | Acyclovir |
| Patient 9 | PCC pneumonia | 4 | 149 | Cotrimoxazole |
| Patient 9 | HSV esophagitis | 4 | 149 | Acyclovir |
| Patient 11 | VZV varicella | 9 | 308 | Acyclovir |
| Patient 14 | VZV varicella | 3 | 81 | Acyclovir |
| Patient 22 | VZV retinitis | 8 | 163 | Acyclovir |
| Unselected group | ||||
| Patient 13 | CNS toxoplasmosis | 9 | 480 | Pyrimethamine-sulfadiazine |
| Patient 17 | HSV pneumonia | 4 | 444 | Acyclovir |
| Patient 28 | VZV zoster | 4 | 508 | Acyclovir |
| . | Type of infection . | Mo of onset . | CD4+CD3+ cells,*cells/mm3 . | Treatment . |
|---|---|---|---|---|
| CD34+-selected group | ||||
| Patient 4 | VZV pancreatitis | 24 | 234 | Acyclovir |
| Patient 9 | PCC pneumonia | 4 | 149 | Cotrimoxazole |
| Patient 9 | HSV esophagitis | 4 | 149 | Acyclovir |
| Patient 11 | VZV varicella | 9 | 308 | Acyclovir |
| Patient 14 | VZV varicella | 3 | 81 | Acyclovir |
| Patient 22 | VZV retinitis | 8 | 163 | Acyclovir |
| Unselected group | ||||
| Patient 13 | CNS toxoplasmosis | 9 | 480 | Pyrimethamine-sulfadiazine |
| Patient 17 | HSV pneumonia | 4 | 444 | Acyclovir |
| Patient 28 | VZV zoster | 4 | 508 | Acyclovir |
VZV indicates varicella zoster virus; PCC, Pneumocystis carinii; HSV, herpes simplex virus; and CNS, central nervous system.
Evaluated at the time or in the month before the onset of infection.
In patients who had opportunistic infection, no significant changes in the immune reconstitution profile were noted after the occurrence of the infection and its treatment (data not shown). Of note, these patients did not experience further opportunistic events, with the exception of patient 4, who developed secondary Hodgkin disease associated with autoimmune hemolytic anemia 4 years after transplantation.
Myeloma progression.
During the 2-year follow-up of the study no patient had significant myeloma progression. As a consequence, no patient received additional therapy such as chemotherapeutic agents, interferon, or thalidomide that could have interacted with their immune reconstitution profile during the study period.
Discussion
In the present study, we evaluated the mechanisms of T-cell reconstitution occurring after autologous transplantation of CD34+-selected versus unselected PBSC grafts reinfused after TBI-containing HDT in adults with multiple myeloma. We showed that overall the CD4+ T-cell reconstitution was significantly delayed during the first year in the selected group, but eventually led to similar counts as in the unselected group. This reconstitution rapidly involves the residual mature memory T cells after unselected grafts that might negatively regulate the thymic differentiation in that setting. In contrast, the immune reconstitution of thymic origin was enhanced in the selected group compared with the unselected group, demonstrating that TBI in adults does not impair thymic function, which is regulated by peripheral lymphocyte emptiness. Age was identified as an independent adverse factor for CD4+ and naive CD4+ T-cell reconstitution.
In this limited population of patients, differences observed in immune reconstitution did not translate into significant differences in the incidence of opportunistic infections. Analysis of the whole study clinical parameters is in progress. Preliminary results appeared to show a higher rate of opportunistic infections in the CD34+-selected group (data not shown here).16This would suggest that not restoring rapidly protective memory T cells against pathogens would be associated with a higher incidence of infections, despite further recovery of the T-cell pool from thymic emigrants observed in this patient group.
Immunodeficiency has been described after allogeneic transplantation, especially after T-cell depletion of the graft.17 However, in the allogeneic setting, immunologic conflict and immunosuppressive therapy are likely to impair not only thymic production, but also T-cell proliferation. Of interest, very long-term T-cell reconstitution (20-27 years after allogeneic transplantation) was evaluated using TRECs quantification.18 The main result was that a late and not clinically relevant deficiency in the naive T-cell pool was only observed in patients receiving transplants in adulthood and not in those undergoing transplantation in childhood. Autologous setting remains, however, preferable to study thymic activity, especially when using CD34-selected graft in which the purging procedure has eliminated mature T cells that could undergo peripheral expansion.
Our results confirm that both known regeneration pathways account for T-cell reconstitution in these patients. The initial rapid increase in CD4+ T-cell count appears to involve peripheral T-cell expansion in both groups. Indeed, proliferation of mature T cells was evidenced by the high level of Ki67+CD4+ T cells as well as the high proportion of memory-type cells within the CD4+ T-cell subset. A second mechanism takes place after 6 months, with differences between both groups, however. Indeed, naive CD62L+CD45RA+CD4+ T cells were restored faster in the selected group compared with the unselected one. Of note, we found a very high proportion of CD45RA+CD4+ T cells, which did not express the CD62L antigen in patients from the unselected group. This was not observed in patients from the CD34-selected group. Such high proportions are quite exceptional and have not been observed in healthy controls or during HIV infection. One explanation might be the reversion of the CD45RO phenotype after peripheral expansion of the mature CD45R0+ memory T cells reinfused with unselected graft.19 These CD62L−CD45RA+CD4+ T cells should therefore belong to the memory rather than to the naive subset. Our results were corroborated by our TRECs findings, which were correlated to the CD62L expression. Altogether, these results show that thymic production in the selected group might be stimulated by some feedback mechanisms from the periphery where the mature T cells infused with the graft were unable to reconstitute a sufficient T-cell pool. Thus, a relative emptiness of the peripheral pool secondary to T cell–depleted HSCT reinfusion is capable of promoting naive T-cell production. This has to be related to the “thymic rebound” described after chemotherapy in children.7 A delay is, however, observed and might be due to transient thymic dysfunction after thymic irradiation. Another explanation could be that even if few naive T cells are generated early, they undergo rapid peripheral expansion due to T-cell pool emptiness.
Immune reconstitution after autologous HSCT has already been evaluated by several groups.2,13,20 In the study of Bomberger et al, immune reconstitution was reported in 9 patients with poor-risk non-Hodgkin lymphomas receiving autologous CD34+-selected cells.2 Immunoaffinity purification followed by high-speed fluorescence-activated cell sorting was used to enrich the CD34+-selected graft. As in the present study, a delayed CD4+ T-cell reconstitution was observed. Surprisingly, in this study, CD4+ T-cell reconstitution was deeply impaired in both groups and remained below 200/mm3 at 6 months even in the unmanipulated group. This could be due to alteration of progenitors by prior chemotherapy before PBSC harvesting. In our study, reinfused T-cell progenitors were not previously exposed to high cumulative dosage of chemotherapy that may impair their ability to mature. In the study of Douek et al, the ability of the thymus to generate naive T cells was already shown in 40 patients with multiple myeloma who underwent autologous transplantation with CD34+-enriched PBSCs.13 The main difference with the design of our present study was the lack of TBI. The main nonconcordant result was the lack of significant delay in CD4+CD3+ T-cell reconstitution compared with patients receiving unmanipulated PBSCs, whereas we found a significant delay from month 3 to 9 in our study. Because peripheral expansion of residual or reinfused mature T cells is proposed as the main mechanism for initial T-cell pool reconstitution, this nonconcordant result thus suggests that TBI exposure could lead to a reduced peripheral expansion after grafting through alterations of the host's microenvironment or residual mature T cells. In the study of Rutella et al conducted in patients with various lymphoid malignancies (probably heavily pretreated, as suggested by the reduced baseline level in CD4+ cells), the very short-term T-cell reconstitution was evaluated for 4 months only after non-TBI–containing autologous CD34+-enriched PBSC transplants.20 A transient and very early deficiency in CD4+CD3+ (and at a lower degree in CD8+CD3+) cells was observed when compared with patients who received unselected transplants. Again, an inverse relationship between the number of mature T cells reinfused and the duration of the deficiency after grafting strongly supported the major role of peripheral expansion of reinfused mature T cells. As mentioned, the lack of TBI may explain that no deficiency in immune reconstitution was observed in these patients after month 2.
Thymic irradiation has been proposed to deeply exacerbate posttransplantation immunodeficiency after CD34+-selected HSCT.3 21 We insist here on the ability of the thymus to produce naive T cells in grafted adults despite irradiation because the TREC proportions observed in the CD45RA+ subset were identical to that of healthy donors. Therefore our results are the first to demonstrate that thymic production of new naive T cells can occur in adults after thymic irradiation. In addition, we show it is positively influenced by low peripheral blood T-cell numbers when the peripheral expansion of mature residual T cells is limited due to T-cell graft depletion. One can also suggest that depletion of NK cells and monocytes might influence the rate and mechanism of T-cell reconstitution after TBI in adults receiving CD34+positively selected grafts. Interestingly, the earlier NK-cell reconstitution observed after CD34+-selected grafts suggests a de novo production of NK cells rather than an expansion of residual NK cells from unselected grafts. Such a phenomenon does not alter thymic regeneration, but might also reflect the influence of the peripheral emptiness on the mechanism of immune reconstitution.
In addition to peripheral emptiness, age is a major factor influencing restoration of naive CD4+ T-cell production, as already proposed in the allogeneic as well as in the autologous setting.13,18 The influence of age also suggests that this naive T-cell production comes from the thymus.22 23
Altogether, our results demonstrate that infusing T-cell–depleted PBSCs in irradiated adults delays the overall T-cell reconstitution in the early period, but accelerates the thymic regeneration process and eventually leads to levels of CD4+ T-cell reconstitution similar to those in unmanipulated HSCT.
We are indebted to Dr Sabine Bréchignac for assistance in blood sample collection and to Dr Hervé Dombret for careful revision of the manuscript and editorial assistance.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-06-1929.
Supported by a grant from the Ligue Nationale Contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brigitte Autran, Laboratoire d'Immunologie Cellulaire et Tissulaire, URA CNRS 625 CERVI, Hôpital Pitié-Salpétrière, 47 Boulevard de l'Hôpital, 75013 Paris, France; e-mail:brigitte.autran@psl.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal