Infection of bovine T cells and B cells with the intracellular protozoan parasite Theileria parva induces a transformed phenotype with characteristics comparable to leukemic cells. The transformed phenotype reverts on drug-induced parasite death, and the cured lymphocytes acquire a resting phenotype and eventually die by apoptosis if not further stimulated. Here, we show that both lymphocyte proliferation and activation of the transcription factor AP-1 are mediated by Src-family protein tyrosine kinases (PTKs) in a parasite-dependent fashion. Src-family PTKs are known to be present in glycolipid-enriched microdomains (GEMs), also called lipid rafts, and to be negatively regulated by PTK Csk complexed to tyrosine-phosphorylated transmembrane adapter protein PAG (phosphoprotein associated with GEMs) also called Cbp (Csk-binding protein). We, therefore, purified GEMs from proliferating infected B cells and from growth-arrested cells that had been drug-cured of parasites. Proliferation arrest led to a striking increase of PAG/Cbp expression; correspondingly, the amount of Csk associated with PAG/Cbp in GEMs increased markedly, whereas PTK Hck accumulation in GEM fractions did not alter on growth arrest. We propose thatTheileria-induced lymphocyte proliferation and permanent activation of Hck stems from down-regulation of PAG/Cbp and the concomitant constitutive loss of the negative regulator Csk from the GEMs of transformed B cells.
Introduction
Infection of bovine T cells and B cells with the intracellular protozoan parasite Theileria parva induces a transformed phenotype with characteristics comparable to tumor cells. Hallmarks of transformation are the capacity of infected cells to proliferate in vitro in the absence of exogenous growth factors or antigenic stimulation.1 Moreover,Theileria-infected leukocytes behave as invasive tumors in both scid2 and in athymic, irradiated mice,3,4 and they can form colonies in soft agar. Parasite-dependent regulation of host cell signal transduction pathways is thought to be critical in establishing the transformed phenotype of infected leukocytes.5,6 Consistently, host cell c-Jun N-terminal kinase (JNK), but not Erk or p38 mitogen-activated protein (MAP) kinases, is activated constitutively in infected leukocytes.7,8 JNK activation results in permanent up-regulation of Jun and Fos family proteins and induction of the transcription factors AP-18 and ATF-2.9Importantly, Theileria-induced transformation can be reverted by treatment with the theilericidal drug BW720c,10 and the leukocytes then acquire a resting phenotype, or die of apoptosis, as reviewed in Dobbelaere and Heussler.11 Reversion of the transformed state is an unusual feature for tumor cells, as cellular transformation is usually associated with genomic alterations (rearrangements/mutations) or stable insertion of foreign DNA.12 Because the “transforming” stimulus can be eliminated by drug treatment, lymphocytes infected with T parva offer a rare opportunity to compare proliferative and antiapoptotic signaling pathways in transformed cells with those of isogenic resting cells.
Src family members are a group of 9 nonreceptor tyrosine kinases that share a high degree of structural homology. Their molecules are organized into 3 major domains, which from the N- to the C-terminus, are a Src homology domain 3 (SH3) domain, a SH2 domain, and the tyrosine kinase (SH1) domain.13 Src family kinases are widely expressed, but with cell type-specific expression patterns that differ between individual members. Src kinases are activated by a large variety of receptors such as immunoreceptors (T-cell receptor [TCR], B-cell receptor [BCR], Fc receptor [FcR]),14 growth factor receptors,15 cytokine receptors,16G-protein–linked receptors,17 or integrins.18 Their function is to convey extracellular signals through membrane-proximal compartments to cellular effector pathways; for review, see Brown and Cooper.19
Src family kinases are modified by myristoylation and palmitoylation at their N-termini; these fatty acid residues direct the enzymes to membrane compartments20 and particularly toGlycolipid Enriched Microdomains (GEMs), also called membrane rafts.21 Recruitment to nonlipidic subcellular locations, such as the cytoskeleton, requires SH3 or SH2 domain-dependent interactions, which are thus important in bringing the kinase into proximity with specific substrates, but also in regulating their state of activation.22,23 Src family kinases are negatively regulated by induction of a closed, inactive conformation.13 Inhibitory phosphorylation of the C-terminal tyrosine24 by the C-terminal Src kinase Csk25-27 promotes binding of the SH2 domain and because of steric hindrance inactivates the kinase as reviewed in Shalloway and Taylor.28 Intramolecular interactions between the SH3 domain and the stretch of amino acids that link SH2 and catalytic domains further reinforces the closed conformation.13Activation is a stepwise process initiated by C-terminal dephosphorylation,29,30 or SH3 domain displacement,17,22 resulting in an open conformation. Opening of the catalytic cleft results in autophosphorylation of the tyrosine in the activation loop within the kinase domain and full activation of the kinase. In resting cells, or under conditions in which activity is not required, Src kinases are maintained in an inactive state through C-terminal phosphorylation by cytoplasmic tyrosine kinase Csk.27,31 Csk is recruited to membrane and activated by binding to the transmembrane adaptor protein called PAG/Cbp phosphoprotine-associated with glycosphingolipid-enriched microdomains/Csk-binding protein).32-34
In lymphocytes, activation of Src family kinases is a prerequisite to activate a plethora of signaling pathways downstream of immune receptors, cytokine receptors, and adhesion molecules, reviewed in Thomas and Brugge.35 Their role inTheileria-induced lymphocyte transformation, however, is only poorly understood. Decreased electrophoretic mobility of Fyn and Lck isolated from T parva–transformed T cells has been suggested to reflect increased catalytic activity of these kinases.5 Furthermore, parasite-dependent regulation of Lyn, Fyn, and Lck has been observed in T parva–infected T-cell lines, with Fyn as the predominant Src kinase activity detected.36 Permanent activation of lymphocytes byTheileria parasites induces production and secretion of cytokines,37-39 and cell-cell contact is required for infected cell growth,40 raising the possibility that surface receptor–activated Src kinases are involved in this process. We have demonstrated that proliferation ofTheileria-transformed lymphocytes is phosphatidylinositiol-3-kinase (PI3-K) dependent.39 As Src kinases can regulate both proliferation41-43 and cellular transformation through activation of PI3-K,44 we decided to examine the role of Src kinases in the proliferation ofTheileria-transformed B cells. We show that the parasite-dependent uncontrolled proliferation of infected B cells is associated with constitutive activation of the protein tyrosine kinase (PTK) Hck apparently caused by exclusion of its negative regulator, Csk, from Hck-positive GEMs.
Materials and methods
Cells, cell culture
TpMD409.B2 is a Theileria parva Muguga–infected B-cell clone (B2) described elsewhere45 and is further referred to in this paper as simply TpM409. The B-cell characteristics of this line grown in our laboratory have been confirmed,46 and its cultivation has previously been described.8 To eliminate the parasite, the hydroxynaphtoquinone derivative BW720c10 was added at 30 ng/mL. BW720c treatment was performed for 48 hours if not otherwise stated, and cell viability was routinely tested by Trypan Blue exclusion (BW720c-treated cells are also referred to as “cured”).
Preparation of resting B cells
Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation (1.083 g/mL; Histopaque; Sigma Chemical, Poole, United Kingdom). If not otherwise stated, cells were resuspended in tissue culture medium (TCM) consisting of RPMI 1640 medium with Glutamax I (Life Technologies, Paisley, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum, 5 × 10−5 M 2-mercaptoethanol, and 100 IU/mL gentamycin (Sigma Chemical). B cells (Bcs) were isolated from PBMCs after staining with monoclonal antibody (mAb) to CD21. Thereafter, cells were incubated with antimouse immunoglobulin G1 (IgG1) superparamagnetic particles (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany), and labeled cells were isolated using MiniMacs columns (Miltenyi Biotech) following the manufacturer's instructions. The purity of the cells was evaluated by flow cytometry and shown to be more than 98%. Cell viability, assessed by exclusion of trypan blue, was more than 95%. Sorted Bcs were either left untreated or stimulated in medium supplemented with 50 ng/mL phorbol 12 myristate 13-acetate (PMA) and 1 μg/mL ionomycin for 4 hours, for the last 4 hours of the 24-hour incubation period, or for the last 24 hours of the 48-hour incubation period. After the times indicated, cells were pelleted (6000g, 2 minutes, 4°C), and washed twice with ice-cold phosphate-buffered saline (PBS; 6000g, 2 minutes, 4°C). Thereafter, the supernatant was removed, and the pellets were stored at −80°C until analysis.
Antibodies
Antibodies (used in Western blotting usually at 1:1000 dilution) were the following: Hck, rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, Sc-72) or mouse monoclonal (Transduction Laboratories); phosphotyrosine, mouse monoclonal 4G10 (Transduction Laboratories) and mouse monoclonal P-TYR-01 (the Prague laboratory); PAG/Cbp, rabbit polyclonal to human PAG (produced in the Prague laboratory); Csk, rabbit polyclonal (Santa Cruz Biotechnology). Mouse mAb PAG/Cbp-C6 (IgG2b) was prepared in the Prague laboratory; it is directed against the C-terminal peptide of human PAG/Cbp and cross-reacts with bovine PAG/Cbp. The hybridoma line secreting mAb CC21 recognizing the bovine CD21 molecule was purchased from the American Type Culture Collection (ATCC, Manassas, VA), and mAb was produced in the Bern lab.
Proliferation assays
Src kinase inhibitor PP2 (Calbiochem) was resuspended in dimethyl sulfoxide (DMSO) and used at different concentrations as indicated in figure legends. For DNA synthesis measurements, cells were washed in PBS and resuspended at 5 × 104 cells/mL in culture medium containing PP2 at different concentrations or solvent alone. [3H]-thymidine (1 μCi [0.037 MBq]) was added per sample for 17 hours, and incorporated radioactivity was quantified by β-counting. When indicated, PMA (100 ng/mL) was added with solvent or together with PP2.
Transient transfection experiments, luciferase and β-Gal assays
Exponentially growing TpM409 cells were harvested at 3.5 × 105 cells/mL and washed once in RPMI 1640. Cells (5 × 106) collected in 500 μL RPMI 1640 medium containing 1 mM dithiothreitol and 1 mM sucrose were transfected by electroporation performed in 4-mm gap cuvettes (500 μF, 280 V, 2 pulses at room temperature in RPMI 1640 medium) followed by 15 minutes of incubation at room temperature. Cells were then resuspended in 5 mL prewarmed cell culture medium, and luciferase assays were performed after 48-hour incubation at 37°C. pEF-BOS vectors were used to express wild-type (wt) and mutant murine Hck (provided by G. Scholz et al47). The pEF-BOS-mHck499F construct encodes a constitutively active Hck (negative regulatory Tyr499 is replaced by Phe), whereas pEF-BOS-mHck267M-499F encodes a kinase-dead Hck mutant (Lys267 replaced by Met and Tyr499 by Phe). PI3-K expression vectors are described elsewhere.39 Kinase expression vectors (20 μg per sample) were cotransfected with 20 μg 3xTRE-luciferase reporter plasmid.8 Cytomegalovirus–driven β-galactosidase-expression vector (5 μg) for standardization of transfection efficiency was included in each sample. Luciferase assays were performed following the manufacturer's (Promega) instructions. Transfection efficiency was standardized on the basis of β-galactosidase activity in each individual transfection: 10 μL total cell lysate was mixed with 100 μL H2O, 700 μL Z-buffer (100 mM Na-phosphate pH 7.5, 100 mM KCl, 1mM MgSO4, 50 mM β-mercaptoethanol), and 200 μL ONPG (4 mg/mL o-nitrophenyl β-D-galactopyranoside in 100 mM Na-phosphate buffer, pH 7.5) and incubated for 1 to several hours at 37°C. Reactions were stopped by the addition of 500 μL 1 M Na2CO3 and quantified by spectrophotometry at 420 nm. Results shown in figures are means of at least 3 independent experiments.
Detergent lysates and membrane preparation, kinase assays, SDS-PAGE, and Western blotting
Crude plasma membranes were prepared by using the method based on gentle cell disruption in hypotonic buffer as described in detail elsewhere.48 49 For kinase assays, 2 × 107PBS-washed TpM409 or cured cells or 1.5 × 108 Bcs or stimulated Bcs were lysed in ice-cold laurylmaltoside lysis buffer (150 mM NaCl, 50 mM Tris (tris(hydroxymethyl)aminomethane) [pH 7.5]) 1% laurylmaltoside [dodecylmaltoside; Calbiochem], 20 μg/mL leupeptin, 20 μg/mL aprotinin, 1 mM pefabloc, 1 mM Na-orthovanadate, 25 mM NaF) for 30 minutes on ice. Lysates were cleared by centrifugation and precleared with Protein A–Sepharose for 30 minutes at 4°C, and Hck was immunoprecipitated with 1.5 μg antibody per sample (Santa Cruz Biotechnology, SC-72) for 2 hours under rotation at 4°C. Immune complexes were fixed on Protein A–Sepharose for 30 minutes at 4°C and then washed as follows: 3 times in lysis buffer, once in ice-cold double-distilled H2O, and once in kinase buffer (20 mM Tris [pH 7.4]), 10 mM MgCl2). PP2 was added during the last 2 washing steps and during kinase reaction at 10 μM final concentration as indicated. Kinase assays were performed in the presence of 5 μM cold adenosine triphosphate (ATP), 10 μCi (0.37 MBq) [32P] γ-ATP, and 1.5 μg/sample acid-activated enolase at 30°C for 15 minutes. Reactions were stopped by adding 5× SDS-loading buffer. Proteins were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in 8% gels and electrophoretically transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), which allows quantification of kinase activity and protein detection by Western blotting on the same support. Incorporation of [32P] γ-ATP was detected by phosphor imaging and analyzed and quantified by using Image-quant software or exposure to X-ray films (Amersham). Equal loading was confirmed by Western blotting using Hck-specific antibodies, and tyrosine phosphorylation status was determined with phosphotyrosine-specific antibodies (for antibodies, see “Antibodies”). For Western blotting, the blots were incubated with antibodies diluted in 5% nonfat milk (1% bovine serum albumin [BSA]) when antiphosphotyrosine antibodies were used) in PBS, 0.1% Tween-20. Proteins were detected by horseradish peroxidase–conjugated secondary antibodies and chemiluminescence-based visualization (Super Signal detection kit, Interchim).
Quantitative immunoisolation of PAG/Cbp from plasma membranes
Plasma membranes obtained from 3 × 107 cells were solubilized in 400 μL ice-cold laurylmaltoside (LM) lysis buffer (100 mM NaCl, 20 mM Tris buffer pH 8.2, 50 mM NaF, 10 mM Na-pyrophosphate, 10 mM EDTA [ethylenediaminetetraacetic acid], 1 mM Na-orthovanadate, 1 mM phenylmethyl sulfonyl fluoride [PMSF], 1% laurylmaltoside [dodecylmaltoside; Calbiochem]). The supernatant obtained after 3 minutes of centrifugation at 14 000 rpm was passed through 100-μL minicolumns of PAG-C6 mAb covalently immobilized (at 2 mg/mL gel) on CNBr-Sepharose (Pharmacia), the flow-through fractions were collected, the columns were washed with 1 mL lysis buffer, and the bound material was released by 2 column volumes of SDS sample buffer. The original cell lysates, flow-through, and SDS-eluted fractions were analyzed by SDS-PAGE and Western blotting.
Ultracentrifugation in sucrose gradients (preparation of GEMs)
TpM409 or cured cells (2 × 107) were collected and washed once in PBS. A volume of 500 μL MBS-Triton X-100 (Tx-100) (25 mM morpholinoethanesulfonic acid [MES], 150 NaCl [pH6.5], 0.5% Tx-100, 1 mM PMSF, 4 μg/μL PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mM orthovanadate, 10 mM NaF,) was added to cell pellet, and cells were lysed for 30 minutes on ice. An equal volume of 85% sucrose solution in metabisulfite (MBS) was gently mixed with the cellular lysate to generate a 42.5% solution (1 mL) at the bottom of an SW55ti ultracentrifugation tube. The lysate/sucrose mixture was subsequently overlayed on ice with 35% (2 mL) and 5% (1 mL) sucrose-MBS containing protease and phosphatase inhibitors described for MBS-T. Ultracentrifugation was performed in a SW55 Ti rotor at 47 000 rpm (200 000g) for 6 hours at 4°C. Samples (300 μL) were taken from the top to the bottom and fractionated by SDS-PAGE. The visible band at the 5% to 35% sucrose interface containing the GEMs corresponded to fraction 4.
Results
Src kinase activity is essential for proliferation of T parva–transformed B cells
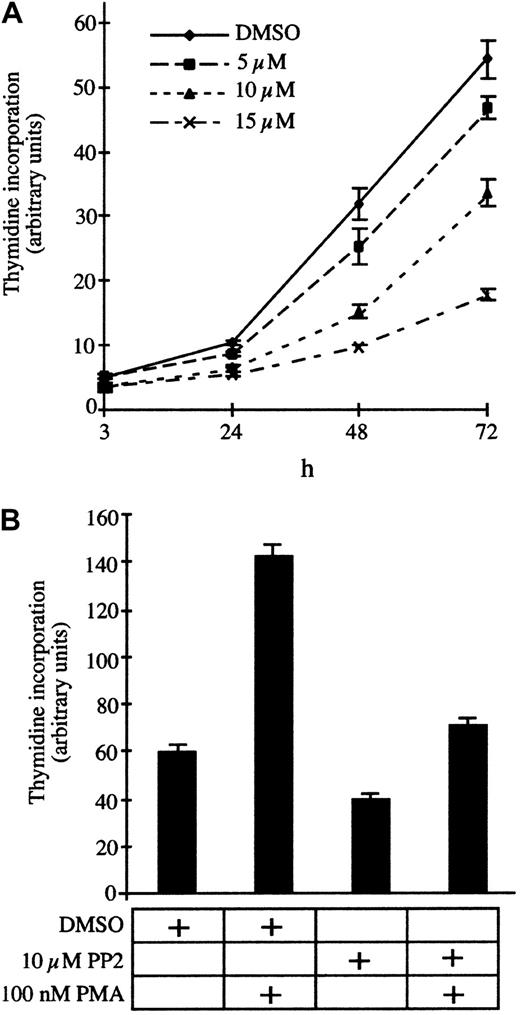
To test the contribution of Src family kinases to the permanent proliferation of T parva–transformed B cells, we incubated the infected B cells with increasing concentrations of the specific inhibitor PP2 and measured DNA synthesis after 24, 48, and 72 hours of treatment. PP2 significantly reduces DNA synthesis in a dose-dependent manner with an IC50 (concentration that inhibits 50%) of approximately 10 μM (Figure 1A). Phorbol-ester (PMA) stimulation rescues the PP2-induced growth arrest (Figure 1B), albeit not to levels observed with PMA alone, which suggests that Src kinases do not stimulate proliferation through a PKC-dependent mechanism. Consistently, the PKC inhibitor bis-indolylmalemide (BIM) does not induce proliferation arrest ofT parva–transformed B cells (data not shown) or T cells.7 We also investigated whether growth inhibition was due to induction of programmed cell death and found that PP2 treatment for 12 or 24 hours did not significantly increase annexin-V binding (data not shown). Thus, Src activity contributes to proliferation ofTheileria-transformed lymphocytes, and, similar to PI3-K,39 the kinase inhibition does not result in apoptosis.
Proliferation of TpM409 is Src kinase dependent.
(A) TpM409 cells were treated with increasing concentrations of PP2, and thymidine incorporation was measured after 3, 24, 48, and 72 hours. (B) PP2-induced growth arrest is overcome by phorbol-ester stimulation. Cells were treated with PP2 and PMA as indicated, and thymidine incorporation was quantified after 24 hours. Error bars indicate mean and standard deviation of 3 independent experiments.
Proliferation of TpM409 is Src kinase dependent.
(A) TpM409 cells were treated with increasing concentrations of PP2, and thymidine incorporation was measured after 3, 24, 48, and 72 hours. (B) PP2-induced growth arrest is overcome by phorbol-ester stimulation. Cells were treated with PP2 and PMA as indicated, and thymidine incorporation was quantified after 24 hours. Error bars indicate mean and standard deviation of 3 independent experiments.
Hck is constitutively active in Theileria-transformed B cells
The Src family kinase Hck is specifically expressed in cells of hemopoietic origin (reviewed in Thomas and Brugge35), and Western blot analysis revealed particularly high expression of Hck inT parva–transformed B lymphocytes (data not shown). We, thus, examined whether Hck might be responsible for proliferation of the infected B cells. To this end, we immunoprecipitated Hck from total cell extracts of TpM409 and determined Hck kinase activity in an in vitro kinase assay. Hck displayed significant kinase activity as estimated by autophosphorylation and by its capacity to phosphorylate enolase (Figure 2A). In parallel, we performed in vitro kinase assays in the presence of the Src kinase inhibitor PP2 to verify specificity of the kinase reaction (middle panel) and to determine tyrosine phosphorylation state of endogenous Hck prior to autophosphorylation (bottom panel). As expected, PP2 treatment completely blocked Hck autophosphorylation and phosphorylation of exogenous substrate enolase; tyrosine phosphorylation of endogenous Hck revealed by the PP2 treatment was relatively low. Another Src-family kinase expressed abundantly in the B cells, Lyn, was inactive under the conditions of the in vitro kinase assay and its basal level of tyrosine phosphorylation was high (M.B., unpublished data, 1999). Transformation of B cells, constitutive activation of signaling pathways, and permanent proliferation of the infected cells is dependent on the presence of live T parva in the host cell cytoplasm. To investigate whether Hck activity is increased by the intracellular parasite, we performed kinase assays with Hck immunoprecipitated from 2 × 107 transformed (TpM) or cured B cells (Figure 2B) and from 1.5 × 108 resting B cells purified from bovine blood (Figure 2C). Elimination of T parva clearly results in a decrease in Hck kinase activity, which is comparable to the inhibition of DNA synthesis in the presence of Src kinase inhibitor PP2 (Figure 1B), rescued by phorbol ester stimulation (Figure 2B). Compared with TpM, a much lower level of Hck kinase activity was detectable in resting B cells, and, as with cured cells, Hck activity is enhanced by PMA stimulation (Figure 2C). These results suggest that, although active in purified resting B cells, Hck activity is significantly higher in T parva–transformed B cells or B cells stimulated with PMA and ionomycin.
Hck activity is markedly different in untreated versus cured TpM409 cells and in resting versus stimulated bovine B cells.
(A) Kinase activity of Hck immunoprecipitated (IP) from total TpM409 extracts was determined by in vitro kinase assays. Endogenous tyrosine phosphorylation status of Hck was investigated by performing kinase assays with (+) or without (−) 10 μM Src kinase inhibitor PP2. In the absence of PP2, Hck phosphorylates the enolase substrate (middle panel). Antiphosphotyrosine Western blotting of the kinase assay products (bottom panel) shows that Hck is only poorly tyrosine phosphorylated in the absence of in vitro kinase activity. Equal loading was confirmed by Western blot using Hck-specific antibodies (top panel). (B) Hck kinase activity immunoprecipitated from 2 × 107 untreated, BW720c-cured TpM409 cells or cured TpM409 cells stimulated for 24 hours with PMA/ionomycin was determined as in panel A (p-Hck, autophosphorylated Hck; p-enolase, phosphorylated enolase). (C) Hck kinase activity immunoprecipitated from 1.5 × 108 resting B cells or the same number of B cells stimulated for 24 hours with PMA/ionomycin was determined as described in panel A. A comparable quantity of Hck is expressed in resting or stimulated B cells (top panel), whereas stimulation increases Hck kinase activity (bottom panel).
Hck activity is markedly different in untreated versus cured TpM409 cells and in resting versus stimulated bovine B cells.
(A) Kinase activity of Hck immunoprecipitated (IP) from total TpM409 extracts was determined by in vitro kinase assays. Endogenous tyrosine phosphorylation status of Hck was investigated by performing kinase assays with (+) or without (−) 10 μM Src kinase inhibitor PP2. In the absence of PP2, Hck phosphorylates the enolase substrate (middle panel). Antiphosphotyrosine Western blotting of the kinase assay products (bottom panel) shows that Hck is only poorly tyrosine phosphorylated in the absence of in vitro kinase activity. Equal loading was confirmed by Western blot using Hck-specific antibodies (top panel). (B) Hck kinase activity immunoprecipitated from 2 × 107 untreated, BW720c-cured TpM409 cells or cured TpM409 cells stimulated for 24 hours with PMA/ionomycin was determined as in panel A (p-Hck, autophosphorylated Hck; p-enolase, phosphorylated enolase). (C) Hck kinase activity immunoprecipitated from 1.5 × 108 resting B cells or the same number of B cells stimulated for 24 hours with PMA/ionomycin was determined as described in panel A. A comparable quantity of Hck is expressed in resting or stimulated B cells (top panel), whereas stimulation increases Hck kinase activity (bottom panel).
Hck kinase activity is required for constitutive activation of the transcription factor AP-1
The transcription factor AP-1 is constitutively induced in T parva–transformed B cells,8 and its transcriptional activity is regulated by PI3-K.39 As Src family kinases can contribute to AP-1 activation,43 50 we asked whether Hck is involved in activation of AP-1 inTheileria-transformed lymphocytes. To address this point, an AP-1–driven luciferase reporter construct was cotransfected into the TpM409 cells together with either kinase-inactive (267M/499F) or constitutively active (499F) Hck expression vectors (Figure3). Transient expression of the kinase-dead Hck267M/499F reduced AP-1–driven luciferase activity by more than 50%, whereas its constitutively active counterpart 499F induced AP-1 activation significantly (Figure 3). These results imply that Hck contributes to AP-1 activation inTheileria-transformed lymphocytes.
Hck kinase activity contributes to AP-1 transcriptional activation; Hck and PI3-K activated pathways converge upstream of AP-1.
Three times TRE-luciferase AP-1 reporter construct was transfected either alone (control), or together with kinase-dead Hck (267M/499F), or with constitutive active Hck (499F). Relative luciferase activity compared with control is shown. Coexpression of Hck267M/499F significantly reduces the constitutive AP-1 activity, whereas Hck499F increases AP-1 activation. Hck and PI3-K–activated pathways converge upstream of AP-1. Coexpression of membrane-targeted constitutive active PI3-K (p110) failed to rescue the Hck267M/499F-induced block in AP-1 activity (p110 + 267M/499F). The increased AP-1 activity induced by Hck 499F expression, however, was ablated by kinase-dead p110 (Δp110 + 499F). Thus, both Hck and PI3-K pathways converge to induce AP-1 transactivation. Mean and standard deviation of at least 3 independent experiments are shown. The error bars indicate the mean and standard deviation relative to vector control of 3 independent experiments.
Hck kinase activity contributes to AP-1 transcriptional activation; Hck and PI3-K activated pathways converge upstream of AP-1.
Three times TRE-luciferase AP-1 reporter construct was transfected either alone (control), or together with kinase-dead Hck (267M/499F), or with constitutive active Hck (499F). Relative luciferase activity compared with control is shown. Coexpression of Hck267M/499F significantly reduces the constitutive AP-1 activity, whereas Hck499F increases AP-1 activation. Hck and PI3-K–activated pathways converge upstream of AP-1. Coexpression of membrane-targeted constitutive active PI3-K (p110) failed to rescue the Hck267M/499F-induced block in AP-1 activity (p110 + 267M/499F). The increased AP-1 activity induced by Hck 499F expression, however, was ablated by kinase-dead p110 (Δp110 + 499F). Thus, both Hck and PI3-K pathways converge to induce AP-1 transactivation. Mean and standard deviation of at least 3 independent experiments are shown. The error bars indicate the mean and standard deviation relative to vector control of 3 independent experiments.
Coordinated action of Hck and PI3-K is required for full AP-1 activation
We have previously shown that permanent activation of AP-1 inTheileria-transformed B cells is under the control of PI3-K, as it can be blocked by overexpression of kinase inactive PI-3K39 and depends exclusively on the JNK kinase pathway.8 As Src family kinases can activate PI3-K directly,51 we examined whether Hck activates AP-1 in PI3-K–dependent manner. Transient expression of Hck499F in the TpM409 cells along with dominant-negative PI3-K (Δp110) suppressed Hck-induced AP-1 activity (Figure 3). This finding is consistent with PI3-K being directly downstream of Hck in a pathway leading to AP-1 induction. However, parallel expression of a constitutively active form of PI3-K (p110) did not overcome inhibition of AP-1 transcriptional activity caused by Hck267M/499F (Figure 3). This result indicates that the Hck- and PI3-K–mediated signals rather converge on an upstream activator of the JNK pathway and that both kinases are at least partially independently important in the induction of AP-1 transcriptional activity in the B cells transformed by T parva. This hypothesis is compatible with the notion that activation of PI3-K alone is not sufficient to induce AP-1 activation in T cells.52
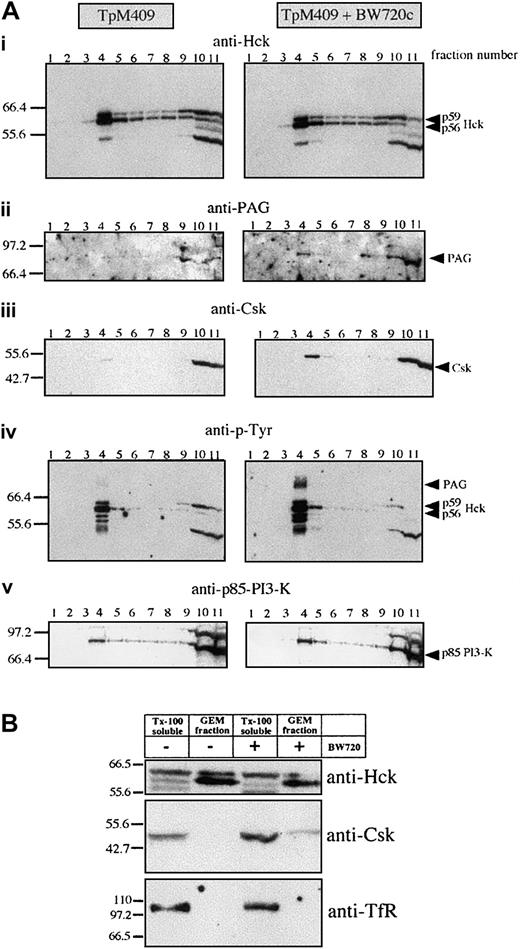
Parasite-dependent exclusion of Csk from Hck-positive GEMs
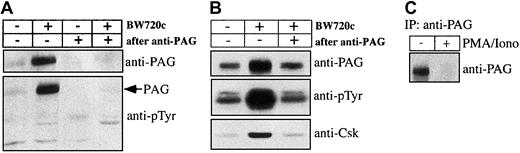
Hck activation, proliferation, and AP-1 activation ofTheileria-infected lymphocytes are parasite dependent and as both appear to be mediated by Hck, we asked by what mechanism the parasite could regulate Hck activity. An obvious possibility was regulation via the PAG/Cbp-Csk complex32 33 present, together with Hck, in GEMs. GEMs purified fromTheileria-infected B cells and from B cells that had been drug-cured of parasites were analyzed by Western blotting. As demonstrated in Figure 4, Hck (and particularly the p56 isoform) was similarly concentrated in the GEM fractions prepared from both infected and BW720c drug-cured B lymphocytes, suggesting that parasite-mediated regulation of Hck does not involve its recruitment into an active signaling complex in the GEMs (Figure 4Ai). We confirmed the selectivity of the sucrose gradient purification by probing the GEM fraction with an antibody directed against the plasma membrane marker transferrin receptor (TfR), which is only detectable in the Tx-100 soluble fractions of TpM409 and cured cells but not in their GEM fractions (Figure 4B). Importantly, drug-induced parasite death leads to recruitment of Csk to the GEMs (Figure 4Aiii), to increased tyrosine-phosphorylation of Hck (Figure 4Aiv), and to marked increase of a tyrosine-phosphorylated protein of 80 kDa (Figure 4Aiv). This phosphoprotein was unambiguously identified as PAG/Cbp (Figure 4Aii,5A). Interestingly, the total amount of PAG/Cbp in the plasma membrane, rather than just the level of its tyrosine-phosphorylation, markedly increased as a result of the drug treatment; the amount of Csk associated with PAG/Cbp also increased correspondingly (Figure 5B). The effect of cellular activation on PAG/Cbp abundance and Csk recruitment can be mimicked by stimulating cured B cells with PMA/ionomycin, resulting in decreased PAG/Cbp expression and dissociation of Csk (Figure 5B). As expected and analogous to cellular activation by the parasite and decreased PAG/Cbp expression, stimulation of resting B cells with PMA/ionomycin results in PAG/Cbp downregulation (Figure 5C) and Hck activation (Figure2C).
Parasite-dependent exclusion of Csk from Hck-positive GEMs.
(Ai-v) GEMs were isolated from untreated or BW720c-cured TpM409 cells by Tx-100 solubilization and sucrose gradient centrifugation. Eleven fractions of the sucrose gradient were analyzed by Western blotting. The GEMs concentrate mainly in fraction 4, whereas Tx-100–soluble material accumulates in fractions 10 and 11. The antibodies used for Western blotting are indicated at the top of each panel (identical membrane was used for each panel shown). (B) GEM fractions are free of plasma membrane marker-transferrin receptor (TfR): Fractions 4 (GEM fraction) and 11 (Tx-100 soluble) were probed with anti-Hck (top), Csk (middle), and TfR (bottom).
Parasite-dependent exclusion of Csk from Hck-positive GEMs.
(Ai-v) GEMs were isolated from untreated or BW720c-cured TpM409 cells by Tx-100 solubilization and sucrose gradient centrifugation. Eleven fractions of the sucrose gradient were analyzed by Western blotting. The GEMs concentrate mainly in fraction 4, whereas Tx-100–soluble material accumulates in fractions 10 and 11. The antibodies used for Western blotting are indicated at the top of each panel (identical membrane was used for each panel shown). (B) GEM fractions are free of plasma membrane marker-transferrin receptor (TfR): Fractions 4 (GEM fraction) and 11 (Tx-100 soluble) were probed with anti-Hck (top), Csk (middle), and TfR (bottom).
Tyrosine-phosphorylated PAG recruits Csk in cured cells.
(A) Crude membrane extracts or membrane extracts after passing through anti-PAG immunosorbent from untreated or BW720c-treated TpM409 cells were prepared and probed by Western blotting with antibodies directed against PAG/Cbp (top) and against phosphotyrosine (bottom). (B) PAG was immunoprecipitated from membrane extracts of untreated or BW720c-treated TpM409 cells, and PAG abundance (top), tyrosine phosphorylation (middle), and Csk association (bottom) were determined by Western blotting. (C) Increased PAG expression in resting B cells is reverted by growth stimulation: PAG was immunoprecipitated from bovine B cells or bovine B cells stimulated with PMA/ionomycin for 4 hours and probed by Western blotting with anti-PAG antibodies.
Tyrosine-phosphorylated PAG recruits Csk in cured cells.
(A) Crude membrane extracts or membrane extracts after passing through anti-PAG immunosorbent from untreated or BW720c-treated TpM409 cells were prepared and probed by Western blotting with antibodies directed against PAG/Cbp (top) and against phosphotyrosine (bottom). (B) PAG was immunoprecipitated from membrane extracts of untreated or BW720c-treated TpM409 cells, and PAG abundance (top), tyrosine phosphorylation (middle), and Csk association (bottom) were determined by Western blotting. (C) Increased PAG expression in resting B cells is reverted by growth stimulation: PAG was immunoprecipitated from bovine B cells or bovine B cells stimulated with PMA/ionomycin for 4 hours and probed by Western blotting with anti-PAG antibodies.
Reversible Csk recruitment to PAG/Cbp regulates AP-1 activation
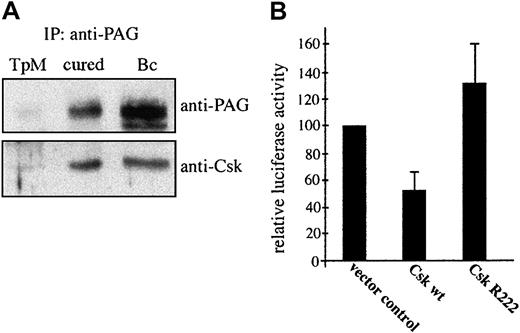
As Hck contributes to parasite-induced AP-1 activation (Figure 3) and most likely plays a key role in Src kinase-controlled proliferation of infected cells (Figure 1), we hypothesized that it is Csk exclusion from GEMs positive for Hck in transformed or PMA/ionomycin-stimulated cells that increases Hck activity (Figure 2). A comparison of Csk association with PAG/Cbp revealed comparable levels in cured and resting B cells, further arguing for a negative regulatory role of Csk in Hck activation (Figure 6A). We next established functional significance of Csk in negatively regulating transcriptional activation of AP-1 by assaying AP-1–driven luciferase activity following transfection of both wild-type and dominant-negative forms of Csk (Figure 6B). Csk expression clearly leads to a significant (53%) inhibition of the AP-1 activation. As both Hck and PI3-K are involved in signaling to AP-1, we also monitored the distribution of the p85 regulatory subunit and found that it remained largely unchanged in drug-cured lymphocytes (Figure 4Av). This finding is consistent with our previous observation that parasite death leads to a decrease in PI3-K activity, without any reduction in the amount of p85 associated with the plasma membrane.39
Parasite death leads to Csk recruitment to GEMs, which accounts for down-regulation of AP-1 transactivation.
(A) PAG was immunoprecipitated from lysates of untreated TpM409 (TpM), of cured cells (cured), or of resting B cells (Bc) and probed by Western blotting with anti-PAG (top) and anti-Csk (bottom) antibodies. (B) TpM409 cells were cotransfected with wild-type (wt) Csk, or a kinase-dead mutant of Csk (R222), together with the 3xTRE-luciferase AP1 reporter construct. Effects of transient expression of wt Csk and CskR222 on AP-1 activation were assayed by monitoring luciferase activity in lysates of transfected cells. Percentage of luciferase activity and standard deviation relative to vector control of 3 experiments is shown.
Parasite death leads to Csk recruitment to GEMs, which accounts for down-regulation of AP-1 transactivation.
(A) PAG was immunoprecipitated from lysates of untreated TpM409 (TpM), of cured cells (cured), or of resting B cells (Bc) and probed by Western blotting with anti-PAG (top) and anti-Csk (bottom) antibodies. (B) TpM409 cells were cotransfected with wild-type (wt) Csk, or a kinase-dead mutant of Csk (R222), together with the 3xTRE-luciferase AP1 reporter construct. Effects of transient expression of wt Csk and CskR222 on AP-1 activation were assayed by monitoring luciferase activity in lysates of transfected cells. Percentage of luciferase activity and standard deviation relative to vector control of 3 experiments is shown.
Discussion
T parva orchestrates specific alterations in upstream signaling modules that lead to permanent activation of the transcription factor AP-1 in infected B cells,6,8,39 and we previously found that proliferation of these cells depends on parasite-induced PI3-K activity.39 In addition to directly demonstrating regulation of AP-1 by constitutively activated Hck inT parva–infected cells, the data presented herein reveal that Src family kinase activation is required for proliferation of these cells as well. Constitutive activation of Hck is parasite dependent, and elimination of the parasite leads to a drop in Hck activity to a level comparable to resting B cells (Figure 2). This minimal Hck kinase activity in both cured and resting cells is closely linked to the level of PAG/Cbp expression and phosphorylation and to the association of Csk with PAG/Cbp (Figure 5). Analogous to transient exclusion of Csk from GEMs in activated T cells,32,53,54Csk associates with tyrosine-phosphorylated PAG/Cbp and accumulates in GEMs only in the absence of the transforming parasite, whereas Hck targeting in these particular membrane compartments appears to be independent of the activation status of the cell (Figures 4-5). Elimination of T parva, which results in growth arrest and decreased AP-1 activity in B cells,39 leads to increased expression of properly phosphorylated PAG/Cbp mediating clear redistribution of Csk into GEMs (Figures 4-5). Hck activation and PAG/Cbp expression can be mimicked by PMA/ionomycin stimulation of resting B cells, suggesting that PAG/Cbp is a critical mediator of B cell activation (Figure 5). Moreover, these data argue that inTheileria-transformed B cells constitutive Hck activation is due to low expression of PAG/Cbp, causing relative exclusion of Csk from GEMs leading further to decreased phosphorylation of negative regulatory C-terminal tyrosine of Hck and thus increased activity of this PTK. Increased phosphorylation of negative regulatory C-terminal tyrosine of Hck by Csk recruited to GEMs is likely to cause inhibition of Hck, which may be crucial in reversion of the transformed phenotype on drug-mediated parasite death. This notion is further underscored by the observation that in infected cells interruption of the spatial separation of Hck and its regulator Csk, by Csk overexpression, results in decreased AP-1 activation. We thus propose that in T parva–transformed B cells, Hck is constitutively and functionally active predominately in GEMs and most probably as a result of permanent exclusion of Csk from this membrane compartment. It cannot be ruled out, however, that additional regulatory mechanisms contribute to the final outcome of Hck kinase activity in infected cells. The relatively low level of endogenous Hck tyrosine phosphorylation (Figure 2) suggests that, in addition to Csk, a phosphotyrosyl-specific phosphatase is permanently involved in Hck regulation.
A fraction of PI3-K, which is required in synergy with Hck to induce full activation of AP-1 (Figure 3), colocalizes with Hck in GEMs (Figure 4). Concomitant accumulation of activated Hck and PI3-K in GEMs may thus result in permanent activation of AP-1 described herein and in previous studies.8,39 Because activation of PI3-K and Src family kinases and induction of AP-1 appears to be a common feature ofTheileria-infected leukocytes,8,9,11,36,39 it is tempting to speculate that regulation of these kinases in GEMs is a key mechanism of host cell transformation induced byTheileria parasites. Importantly, activities of both these kinases may be jointly regulated by means of the PAG/Cbp-Csk system, as PI3-K is activated by Src PTKs.51 Thus, a crucial step inTheileria-mediated lymphocyte transformation may be down-modulation of PAG/Cbp expression, which results in relaxation of Csk-mediated Src family PTK inhibition and subsequently also Src-family PTK (Hck in B cells)–mediated PI3-K activation. Activated Hck and PI3-K then apparently jointly substantially contribute to the transformed phenotype of the infected cells. Another potential contribution to the transformed phenotype related to modified PAG/Cbp expression could be mediated by the recently described interaction of the C-terminus of PAG/Cbp with a cytoskeleton linker EBP50.54 55
Taken together, our data exemplify that the same molecular mechanisms regulating the fate of B lymphocytes in health may become a target of pathologic alterations caused by an intracellular parasite, and they illustrate how B-lymphocyte activation may be subjected to fine-tuned regulation that is not a direct result of extracellular stimuli. Future work will be directed toward understanding how Theileriaparasites regulate expression of PAG/Cbp, as one can speculate that down-modulation of PAG/Cbp expression (or altered phosphorylation) might be involved in other types of B-lymphocyte transformation.
The authors are grateful to Glen Scholz for kindly providing Hck expression vectors and helpful advice. We thank Marie-Françoise Moreau and Brigitte Blumen for technical support and Marie Chaussepied and Alphonse Garcia for stimulating discussions.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/ blood-2002-02-0456.
Supported by fellowships of the Swiss National Science Foundation and the Roche Research Foundation (M.B.) and by the Center of Molecular and Cellular Immunology LN00A026 (Ministry of Education of the Czech Republic; V.H.) and The Wellcome Trust (J1116W24Z; V.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gordon Langsley, Laboratoire de Signalisation Immunoparasitaire, Departement d'Immunologie, Institut Pasteur, 25-28 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail:langsley@pasteur.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal