Host response to injury and infection is accompanied by a rapid rise in the blood of acute-phase proteins such as serum amyloid A (SAA). Although SAA has been used as a marker for inflammatory diseases, its role in the modulation of inflammation and immunity has not been defined. Human neutrophils respond to SAA with secretion of the proinflammatory cytokines interleukin 8 (IL-8) and, to a lesser extent, tumor necrosis factor α (TNF-α). The induction of IL-8 secretion by SAA involves both transcription and translation and correlates with activation of nuclear factor κB (NF-κB). The proximal signaling events induced by SAA include mobilization of intracellular Ca2+ and activation of the mitogen-activated protein kinases ERK1/2 and p38, both required for the induced IL-8 secretion. Pertussis toxin effectively blocks SAA-induced IL-8 secretion indicating involvement of a Gi-coupled receptor. Overexpression of FPRL1/LXA4R in HeLa cells results in a significant increase of the expression of NF-κB and IL-8 luciferase reporters by SAA, and an antibody against the N-terminal domain of FPRL1/LXA4R inhibits IL-8 secretion. Lipoxin A4, which binds to FPRL1/LXA4R specifically, decreases SAA-induced IL-8 secretion significantly. Collectively, these results indicate that the cytokine-like property of SAA is manifested through activation of the Gi-coupled FPRL1/LXA4R, which has been known to mediate the anti-inflammatory effects of lipoxin A4. The ability of FPRL1/LXA4R to mediate 2 drastically different and opposite functions suggests that it plays a role in the modulation of inflammatory and immune responses.
Introduction
Serum amyloid A (SAA) is a major acute-phase protein released to circulation in response to infection and injury. Within the first 24 to 36 hours after infection or injury, the blood concentration of SAA can increase by as much as 1000-fold over basal level, reaching a concentration of 80 μM or 1 mg/mL.1,2The liver is a major source of acute-phase SAA, but extrahepatic expression of SAA has also been documented and is known to involve cells of atherosclerotic lesions, that is, smooth muscle cells, endothelial cells, and monocytes/macrophages.3,4Inflammatory cytokines such as interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and IL-6 are potent inducers of SAA expression by hepatocytes and, to various degrees, by macrophages and synoviocytes.2,5-7 In circulation, SAA is associated with high-density lipoproteins (HDLs) at lower concentrations, but it dissociates from HDLs at higher concentrations.8,9 Free SAA is also found in the inflammatory sites,3 4 suggesting a role of SAA in local inflammation.
The marked increase of SAA has been used as an important indicator for diagnosis and prognosis of inflammatory diseases.7,10 In addition, SAA is implicated as both a beneficial and harmful factor in the inflammatory process. Potential beneficial roles include reverse transport of cholesterol at sites of inflammation, through its ability to displace cholesterol from HDL.11,12 With respect to being a harmful factor, SAA is the precursor of amyloid A, the deposit of which causes amyloidosis.7,13 The findings that SAA is produced locally in atherosclerotic lesions and in arthritic joints suggest a potential role of this acute-phase protein in chronic inflammatory diseases such as atherosclerotic and rheumatoid arthritis.13-15
Despite these important findings, a precise function of SAA in acute inflammation has not been defined. It is notable that a number of studies suggest a link between SAA and leukocyte infiltration. SAA is chemotactic to leukocytes including monocytes, mast cells, and T lymphocytes at concentrations attained in the blood during an acute-phase response.16-18 These early observations have led to the recent identification of a cell surface receptor that mediates SAA-stimulated chemotaxis in monocytes.19 There is also accumulating evidence suggesting that SAA possesses cytokinelike activities and is able to induce the production of matrix metalloproteinases (MMPs),20 cytokines, and cytokine receptors including IL-1β, interleukin-1 receptor antagonist (IL-1ra), and soluble TNF-α type II receptor (sTNFr-II).21 Neutrophils that are stimulated by SAA for 24 hours release TNF-α, IL-1β, and IL-8 into culture medium.22 However, it is not clear whether this is a primary response to SAA or a secondary response to other secreted cytokines because of the long incubation time. The receptor that mediates this function of SAA has not been identified.
In this study, we investigated whether SAA induces primary cytokine responses in neutrophils, what proximal signaling events are associated with these responses, and which receptor is responsible for the cytokinelike activity of SAA. Our results indicate that SAA stimulates a rapid and potent secretion of IL-8 from neutrophils. SAA-induced IL-8 secretion correlates with nuclear factor κB (NF-κB) activation and IL-8 gene expression and requires both calcium mobilization and activation of the mitogen-activated protein (MAP) kinases ERK1/2 and p38. Finally, we demonstrate that formyl peptide receptor-like 1/lipoxin A4 receptor (FPRL1/LXA4R), which has been previously shown to mediate the anti-inflammatory functions of lipoxin A4, is a receptor for SAA-induced IL-8 secretion.
Materials and methods
Reagents
Recombinant human SAA was purchased from Peprotech (Rocky Hill, NJ). The endotoxin level is less than 0.1 ng/μg protein. Lipoxin A4 (LXA4), the N-formyl peptide fMLF, and cycloheximide (CHX) were obtained from Sigma (St Louis, MO). The surrogate agonist for FPRL1/LXA4R, MMK-1, (LESIFRSLLFRVM; > 90% purity) was synthesized and purified by Macromolecular Resources (Fort Collins, CO). Indo-1/am and BAPTA/am were obtained from Molecular Probes (Eugene, OR). U0126, SB202190, actinomycin D (ActD), and lipopolysaccharide (LPS) fromEscherichia coli 0111:B4 or Salmonella minnesotaRe595 were from Calbiochem (San Diego, CA). Pertussis toxin (PTX) was purchased from List Laboratories (Campbell, CA). The anti-ERK1/2 and antiphospho-ERK1/2 polyclonal antibodies were obtained from Cell Signaling Technologies (Beverly, MA), and anti-IκBα antibody was from Torrey Pines Biolabs (Houston, TX). The polyclonal antibody directly against p38 was from Santa Cruz Biotechnology (Santa Cruz, CA) and antiphospho-p38 polyclonal antibody was obtained from Biosource (Camarillo, CA). The human FPRL1/LXA4R expression construct was prepared by cloning of its cDNA23 into the pRK5 expression vector (BD Pharmingen, San Diego, CA) or into the SFFV.neo expression vector that contains a neomycin-resistance cassette.24
Cell culture
HeLa cells were maintained at 37°C with 5% CO2 in Dulbecco modified Eagle medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS). HL-60 and HEK293 cells were maintained in RPMI 1640 medium containing 10% FBS. All the cell culture mediums contain 2 mM l-glutamine, 100 IU/mL penicillin, and 50 μg/mL streptomycin. The human FPR- and FPRL1/LXA4R-transfected RBL-2H3 cells were generated as described previously.25Stable transfectants were selected by G418 (Invitrogen, Carlsbad, CA).
Preparation of human neutrophils
Peripheral blood was drawn from healthy donors using a protocol approved by the institutional review board at the University of Illinois at Chicago. Neutrophils were prepared using Percoll gradient (Amersham Pharmacia, Piscataway, NJ), based on the method of Ulmer and Flad.26 In brief, erythrocytes were sedimented by adding 0.5 volume of 6% hetastarch (Abbott Laboratories, North Chicago, IL) at room temperature for 45 minutes. The erythrocyte-depleted supernatants were then layered on 55% isotonic Percoll containing a 74% cushion and centrifuged at 450g at 12°C for 60 minutes. Neutrophils were collected from the cushion interface, diluted 4-fold in ice-cold phosphate-buffered saline (PBS) and washed twice by centrifugation at 500g. Contaminated erythrocytes were lysed after a brief (< 30 seconds) treatment with H2O. Neutrophils were then resuspended in serum-free RPMI 1640 medium at a density of 2 × 106 cells/mL and maintained at 37°C. The cells prepared using this procedure contain approximately 97% neutrophils with viability higher than 98%.
Measurement of cytokine secretion
Neutrophils (4-5 × 105 cells/0.2 mL) were placed in serum-free medium in 96-well plates and kept in a CO2atmosphere (5%) at 37°C with or without stimulants. After stimulation, cell-free supernatants were collected by centrifugation at 400g for 5 minutes and assayed for TNF-α, IL-1β, IL-6, and IL-8 with enzyme-linked immunosorbent assay (ELISA) kits (Biosource) according to the instructions of the vender. The pellets were suspended in 0.2 mL RPMI with 0.1% Tween 20 and were lysed by 3 freeze-thaw cycles. The cell lysates were assayed for cytokines using ELISA.27
Measurement of IL-8 transcripts
Neutrophils (∼1 × 106 cells) were stimulated with 1 μM SAA in a total volume of 0.2 mL for the indicated times. Total RNA was extracted using TriZol reagent (Invitrogen) followed by DNase treatment (RNase-free DNase; Invitrogen). cDNA was prepared with Superscript reverse transcriptase (Invitrogen). Amplification of IL-8 transcripts was accomplished with primers from Ambion (Austin, TX), generating a 279-bp fragment. The housekeeping gene fragment of glyceraldehyde-3-phosphate dehydrogenase (G3PDH; 452 bp) was used for verification of equal loading and of reverse transcription-polymerase chain reaction (RT-PCR) efficiency.
Electrophoretic mobility shift assay
Nuclear extracts were isolated using the method of Dignam et al.28 Briefly, 1 × 106 neutrophils were homogenized in NEBA buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.8, 10 mM KCl, 0.1 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM EGTA [ethyleneglycoltetraacetic acid], 1 mM dithiothreitol) containing 2 μM diisopropyl fluorophosphate, protease inhibitors (1 × Protease Inhibitor Mixture Set I from Calbiochem) and 1 mM phenylmethylsulfonyl fluoride. Total cytosolic proteins were lysed in NEBA buffer with 0.6% NP-40 and removed after centrifugation at 11 000g for 10 seconds. Following homogenization of nuclei in NEBB buffer (20 mM HEPES, pH 7.8, 0.4 M NaCl, 1 mM EDTA, and 1 mM EGTA), solubilized nuclear extracts were obtained after removing nuclear debris by centrifugation at 11 000g for 30 seconds. Radiolabeling of the NF-κB probe, binding reactions, electrophoresis, and autoradiography were conducted as described previously.29
Calcium mobilization assay
Calcium mobilization was detected with Indo-1/am–labeled neutrophils, according to a previously described procedure.25 Intracellular Ca2+level was expressed as relative fluorescence, calculated based on the ratio of Indo-1 fluorescence (405:485 nm).
Preparation of antiserum and flow cytometry analysis
Rabbit antiserum was prepared against a synthetic peptide, corresponding to amino acids 2-21 of FPRL1/LXA4R (ETNFSTPLNEYEEVSYESAG) plus a cysteine at the C-terminus. Flow cytometry analyses were performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Cells were first incubated with preimmune serum or antiserum for FPRL1/LXAR4, washed in PBS containing 0.2% bovine serum albumin (BSA), and then incubated with fluorescein isothiocyanate (FITC)–conjugated goat antirabbit IgG (1:200). All incubations were carried out on ice for 60 minutes. The cells were then washed 3 times in PBS containing 0.2% BSA, between incubations and prior to flow cytometry analysis.
Transient transfection and luciferase assay
Transient transfection of HeLa and HEK293 cells with pRK5/FPRL1 was achieved by using LipofectAMINE Plus reagent (Invitrogen) according to the manufacturer's instruction. For luciferase reporter assay, HeLa cells were transiently transfected in 6-well cell culture plates with 0.2 μg of a 3 × NF-κB luciferase reporter30 or a IL-8 luciferase reporter (−272 IL-8 luc; a gift from Dr N. Mukaida, Knazawa University, Japan), together with 0.02 μg plasmid coding for β-galactosidase (pCMVβ) and 0.2 μg FPRL1/LXA4R expression vector. When necessary, additional DNA (pRK5 vector) was added to make total DNA equal in each well. HL-60 cells were transiently transfected by electroporation as described previously.31 Each transfection contained 15 μg NF-κB luciferase reporter or IL-8 luciferase reporter and 1.5 μg pCMVβ. Vector DNA (pRK5) was added to bring total DNA to 50 μg. Twenty-four hours after transfection, cells were starved in serum-free medium for 16 hours (for HeLa) and then stimulated with control solvent or 10 μM SAA for 4 to 5 hours. The NF-κB– or IL-8–directed expression of firefly luciferase was determined using luciferase assay reagents from Promega (Madison, WI) and a Femtomaster FB12 luminometer (Berthold Detection Systems, Pforzheim, Germany). The β-galactosidase activity in each sample was also determined and used for normalization of transfection/protein expression efficiency that may differ among samples.
Results
SAA induces secretion of IL-8 by human neutrophils
Neutrophils were stimulated with SAA, and the concentrations of selected proinflammatory cytokines in the culture medium and in the cell lysates were measured (Figure 1A). To minimize the autocrine and paracrine stimulation that may have contributed to the elevated cytokine levels in a previous study,22 the duration of stimulation was limited to 4 hours. SAA induced a potent production of IL-8 that reached a level of 1500 pg/mL/106 cells. SAA also stimulated TNF-α production to a lesser extent (250 pg/mL/106 cells), but induced only minimal secretion of IL-6 and IL-1β. Although there was spontaneous production of IL-8 and TNF-α, SAA stimulation induced a 2- to 5-fold increase of IL-8 and a 2- to 3-fold increase of TNF-α in neutrophils from 12 different blood donors (data not shown).
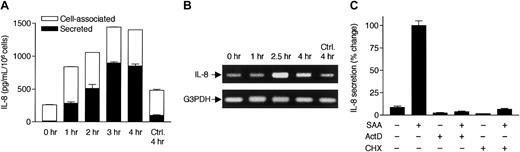
SAA induces rapid secretion of IL-8 from neutrophils.
(A) Freshly prepared neutrophils were stimulated with 2 μM SAA at 37°C for 4 hours in serum-free medium. The secreted (from supernatant; closed bars) and cell-associated (from cell lysate; open bars) cytokines were detected by ELISA. The percentage of cell-associated cytokines in SAA-stimulated samples are 22% (IL-1β), 20% (IL-6), 3% (TNF-α), and 36% (IL-8). In unstimulated neutrophils, 77% of IL-8 is present in cell-associated form. (B) Neutrophils were similarly stimulated with LPS from E coli0111:B4 (LPS1) and S minnesota Re595 (LPS2), SAA, and TNF-α for 4 hours, at the indicated concentrations. Cell-associated IL-8 (open bars) and secreted IL-8 (closed bars) were determined by ELISA. All data are presented as mean ± SEM of 3 independent experiments, each performed in duplicate.
SAA induces rapid secretion of IL-8 from neutrophils.
(A) Freshly prepared neutrophils were stimulated with 2 μM SAA at 37°C for 4 hours in serum-free medium. The secreted (from supernatant; closed bars) and cell-associated (from cell lysate; open bars) cytokines were detected by ELISA. The percentage of cell-associated cytokines in SAA-stimulated samples are 22% (IL-1β), 20% (IL-6), 3% (TNF-α), and 36% (IL-8). In unstimulated neutrophils, 77% of IL-8 is present in cell-associated form. (B) Neutrophils were similarly stimulated with LPS from E coli0111:B4 (LPS1) and S minnesota Re595 (LPS2), SAA, and TNF-α for 4 hours, at the indicated concentrations. Cell-associated IL-8 (open bars) and secreted IL-8 (closed bars) were determined by ELISA. All data are presented as mean ± SEM of 3 independent experiments, each performed in duplicate.
The potential effects of contaminating LPS in the SAA preparation and of the secreted TNF-α on IL-8 production were examined. Stimulation of neutrophils with LPS from 2 different sources, at the concentrations equivalent to those contained in 4 μM SAA (5 ng/mL LPS), did not induce significant production of IL-8 (Figure 1B). Similarly, no IL-8 production was induced by TNF-α at a concentration equivalent to that of TNF-α secreted by neutrophils in 4 hours (250 pg/mL; data not shown), although TNF-α was able to stimulate a substantial increase in IL-8 production at 200-fold or higher concentrations (Figure 1B). Therefore, the observed increase of IL-8 production results primarily from SAA stimulation.
At resting state, cell-associated IL-8 constitutes 75% to 90% of the total IL-8 detected by ELISA (Figures 1B and2A). Stimulation with SAA or other tested agonists leads to increases of IL-8 secretion through a time course of 4 hours, whereas the cell-associated IL-8 remains relatively constant. Thus, measurement of the secreted IL-8 accurately reflects the induction by SAA and was used in subsequent studies. The SAA-induced secretion of IL-8 became obvious after 1 hour and reached a peak between 3 and 4 hours (Figure 2A), suggesting that IL-8 is a primary (as opposed to secondary) cytokine induced by SAA.
SAA-induced IL-8 secretion requires transcription and de novo protein synthesis.
(A) Time course of SAA-induced IL-8 production. Neutrophils were stimulated with 2 μM SAA, and the secreted and cell-associated IL-8 concentrations were determined by ELISA after 1, 2, 3, and 4 hours. All data are presented as mean ± SEM of 3 independent experiments, each performed in duplicate. (B) Neutrophils, stimulated with 2 μM SAA for 1, 2.5, and 4 hours or incubated in the absence of SAA for 4 hours, were harvested for RNA preparation. RT-PCR was performed with specific primers for human IL-8 and G3PDH. The PCR products were resolved on a 1% agarose gel by electrophoresis and stained with ethidium bromide. Data shown are from one representative experiment of a total of 3, all with similar results. (C) Neutrophils were preincubated for 1 hour with or without actinomycin D (ActD; 10 μg/mL) or cycloheximide (CHX; 100 μM). The cells were then stimulated with 1 μM SAA for 4 hours. Secreted IL-8 was measured by ELISA as described. Maximal production of IL-8 (∼800 pg/106 cells/mL) was observed in the absence of either inhibitor and was set as 100%, against which the effects of ActD and CHX were measured. Data shown are means ± SEM derived from 3 independent experiments, each performed in duplicate.
SAA-induced IL-8 secretion requires transcription and de novo protein synthesis.
(A) Time course of SAA-induced IL-8 production. Neutrophils were stimulated with 2 μM SAA, and the secreted and cell-associated IL-8 concentrations were determined by ELISA after 1, 2, 3, and 4 hours. All data are presented as mean ± SEM of 3 independent experiments, each performed in duplicate. (B) Neutrophils, stimulated with 2 μM SAA for 1, 2.5, and 4 hours or incubated in the absence of SAA for 4 hours, were harvested for RNA preparation. RT-PCR was performed with specific primers for human IL-8 and G3PDH. The PCR products were resolved on a 1% agarose gel by electrophoresis and stained with ethidium bromide. Data shown are from one representative experiment of a total of 3, all with similar results. (C) Neutrophils were preincubated for 1 hour with or without actinomycin D (ActD; 10 μg/mL) or cycloheximide (CHX; 100 μM). The cells were then stimulated with 1 μM SAA for 4 hours. Secreted IL-8 was measured by ELISA as described. Maximal production of IL-8 (∼800 pg/106 cells/mL) was observed in the absence of either inhibitor and was set as 100%, against which the effects of ActD and CHX were measured. Data shown are means ± SEM derived from 3 independent experiments, each performed in duplicate.
SAA-induced IL-8 secretion requires transcriptional activation and de novo protein synthesis
To investigate the mechanism for SAA-induced IL-8 production, we treated neutrophils with the transcription inhibitor ActD and the protein synthesis inhibitory CHX. The treated cells were then incubated in the absence or presence of SAA for 4 hours. Both ActD and CHX markedly reduced the basal and induced IL-8 secretion in the unstimulated and stimulated cells, respectively (Figure 2C). The same treatments also reduced cell-associated IL-8 to a similar extent (data not shown). These results suggest that transcription and de novo protein synthesis are required for SAA-induced IL-8 secretion. We next determined the level of IL-8 mRNA in SAA-stimulated neutrophils. Reverse transcription of total RNA was followed by PCR with specific primers for human IL-8 and control primers for the housekeeping gene coding for G3PDH. Elevation of the IL-8 mRNA level was observed after 1 hour of SAA stimulation. The induced IL-8 mRNA reached maximum (300% of basal level) after 2.5 hours and slightly declined by 4 hours (Figure 2B). In comparison, little increase of IL-8 mRNA was detected in cells incubated with medium over 4 hours. The time course of IL-8 mRNA accumulation is consistent with IL-8 production that peaked at 3 to 4 hours following SAA stimulation (Figure 2A).
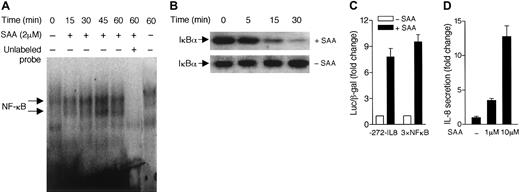
Because expression of the gene for IL-8 requires activation of NF-κB,32 we examined whether SAA can activate this transcription factor in neutrophils. Using electrophoresis mobility shift assay, we detected formation of NF-κB/DNA complexes in SAA-stimulated neutrophils (Figure 3A). The NF-κB DNA-binding activity peaked at approximately 45 minutes after SAA stimulation. Because NF-κB activation is often accompanied by IκBα degradation, we examined the cytosolic IκBα level in SAA-stimulated neutrophils. Using Western blotting with an anti-IκBα antibody, we found that neutrophil IκBα started to degrade 15 minutes after SAA stimulation (Figure 3B). In unstimulated cells, the IκBα level remained stable over the same period of time. We next examined whether the increased NF-κB–binding activity correlates with induction of IL-8 gene expression. The human HL-60 promyelocytic cell line has been shown to express FPRL1/LXA4R.23 SAA stimulation of transiently transfected HL-60 cells, expressing either the −272–IL-8 luciferase reporter33 or a 3 × NF-κB luciferase reporter,30 resulted in 8-fold and 10-fold induction of the luciferase activities, respectively (Figure 3C). Accordingly, SAA increased secretion of IL-8 from the stimulated HL-60 cells (Figure3D). These results demonstrate that SAA is able to stimulate NF-κB activation, which likely contributes to induction of IL-8 production.
SAA stimulates NF-κB activation.
(A) Neutrophils were stimulated with 2 μM SAA for different periods of time. Nuclear proteins were extracted and electrophoretic mobility shift assay was performed as described in “Materials and methods.” The NF-κB/DNA complex was detected by32P-labeled NF-κB oligonucleotide probe. Unlabeled NF-κB probe (100 × in excess) was used as a competitor to determine the specificity of DNA binding (lane 6). (B) Western blotting analysis was used to detect the cytoplasmic level of IκBα in neutrophils. Time-dependent degradation of IκBα was observed in SAA-treated (2 μM) but not the untreated neutrophils. Data shown are from one representative experiment of a total of 3, all with similar results. (C) HL-60 cells were transiently transfected with either a IL-8 luciferase reporter (−272–IL-8) or an NF-κB luciferase reporter (3 × NF-κB) as described in “Materials and methods.” The luciferase assays were performed after stimulation with 10 μM SAA for 4 hours at 37°C. Relative luciferase activities are expressed as fold induction over basal, after normalization of data against coexpressed β-galactosidase activities. (D) HL-60 cells (5 × 105 cells/200 μL) were cultured in serum-free medium with or without SAA for 4 hours. The secreted IL-8 was detected by ELISA. The fold increases of IL-8 production by the stimulated cells over unstimulated cells are shown. All data are presented as mean ± SEM, from 3 independent experiments, each performed in duplicate.
SAA stimulates NF-κB activation.
(A) Neutrophils were stimulated with 2 μM SAA for different periods of time. Nuclear proteins were extracted and electrophoretic mobility shift assay was performed as described in “Materials and methods.” The NF-κB/DNA complex was detected by32P-labeled NF-κB oligonucleotide probe. Unlabeled NF-κB probe (100 × in excess) was used as a competitor to determine the specificity of DNA binding (lane 6). (B) Western blotting analysis was used to detect the cytoplasmic level of IκBα in neutrophils. Time-dependent degradation of IκBα was observed in SAA-treated (2 μM) but not the untreated neutrophils. Data shown are from one representative experiment of a total of 3, all with similar results. (C) HL-60 cells were transiently transfected with either a IL-8 luciferase reporter (−272–IL-8) or an NF-κB luciferase reporter (3 × NF-κB) as described in “Materials and methods.” The luciferase assays were performed after stimulation with 10 μM SAA for 4 hours at 37°C. Relative luciferase activities are expressed as fold induction over basal, after normalization of data against coexpressed β-galactosidase activities. (D) HL-60 cells (5 × 105 cells/200 μL) were cultured in serum-free medium with or without SAA for 4 hours. The secreted IL-8 was detected by ELISA. The fold increases of IL-8 production by the stimulated cells over unstimulated cells are shown. All data are presented as mean ± SEM, from 3 independent experiments, each performed in duplicate.
The MAP kinases ERK1/2 and p38 play a role in SAA-induced IL-8 expression
Activation of the MAP kinases ERK1/2 and p38 results from stimulation of neutrophils with chemoattractants.34 35These kinases play important roles in transcriptional regulation. We therefore tested whether SAA could induce these MAP kinases and whether their activation contributes to the induced IL-8 expression. Neutrophils responded to 2 μM SAA with phosphorylation of ERK1/2 that was detectable after 2 minutes and peaked at 5 to 10 minutes (Figure4A, upper panel). SAA also stimulated phosphorylation of p38 with similar kinetics (Figure 4A, lower panel). Treatment of neutrophils with pharmacologic inhibitors for the ERK activation pathway (U0126) and for p38 (SB202190) effectively blocked the SAA-induced IL-8 secretion (Figure 4B). These data suggest that ERK1/2 and p38 play a role in SAA-induced production of IL-8.
Phosphorylation of ERK1/2 and p38 and their potential involvement in SAA-induced IL-8 secretion.
(A) Neutrophils (4 × 105 cells) were stimulated with 2 μM SAA for indicated times. Phosphorylation of ERK1/2 and p38 was determined by Western blotting, using specific antibodies against phospho-ERK1/2 and phospho-p38. The unphosphorylated ERK1/2 and p38 were also determined. Three experiments were performed and a representative set of data is shown. (B) Neutrophils were treated with the MEK inhibitor U0126 or the p38 inhibitor SB202190 for 1 hour prior to SAA (2 μM) stimulation. The secreted IL-8 was measured after 4 hours and expressed as percentage changes. Maximal secretion of IL-8 (1090 pg/mL/106 cells) was obtained in the absence of inhibitor and was set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate.
Phosphorylation of ERK1/2 and p38 and their potential involvement in SAA-induced IL-8 secretion.
(A) Neutrophils (4 × 105 cells) were stimulated with 2 μM SAA for indicated times. Phosphorylation of ERK1/2 and p38 was determined by Western blotting, using specific antibodies against phospho-ERK1/2 and phospho-p38. The unphosphorylated ERK1/2 and p38 were also determined. Three experiments were performed and a representative set of data is shown. (B) Neutrophils were treated with the MEK inhibitor U0126 or the p38 inhibitor SB202190 for 1 hour prior to SAA (2 μM) stimulation. The secreted IL-8 was measured after 4 hours and expressed as percentage changes. Maximal secretion of IL-8 (1090 pg/mL/106 cells) was obtained in the absence of inhibitor and was set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate.
Requirement of Ca2+ mobilization for the induced IL-8 secretion
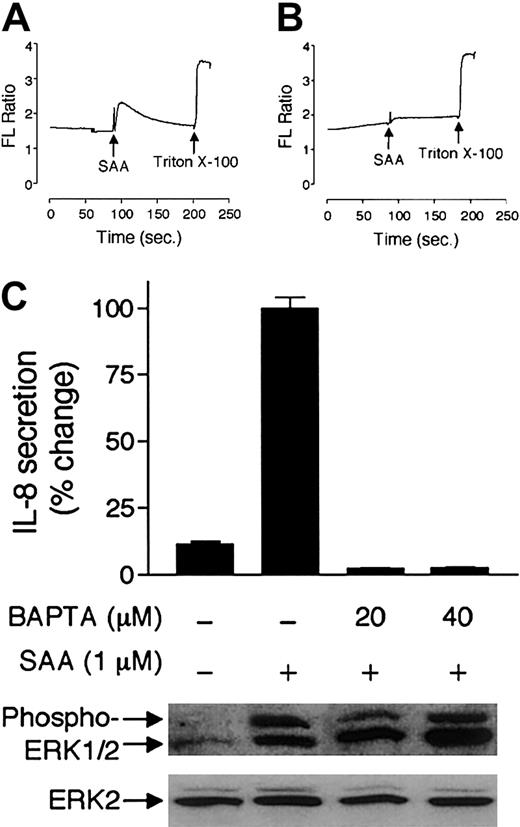
To characterize the proximal signaling events associated with SAA-induced IL-8 expression, neutrophils were loaded with the Ca2+-sensitive fluorescence probe Indo-1/amprior to SAA stimulation. A rapid increase of intracellular Ca2+ was observed in the stimulated neutrophils (Figure5A). Pretreatment of neutrophils with BAPTA/am, which buffers the rise of intracellular Ca2+, effectively blocked SAA-induced Ca2+mobilization (Figure 5B). BAPTA/am treatment also blocked SAA-induced IL-8 secretion by neutrophils (Figure 5C). It has been previously reported that calcium mobilization is required for the induction of IL-8 secretion by other stimuli.36 37Therefore, the inhibitory effect of BAPTA/am may not be specific for SAA-induced IL-8 expression. In reporter-based assays, BAPTA/am also completely blocked SAA-induced NF-κB activation and inhibited TNF-α–induced NF-κB activation by 40% (data not shown). However, it did not affect SAA-stimulated ERK1/2 phosphorylation (Figure 5C), suggesting that these signaling events are regulated differently.
Elevation of intracellular Ca2+ is required for IL-8 secretion.
(A-B) Indo-1/am–labeled neutrophils were stimulated with SAA (1 μM) in the absence (A) or presence (B) of BAPTA/am (20 μM). Triton X-100 was then added to 0.1% for measurement of total intracellular Ca2+, an indicator of Indo-1/am equal loading. Relative intracellular Ca2+ concentration was expressed as fluorescence ratio (405:485 nm). (C) Secretion of IL-8 and phosphorylation of ERK1/2 by SAA-stimulated neutrophils were determined in the absence or presence of BAPTA/am (20 μM and 40 μM). Maximal secretion (580 pg/mL/106 cells) was set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate.
Elevation of intracellular Ca2+ is required for IL-8 secretion.
(A-B) Indo-1/am–labeled neutrophils were stimulated with SAA (1 μM) in the absence (A) or presence (B) of BAPTA/am (20 μM). Triton X-100 was then added to 0.1% for measurement of total intracellular Ca2+, an indicator of Indo-1/am equal loading. Relative intracellular Ca2+ concentration was expressed as fluorescence ratio (405:485 nm). (C) Secretion of IL-8 and phosphorylation of ERK1/2 by SAA-stimulated neutrophils were determined in the absence or presence of BAPTA/am (20 μM and 40 μM). Maximal secretion (580 pg/mL/106 cells) was set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate.
FPRL1/LXA4R mediates SAA-induced IL-8 secretion
To identify a functional receptor that mediates the cytokine-like activity of SAA, we first tested whether PTX has an effect on the induction of IL-8 by SAA. PTX mediates adenosine diphosphate (ADP) ribosylation of the Gi class of G proteins that are known for coupling most chemoattractant receptors. Pretreatment of neutrophils by PTX led to a marked decrease of IL-8 secretion (Figure 6A). In addition, PTX treatment inhibited SAA-stimulated phosphorylation of ERK1/2 and p38 (Figure 6B), suggesting that SAA uses a receptor that functionally couples to a Gi protein.38
Inhibition of SAA-induced IL-8 secretion and MAP kinase phosphorylation by PTX.
(A) Neutrophils were preincubated with or without either 100 ng/mL or 500 ng/mL pertussis toxin (PTX) at 37°C for 1 hour and then stimulated with 2 μM SAA for 3 hours. The secreted IL-8 was measured by ELISA and expressed as percent of change, with maximal IL-8 (980 pg/mL/106 cells) set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate. (B) Neutrophils (4 × 105 cells) were incubated with or without PTX (400 ng/mL) for 30 minutes, and then stimulated with 2 μM SAA for indicated times. Induction of both ERK1/2 and p38 phosphorylation was determined by Western blotting. Three experiments were performed and a representative set of results is shown.
Inhibition of SAA-induced IL-8 secretion and MAP kinase phosphorylation by PTX.
(A) Neutrophils were preincubated with or without either 100 ng/mL or 500 ng/mL pertussis toxin (PTX) at 37°C for 1 hour and then stimulated with 2 μM SAA for 3 hours. The secreted IL-8 was measured by ELISA and expressed as percent of change, with maximal IL-8 (980 pg/mL/106 cells) set as 100%. Data are presented as means ± SEM of 3 independent experiments, each performed in duplicate. (B) Neutrophils (4 × 105 cells) were incubated with or without PTX (400 ng/mL) for 30 minutes, and then stimulated with 2 μM SAA for indicated times. Induction of both ERK1/2 and p38 phosphorylation was determined by Western blotting. Three experiments were performed and a representative set of results is shown.
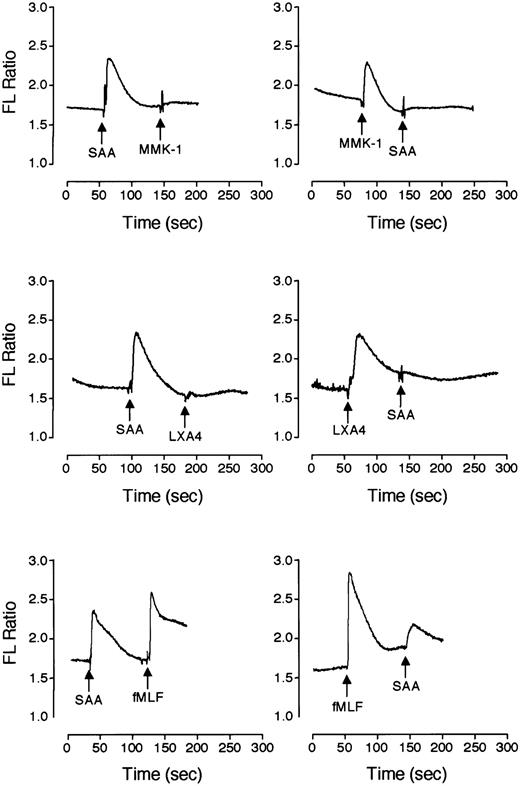
SAA induces monocyte chemotaxis through a 7-transmembrane domain receptor, FPRL1/LXA4R,19 that binds LXA4 as well as a number of peptide ligands such as MMK-1.39-41 Using a calcium mobilization assay, we determined that neutrophils express the same receptor. As shown in Figure 7, SAA-induced calcium signaling could desensitize either MMK-1– or LXA4-induced calcium mobilization, whereas these ligands also desensitized SAA-induced calcium mobilization suggesting that they share the same receptor. The calcium signal triggered by SAA partially desensitized fMLF-induced calcium mobilization and vice versa (Figure 7E-F). This most likely results from heterologous desensitization because SAA could not induce calcium mobilization through FPR (Figure8A) and fMLF binding to FPRL1/LXA4R would be minimal at a concentration of 10 nM.42 We concluded that SAA uses FPRL1/LXA4R, but not FPR, as a functional receptor. To verify this notion, FPRL1/LXA4R was transfected into the rat basophilic leukemia cell line RBL-2H3. Stable transfectants responded to SAA and MMK-1 stimulation with a rapid rise of intracellular calcium (Figure8A). LXA4, at concentrations of up to 2 μM, also induced calcium mobilization in these cells (data not shown). The untransfected cells (RBL) and FPR-transfected cells (RBL/FPR) did not respond to SAA with calcium mobilization (Figure 8A). In addition, both SAA and the FPRL1/LXA4R-specific agonist MMK-139 stimulated phosphorylation of the MAP kinases ERK1/2 in FPRL1/LXA4R-transfected RBL but not the untransfected RBL cells (Figure 8B).
SAA induces Ca2+ mobilization through PFRL1/LXA4R in neutrophils.
Neutrophils labeled with Indo-1 were stimulated with SAA (1 μM), MMK-1 (10 nM), LXA4 (1.4 μM), and fMLF (10 nM) in different sequence combinations to determine cross-desensitization. Changes of intracellular Ca2+ are expressed as relative fluorescence ratio (405:485 nm). Results shown are representative of 1 of the 2 experiments.
SAA induces Ca2+ mobilization through PFRL1/LXA4R in neutrophils.
Neutrophils labeled with Indo-1 were stimulated with SAA (1 μM), MMK-1 (10 nM), LXA4 (1.4 μM), and fMLF (10 nM) in different sequence combinations to determine cross-desensitization. Changes of intracellular Ca2+ are expressed as relative fluorescence ratio (405:485 nm). Results shown are representative of 1 of the 2 experiments.
SAA induces Ca2+ mobilization and ERK1/2 phosphorylation in FPRL1/LXA4R-transfected cells.
(A) Stable transfectants, or the untransfected RBL-2H3 cells, were loaded with Indo-1/am and stimulated with SAA (2 μM) or MMK-1 (100 nM). Elevation of intracellular Ca2+ was observed in FPRL1/LXA4R-transfected but not the untransfected RBL or FPR-transfected RBL cells. (B) SAA and MMK-1 stimulate activation of ERK1/2 in FPRL1/LXA4R-transfected cells but not in the untransfected cells, as measured by Western blotting using an antiphospho-ERK1/2 antibody (upper blot). Equal loading of samples was determined by an antibody against unphosphorylated ERK1/2 (lower blot).
SAA induces Ca2+ mobilization and ERK1/2 phosphorylation in FPRL1/LXA4R-transfected cells.
(A) Stable transfectants, or the untransfected RBL-2H3 cells, were loaded with Indo-1/am and stimulated with SAA (2 μM) or MMK-1 (100 nM). Elevation of intracellular Ca2+ was observed in FPRL1/LXA4R-transfected but not the untransfected RBL or FPR-transfected RBL cells. (B) SAA and MMK-1 stimulate activation of ERK1/2 in FPRL1/LXA4R-transfected cells but not in the untransfected cells, as measured by Western blotting using an antiphospho-ERK1/2 antibody (upper blot). Equal loading of samples was determined by an antibody against unphosphorylated ERK1/2 (lower blot).
Because no FPRL1/LXA4R antagonist is currently available, we sought to use a receptor-blocking antibody to determine whether FPRL1/LXA4R mediates SAA-induced IL-8 secretion. A rabbit antiserum was raised against an N-terminal domain of FPRL1/LXA4R, as described in “Materials and methods.” It could recognize cell surface expression of FPRL1/LXA4R in both neutrophils (Figure9A) and RBL-2H3 and HEK293 cells that were transfected to express FPRL1/LXA4R (Figure 9B). The antiserum did not recognize FPR in transfected RBL cells. Incubation of neutrophils with anti-FPRL1 resulted in an inhibition of SAA-induced calcium mobilization (Figure 9C). In addition, preincubation of neutrophils with the antiserum reduced SAA-stimulated IL-8 secretion by approximately 70% (Figure 9D). In comparison, the control (preimmune) serum failed to block SAA-induced IL-8 secretion. Both the preimmune serum and the FPRL1/LXA4R antiserum also slightly increased IL-8 release, presumably due to binding to the Fc receptors on cell surface.43
Characterization of rabbit antiserum against FPRL1/LXA4R.
(A) Histogram of flow cytometry demonstrating binding of the rabbit antiserum (1:50; solid line) as compared with preimmune serum (same dilution; shaded curve). The original data of side scattering versus FITC (from the secondary antibody) are shown in dot plots below. Mean fluorescence intensities are 14 ± 3.31 with preimmune serum and 27 ± 2.41 with anti-FPRL1. Data presented are from 1 representative experiment of a total of 3. (B) RBL-2H3 cells, the stable transfectants RBL/FPR and RBL/FPRL1, and the HEK293-transient transfectants 293/FPRL1 were incubated with preimmune serum (1:50 dilution; shaded curve) or anti-FPRL1 (same dilution; solid line), followed by FITC-conjugated secondary antibody. The histograms of flow cytometry were shown. Data are representative of 1 of the 2 experiments. (C) Neutrophils were preincubated with either preimmune serum or anti-FPRL1 (1:50 dilution) for 30 minutes at 37°C, and loaded with Indo-1/am. Elevation of intracellular Ca2+ by 1 μM SAA stimulation was blocked by anti-FPRL1. The results are representative of 1 experiment of a total of 3. (D) Inhibition of IL-8 secretion. Neutrophils were preincubated with or without either preimmune serum or anti-FPRL1 (1:50 dilution each) for 30 minutes at 37°C, and then stimulated with 1 μM SAA for 4 hours. IL-8 secretion was measured by ELISA. The secreted IL-8 (1060 pg/106/mL) by 1 μM SAA without serum pretreatment was set as 100%. Data are presented as means ± SEM of 2 independent experiments each performed in duplicate.
Characterization of rabbit antiserum against FPRL1/LXA4R.
(A) Histogram of flow cytometry demonstrating binding of the rabbit antiserum (1:50; solid line) as compared with preimmune serum (same dilution; shaded curve). The original data of side scattering versus FITC (from the secondary antibody) are shown in dot plots below. Mean fluorescence intensities are 14 ± 3.31 with preimmune serum and 27 ± 2.41 with anti-FPRL1. Data presented are from 1 representative experiment of a total of 3. (B) RBL-2H3 cells, the stable transfectants RBL/FPR and RBL/FPRL1, and the HEK293-transient transfectants 293/FPRL1 were incubated with preimmune serum (1:50 dilution; shaded curve) or anti-FPRL1 (same dilution; solid line), followed by FITC-conjugated secondary antibody. The histograms of flow cytometry were shown. Data are representative of 1 of the 2 experiments. (C) Neutrophils were preincubated with either preimmune serum or anti-FPRL1 (1:50 dilution) for 30 minutes at 37°C, and loaded with Indo-1/am. Elevation of intracellular Ca2+ by 1 μM SAA stimulation was blocked by anti-FPRL1. The results are representative of 1 experiment of a total of 3. (D) Inhibition of IL-8 secretion. Neutrophils were preincubated with or without either preimmune serum or anti-FPRL1 (1:50 dilution each) for 30 minutes at 37°C, and then stimulated with 1 μM SAA for 4 hours. IL-8 secretion was measured by ELISA. The secreted IL-8 (1060 pg/106/mL) by 1 μM SAA without serum pretreatment was set as 100%. Data are presented as means ± SEM of 2 independent experiments each performed in duplicate.
To further establish a correlation between FPRL1/LXA4R and SAA-induced IL-8 expression, HeLa cells were transiently transfected with a FPRL1/LXA4R cDNA expression construct, and either a 3 × NF-κB luciferase reporter or an IL-8 luciferase reporter (−272-IL-8). The untranfected HeLa cells do not express FPRL1/LXA4R as determined by RT-PCR (data not shown). After transient transfection, a small population of cells expressed the receptor as detected by the anti-FPRL1/LXA4R antiserum using flow cytometry (Figure10B). Stimulation of transfected HeLa cells with SAA led to induced expression of the NF-κB reporter (170% of basal) and the IL-8 reporter (240% of basal), as compared with mock-transfected cells (Figure 10A). Therefore, data from both gain-of-function and loss-of-function experiments support a role of FPRL1/LXA4R in mediating SAA-induced IL-8 expression.
Induction of IL-8 expression in FPRL1/LXA4R-transfected cells.
(A) HeLa cells were transiently transfected with vector (mock) or a FPRL1/LXA4R expression construct, together with one of the luciferase reporters as indicated. Two days after transfection, cells were stimulated with SAA and the expressed luciferase activities were determined. Data shown are mean ± SEM from 3 separate experiments, each performed in duplicate. (B) The cell surface expression of the receptor was confirmed in FPRL-transfected HeLa cells (open curve), but not in vector (pRK5)–transfected HeLa cells (shaded curve), by flow cytometry using anti-FPRL1 and FITC-conjugated secondary antibody.
Induction of IL-8 expression in FPRL1/LXA4R-transfected cells.
(A) HeLa cells were transiently transfected with vector (mock) or a FPRL1/LXA4R expression construct, together with one of the luciferase reporters as indicated. Two days after transfection, cells were stimulated with SAA and the expressed luciferase activities were determined. Data shown are mean ± SEM from 3 separate experiments, each performed in duplicate. (B) The cell surface expression of the receptor was confirmed in FPRL-transfected HeLa cells (open curve), but not in vector (pRK5)–transfected HeLa cells (shaded curve), by flow cytometry using anti-FPRL1 and FITC-conjugated secondary antibody.
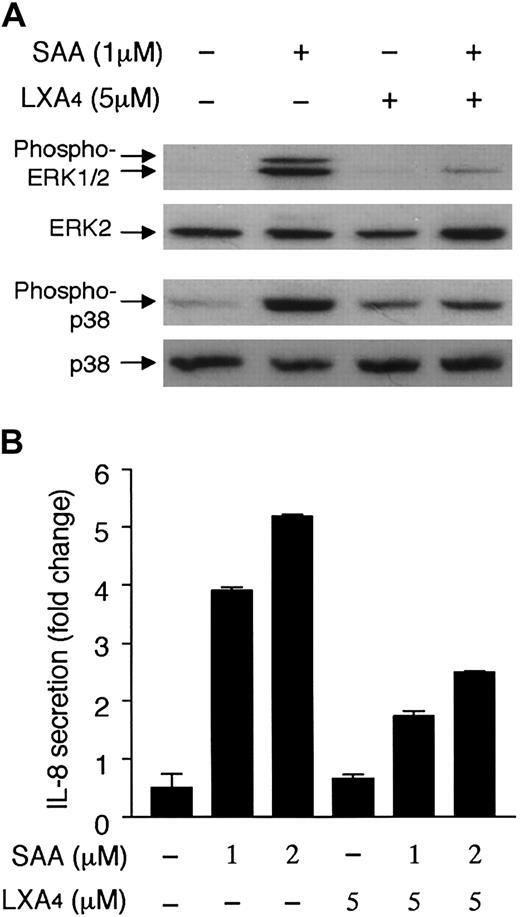
LXA4 is a lipid ligand for FPRL1/LXA4R with anti-inflammatory properties.44 We compared LXA4 with SAA for their effects on cell signaling and induction of IL-8 expression. LXA4, at nanomolar to lower micromolar concentrations (up to 5 μM), failed to induce phosphorylation of ERK1/2 in neutrophils (Figure11A). Furthermore, treatment of neutrophils with LXA4 resulted in a marked reduction of SAA-induced ERK1/2 phosphorylation and a small inhibition of SAA-induced p38 phosphorylation. There was a small increase in p38 phosphorylation in LXA4-stimulated cells (Figure 11A). This apparently was insufficient for the induction of significant IL-8 secretion (Figure 11B). On the contrary, treatment of neutrophils with LXA4 partially inhibited SAA-induced IL-8 secretion. These results demonstrate, for the first time, the ability of FPRL1/LXA4R to mediate 2 drastically different cytokine responses in neutrophils.
Inhibition of MAP kinase phosphorylation and IL-8 secretion by LXA4.
(A) Neutrophils were stimulated with 1 μM SAA or 5 μM LXA4, or with SAA and LXA4. After 15 minutes, samples were collected for detection of ERK1/2 and p38 phosphorylation by antiphospho antibodies against these kinases. The results are from a representative experiment of 3 separate experiments. (B) Neutrophils were stimulated with SAA, in the presence or absence of LXA4 as indicated. After 4 hours, the secreted IL-8 was measured by ELISA. The induction of IL-8 in the absence and presence of LXA4 was compared. Data are shown as mean ± SEM of 3 independent experiments, each performed in duplicate.
Inhibition of MAP kinase phosphorylation and IL-8 secretion by LXA4.
(A) Neutrophils were stimulated with 1 μM SAA or 5 μM LXA4, or with SAA and LXA4. After 15 minutes, samples were collected for detection of ERK1/2 and p38 phosphorylation by antiphospho antibodies against these kinases. The results are from a representative experiment of 3 separate experiments. (B) Neutrophils were stimulated with SAA, in the presence or absence of LXA4 as indicated. After 4 hours, the secreted IL-8 was measured by ELISA. The induction of IL-8 in the absence and presence of LXA4 was compared. Data are shown as mean ± SEM of 3 independent experiments, each performed in duplicate.
Discussion
Emerging evidence suggests that the acute-phase protein SAA has cytokinelike properties and can induce the secretion of proinflammatory cytokines including IL-1β, TNF-α, and IL-8.21 22 This function of SAA, combined with its rapid release into circulation after injury and infection, suggests that modulation of cytokine production by SAA may influence the course of infection and inflammation. Indeed, local production of SAA in atherosclerotic sites and arthritic joints has been associated with the progression of these inflammatory diseases. In this work, we sought to identify the receptor that mediates this function of SAA and to delineate the proximal signaling pathways associated with SAA-induced IL-8 production. Our study using human neutrophils demonstrates that SAA stimulates a rapid (within hours) and potent synthesis of IL-8. This is a primary response to SAA that requires de novo protein synthesis and transcription of the IL-8 gene. There is also direct evidence for an association between activation of the MAP kinases (ERK1/2 and p38) and SAA-induced IL-8 secretion. Like other proinflammatory cytokines such as TNF-α and IL-1β, SAA stimulates activation of NF-κB that has been known as one of the major transcription factors for IL-8 gene expression.
We have shown, for the first time, that the G protein–coupled receptor FPRL1/LXA4R mediates the cytokinelike properties of SAA. FPRL1/LXA4R was originally identified as a low-affinity receptor forN-formyl-Met-Leu-Phe that shares 69% sequence identity with the high-affinity formyl peptide receptor FPR.23,45-47 It was subsequently reported that LXA4 and aspirin-triggered 15-epi-LXA4 are endogenous ligands for FPRL1/LXA4R and exert their anti-inflammatory functions through this receptor.44For example, LXA4 suppression of TNF-α–induced IL-8 production by enterocytes is mediated by FPRL1/LXA4R.48 This receptor also mediates the anti-inflammatory function of LXA4 in synoviocytes.49 Thus, our finding that SAA stimulates FPRL1/LXA4R and induces NF-κB activation as well as IL-8 secretion by neutrophils is the first report that a 7-transmembrane domain receptor can either activate or inhibit proinflammatory cytokine synthesis depending on the specific ligands that bind to the receptor. Exactly how FPRL1/LXA4R differentially responds to these ligands remains enigmatic. All FPRL1/LXA4R agonists so far identified can induce calcium signaling as measured by elevation of intracellular Ca2+, a function that appears to be required for IL-8 production in neutrophils. Although LXA4 also stimulates calcium mobilization (Figure 7) and limited phosphorylation of p38, it does not induce IL-8 secretion. It is possible that the inability of LXA4 to activate certain signaling events, such as phosphorylation of ERK1/2, contributes to the lack of IL-8 induction. Our data do not exclude the likelihood that LXA4 sends a negative signal that inhibits IL-8 production by other cytokines. Indeed, published data demonstrate that LXA4 can block IL-8 secretion induced by other cytokines such as TNF-α.48
The structural basis for the divergent signaling mechanisms originating from FPRL1/LXA4R remains to be delineated. It appears that SAA binding to FPRL1/LXA4R is blocked by an antibody against the N-terminal domain of this receptor. The N-terminal domain contains one of theN-glycosylation sites. N-glycosylation has been shown to be necessary for binding of peptide ligands, but not LXA4, by FPRL1/LXA4R.40 Thus, LXA4 and peptide agonists such as SAA may occupy different binding domains on the FPRL1/LXA4R, leading to different receptor conformational changes. Defining the ligand-binding pocket of FPRL1/LXA4R will help to understand the discrepancy in transmembrane signaling.
Among the cytokines measured from SAA-stimulated neutrophils, IL-8 is most abundant. In comparison, SAA induced very limited secretion of IL-6 and IL-1β and a modest production of TNF-α. Given that a terminally differentiated neutrophil is a relatively inefficient protein synthesis machine, the level of IL-8 that is induced by SAA in these cells is relatively high. It is unclear why SAA selectively promotes IL-8 synthesis in neutrophils, although it has been known that neutrophils have a limited protein synthesis profile.50One possibility is that SAA preferentially activates the transcription factors required for IL-8 synthesis. The IL-8 gene containscis-regulatory elements for NF-κB, AP-1, and NF–IL-6.33 Of these transcription factors, NF-κB plays a key role in the induced expression of IL-8.32Accordingly, we found that SAA stimulates NF-κB activation and induces transcription of the IL-8 gene. In our preliminary studies, we also observed SAA induction of an AP-1 luciferase reporter in transiently transfected HeLa cells that express FPRL1/LXA4R (data not shown), a finding consistent with the role of AP-1 in the enhancement of IL-8 gene expression.33 However, SAA induces minimal expression of IL-6. Thus, the preferential expression of IL-8 in SAA-stimulated neutrophils may be both cell specific and ligand specific.
There is apparently spontaneous synthesis of IL-8 in unstimulated neutrophils because the basal level of IL-8 is 10- to 50-fold higher than that of IL-1β and TNF-α in 24 hours.22 In this study, we have observed that a significant amount of the spontaneously produced IL-8 is cell associated. These may include newly synthesized IL-8 and IL-8 bound to and internalized through their receptors. Stimulation with SAA increased total production and the secretion of IL-8, without a drastic alteration of the proportion of cell-associated IL-8. This observation, along with inhibition by CHX and BAPTA/am, demonstrate de novo synthesis of IL-8 in response to SAA stimulation. Calcium mobilization appears to be required, but certainly not sufficient, for IL-8 synthesis. For example, induction of calcium mobilization by LXA4 is insufficient for IL-8 production (Figures 7 and 11). BAPTA/am treatment reduced IL-8 secretion to a level below that of unstimulated cells (Figure 5C), indicating a general inhibitory mechanism36 37as opposed to an SAA-specific inhibitory mechanism. BAPTA/am did not inhibit SAA-induced phosphorylation of ERK1/2. This unexpected finding suggests that ERK1/2 phosphorylation is a signaling event that does not require calcium mobilization and may originate from a different branch of the FPRL1/LXA4R signaling mechanism.
Because neutrophils are the predominant cell type in acute inflammation, the effect of SAA on neutrophil cytokine synthesis is consistent with its potential role in regulating the inflammatory and immune processes in vivo. IL-8 is a potent chemokine for various leukocytes that express the IL-8 receptors CXCR1 and CXCR2. The important function of IL-8 in acute inflammation has been well documented and supported by in vivo data.51 Thus, generation of IL-8 at the site of inflammation may sensitize and activate neutrophils in an autocrine fashion. In addition, neutrophil-produced IL-8 can also attract monocytes and a subpopulation of T cells that are important for cell-mediated immune response.
In summary, the current study provides direct evidence for a cytokinelike property of SAA and demonstrates that the G protein–coupled FPRL1/LXA4R is a receptor that mediates this function. We have also shown that SAA stimulates NF-κB activation, phosphorylation of ERK1/2 and p38, and transcription of the IL-8 gene. Combined with the previous observation that SAA is chemotactic for phagocytes, this acute-phase protein has all the functional features of a chemotactic cytokine even though it does not have the characteristic localization of cysteine residues found in a typical chemokine. It will be of great interest to determine in future studies whether other inflammatory cells can also respond to SAA with cytokine production and how FPRL1/LXA4R transduces different signals generated by SAA and LXA4.
We thank Dr Naofumi Mukaida for the IL-8 luciferase reporter construct, and Mr Joseph Schober for drawing blood.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-05-1431.
Supported in part by National Institutes of Health grants AI33503 and AI40176 (to R.D.Y.) and by a postdoctoral fellowship from American Heart Association, Midwest Chapter (to R.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard D. Ye, Department of Pharmacology, M/C 868, University of Illinois at Chicago, 835 S Wolcott Ave, Chicago, IL 60012; e-mail: yer@uic.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal