Ubiquitin is suggested to play a key role in essential intracellular functions, such as heat shock response, protein breakdown, and regulation of immune responses. Ubiquitin has also been detected in the extracellular space, but the function and biologic significance is unclear. We describe a new function of extracellular ubiquitin and show that extracellular ubiquitin specifically inhibits ex vivo secretion of tumor necrosis factor-α (TNF-α) and TNF-α mRNA expression from peripheral blood mononuclear cells (PBMNCs) in response to endotoxin in a dose-dependent manner. In contrast, the TNF-α response to zymosan or Staphylococcus aureus as well as the interleukin-6 (IL-6) and IL-8 responses to endotoxin were unaffected by ubiquitin. Measurement of serum ubiquitin levels showed a significant 5- to 7-fold increase in sepsis and trauma patients, to the level required for inhibition of the PBMNC TNF-α response to endotoxin by ubiquitin. Elevated ubiquitin levels in serum were significantly correlated with a reduced TNF-α production. Antibodies to ubiquitin were able to (1) significantly increase (2- to 5-fold) the TNF-α response to endotoxin in whole blood from trauma and sepsis patients, (2) completely neutralize the inhibitory effect of trauma patients' serum on healthy donors' TNF-α production, and (3) partially neutralize the inhibitory effect of sepsis patients' serum on healthy donors' TNF-α production. Ubiquitin-depleted serum from trauma patients lost the inhibitory activity for TNF-α production, whereas extracted endogenous ubiquitin exerts the inhibitory activity. The results demonstrate that extracellular ubiquitin acts as a cytokinelike protein with anti-inflammatory properties and indicate that extracellular ubiquitin is involved in the regulation of immunodepression in critical illness.
Introduction
Ubiquitin, a small (8.6-kDa), heat-stable, and highly conserved 76–amino acid protein in all eukaryotic cells has originally been discovered as an immunopoietic polypeptide from thymocytes.1,2 Further research has suggested that ubiquitin plays a key role in essential intracellular functions such as cell differentiation, cell cycle control, heat shock response, and regulation of immune responses.3-5 Traditionally, the most important function of ubiquitin is believed to be regulation of protein turnover by the ubiquitin-proteasome pathway.3-6Furthermore, ubiquitin has also been detected in the extracellular space. Compared with the normal range, significantly increased ubiquitin levels have been described in serum or plasma during parasitic infections,7 in alcoholic liver cirrhosis,8 in type 2 diabetes,9 in hairy cell leukemia,10 and in patients with renal failure and hemodialysis treatment,11,12 but data on the potential biologic functions of extracellular ubiquitin are rare. Besides possible lymphocyte-differentiating properties, extracellular ubiquitin has been reported to inhibit platelet activities13 and IgG production in splenocyte cultures,14 to synergistically effect lipopolysaccharide (endotoxin; LPS)–induced tumor necrosis factor-α (TNF-α) production in a murine macrophage cell line,15 and to be involved in growth regulation and induction of apoptosis in human hematopoietic cell lines.10 16 Nevertheless, the biologic significance of extracellular ubiquitin is almost enigmatic.
In the present study, we describe a new biologic function of extracellular ubiquitin and show that exogenous ubiquitin specifically inhibits the TNF-α response to LPS of human peripheral blood mononuclear cells (PBMNCs) at physiologically relevant concentrations. Because this effect mimics alterations in leukocyte function, which are commonly referred to as “immunoparalysis” in critical illness,17 18 the role of endogenous extracellular ubiquitin in blood of critically ill patients was further studied. High serum ubiquitin levels were accompanied by a low TNF-α response to LPS. Endogenous extracellular ubiquitin inhibited TNF-α secretion of LPS-stimulated PBMNCs and neutralization of endogenous extracellular ubiquitin and separation from critically ill patients' blood resolved endotoxin hyporesponsiveness. Therefore, evidence is provided for the biologic significance of this finding, indicating that extracellular ubiquitin is involved in regulation of immunodepression in critically ill patients.
Patients, materials, and methods
Healthy blood donors and critically ill patients
To study mechanisms of infectious and noninfectious immunodepression in critically ill patients, we decided to examine multiply injured patients sustaining blunt trauma (trauma group) and patients with sepsis (sepsis group). In both trauma and sepsis patients, a pronounced depression of cellular immune functions17,18 is detectable. We studied blood or urine samples (or both) from a total of 34 healthy adult blood donors, 20 multiply injured blunt trauma patients, and 24 patients with sepsis from an interdisciplinary intensive care unit. The protocol used was approved by the local ethics committee. All patients and blood donors were white. The age of the healthy and uninjured blood donors (13 women, 21 men) was 31 ± 7 years (mean ± SD). Blood donors had no signs of infectious diseases 4 weeks prior to blood collection. Trauma patients (8 women, 12 men) fulfilled the following criteria: (1) no penetrating injuries, (2) a severity of injury with an injury severity score (ISS)19 of more than 16 points, and (3) no pre-existing chronic illness. The trauma patients were assigned an ISS by independent evaluators. Injuries of the various body regions (head and neck, face, thorax, abdomen, extremities, skin) were classified using the Abbreviated Injury Scale (AIS),19which ranges from 0 (no injury of the body region) to 6 (fatal injury of the body region) for each body region. The age of the trauma patients was 39 ± 18 years (mean ± SD) and the ISS was 27 ± 10 (mean ± SD) points (AIS head/neck: 2.1 ± 1.5; AIS face: 0.9 ± 1.5; AIS thorax: 2.6 ± 1.7; AIS abdomen: 0.7 ± 1.4; AIS extremities: 2.2 ± 1.7; AIS skin: 0.2 ± 0.6). Five trauma patients died.
Sepsis patients (8 women, 16 men) fulfilled the Criteria of the American College of Chest Physicians/Society of Critical Care Medicine consensus conference20 (10 patients for sepsis, 7 patients for severe sepsis, and 7 patients for septic shock). The age of the sepsis patients was 52 ± 18 years (mean ± SD). The source of infection was pneumonia in 17 patients, peritonitis in 6 patients, and pancreatitis in 1 patient. Five patients with septic shock died. All patients requiring surgical intervention received standard surgical care and postoperative intensive care unit treatment. Blood was collected in plastic tubes (NH4-heparin (9 mL) and serum (9 mL) tubes, Sarsted, Nümbrecht, Germany) along with the routine baseline laboratory work-up. Whole blood collected in a serum tube was separated and the sera were aliquoted and stored frozen at −70°C.
Furthermore, blood from mongrel swine (n = 3, 35-55 kg body weight; kindly provided by Prof Dr K. G. Proctor, Department of Surgery, Ryder Trauma Center, University of Miami, FL) and mice (n = 3, 25-35 g body weight; kindly provided by Dr A. El-Haddad, Department of Surgery, University of Miami) was collected in an NH4-heparin tube. Whole blood collected in an NH4-heparin tube was immediately used for culture experiments and for isolation of PBMNCs.
Isolation of PBMNCs and cell cultures
PBMNCs were isolated by density centrifugation of heparinized blood diluted 1:1 (vol/vol) in phosphate-buffered saline (PBS) over a Lymphoprep (Nycomed Pharma, Oslo, Norway) density gradient and were used for endotoxin stimulation immediately after isolation. Whole blood mixed 1:3 (vol/vol) with cell culture medium (RPMI 1640 or 105 PBMNCs resuspended in cell culture medium RPMI 1640; Gibco BRL, Eggenstein, Germany) containing 10% serum were transferred to microtiter plates (Greiner Bio One, Greiner, Germany). Samples were prepared in duplicate. The mixtures were incubated at 37°C and 5% CO2 with LPS (100 ng/mL; fromSalmonella abortus equi; Sigma, Taufkirchen, Germany). Control mixtures were incubated without LPS. After incubation the supernatants were separated and stored frozen at −20°C. Following endotoxin stimulation, PBMNCs were tested for viability by incorporation of 3-(4,5 dimethylthiazol-2-yl)-2,5, diphenyl-tetrazolium bromide (MTT; Sigma).21
Proteins and antibodies
Ubiquitin was purchased from Sigma (U6253). Prior to use in cell cultures, ubiquitin was boiled for 5 minutes, placed on ice for 5 minutes, and centrifuged. The pellet was removed and the supernatant, which contains the heat-stable ubiquitin, was further used. Human recombinant interleukin-10 (IL-10; I9276) was purchased from Sigma. Rabbit antiubiquitin antiserum (AS; U5379), ubiquitin-fluorescein conjugate (U5504), and goat antiserum to rabbit IgG (R8633) were purchased from Sigma. Monoclonal mouse antiubiquitin antibody (UbP4D1) and goat polyclonal antiubiquitin antibody (UbN19) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Peroxidase-linked antirabbit and antimouse IgG was obtained from Amersham-Pharmacia (Freiburg, Germany).
Immunoassays
Ubiquitin.
Quantification of ubiquitin concentrations in serum and urine specimens was performed with a competitive-binding immunoassay, in which ubiquitin-fluorescein conjugate and ubiquitin in the test sample compete for a limited number of binding sites in the antiubiquitin antiserum. Two to 4 dilutions of each serum/urine sample were measured in duplicate. In brief, 100 μL ubiquitin-fluorescein conjugate, 100 μL test sample, and 100 μL rabbit antiubiquitin antiserum were transferred to plastic tubes, mixed, and incubated for 60 minutes at room temperature in the dark. After incubation, 1 mL goat antiserum to rabbit IgG was added to the test tubes, the solution was centrifuged for 15 minutes at 4°C, and the supernatant was removed. The pellet was resuspended in 2 mL 0.1 N NaOH and 2% sodium dodecyl sulfate (SDS), and the fluorescence (λ excitation 485 nm, λ emission 535 nm) was measured in a Genios microreader (Tecan, Germany). The ubiquitin concentration in the test sample was calculated from a nonlinear regression analysis using ubiquitin as standard (0-1000 ng/mL). The nonlinear regression analysis (one-phase exponential decay) was calculated with the GraphPad Prism program (GraphPad, San Diego, CA). The correlation coefficients for each standard curve were 0.95 to 1. The lower detection limit was determined to be 17 ng ubiquitin/mL.
TNF-α, IL-6, and IL-8.
Quantification of TNF-α, IL-6, and IL-8 concentrations in cell cultures were performed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (human: Millenia Biotech, Bad Nauheim, Germany; porcine and murine: R & D Systems, Wiesbaden, Germany) according to the manufacturer's instructions. The lower detection limits of the ELISAs were 10 pg/mL for human TNF-α, 1.2 pg/mL for human IL-6, 3.5 pg /mL for human IL-8, 5 pg/mL for porcine TNF-α, and 5 pg/mL for murine TNF-α.
Immunoblotting
Following SDS–polyacrylamide gel electrophoresis (SDS-PAGE), serum or urine samples were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Hybond-P; Amersham-Pharmacia). After blocking residual binding sites on the membrane with 5% (wt/vol) nonfat dried milk powder (Milupa, Friedrichsdorf, Germany), 0.1% Tween 20 (Sigma) in PBS immunoblotting was performed with antiubiquitin AS (1:200, vol/vol) and monoclonal UbP4D1 (1:500, vol/vol) using a corresponding second horseradish peroxidase–labeled antibody (1:10 000, vol/vol, and 1:5000, vol/vol), respectively; Amersham Biosource, Freiburg, Germany). Immunoreactive proteins were visualized with a enhanced chemiluminescence (ECL-Plus) detection system (Amersham Pharmacia) using the ImageMaster VDS-CL video system (Amersham Pharmacia).
Affinity chromatography
Antiubiquitin affinity chromatography was performed using the rabbit antiubiquitin AS (Sigma). HiTrap NHS-activated columns (1-mL column, 7-mm inner diameter × 25-mm column height; Amersham Pharmacia) were incubated with rabbit antiubiquitin AS (4 mg/mL in 0.2 M NaHCO3, 0.5 M NaCl, pH 8.3) for 30 minutes at ambient temperature. After incubation, the column was washed and deactivated with several volumes of 0.5 M ethanolamine, 0.5 M NaCl, pH 8.3 (buffer A), 0.1 M acetate, 0.5 M NaCl, pH 4 (buffer B), and again with buffer A with a flow rate of 1 mL/min. Following 25 minutes of incubation in buffer A at ambient temperature, the column was washed again and was then equilibrated with RPMI 1640 (Gibco BRL). Patient serum 1:1 (vol/vol) in RPMI 1640 was applied to the column and was incubated for 30 minutes. The run-through was collected and the column was washed with several volumes of RPMI 1640. The column was eluted with a 5-step pH gradient of each 2-column volumes of 0.2 M glycine at pH 7, pH 6, pH 5, pH 4, and pH 3 with a flow rate of 1 mL/min. Fractions of 1 mL were collected. Immediately after elution, the fractions were neutralized to pH 7.5 and were used in cell culture experiments.
mRNA quantification
TNF-α mRNA levels in endotoxin-stimulated PBMNCs (106 PBMNC/mL) were quantified using a commercially available colorimetric microplate assay kit (Qantikine mRNA; R & D Systems) according to the manufacturer's instructions. The lower detection limit is 3.2 amol TNF-α mRNA/mL.
Other procedures and substances
Protein was determined with a protein assay kit (P5656, Sigma) using bovine serum albumin as standard. Protein standards for gel electrophoresis were purchased from Amersham Pharmacia. Zymosan A (Z4250) was purchased from Sigma. Heat-killed Staphylococcus aureus (clinical isolates, autoclaved) was kindly provided by Prof Dr H. Hof (Institute of Medical Microbiology, University Hospital Mannheim, Germany). Cells were counted with a XR-21 automatic multichannel hematology cell counter (Sysmex, Norderstadt, Germany).
Statistics
If not otherwise mentioned, data are expressed as the mean ± SEM. Spearman correlation coefficient (rs), Student t test, and the Bonferroni corrected one-way analysis of variance (ANOVA) for multiple comparisons were calculated with the SPSS for Windows Release 10.0.7 program (SPSS, Chicago, IL). A 2-tailed P < .05 was considered significant. Standard curves of the assays and dose-related effects of exogenous ubiquitin were analyzed by linear and nonlinear regression analysis using the GraphPad Prism program (version 1.0, 1994, GraphPad Software).
Results
Exogenous ubiquitin inhibits LPS-induced TNF-α production of human whole blood and PBMNCs
We measured the effect of exogenous ubiquitin on TNF-α secretion of human whole blood and PBMNC cultures stimulated with and without LPS. TNF-α was not detectable in LPS free whole blood and PBMNC cultures incubated with 0 to 1 μg/mL exogenous ubiquitin (not shown). As estimated with the MTT assay viability was more than 90% in all PBMNC cultures (not shown). In whole blood and PBMNC cultures with LPS, exogenous ubiquitin significantly inhibited the TNF-α secretion in a dose-dependent manner (correlation coefficients: whole blood rs = 0.92; PBMNC rs = 0.96; Figure1). Maximal inhibition of the TNF-α production was found at a concentration of 500 ng/mL exogenous ubiquitin in both whole blood and PBMNC cultures. As expected, no difference in inhibitory activity between boiled and untreated ubiquitin was detectable (Table 1). Therefore, boiled ubiquitin was used throughout the study to heat-inactivate and exclude possible biologically active compounds at concentrations below the detection limit of SDS-PAGE in the ubiquitin solution. Kinetics of the LPS-stimulated TNF-α production of whole blood and PBMNCs showed that exogenous ubiquitin did not influence the time course of the TNF-α secretion within an incubation period of 0 to 16 hours. To exclude interference of exogenous ubiquitin with the immunologic detection of TNF-α in the cell cultures, ubiquitin was added to whole blood and PBMNC cultures (n = 8) after 4 hours of LPS stimulation. Compared with the control measurements without ubiquitin, in the presence of 500 ng/mL and 1000 ng/mL ubiquitin, the recovery of TNF-α was 97% ± 1.7% (mean ± SEM) and 96% ± 3% (mean ± SEM), respectively.
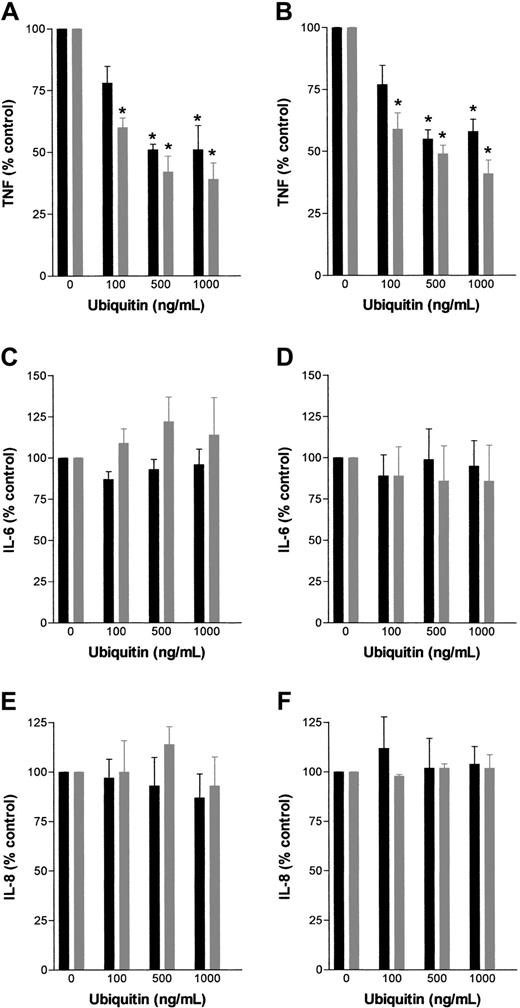
Exogenous ubiquitin inhibits TNF-α secretion of blood and PBMNCs.
(A) Dose-dependent inhibition of TNF-α secretion of human whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates) from healthy donors (n = 13-18) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. (B) Dose-dependent inhibition of TNF-α secretion of human PBMNCs by exogenous ubiquitin. PBMNC cultures (in duplicates) from healthy donors (n = 10-15) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. (C) Kinetics of the LPS-stimulated TNF-α secretion of human whole blood in the presence of 0 (■), 500 ng/mL (▪), and 1000 ng/mL (●) exogenous ubiquitin. Cultures (in duplicates) were incubated for 2, 4, 8, and 16 hours. (D) Kinetics of the LPS-stimulated TNF-α secretion of 105 human PBMNCs in the presence of 0 (■), 500 ng/mL (▪), and 1000 ng/mL (●) exogenous ubiquitin. Cultures (in duplicates) were incubated for 2, 4, 8, and 16 hours. (E) TNF-α mRNA levels in human PBMNCs stimulated with 100 ng/mL LPS in the presence of 0, 500, and 1000 ng/mL ubiquitin for 2 hours. *P < .05 versus stimulation without ubiquitin. (F) Dose-dependent inhibition of TNF-α secretion of porcine (▪) and murine ( ) whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates; n = 3) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL (porcine) and 1 μg/mL (murine) LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin.
) whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates; n = 3) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL (porcine) and 1 μg/mL (murine) LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin.
Exogenous ubiquitin inhibits TNF-α secretion of blood and PBMNCs.
(A) Dose-dependent inhibition of TNF-α secretion of human whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates) from healthy donors (n = 13-18) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. (B) Dose-dependent inhibition of TNF-α secretion of human PBMNCs by exogenous ubiquitin. PBMNC cultures (in duplicates) from healthy donors (n = 10-15) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. (C) Kinetics of the LPS-stimulated TNF-α secretion of human whole blood in the presence of 0 (■), 500 ng/mL (▪), and 1000 ng/mL (●) exogenous ubiquitin. Cultures (in duplicates) were incubated for 2, 4, 8, and 16 hours. (D) Kinetics of the LPS-stimulated TNF-α secretion of 105 human PBMNCs in the presence of 0 (■), 500 ng/mL (▪), and 1000 ng/mL (●) exogenous ubiquitin. Cultures (in duplicates) were incubated for 2, 4, 8, and 16 hours. (E) TNF-α mRNA levels in human PBMNCs stimulated with 100 ng/mL LPS in the presence of 0, 500, and 1000 ng/mL ubiquitin for 2 hours. *P < .05 versus stimulation without ubiquitin. (F) Dose-dependent inhibition of TNF-α secretion of porcine (▪) and murine ( ) whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates; n = 3) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL (porcine) and 1 μg/mL (murine) LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin.
) whole blood by exogenous ubiquitin. Whole-blood cultures (in duplicates; n = 3) were incubated for 4 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL (porcine) and 1 μg/mL (murine) LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin.
Comparison of boiled and untreated ubiquitin on LPS-stimulated TNF-α secretion of whole blood and PBMNCs
| Ubiquitin, ng/mL . | WB TNF-α production, % control . | PBMNC TNF-α production, % control . | ||
|---|---|---|---|---|
| Boiled . | Native . | Boiled . | Native . | |
| 0 | 100 | 100 | 100 | 100 |
| 500 | 66 ± 5.9 | 59 ± 4.6 | 61 ± 4.6 | 62 ± 3.6 |
| 1000 | 55 ± 4.1 | 57 ± 7.4 | 56 ± 4.1 | 57 ± 4.5 |
| Ubiquitin, ng/mL . | WB TNF-α production, % control . | PBMNC TNF-α production, % control . | ||
|---|---|---|---|---|
| Boiled . | Native . | Boiled . | Native . | |
| 0 | 100 | 100 | 100 | 100 |
| 500 | 66 ± 5.9 | 59 ± 4.6 | 61 ± 4.6 | 62 ± 3.6 |
| 1000 | 55 ± 4.1 | 57 ± 7.4 | 56 ± 4.1 | 57 ± 4.5 |
Whole blood (WB) and PBMNC cultures (n = 4) from healthy donors were incubated for 4 hours with 0, 500, and 1000 ng/mL exogenous boiled or native ubiquitin in the presence of LPS (100 ng/mL). Percent control is percent TNF-α secretion of the cultures incubated without exogenous ubiquitin (= 100%). Data represent mean ± SEM.
To further confirm the inhibitory effect of exogenously added ubiquitin on LPS-stimulated TNF-α production, TNF-α mRNA levels were quantified. As shown in Figure 1E, similar to the LPS-induced TNF-α secretion, exogenous ubiquitin produced a dose-related inhibition of the LPS- evoked mRNA expression of human PBMNCs.
In line with the findings in human whole blood, exogenous ubiquitin was found to inhibit the LPS- induced TNF-α response in both murine and porcine whole blood in a dose-dependent manner (Figure 1F).
In contrast to the LPS-evoked TNF-α production, ubiquitin did not alter TNF-α secretion after stimulation with zymosan A or S aureus (Table 2). Furthermore, we could not determine any effect of exogenous ubiquitin on LPS-stimulated IL-6 or IL-8 production of whole blood or PBMNCs (Figure 2) cultured for up to 24 hours.
Effect of exogenous ubiquitin on zymosan- and S aureus-induced TNF-α production of human PBMNCs and whole blood
| Ubiquitin, ng/mL . | Zymosan-induced TNF-α production . | S aureus-induced TNF-α production . | ||
|---|---|---|---|---|
| WB, pg/mL . | PBMNC, pg/106PBMNC . | WB, pg/mL . | PBMNC, pg/106 PBMNC . | |
| 0 | 2960 ± 446 | 2516 ± 810 | 1474 ± 366 | 4872 ± 1080 |
| 100 | 2798 ± 535 | 2304 ± 630 | 1359 ± 295 | 5018 ± 1108 |
| 500 | 2466 ± 443 | 2560 ± 750 | 1467 ± 406 | 5118 ± 992 |
| 1000 | 2676 ± 592 | 2340 ± 648 | 1549 ± 277 | 4972 ± 808 |
| Ubiquitin, ng/mL . | Zymosan-induced TNF-α production . | S aureus-induced TNF-α production . | ||
|---|---|---|---|---|
| WB, pg/mL . | PBMNC, pg/106PBMNC . | WB, pg/mL . | PBMNC, pg/106 PBMNC . | |
| 0 | 2960 ± 446 | 2516 ± 810 | 1474 ± 366 | 4872 ± 1080 |
| 100 | 2798 ± 535 | 2304 ± 630 | 1359 ± 295 | 5018 ± 1108 |
| 500 | 2466 ± 443 | 2560 ± 750 | 1467 ± 406 | 5118 ± 992 |
| 1000 | 2676 ± 592 | 2340 ± 648 | 1549 ± 277 | 4972 ± 808 |
Whole blood (WB) and PBMNC cultures (n = 6) from healthy donors were incubated for 4 hours with 0, 100, 500, and 1000 ng/mL exogenous ubiquitin in the presence of zymosan (100 μg/mL) or heat killed S aureus (105 cells/mL). Data represent mean ± SEM.
Effect of exogenous ubiquitin on LPS-induced TNF-α, IL-6, and IL-8 secretion of human whole blood and PBMNCs.
Whole blood (▪) and PBMNC ( ) cultures (in duplicates) from healthy donors (n = 3) were incubated for 4 hours and 24 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. TNF-α/IL-6/IL-8 production (% control) is percent of TNF-α/IL-6/IL-8 secretion in cultures without exogenous ubiquitin. (A) TNF-α secretion, 4 hours of incubation. (B) TNF-α secretion, 24 hours of incubation. (C) IL-6 secretion, 4 hours of incubation. (D) IL-6 secretion, 24 hours of incubation. (E) IL-8 secretion, 4 hours of incubation. (F) IL-8 secretion, 24 hours of incubation.
) cultures (in duplicates) from healthy donors (n = 3) were incubated for 4 hours and 24 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. TNF-α/IL-6/IL-8 production (% control) is percent of TNF-α/IL-6/IL-8 secretion in cultures without exogenous ubiquitin. (A) TNF-α secretion, 4 hours of incubation. (B) TNF-α secretion, 24 hours of incubation. (C) IL-6 secretion, 4 hours of incubation. (D) IL-6 secretion, 24 hours of incubation. (E) IL-8 secretion, 4 hours of incubation. (F) IL-8 secretion, 24 hours of incubation.
Effect of exogenous ubiquitin on LPS-induced TNF-α, IL-6, and IL-8 secretion of human whole blood and PBMNCs.
Whole blood (▪) and PBMNC ( ) cultures (in duplicates) from healthy donors (n = 3) were incubated for 4 hours and 24 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. TNF-α/IL-6/IL-8 production (% control) is percent of TNF-α/IL-6/IL-8 secretion in cultures without exogenous ubiquitin. (A) TNF-α secretion, 4 hours of incubation. (B) TNF-α secretion, 24 hours of incubation. (C) IL-6 secretion, 4 hours of incubation. (D) IL-6 secretion, 24 hours of incubation. (E) IL-8 secretion, 4 hours of incubation. (F) IL-8 secretion, 24 hours of incubation.
) cultures (in duplicates) from healthy donors (n = 3) were incubated for 4 hours and 24 hours with 0 to 1 μg/mL exogenous ubiquitin in the presence of 100 ng/mL LPS. Data represent means ± SEM. *P < .05 versus cultures without ubiquitin. TNF-α/IL-6/IL-8 production (% control) is percent of TNF-α/IL-6/IL-8 secretion in cultures without exogenous ubiquitin. (A) TNF-α secretion, 4 hours of incubation. (B) TNF-α secretion, 24 hours of incubation. (C) IL-6 secretion, 4 hours of incubation. (D) IL-6 secretion, 24 hours of incubation. (E) IL-8 secretion, 4 hours of incubation. (F) IL-8 secretion, 24 hours of incubation.
Ubiquitin serum and urine concentrations in healthy individuals and critically ill patients
Determination of ubiquitin levels in serum, plasma, and whole blood derived from the same blood specimen revealed equal concentrations in each sample (Table 3), indicating no relevant ubiquitin release during blood clotting or sample preparation. This is corroborated by the finding that ubiquitin serum concentrations in a donor's blood specimen were determined to be 84 ng/mL when serum was separated immediately, 90 ng/mL when serum was separated 1 hour after collecting the blood in a serum tube, and 90 ng/mL after 4 hours, respectively.
Comparison of ubiquitin concentrations in serum, plasma, and whole blood
| . | Serum, ng/mL . | Plasma, ng/mL . | Whole blood, ng/mL . |
|---|---|---|---|
| Patient 1 | 338 | 293 | 322 |
| Patient 2 | 256 | 247 | 264 |
| Healthy donor | < 17 | < 17 | < 17 |
| . | Serum, ng/mL . | Plasma, ng/mL . | Whole blood, ng/mL . |
|---|---|---|---|
| Patient 1 | 338 | 293 | 322 |
| Patient 2 | 256 | 247 | 264 |
| Healthy donor | < 17 | < 17 | < 17 |
Patient 1 was a man, aged 75 years, with septic shock. Patient 2 was a man, aged 44 years, at trauma day 0, ISS 29. The healthy donor was a 31-year-old man.
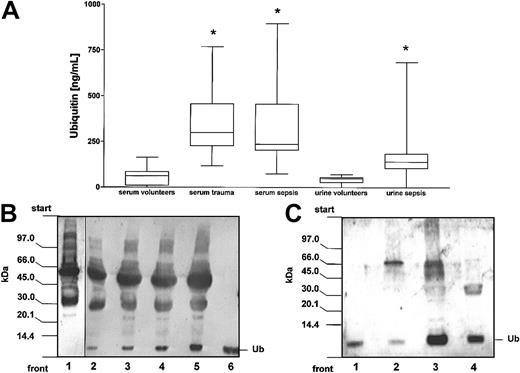
Ubiquitin was detectable in 27 of the 35 serum samples from healthy blood donors, in all serum samples from multiply injured patients on day 0 and 1 after trauma, and in all serum samples from sepsis patients. In healthy individuals ubiquitin serum concentrations were determined to be 58 ± 48 ng/mL (mean ± SD). Compared with healthy individuals, the ubiquitin serum concentrations were found to be 6-fold elevated in multiply injured patients (n = 23) on day 0 after trauma (359 ± 177 ng/mL, mean ± SD) as well as in 24 sepsis patients (327 ± 203 ng/mL, mean ± SD; Figure3A).
Detection of ubiquitin in serum and urine.
(A) Ubiquitin serum and urine concentrations in healthy volunteers and critically ill patients. The boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the range of data. Data are measurements of ubiquitin concentrations in serum samples from 35 healthy uninjured donors, 23 severely injured blunt trauma patients on the day of admission, and 24 patients with sepsis. Ubiquitin urine concentrations were determined in specimens from 19 sepsis patients and 10 healthy individuals. *P < .05 versus concentrations in specimens from healthy volunteers. (B) Detection of free ubiquitin in serum specimen by immunoblotting. Serum proteins were separated by SDS-PAGE, transferred to PVDF membranes, and probed for ubiquitin with antiubiquitin AS (1:200). Lane 1, healthy donors' serum (15 μg); lanes 2-5, patients' serum (lane 2, 10 μg; lane 3, 15 μg; lane 4, 20 μg; lane 5, 25 μg); lane 6, ubiquitin (Ub; 10 ng). (C) Detection of free ubiquitin in urine specimen (10 μL) by immunoblotting. Lane 1, ubiquitin (Ub; 5 ng); lane 2, healthy donors' specimen; lanes 3 and 4, patients' specimens.
Detection of ubiquitin in serum and urine.
(A) Ubiquitin serum and urine concentrations in healthy volunteers and critically ill patients. The boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the range of data. Data are measurements of ubiquitin concentrations in serum samples from 35 healthy uninjured donors, 23 severely injured blunt trauma patients on the day of admission, and 24 patients with sepsis. Ubiquitin urine concentrations were determined in specimens from 19 sepsis patients and 10 healthy individuals. *P < .05 versus concentrations in specimens from healthy volunteers. (B) Detection of free ubiquitin in serum specimen by immunoblotting. Serum proteins were separated by SDS-PAGE, transferred to PVDF membranes, and probed for ubiquitin with antiubiquitin AS (1:200). Lane 1, healthy donors' serum (15 μg); lanes 2-5, patients' serum (lane 2, 10 μg; lane 3, 15 μg; lane 4, 20 μg; lane 5, 25 μg); lane 6, ubiquitin (Ub; 10 ng). (C) Detection of free ubiquitin in urine specimen (10 μL) by immunoblotting. Lane 1, ubiquitin (Ub; 5 ng); lane 2, healthy donors' specimen; lanes 3 and 4, patients' specimens.
Furthermore, we detected ubiquitin concentrations in urine specimens. Similar to the findings in serum specimens, the urine ubiquitin concentrations were found to be in the same range of magnitude with an urine ubiquitin concentration of 41 ± 22 ng/mL (mean ± SD) in healthy volunteers and a 4.5-fold increased urine ubiquitin concentration (180 ± 166 ng/mL, mean ± SD) in sepsis patients.
In addition, we performed immunoblot analysis of patient and healthy donor serum and urine specimens. As determined from control experiments using ubiquitin as a standard, the detection limit was 1 ng ubiquitin using the antiubiquitin AS and 20 ng ubiquitin using the monoclonal UbP4D1 (not shown). Using both antibodies, patterns of detectable ubiquitin immunoreactive proteins were found to be identical (Figure 5B, lanes 1 and 5, lanes 2 and 6, and lanes 3 and 8). As shown in Figure 3, no or only a faint band corresponding to free ubiquitin was detectable in healthy donor samples (Figures 3B lane 1 and 3C lane 2), whereas patient serum and urine samples contained a strong band corresponding to free ubiquitin (Figure 3B-C). Although unspecific binding cannot be excluded for each of the numerous high-molecular-weight bands visualized using both the antiubiquitin AS and the monoclonal UbP4D1, obvious differences between patient and healthy donor serum samples were not detectable except for free endogenous ubiquitin (Figures 3B lanes 1 and 3 and 5B lane 1).
Comparison of ubiquitin serum concentrations with the LPS-induced whole blood TNF-α production in healthy volunteers and critically ill patients
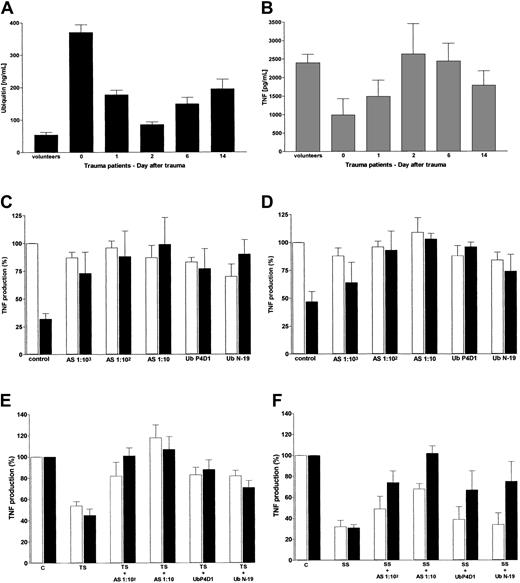
Because ubiquitin serum levels in multiply injured blunt trauma patients were determined to be in the same range of magnitude as determined for the maximal inhibitory activity of exogenous ubiquitin on LPS-stimulated TNF-α production, we compared ubiquitin serum concentrations with the whole blood TNF-α response to LPS in healthy individuals and trauma patients. As shown in Figure4A, high ubiquitin serum concentrations are significantly associated with low TNF-α concentrations in LPS-stimulated whole blood from healthy donors and severely injured patients (n = 62, rs = −0.263; P = .018). In severely injured trauma patients, the ubiquitin serum concentrations on days 0 to 14 resemble a mirror image of the LPS-induced whole blood TNF-α production.
Comparison of ubiquitin serum levels with LPS-stimulated whole blood TNF-α production and neutralization of the inhibitory activity for TNF-α production in patients' serum with antiubiquitin antibody.
(A) Ubiquitin serum concentrations in volunteers (n = 12) and trauma patients (n = 10) during 14 days after trauma. Data represent means ± SEM. (B) LPS-induced whole blood TNF-α secretion. Volunteers and trauma patients are the same as described in panel A. Whole blood cultures were incubated for 4 hours in the presence of 100 ng/mL LPS. Data represent means ± SEM. (C-D) Antiubiquitin antibodies neutralize the inhibitory activity of ubiquitin on LPS-induced whole blood (C) and PBMNCs (D) TNF-α production. TNF-α production (%) indicates percent of the TNF-α secretion in cultures without exogenous ubiquitin and without antibodies. Data represent means ± SEM from 3 different whole blood and PBMNC cultures obtained from healthy volunteers. Cultures without (■) or with 500 ng/mL exogenous ubiquitin (▪) in the presence of LPS (100 ng/mL for 4 hours). Control indicates cultures without addition of antibody. AS: antiubiquitin AS diluted 1:103, 1:102, and 1:10 in the cell cultures. UbP4D1 and UbN-19: diluted 1:103 in the cell cultures. (E-F) Effect of antiubiquitin antibody on the inhibitory activity of trauma (E) and sepsis (F) patients' serum on LPS-induced TNF-α production of whole blood and PBMNCs. Whole blood (■) and PBMNCs (▪) were cultured with 100 ng/mL LPS for 4 hours. TNF production (%) indicates percent of the TNF-α secretion in cultures containing additional healthy volunteers' serum (30%, vol/vol, in the cell culture mixture) without antibodies. Data represent means ± SEM from 4 different cultures obtained from healthy volunteers. C indicates control, healthy volunteers' serum; TS, trauma patients' serum (n = 4, 30%, vol/vol, in the cell culture mixture); SS, sepsis patients' serum (n = 4, 30%, vol/vol, in the cell culture mixture). Antiubiquitin AS diluted 1:102 and 1:10 in the cell cultures. UbP4D1 and UbN19 were diluted 1:103 in the cell cultures.
Comparison of ubiquitin serum levels with LPS-stimulated whole blood TNF-α production and neutralization of the inhibitory activity for TNF-α production in patients' serum with antiubiquitin antibody.
(A) Ubiquitin serum concentrations in volunteers (n = 12) and trauma patients (n = 10) during 14 days after trauma. Data represent means ± SEM. (B) LPS-induced whole blood TNF-α secretion. Volunteers and trauma patients are the same as described in panel A. Whole blood cultures were incubated for 4 hours in the presence of 100 ng/mL LPS. Data represent means ± SEM. (C-D) Antiubiquitin antibodies neutralize the inhibitory activity of ubiquitin on LPS-induced whole blood (C) and PBMNCs (D) TNF-α production. TNF-α production (%) indicates percent of the TNF-α secretion in cultures without exogenous ubiquitin and without antibodies. Data represent means ± SEM from 3 different whole blood and PBMNC cultures obtained from healthy volunteers. Cultures without (■) or with 500 ng/mL exogenous ubiquitin (▪) in the presence of LPS (100 ng/mL for 4 hours). Control indicates cultures without addition of antibody. AS: antiubiquitin AS diluted 1:103, 1:102, and 1:10 in the cell cultures. UbP4D1 and UbN-19: diluted 1:103 in the cell cultures. (E-F) Effect of antiubiquitin antibody on the inhibitory activity of trauma (E) and sepsis (F) patients' serum on LPS-induced TNF-α production of whole blood and PBMNCs. Whole blood (■) and PBMNCs (▪) were cultured with 100 ng/mL LPS for 4 hours. TNF production (%) indicates percent of the TNF-α secretion in cultures containing additional healthy volunteers' serum (30%, vol/vol, in the cell culture mixture) without antibodies. Data represent means ± SEM from 4 different cultures obtained from healthy volunteers. C indicates control, healthy volunteers' serum; TS, trauma patients' serum (n = 4, 30%, vol/vol, in the cell culture mixture); SS, sepsis patients' serum (n = 4, 30%, vol/vol, in the cell culture mixture). Antiubiquitin AS diluted 1:102 and 1:10 in the cell cultures. UbP4D1 and UbN19 were diluted 1:103 in the cell cultures.
Antiubiquitin antibodies neutralize the inhibitory activity for TNF-α production in patient serum
Serum from trauma and sepsis patients is known to mediate immunodepression and to depress the TNF-α–producing capacity of volunteers' whole blood and PBMNCs.22-25 To address the involvement of ubiquitin in this context, we tested the effect of antiubiquitin antibodies in whole blood and PBMNC cultures incubated with and without patients' serum (Figure 4). In a first series of cell culture experiments, we examined the potential neutralizing effect of antiubiquitin antibodies on the inhibitory activity of ubiquitin on the LPS-induced TNF-α secretion. Antiubiquitin AS was found to neutralize the effect of ubiquitin dose dependently at a dilution of 1:100 and 1:10 without effects on whole blood and PBMNCs cultured in the absence of exogenous ubiquitin. Moreover, the tested monoclonal (UbP4D1 diluted 1:1000) and polyclonal antiubiquitin antibody (UbN19 diluted 1:1000) neutralized the inhibitory effect of exogenous ubiquitin on the LPS-stimulated TNF-α release in whole blood and PBMNC cultures. None of these antibodies affected the TNF-α secretion of cell cultures without exogenous ubiquitin. To further exclude unspecific stimulation induced by immune complexes, the LPS-induced TNF-α secretion of whole blood and PBMNCs was tested in cocultures with exogenous human recombinant IL-10 and antiubiquitin antibodies. The antiubiquitin antibodies did not influence the IL-10–induced inhibition of the LPS-stimulated TNF-α secretion (not shown). In the second series of experiments, whole blood and PBMNCs from healthy donors were cultured in the presence of serum from trauma (Figure 4E) and sepsis (Figure 4F) patients and the effect of the antiubiquitin antibodies was examined.
As expected, serum from trauma patients (mean ubiquitin level, 330 ± 99 [SD] ng/mL) reduced LPS-stimulated TNF-α secretion to 40% to 50% (Figure 4E). Addition of antiubiquitin AS and monoclonal and polyclonal antibodies neutralized the inhibitory effect of trauma patients' serum on whole blood and PBMNCs.
Incubating whole blood and PBMNCs in the presence of serum from sepsis patients (mean ubiquitin level, 393 ± 179 [SD] ng/mL) inhibited the LPS-induced TNF-α secretion to 30% of the TNF-α secretion in the presence of serum from healthy volunteers (Figure 4F). Similar to serum from trauma patients, in PBMNC cultures the inhibition induced by serum from sepsis patients was neutralized by antiubiquitin antiserum dose dependently. Compared to serum from trauma patients, the neutralizing effects of UbP4D1 and UbN19 were attenuated on PBMNCs incubated with serum from sepsis patients.
In contrast to PBMNC cultures, the neutralizing effect of antiubiquitin AS on the inhibition induced by serum from sepsis patients on LPS-induced whole blood TNF-α production was diminished. Furthermore, the neutralizing effects of UbP4D1 and UbN19 detected in PBMNC cultures were abolished in whole blood cultures incubated in the presence of serum from sepsis patients.
Endogenous ubiquitin regulates the inhibitory activity for TNF-α production in patient serum
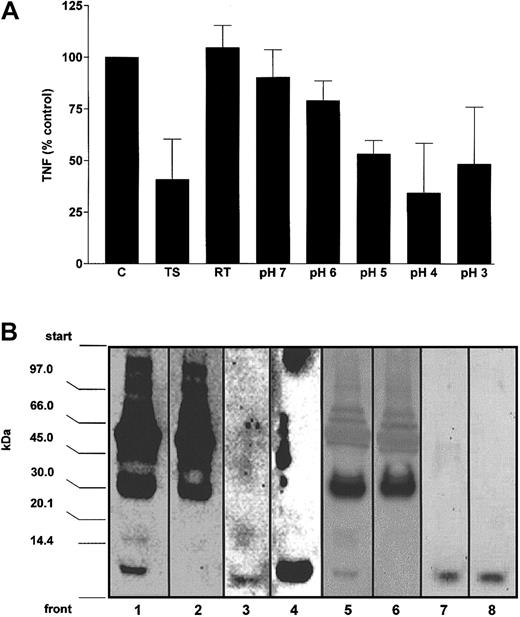
To obtain direct evidence for the immunomodulatory functions of extracellular ubiquitin, we used antiubiquitin affinity chromatography for the depletion and fractionation of endogenous ubiquitin from trauma patient serum. As shown in Figure 5, in the unadsorbed fraction (run-through) the inhibitory activity for LPS-induced TNF-α secretion was abolished. In line with the biologic activity, immunoblot analysis of the run-through showed that free endogenous ubiquitin was removed from the patients' serum. Elution of bound proteins from the antiubiquitin column was performed by acidification. Inhibitory activity for LPS-induced TNF-α secretion was found in the eluted fractions, with a maximal inhibitory effect of the fractions at pH 4. The inhibitory activity measured in fraction 4 was similar to the effect of patients' serum. Immunoblotting of the fractions containing the maximal inhibitory activity showed a single band corresponding to free ubiquitin, whereas the high-molecular-weight bands were detectable in the unadsorbed fractions.
Endogenous ubiquitin regulates the inhibitory activity for TNF-α production in trauma patients' serum.
(A) Trauma patients' serum was applied to an antiubiquitin antibody column and the adsorbed protein was eluted by acidification. Run-through and elutions were collected and tested for inhibitory activity of LPS-induced TNF-α production in healthy donors' whole blood. Whole blood cultures were incubated with the fractions (50%, vol/vol, in the cell culture mixtures) obtained by affinity chromatography in the presence of LPS for 4 hours in a constant volume of 200 μL. Data (percent control) are means ± SD of the TNF-α secretion in the cell culture supernatants from 2 experiments (in duplicates). C indicates control cell culture in the presence of 25% additional volunteers' serum in a constant volume of 200 μL; TS, cell culture in the presence of 25% trauma patients' serum in a constant volume of 200 μL; RT, cell cultures containing the run-through fraction. pH 7 to pH 3, cell cultures containing the eluted fractions. (B) Immunoblot analysis of the fractions obtained by antiubiquitin affinity chromatography. Fractions were separated by SDS-PAGE, transferred to PVDF membranes, and probed for ubiquitin with antiubiquitin AS (1:200; lanes 1-4) and monoclonal UbP4D1 (1:500; lanes 5-8). Lane 1, patients' serum, 10 μg; lane 2, run-through, 20 μg; lane 3, pH 3/4 eluate, 20 μL; lane 4, ubiquitin, 10 ng; lane 5, patients' serum, 50 μg; lane 6, run-through, 50 μg; lane 7, pH3/4 eluate, 200 μL, pH3/4 eluate 10-fold concentrated by boiling; lane 8, ubiquitin 80 ng.
Endogenous ubiquitin regulates the inhibitory activity for TNF-α production in trauma patients' serum.
(A) Trauma patients' serum was applied to an antiubiquitin antibody column and the adsorbed protein was eluted by acidification. Run-through and elutions were collected and tested for inhibitory activity of LPS-induced TNF-α production in healthy donors' whole blood. Whole blood cultures were incubated with the fractions (50%, vol/vol, in the cell culture mixtures) obtained by affinity chromatography in the presence of LPS for 4 hours in a constant volume of 200 μL. Data (percent control) are means ± SD of the TNF-α secretion in the cell culture supernatants from 2 experiments (in duplicates). C indicates control cell culture in the presence of 25% additional volunteers' serum in a constant volume of 200 μL; TS, cell culture in the presence of 25% trauma patients' serum in a constant volume of 200 μL; RT, cell cultures containing the run-through fraction. pH 7 to pH 3, cell cultures containing the eluted fractions. (B) Immunoblot analysis of the fractions obtained by antiubiquitin affinity chromatography. Fractions were separated by SDS-PAGE, transferred to PVDF membranes, and probed for ubiquitin with antiubiquitin AS (1:200; lanes 1-4) and monoclonal UbP4D1 (1:500; lanes 5-8). Lane 1, patients' serum, 10 μg; lane 2, run-through, 20 μg; lane 3, pH 3/4 eluate, 20 μL; lane 4, ubiquitin, 10 ng; lane 5, patients' serum, 50 μg; lane 6, run-through, 50 μg; lane 7, pH3/4 eluate, 200 μL, pH3/4 eluate 10-fold concentrated by boiling; lane 8, ubiquitin 80 ng.
Antiubiquitin antibodies restore reduced TNF-α–producing capacities in blood from trauma and sepsis patients
We further examined whether neutralization of endogenous ubiquitin in whole blood from critically ill patients normalizes the reduced TNF-α–producing capacities (Figure 6).
Antiubiquitin antibodies restore reduced TNF-α–producing capacities in blood from trauma and sepsis patients.
(A-B) Effect of antiubiquitin antibodies on LPS-induced TNF-α secretion in blood of multiply-injured (A) and sepsis (B) patients. Whole blood was incubated with LPS (100 ng/mL) for 4 hours. AS indicates antiubiquitin AS diluted 1:102 and 1:10 in the cell cultures; UbP4D1 and Ub N19, diluted 1:103 in the cell cultures; volunteers, whole blood cultures from healthy donors; TNF production (%), percent of the TNF-α secretion in trauma (A) and sepsis (B) patients' whole blood incubated without antibodies. Values are mean ± SEM from 5 healthy donors, 5 trauma patients, and 5 sepsis patients. (C) Effect of antiubiquitin AS (1:10) on LPS-induced TNF-α secretion in blood of uninjured donor and trauma and sepsis patients. Individual values from panels A and B. Data are pg/mL TNF-α.
Antiubiquitin antibodies restore reduced TNF-α–producing capacities in blood from trauma and sepsis patients.
(A-B) Effect of antiubiquitin antibodies on LPS-induced TNF-α secretion in blood of multiply-injured (A) and sepsis (B) patients. Whole blood was incubated with LPS (100 ng/mL) for 4 hours. AS indicates antiubiquitin AS diluted 1:102 and 1:10 in the cell cultures; UbP4D1 and Ub N19, diluted 1:103 in the cell cultures; volunteers, whole blood cultures from healthy donors; TNF production (%), percent of the TNF-α secretion in trauma (A) and sepsis (B) patients' whole blood incubated without antibodies. Values are mean ± SEM from 5 healthy donors, 5 trauma patients, and 5 sepsis patients. (C) Effect of antiubiquitin AS (1:10) on LPS-induced TNF-α secretion in blood of uninjured donor and trauma and sepsis patients. Individual values from panels A and B. Data are pg/mL TNF-α.
In healthy donor blood antiubiquitin antibodies did not influence the TNF-α secretion on LPS stimulation. In contrast, in every trauma patient's blood (n = 5) antiubiquitin AS increased the TNF-α secretion dose dependently 2- to 3-fold. The increase in TNF-α secretion in cultures incubated in the presence of UbP4D1 and UbN19 (1.5- to 2-fold increase) was lower, but constantly detectable. After neutralization of endogenous ubiquitin in blood from trauma patients, mean TNF-α secretion reaches the level of uninjured donors. In blood from sepsis patients, ubiquitin antibodies were able to normalize the reduced LPS-stimulated TNF-α secretion. Again, neutralizing ubiquitin increases LPS-induced TNF-α secretion in every blood sample tested. Compared to trauma patients' blood, in sepsis patients the increase in TNF-α secretion induced by antiubiquitin AS (5- to 6-fold), UbP4D1 antibody (2- to 3-fold), and UbN-19 antibody (2-fold) was higher.
Discussion
In this study we found that exogenous ubiquitin specifically inhibits the LPS-induced TNF-α response of human whole blood and PBMNCs in a dose-dependent manner.
Based on the findings that exogenous ubiquitin influences neither LPS-evoked IL-6 or IL-8 production nor zymosan- or S aureus–stimulated TNF-α production and that inhibition of the TNF-α response of human PBMNCs was detectable at a concentration (0.5 μg/mL [58 nM]) 40- to 200-fold below the concentrations required for growth suppression (20 μg/mL [2 μM])16 and induction of apoptosis (100 μg/mL [12 μM])10 of KT-3 and HL-60 cells, the immunomodulatory action of exogenous ubiquitin appears to be of high specificity.
In contrast to our results is the finding that exogenous ubiquitin augments the LPS (1 μg/mL)–stimulated TNF-α secretion of the murine macrophage cell line Raw 246.7.15 Besides the difference that this effect required a 20-fold higher concentration of exogenous ubiquitin in the cell cultures,15 our own preliminary studies using the murine macrophage cell line J774 showed neither inhibitory nor synergistically effects of exogenous ubiquitin on TNF-α secretion to LPS (U.K. and M.M., unpublished observation, January 1998). In connection with the finding that exogenous ubiquitin inhibits the LPS-evoked TNF-α response of murine and porcine whole blood similar to human blood, cell line–specific mechanisms may explain these differences.
However, the findings in murine macrophage cell lines as well as the unaffected LPS-evoked IL-6 and IL-8 production by ubiquitin indicate that neutralization of LPS by exogenous ubiquitin, for example, by LPS binding, is not accountable for the inhibitory effects in human PBMNCs. Although exogenous ubiquitin at 100 μg/mL inhibited proliferation in several hematopoietic cell lines after 48 hours of incubation, the inhibitory effect, as measured with the MTT assay, was marginal on MOLT-4 cells and human PBMNCs.10 Therefore, our finding that ubiquitin did not affect viability of human PBMNCs after 4 hours of incubation is not contradictory.
Because indirect evidence has been provided for a transport of exogenous ubiquitin into the cell and metabolism via ubiquitylation to target proteins and degradation by the proteasome system,10 a similar mechanism could possibly explain the effects in human PBMNCs. Human PBMNCs were described to contain 50 ng free ubiquitin per cells from 1 mL of blood.26 As estimated from these data, the amount of free ubiquitin approximates 7 fg/cell. In our experiments, the amount of exogenous ubiquitin per PBMNC supplied in the cell cultures was 150- to 300-fold higher. Although the mechanism of ubiquitin transportation into intact cells is unknown, the high extracellular ubiquitin content could possibly explain a significant increase of the intracellular ubiquitin concentration, even if only small proportion of exogenously supplied ubiquitin is transported into the PBMNCs.
The ubiquitin serum concentrations determined in healthy volunteers are in agreement with the normal range determined by others.7,8,26 Compared with healthy volunteers, ubiquitin concentrations were found to be significantly (5- to 7-fold) increased in serum from both trauma and sepsis patients, and to be 4.5-fold increased in urine from sepsis patients. Surprisingly, patients' ubiquitin serum concentrations were on a level with the ubiquitin concentration required for inhibition of the PBMNC TNF-α response to LPS. Because the ubiquitin-induced inhibition of the TNF-α secretion of PBMNCs from healthy donors resembles alterations of leukocyte function in critically ill patients, which are in common referred to as immunoparalysis,17,18 we investigated the role of ubiquitin in blood from trauma and sepsis patients. Various anti-inflammatory cytokines as well as acute-phase proteins have been suggested to be responsible for the reduced TNF-α secretion to LPS in critically ill patients' blood and for the inhibitory activity of patients' serum on PBMNCs from healthy donors; until now, however, none of these mediators has been shown to be decisive.22-25
In contrast to IL-10, IL-4, and transforming growth factor-β (TGF-β) serum levels in trauma patients,25 we found high ubiquitin serum levels to be significantly associated with a low LPS-stimulated TNF-α secretion into blood of trauma patients.
Although comparison of the inhibitory serum activity measured in trauma and sepsis patients' serum (50% inhibition by 30% [vol/vol] serum with a mean ubiquitin concentration of 350 ng/mL) with the dose-dependent effect of exogenous ubiquitin on LPS-evoked TNF-α production showed that the inhibitory activity cannot be explained exclusively by ubiquitin, antiubiquitin antibodies were able to neutralize the inhibitory activity of trauma patients' serum on PBMNCs and whole blood from healthy donors. Furthermore, neutralization of ubiquitin with antiubiquitin AS and monoclonal and polyclonal antibodies in trauma patients' blood restored the TNF-α response to LPS to a level comparable with healthy volunteers. In addition, direct evidence of an involvement of ubiquitin in immunoregulation was provided by the finding that trauma patients' serum lost the inhibitory activity after ubiquitin depletion and that endogenous ubiquitin appears to be inhibitory for TNF-α secretion of LPS-stimulated human blood.
With regard to the higher-molecular-weight bands visualized in serum by immunoblotting using both antiubiquitin AS and monoclonal UbP4D1 antibody, affinity chromatography showed that they were not bound to immobilized antiubiquitin AS, whereas free ubiquitin was retained. Besides low affinity or competitive binding,27 where sample proteins compete with binding sites and are displaced by high-affinity bound free ubiquitin, unspecific binding in immunoblotting could explain that ubiquitin immunoreactive proteins are detectable in the unadsorbed fractions. However, the finding, that the unadsorbed fractions exert no effect on the LPS-evoked TNF-α response indicate that these ubiquitin immunoreactive proteins are not related to the inhibitory activity, which can be neutralized by antiubiquitin antibodies.
Although antiubiquitin AS was able to neutralize the inhibition induced by sepsis patients' serum on PBMNCs, monoclonal and polyclonal antiubiquitin antibodies showed a decreased neutralizing activity on PBMNCs and hardly any capacity in neutralizing the inhibitory effect of sepsis patients' serum on whole blood cultures.
Nevertheless, all used antiubiquitin antibodies were able to revert reduced LPS-stimulated TNF-α secretion in blood from sepsis patients, with a slightly higher neutralizing capacity in blood from sepsis patients than from trauma patients. The finding that antiubiquitin AS was more effective to revert a depressed TNF-α response to LPS than monoclonal UbP4D1 in both trauma and sepsis patients' serum and blood is in line with the higher sensitivity of antiubiquitin AS to detect free ubiquitin by immunoblotting.
Besides the well-described similarity of a reduced TNF-α response to LPS in sepsis and trauma patients' blood, the differences found for the effects of antiubiquitin antibodies in sepsis and trauma patients' serum and blood might be attributable to differences in other regulatory mediators of leukocyte function in infectious and noninfectious immunodepression, which, for example, have been supposed for the involvement of several anti-inflammatory cytokines in regulating the TNF-α response to LPS.22,25 28
Although the origin of extracellular ubiquitin in critically ill patients remains to be determined, secretion of intracellularly synthesized ubiquitin10 as well as liberation of intracellular ubiquitin by tissue damage appears reasonable. In particular, the latter hypothesis could explain the early appearance of reduced leukocyte function and availability of inhibitory serum activity for TNF-α production in trauma patients, which have been shown to be detectable 94 ± 89 minutes (minimum, 25 minutes) after the traumatic event.29 In this model, extracellular ubiquitin could possibly serve as a reservoir for immediate ubiquitin-dependent regulatory immune functions unless the cell is able to maintain a sufficient cytosolic level.
In conclusion, the results demonstrate for the first time that extracellular ubiquitin acts as a cytokinelike protein with anti-inflammatory properties and indicate that extracellular ubiquitin is involved in the regulation of immunodepression in critical illness. The relative contribution of extracellular ubiquitin to immunomodulation in various inflammatory situations as well as its biologic significance in vivo remain to be determined.
We thank Mrs Anja Bistron for her excellent technical support.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-03-0918.
Supported in part by a grant from the Deutsche Forschungsgemeinschaft (DFG, grant Ma 2474/1-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Matthias Majetschak, Department of Trauma Surgery, University Hospital Mannheim, Faculty of Clinical Medicine Mannheim of the Ruprecht-Karls University Heidelberg, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; e-mail:mmajetschak@med.miami.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal