Glycoprotein (GP) VI is a critical platelet collagen receptor, yet the steps involved in GPVI-mediated platelet activation remain incompletely understood. Because activation of Rap1, an abundant small guanosine triphosphatase (GTPase) in platelets, contributes to integrin αIIbβ3 activation, we asked whether and how GPVI signaling activates Rap1 in platelets. Here we show that platelet Rap1 is robustly activated upon addition of convulxin, a GPVI-specific agonist. Using a reconstituted system in RBL-2H3 cells, we found that GPVI-mediated Rap1 activation is dependent on FcRγ but independent of another platelet collagen receptor, α2β1. Interestingly, GPVI-mediated Rap1 activation in human platelets is largely dependent on adenosine diphosphate (ADP) signaling through the P2Y12 and not the P2Y1 receptor. However, experiments with specific ADP receptor antagonists and platelets from knockout mice deficient in P2Y1 or the P2Y12-associated G-protein, Gαi2, indicate that human and murine platelets also have a significant P2Y12-independent component of GPVI-mediated Rap1 activation. The P2Y12-independent component is dependent on phosphatidylinositol 3-kinase and is augmented by epinephrine-mediated signaling. P2Y12-dependent and -independent components are also observed in GPVI-mediated platelet aggregation, further supporting a role for Rap1 in aggregation. These results define mechanisms of GPVI-mediated platelet activation and implicate Rap1 as a key signaling protein in GPVI-induced platelet signaling.
Introduction
Collagen is a major component of the subendothelial matrix and atherosclerotic plaques and is a potent platelet agonist that contributes to thrombus formation upon plaque rupture. Collagen-mediated platelet activation results from a complex set of signals initiated by the coordination of platelet surface proteins, including the immunoglobulin superfamily receptor glycoprotein (GP) VI, the integrin α2β1, and most recently, GPV.1-3
Activation of GPVI causes the assembly of distinct proximal signaling pathways. GPVI is constitutively associated with FcRγ, a protein containing an immunoreceptor tyrosine-based activation motif.4,5 Upon collagen ligation to GPVI, FcRγ becomes phosphorylated by a Src family kinase,6,7 which subsequently allows the recruitment of other signaling proteins such as Syk, Bruton tyrosine kinase, and phospholipase Cγ.6-11
GPVI-mediated signaling is absolutely required for collagen-induced platelet aggregation. Human platelets lacking GPVI, but expressing α2β1, fail to aggregate in response to collagen.12 In addition, GPVI antagonists inhibit collagen-mediated aggregation.13 Although human platelets lacking α2β1 also fail to aggregate in response to collagen,14 fibrillar collagen-mediated platelet aggregation still occurs in β1-deficient murine platelets that express GPVI.15,16 Additionally, GPVI signaling itself can activate α2β1function.15 This finding suggests that not only is GPVI a key signaling component of collagen-mediated platelet activation, but it is also important for enhanced platelet interaction with collagen.
Adenosine diphosphate (ADP) signaling is often required for a complete platelet aggregation response. ADP is released from dense granules in response to agonist stimulation and can synergize with other agonists to activate platelets.17 ADP signaling in platelets is largely dependent on two 7-transmembrane spanning receptors, the Gαq-coupled P2Y1 receptor and the Gαi-coupled P2Y12 receptor. Antithrombogenic drugs such as clopidogrel specifically target the P2Y12 receptor, demonstrating the importance of this receptor in platelet function.18Indeed, murine platelets deficient in P2Y12 show increased bleeding times and reduced aggregation,19 and a human patient with a mutant P2Y12 gene exhibits abnormal bleeding.20 21
One result of collagen- or ADP-mediated platelet activation is the conversion of the platelet fibrinogen receptor, integrin αIIbβ3, to an active conformation. The activation of this integrin is critical for platelet aggregation. Another result of collagen- or ADP-mediated stimulation of platelets is the activation of the small guanosine triphosphatase (GTPase) Rap1,22 an abundant protein in platelets. Recent evidence indicates the importance of Rap1 in promoting αIIbβ3 activation in murine megakaryocytes.23 Moreover, Rap1 positively modulates β1 and β2 family integrins in other cell types.24-26 The ability of Rap1 to activate integrins in platelet precursor cells suggests that this GTPase is critical for platelet function, including platelet aggregation.
Although collagen activates Rap1 in platelets,22 the ability of individual collagen receptors to activate Rap1 and the platelet signaling pathways that facilitate collagen-mediated Rap1 activation are unknown. In this study, we determined that signaling from GPVI leads to robust Rap1 activation in an FcRγ-dependent manner. In human platelets, we found that GPVI-mediated Rap1 activation is largely dependent on ADP receptor signaling. This ADP-dependent component requires signaling through the P2Y12 ADP receptor, whereas no requirement for the P2Y1 ADP receptor was observed. However, a portion of GPVI-mediated Rap1 activation is independent of P2Y12 signaling. Murine platelets deficient in Gαi2 also demonstrate a P2Y12-independent component of GPVI-mediated Rap1 activation. Interestingly, GPVI-mediated murine platelet aggregation also contains P2Y12-dependent and -independent components, suggesting that Rap1 activation may play an important role in platelet aggregation. These results reveal signaling pathways by which GPVI stimulation activates Rap1.
Materials and methods
Plasmids, antibodies, and reagents
The plasmid pGEX 2T (Amersham Biosciences, Piscataway, NJ) containing RalGDS Ras-binding domain (RBD) was kindly provided by Dr Danny Altschuler (University of Pittsburgh, Pittsburgh, PA). Monoclonal antibodies (mAbs) to Rap1 (catalogue no. R22020) were obtained from BD Transduction Labs (Franklin Lakes, NJ) and polyclonal antibodies (catalogue no. sc-65) were from Santa Cruz Biotechnology (Santa Cruz, CA). P1E6 mAbs against the α2 integrin (catalogue no. MAB1950) were from Chemicon International (Temecula, CA). Purified convulxin was a generous gift of Dr Tom Kunicki (Scripps Research Institute, La Jolla, CA).27 Equine type I fibrillar collagen was from Chrono-Log (Havertown, PA). Indomethacin, ADP, adenosine 3′-5′-diphosphate (A3P5P), 2-methylthio-adenosine monophosphate (2-MeSAMP), and epinephrine were obtained from Sigma (St Louis, MO). Latrunculin-A, calpeptin, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), LY294002, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-am), and protease inhibitor cocktail III were from Calbiochem (San Diego, CA). Rat basophilic leukemia (RBL)–2H3 cells transfected with GPVI (GPVI.ori) or a GPVI mutant (GPVI.R272L) were generated as previously described.28
P2Y1- and Gαi2-deficient mice
Generation of GST-RalGDS RBD beads
Glutathione-agarose beads bound to glutathione-S-transferase (GST)–RalGDS RBD were purified using a modified protocol kindly provided by Dr Danny Altschuler. Briefly, LB-carbenicillin (50 μg/mL) was inoculated with BL21Escherichia coli (Stratagene, La Jolla, CA) containing pGEX 2T with RalGDS RBD insert and cultured overnight at 37°C. The culture was diluted 1:10 into fresh LB-carbenicillin and cultured to an OD600 of 0.6 to 1.0. Isopropyl-β-d-thiogalactoside (0.1 mM) was added to induce protein production and cultured for 3 hours. Bacteria were then resuspended in phosphate-buffered saline (PBS) with 1 mM Na3VO4, 0.5 mM dithiothreitol (DTT) and protease inhibitor cocktail III, and lysed via sonication. Triton-X 100 (1%) was added and the lysates were incubated for 30 minutes at 4°C. Glutathione-agarose beads were washed in 2 times lysis buffer (75 mM NaCl, 50 mM Tris [tris(hydroxymethyl)aminomethane]–-HCl, pH 7.4), 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1 mM Na3VO4, and protease inhibitor cocktail III)22 and 5.0 mL beads per 1000 mL culture were added to the clarified lysates and incubated for 1 hour at 4°C. Beads were collected, washed, and diluted to 50% in lysis buffer and the slurry was added 1:1 to glycerol, snap-frozen in liquid nitrogen and stored at −80°C.
Preparation of washed platelets
Blood from consenting healthy human donors was collected in acid citrate dextrose (85 mM sodium citrate, 111 mM glucose, 71.4 mM citric acid) and platelet-rich plasma was collected via centrifugation (200g). Platelets were washed twice (800g) with Tyrode buffer (5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 2.7 mM KCl, 11.9 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgCl2, 0.1% bovine serum albumin, 1% dextrose, 1 U/mL grade I apyrase, 50 U/mL heparin [first wash only]), and prostaglandin I2(PGI2, 50 ng/mL) was only added before each centrifugation to preserve platelet function. The effects of PGI2 were rapidly diminished such that washed platelets were responsive to agonist-induced platelet aggregation and integrin activation. Platelets were finally resuspended in Tyrode buffer without apyrase or heparin and counted on a thrombocounter (Coulter, Hialeah, FL). Mouse platelets were prepared as previously described.29
Precipitation of GTP-Rap1
Active Rap1 was precipitated from human platelet lysates, RBL-2H3 cell lysates, or mouse platelet lysates as described by Franke et al.22 Briefly, 1.0 to 1.5 × 108 human platelets, 0.7 × 107 RBL-2H3 cells, or 2.0 × 107 mouse platelets were stimulated at room temperature with platelet agonists and lysed for 30 minutes at 4°C with 2 × lysis buffer. Glutathione-linked agarose beads prebound to RalGDS RBD fused to GST were washed with ice-cold lysis buffer and 15 μL bead volume was added to cell lysates and incubated for 1 hour at 4°C. The beads were separated from the lysate by centrifugation and the precipitated GTP-Rap1 was detected by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with anti-Rap1–specific antibodies. Rap1 in total platelet lysate was determined by removing a lysate sample prior to bead addition and blotting for Rap1 as described for GTP-Rap1 precipitation. Fold stimulation of active Rap1 was determined by densitometry on a Bio-Rad Fluor-S fluorometer (Hercules, CA) using Quantity One quantitation software (Bio-Rad).
Murine platelet aggregation
Washed murine platelets were used in aggregation studies as previously described.29 Briefly, 0.6 × 108washed mouse platelets were stirred in the presence of 0.4 mg/mL fibrinogen and stimulated with platelet agonists. Inhibitors were added 1 minute prior to stimulation. Aggregation was monitored in an aggregometer (Chrono-Log).
Results
GPVI signaling to Rap1
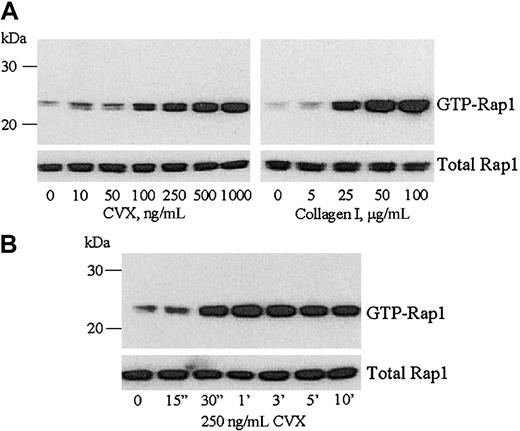
To determine if stimulation of GPVI was sufficient to activate Rap1 in platelets, we used the snake toxin convulxin (CVX). CVX is a potent platelet agonist that specifically induces GPVI signaling even in the absence of α2β1.15,31Precipitation of active Rap1 with GST-linked RalGDS RBD from washed human platelets treated with increasing concentrations of CVX demonstrated that Rap1 was activated in a concentration-dependent manner (Figure 1A left panel). Similarly, type I collagen also induced a concentration-dependent increase in Rap1 activation (Figure 1A right panel) as previously reported.22 CVX-mediated Rap1 activation was rapid, occurring within 30 seconds and lasting for at least 10 minutes (Figure1B). The total amount of Rap1 in each sample remained constant during either the concentration or time course treatments with CVX (Figure 1lower panels). Additionally, GST alone failed to precipitate any GTP-Rap1 (data not shown).
CVX activates Rap1 in platelets in a concentration- and time-dependent manner.
(A) Equal numbers of washed human platelets were stimulated with the indicated concentrations of CVX (left panel) or fibrillar type I collagen (right panel) for 1 minute. The platelets were lysed and GTP-bound Rap1 was precipitated with GST-RalGDS RBD bound to glutathione-agarose beads. The bound Rap1 was separated by SDS-PAGE and detected by immunoblotting with an anti-Rap1 mAb. (B) Washed human platelets were stimulated with 250 ng/mL CVX and equal aliquots were removed and lysed at the indicated time points. Active Rap1 was precipitated from the platelet lysates and detected as described in panel A. In lanes containing both total and GTP-bound Rap1, the presence of a second band may be phosphorylated Rap1, although no change in the ratio of these 2 bands was detected with CVX or collagen treatment in this study. Additionally, Woulfe et al find that inhibition of the protein kinase A pathway, which can phosphorylate Rap1, had no effect on Rap1 activation.38 These results were confirmed in 4 separate experiments.
CVX activates Rap1 in platelets in a concentration- and time-dependent manner.
(A) Equal numbers of washed human platelets were stimulated with the indicated concentrations of CVX (left panel) or fibrillar type I collagen (right panel) for 1 minute. The platelets were lysed and GTP-bound Rap1 was precipitated with GST-RalGDS RBD bound to glutathione-agarose beads. The bound Rap1 was separated by SDS-PAGE and detected by immunoblotting with an anti-Rap1 mAb. (B) Washed human platelets were stimulated with 250 ng/mL CVX and equal aliquots were removed and lysed at the indicated time points. Active Rap1 was precipitated from the platelet lysates and detected as described in panel A. In lanes containing both total and GTP-bound Rap1, the presence of a second band may be phosphorylated Rap1, although no change in the ratio of these 2 bands was detected with CVX or collagen treatment in this study. Additionally, Woulfe et al find that inhibition of the protein kinase A pathway, which can phosphorylate Rap1, had no effect on Rap1 activation.38 These results were confirmed in 4 separate experiments.
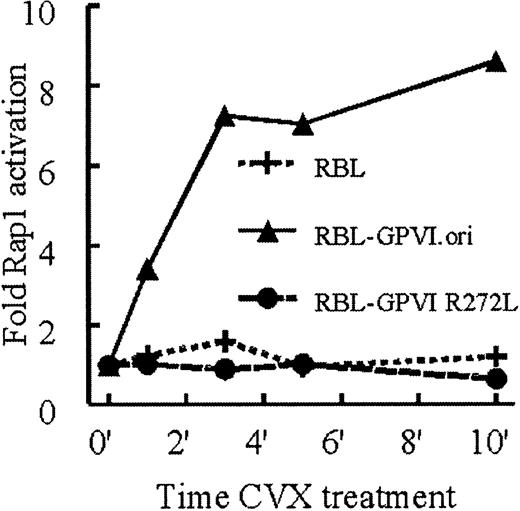
To further confirm GPVI-mediated Rap1 activation, we asked whether this process could be reconstituted in a nonplatelet system. RBL-2H3 cells lack α2β1 and GPVI expression,28 express endogenous FcRγ, and, when stably transfected with GPVI, increase intracellular Ca2+ in response to CVX.28 Therefore, we tested the ability of GPVI-transfected RBL-2H3 cells (GPVI.ori) to activate Rap1 in response to CVX. Indeed, these cells showed a dramatic CVX-induced increase in active Rap1 compared with untransfected RBL-2H3 cells. As in human platelets, Rap1 activation in GPVI.ori cells occurred within 1 minute of CVX treatment and lasted for at least 10 minutes (Figure2). In contrast to platelets, GPVI.ori cells did not activate Rap1 in response to type I collagen (data not shown). Correspondingly, these cells do not adhere well to collagen,32 33 consistent with a deficiency of α2β1. These results suggest that platelets can activate Rap1 in response to collagen because they express both GPVI and α2β1.
CVX, but not collagen, treatment of RBL-2H3 cells stimulates Rap1 activation in an FcRγ-dependent manner.
RBL-2H3 cells stably transfected with wild-type GPVI (GPVI.ori), a mutant GPVI that fails to associate with FcRγ (GPVI.R272L), or untransfected RBL-2H3 cells were stimulated with 10 nM CVX. Aliquots were removed at the indicated time points. Active Rap1 was precipitated and detected as described in Figure 1. Levels of active Rap1 were determined by densitometry and normalized to total Rap1 present in each sample. These results were confirmed in 3 separate experiments.
CVX, but not collagen, treatment of RBL-2H3 cells stimulates Rap1 activation in an FcRγ-dependent manner.
RBL-2H3 cells stably transfected with wild-type GPVI (GPVI.ori), a mutant GPVI that fails to associate with FcRγ (GPVI.R272L), or untransfected RBL-2H3 cells were stimulated with 10 nM CVX. Aliquots were removed at the indicated time points. Active Rap1 was precipitated and detected as described in Figure 1. Levels of active Rap1 were determined by densitometry and normalized to total Rap1 present in each sample. These results were confirmed in 3 separate experiments.
We also determined whether FcRγ was necessary for GPVI-mediated Rap1 activation by using RBL-2H3 cells transfected with a GPVI mutant (GPVI.R272L) that fails to associate with FcRγ.28 Cells expressing this mutant did not exhibit any CVX-mediated Rap1 activation (Figure 2). These results demonstrate that GPVI activates Rap1 in an FcRγ-dependent manner.
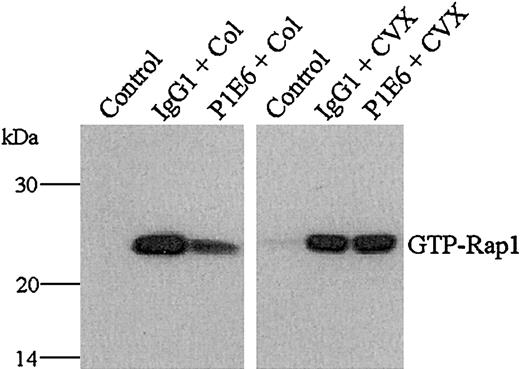
To further confirm the α2β1 dependency of collagen-induced, but not CVX-induced, Rap1 activation, we preincubated collagen- and CVX-treated platelets with the α2-blocking mAb P1E6. P1E6, but not an isotype-matched control IgG, decreased the level of Rap1 activation in collagen, but not in CVX-treated platelets (Figure 3). These data agree with the Rap1 activation patterns seen in GPVI.ori cells, further indicating that collagen requires both α2β1and GPVI to fully activate Rap1. Whether the α2β1 integrin provides a specific signaling component to Rap1 activation remains unclear, but it is apparent that CVX-induced GPVI signaling activates Rap1 independent of α2β1 integrin.
Collagen, but not CVX, requires α2β1 to fully stimulate Rap1 in human platelets.
Washed human platelets were treated with control buffer, 10 μg/mL control IgG1, or 10 μg/mL of the α2-blocking mAb P1E6 for 15 minutes and then stimulated with either fibrillar type I collagen (Col, 50 μg/mL, left panel, middle, right lanes) or CVX (250 ng/mL, right panel, middle, right lanes) for 1 minute. The presence of active Rap1 was determined as described in Figure 1. Note that the inclusion of P1E6 reduced collagen- but not CVX-mediated Rap1 activation. These results were confirmed in 3 separate experiments.
Collagen, but not CVX, requires α2β1 to fully stimulate Rap1 in human platelets.
Washed human platelets were treated with control buffer, 10 μg/mL control IgG1, or 10 μg/mL of the α2-blocking mAb P1E6 for 15 minutes and then stimulated with either fibrillar type I collagen (Col, 50 μg/mL, left panel, middle, right lanes) or CVX (250 ng/mL, right panel, middle, right lanes) for 1 minute. The presence of active Rap1 was determined as described in Figure 1. Note that the inclusion of P1E6 reduced collagen- but not CVX-mediated Rap1 activation. These results were confirmed in 3 separate experiments.
Role of ADP in GPVI-mediated Rap1 activation in platelets
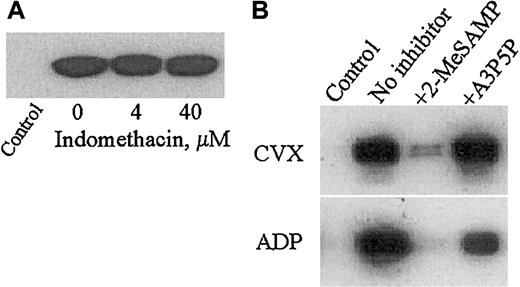
To elucidate downstream pathways involved in GPVI-mediated Rap1 activation in platelets, we inhibited 2 well-recognized secondary platelet activation pathways involving either thromboxane A2 (TXA2) generation or released ADP. Treatment of platelets with the TXA2 inhibitor indomethacin at concentrations that inhibit arachidonic acid–induced platelet aggregation (data not shown) did not affect CVX-induced Rap1 activation (Figure 4A). We then preincubated human platelets with specific inhibitors of the platelet G-protein–coupled ADP receptors, P2Y1 and P2Y12. Both receptors are critical for normal platelet function because P2Y1- and P2Y12-deficient mice each show increased bleeding times and impaired platelet aggregation in response to ADP.19,30Exposing platelets to a P2Y12-specific inhibitor, 2-MeSAMP, drastically reduced but did not eliminate Rap1 activation in CVX-treated platelets, but almost completely inhibited Rap1 activation in ADP-treated platelets. However, preincubation with a P2Y1-specific antagonist, A3P5P, had no effect on CVX-stimulated Rap1 activation, and only partially inhibited ADP-stimulated Rap1 activation (Figure 4B). Additionally, simultaneous application of both ADP receptor antagonists had no greater effect on Rap1 activation in response to CVX or ADP as compared with 2-MeSAMP alone (data not shown). Furthermore, inhibition of CVX-mediated Rap1 activation was constant over a wide range of 2-MeSAMP concentrations (50-500 μM, data not shown), suggesting that P2Y12-binding sites are saturated with inhibitor. Lastly, we asked whether P2Y12 signaling still contributes to Rap1 activation with higher doses of CVX. Interestingly, Rap1 activation induced by up to a 10-fold higher concentration of CVX (2.5 μg/mL) was still significantly inhibited by 2-MeSAMP (data not shown). These results suggest that P2Y12 is a main conduit for GPVI- and ADP-mediated Rap1 activation in human platelets. Consistent with these results, FcγRII-mediated Rap1 activation is P2Y12dependent,34 and GPVI-mediated signaling can enhance the P2Y12 but not the P2Y1 component of ADP-induced platelet aggregation.35
Full CVX-mediated Rap1 activation is dependent on ADP but not TXA2 signaling and specifically requires the P2Y12 ADP receptor in human platelets.
(A) Washed human platelets were pretreated with dimethyl sulfoxide (DMSO; control) or the indicated concentrations of the cyclooxygenase inhibitor indomethacin for 15 minutes. CVX (250 ng/mL) was then added for 1 additional minute. (B) Washed human platelets were pretreated for 15 minutes with Tyrode buffer (control and no inhibitor), the specific P2Y12 ADP receptor antagonist 2-MeSAMP (50 μM), or the specific P2Y1 ADP receptor antagonist A3P5P (100 μM) prior to 1 minute of stimulation with either 250 ng/mL CVX (top panel) or 20 μM ADP (bottom panel). Control lanes are without agonist stimulation. The presence of active Rap1 in treated platelet lysates in panels A and B was detected as described in Figure 1. Note the dramatic inhibition of Rap1 activation by 2-MeSAMP pretreatment. These results were confirmed in 4 separate experiments.
Full CVX-mediated Rap1 activation is dependent on ADP but not TXA2 signaling and specifically requires the P2Y12 ADP receptor in human platelets.
(A) Washed human platelets were pretreated with dimethyl sulfoxide (DMSO; control) or the indicated concentrations of the cyclooxygenase inhibitor indomethacin for 15 minutes. CVX (250 ng/mL) was then added for 1 additional minute. (B) Washed human platelets were pretreated for 15 minutes with Tyrode buffer (control and no inhibitor), the specific P2Y12 ADP receptor antagonist 2-MeSAMP (50 μM), or the specific P2Y1 ADP receptor antagonist A3P5P (100 μM) prior to 1 minute of stimulation with either 250 ng/mL CVX (top panel) or 20 μM ADP (bottom panel). Control lanes are without agonist stimulation. The presence of active Rap1 in treated platelet lysates in panels A and B was detected as described in Figure 1. Note the dramatic inhibition of Rap1 activation by 2-MeSAMP pretreatment. These results were confirmed in 4 separate experiments.
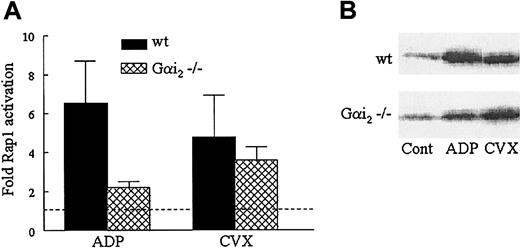
To further confirm the importance of P2Y12 and the dispensability of P2Y1 signaling in GPVI-stimulated Rap1 activation, we analyzed Rap1 activation in mice with defective ADP signaling pathways. To accomplish this, we used platelets from Gαi2−/− or P2Y1−/− mice. Gαi2 couples to P2Y12, and like P2Y12-deficient murine platelets, Gαi2-deficient platelets show impaired ADP-mediated platelet aggregation and impaired cAMP inhibition.19 29 Wild-type murine platelets exhibited significant Rap1 activation in response to either ADP or CVX. However, Rap1 activation in Gαi2-deficient murine platelets was diminished in response to ADP but not in response to CVX (Figure5). In contrast, no inhibition of either CVX- or ADP-induced Rap1 activation was observed in P2Y1-deficient murine platelets (data not shown). The ability of CVX to induce near wild-type levels of Rap1 activation in platelets lacking Gαi2 suggests that GPVI signaling in mice can bypass either Gαi2 or its associated receptor, indicating the necessity of examining the contributions of the ADP receptors directly.
Differential effects on Rap1 activation in Gαi2−/− murine platelets in response to ADP and CVX.
(A) Washed wild-type (wt) and Gαi2-deficient murine platelets were stimulated with 50 μM ADP or 500 ng/mL CVX for 1 minute before lysis. GTP-bound Rap1 was precipitated and detected as described in Figure 1, except that a polyclonal antibody was used to detect Rap1. Relative quantities of active Rap1 were determined via densitometry and normalized to levels of active Rap1 in unstimulated platelets on the same blot. The wild-type and Gαi2−/− murine platelet results are plotted as an average fold of Rap1 activation ± SEM (n = 6). (B) In a representative blot the GTP-Rap1 bands in the control (Cont) lanes are assigned a fold Rap1 activation value of 1.0, indicated by the dashed line in the histogram.
Differential effects on Rap1 activation in Gαi2−/− murine platelets in response to ADP and CVX.
(A) Washed wild-type (wt) and Gαi2-deficient murine platelets were stimulated with 50 μM ADP or 500 ng/mL CVX for 1 minute before lysis. GTP-bound Rap1 was precipitated and detected as described in Figure 1, except that a polyclonal antibody was used to detect Rap1. Relative quantities of active Rap1 were determined via densitometry and normalized to levels of active Rap1 in unstimulated platelets on the same blot. The wild-type and Gαi2−/− murine platelet results are plotted as an average fold of Rap1 activation ± SEM (n = 6). (B) In a representative blot the GTP-Rap1 bands in the control (Cont) lanes are assigned a fold Rap1 activation value of 1.0, indicated by the dashed line in the histogram.
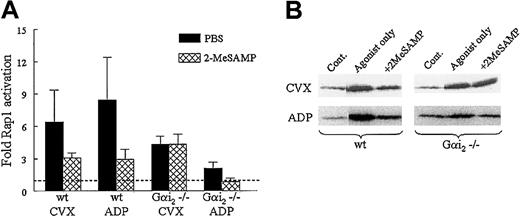
We therefore tested the effects of ADP receptor antagonists on Rap1 activation in murine wild-type and Gαi2 knockout platelets. As with human platelets, CVX- and ADP-mediated Rap1 activation was reduced in wild-type murine platelets treated with 2-MeSAMP (Figure 6) but not with A3P5P (data not shown), suggesting that P2Y12 does indeed contribute to GPVI-mediated Rap1 activation in murine platelets, but perhaps in a manner not solely dependent on Gαi2. Correspondingly, 2-MeSAMP further reduced Rap1 activation in ADP-treated Gαi2−/− platelets (Figure 6), suggesting that another protein may couple to P2Y12 in the absence of Gαi2, thereby contributing to CVX- and ADP-induced Rap1 activation. Finally, 2-MeSAMP had no effect on Rap1 activation in Gαi2−/− platelets in response to CVX (Figure 6). This result suggests the existence of an additional pathway in GPVI-stimulated platelets separate from P2Y12and Gαi2 signaling.
Effect of 2-MeSAMP on Rap1 activation in wild-type and Gαi2−/− platelets stimulated with ADP and CVX.
(A) Washed wild-type (wt) or Gαi2-deficient murine platelets were treated with 2-MeSAMP or PBS for 15 minutes, followed by CVX (500 ng/mL, n = 4) or ADP (50 μg/mL, n = 3) for 1 minute. Platelets were lysed and active Rap1 was precipitated and detected as in Figure 1, except a polyclonal antibody was used to detect Rap1. Relative quantities of active Rap1 were determined via densitometry and normalized to levels of active Rap1 in unstimulated platelets on the same blot. Results are plotted as an average fold Rap1 activation ±SEM. (B) In a representative blot the GTP-Rap1 bands in the control lanes are assigned a fold Rap1 activation value of 1.0, indicated by the dashed line in the histogram.
Effect of 2-MeSAMP on Rap1 activation in wild-type and Gαi2−/− platelets stimulated with ADP and CVX.
(A) Washed wild-type (wt) or Gαi2-deficient murine platelets were treated with 2-MeSAMP or PBS for 15 minutes, followed by CVX (500 ng/mL, n = 4) or ADP (50 μg/mL, n = 3) for 1 minute. Platelets were lysed and active Rap1 was precipitated and detected as in Figure 1, except a polyclonal antibody was used to detect Rap1. Relative quantities of active Rap1 were determined via densitometry and normalized to levels of active Rap1 in unstimulated platelets on the same blot. Results are plotted as an average fold Rap1 activation ±SEM. (B) In a representative blot the GTP-Rap1 bands in the control lanes are assigned a fold Rap1 activation value of 1.0, indicated by the dashed line in the histogram.
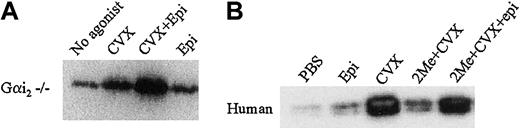
Because GPVI-mediated Rap1 activation in murine platelets appears to have a P2Y12/Gαi2-independent component of Rap1 activation and because human platelets still retain some Rap1 activation even in the presence of P2Y12 antagonists (Figure 4B), we asked if a non-ADP receptor with a different associated Gα subunit could substitute for the P2Y12/Gαi2-dependent component of GPVI-mediated Rap1 activation. The platelet α2-adrenergic receptors are linked to another member of the Gαi family, Gαz.36 We therefore asked whether GPVI signaling could be restored by epinephrine activation of the α2-adrenergic receptor when P2Y12 and Gαi2 signaling were blocked. We found that CVX and epinephrine together induced a synergistic activation of Rap1 in Gαi2-deficient murine platelets compared with CVX or epinephrine alone (Figure 7A). In human platelets, epinephrine alone minimally activated Rap1 as compared with CVX. However, 2-MeSAMP–mediated inhibition of CVX-induced Rap1 activation could be overcome by the addition of epinephrine (Figure 7B). These results suggest that other Gαi family subunits, although alone not potent Rap1 activators, can bypass P2Y12 signaling to Rap1 by synergizing with the P2Y12-independent component of GPVI-mediated Rap1 activation.
Epinephrine augments CVX-mediated Rap1 activation in Gαi2-deficient murine platelets and 2-MeSAMP–treated human platelets.
(A) Washed Gαi2-deficient murine platelets were stimulated with either CVX or epinephrine (2 μM) or CVX and epinephrine for 1 minute. (B) Washed human platelets were pretreated with either PBS or 2-MeSAMP (2Me) for 15 minutes prior to treatment for 1 minute each with control buffers, 2 μM epinephrine (epi), 250 ng/mL CVX, or CVX and epinephrine. GTP-bound Rap1 was precipitated and detected in panels A and B as described in Figure 1, except with a polyclonal Rap1 antibody used in panel A. These results were confirmed in 3 separate experiments.
Epinephrine augments CVX-mediated Rap1 activation in Gαi2-deficient murine platelets and 2-MeSAMP–treated human platelets.
(A) Washed Gαi2-deficient murine platelets were stimulated with either CVX or epinephrine (2 μM) or CVX and epinephrine for 1 minute. (B) Washed human platelets were pretreated with either PBS or 2-MeSAMP (2Me) for 15 minutes prior to treatment for 1 minute each with control buffers, 2 μM epinephrine (epi), 250 ng/mL CVX, or CVX and epinephrine. GTP-bound Rap1 was precipitated and detected in panels A and B as described in Figure 1, except with a polyclonal Rap1 antibody used in panel A. These results were confirmed in 3 separate experiments.
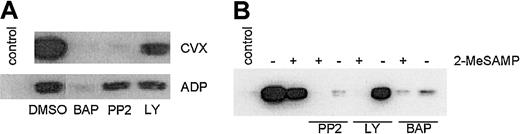
To further define pathways facilitating GPVI-mediated Rap1 activation, we sought to identify additional signaling molecules involved in the P2Y12-dependent and -independent pathways by pretreating CVX- or ADP-stimulated human platelets with inhibitors of key signaling molecules. The intracellular calcium chelator BAPTA-am reduced both CVX- and ADP-stimulated Rap1 activation to near-background levels (Figure8A), suggesting that Ca2+ is a key component of Rap1 activation. Because BAPTA-am also nearly eliminated the P2Y12-independent component of CVX-induced Rap activation as measured in the presence of excess 2-MeSAMP (Figure 8B), Ca2+ is also an essential component of the P2Y12-independent pathway. The Src kinase inhibitor PP2 preferentially inhibited CVX-induced as compared with ADP-induced Rap1 activation (Figure 8A), consistent with the close proximity of these kinases to GPVI in platelets.6 The remaining Rap1 activation in the presence of PP2 was eliminated by 2-MeSAMP, suggesting that residual ADP release occurs even in the presence of PP2. (Figure 8B). Thus, Src family kinases appear capable of affecting the P2Y12-dependent and -independent arms of GPVI-mediated Rap1 activation. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 moderately inhibited both ADP- and GPVI-mediated Rap1 activation (Figure 8A). To determine if this inhibition occurred solely downstream of P2Y12 or also as part of the P2Y12-independent component, we pretreated platelets with both LY294002 and 2-MeSAMP and found CVX-stimulated Rap1 activation was completely inhibited (Figure 8B). These results demonstrate that PI3K is also a major contributor to the P2Y12-independent pathway.
PI3K is a part of the ADP-independent component of GPVI-mediated Rap1 activation.
(A) Human platelets were pretreated with DMSO or BAPTA-am(BAP, 20 μM), PP2 (30 μM), or LY 294002 (LY, 100 μM), 10 minutes prior to a 1-minute stimulation with CVX (250 ng/mL) or ADP (20 μM). (B) Platelets were pretreated with or without 200 μM 2-MeSAMP and the indicated soluble inhibitors prior to 1 minute of CVX treatment. Control lanes are without agonist stimulation. GTP-bound Rap1 was precipitated and detected as described in Figure 1. These results were confirmed in 4 separate experiments. Note that only LY-treated platelets show significantly reduced levels of GTP-Rap1 in the presence of 2-MeSAMP.
PI3K is a part of the ADP-independent component of GPVI-mediated Rap1 activation.
(A) Human platelets were pretreated with DMSO or BAPTA-am(BAP, 20 μM), PP2 (30 μM), or LY 294002 (LY, 100 μM), 10 minutes prior to a 1-minute stimulation with CVX (250 ng/mL) or ADP (20 μM). (B) Platelets were pretreated with or without 200 μM 2-MeSAMP and the indicated soluble inhibitors prior to 1 minute of CVX treatment. Control lanes are without agonist stimulation. GTP-bound Rap1 was precipitated and detected as described in Figure 1. These results were confirmed in 4 separate experiments. Note that only LY-treated platelets show significantly reduced levels of GTP-Rap1 in the presence of 2-MeSAMP.
Finally, latrunculin A and calpeptin had no effect on either CVX- or ADP-induced Rap1 activation (data not shown), suggesting that Rap1 activation occurs independently of the state of actin polymerization and calpain activation.
Correlation of Rap1 activation to platelet aggregation
Because GPVI-induced Rap1 activation is mediated by P2Y12-dependent and -independent components, and because Rap1 activates platelet integrins,23 we next asked whether these separate signaling components also influence GPVI-mediated platelet aggregation. When wild-type murine platelets were pretreated simultaneously with P2Y1 and P2Y12 receptor antagonists, CVX-induced platelet aggregation was reduced, but not eliminated, compared with platelets treated with CVX alone (Figure 9A-B). Similar levels of reduced aggregation were also seen in CVX-stimulated Gαi2-deficient murine platelets (Figure 9C). These results suggest that GPVI-mediated platelet aggregation, like GPVI-mediated Rap1 activation, is a result of both P2Y12-dependent and -independent processes. The correlation between GPVI-mediated platelet aggregation and Rap1 activation further suggests that Rap1 plays a role in platelet aggregation.
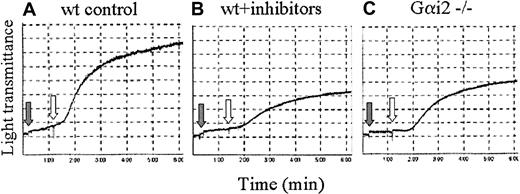
CVX-induced platelet aggregation is mediated by ADP-dependent and -independent components.
Aggregation traces from wild-type (wt, A-B) or Gαi2−/− (C) murine platelets treated with either control buffer (A,C) or the ADP receptor antagonists 2-MeSAMP (50 μM) and A3P5P (100 μM; B; gray arrows) for 1 minute and then stimulated with 250 ng/mL CVX (white arrows). These results were confirmed in 3 separate experiments.
CVX-induced platelet aggregation is mediated by ADP-dependent and -independent components.
Aggregation traces from wild-type (wt, A-B) or Gαi2−/− (C) murine platelets treated with either control buffer (A,C) or the ADP receptor antagonists 2-MeSAMP (50 μM) and A3P5P (100 μM; B; gray arrows) for 1 minute and then stimulated with 250 ng/mL CVX (white arrows). These results were confirmed in 3 separate experiments.
Discussion
Here we show that engagement of GPVI, a platelet collagen receptor required for collagen-mediated platelet activation, robustly activates the small GTPase Rap1 in an FcRγ-dependent manner. In human platelets, GPVI-mediated Rap1 activation is largely dependent on secreted ADP and specifically requires signaling through the P2Y12 ADP receptor, a receptor that is critical for the formation of stable platelet aggregates.37 The connection between a collagen receptor necessary for platelet responsiveness to the extracellular matrix, an ADP receptor critical for stable platelet aggregate formation, and a small GTPase that is readily activated by a number of platelet agonists22 suggests a mechanism by which platelets can coordinate a number of different activation signals.
The discovery that ADP signaling is critical for complete Rap1 activation by GPVI is an intriguing finding. Previous studies indicated that thrombin-induced Rap1 activation was not dependent on ADP because ADP scavengers failed to inhibit Rap1 activation.22 In agreement, we found that ADP receptor antagonists had little effect on thrombin-induced Rap1 activation (data not shown). Although recent studies implicate P2Y12/Gαi signaling in FcγRII-mediated Rap1 activation34 and in ADP-mediated Rap1 activation in platelets,38 the effects of secondary activation pathways on collagen or GPVI-mediated Rap1 activation have not been addressed in any other study. Although our findings demonstrate the importance of ADP signaling in GPVI-mediated platelet Rap1 activation, the ADP-independent activation of Rap1 by thrombin22 and the presence of P2Y12independent pathways as described here suggest that there are several routes of Rap1 activation in platelets.
Interestingly, GPVI signaling was recently shown to enhance a P2Y12/Gαi component of ADP-mediated aggregation.35,39 Our finding that Rap1 is downstream of GPVI and P2Y12 signaling strongly suggests that Rap1 is one of the key effectors of these 2 receptors. In support of this hypothesis, we show that both GPVI-mediated Rap1 activation and GPVI-mediated platelet aggregation contain P2Y12-dependent and -independent components. This finding, in addition to the ability of constitutively active Rap1 to promote and dominant-negative Rap1 to block integrin activation in megakaryocytes23 and other cell types,24 26 supports a critical role for Rap1 in platelet aggregation.
Although we find that complete GPVI-mediated aggregation of murine platelets requires P2Y12 signaling, Quinton et al40 observed an ADP-dependency of CVX-induced aggregation of human platelets only at relatively low concentrations of CVX (< 100 ng/mL); this ADP requirement was overcome at higher concentrations of CVX. Likewise, Atkinson et al39 reported only a slight right shift in the CVX-mediated dose response of human platelet aggregation due to the presence of ADP inhibitors. Although seemingly contradictory to our aggregation results, these discrepancies may reflect differences between human and murine platelet aggregation responses. However, if we treat human platelets with concentrations of CVX that reportedly induce complete platelet aggregation independent of P2Y12,39 40 we still observe a P2Y12-dependent component of Rap1 activation. This may suggest that there is not an exact correlation between the amount of Rap1 activation and extent of platelet aggregation or that the amount of Rap1 required for platelet aggregation is less than the total amount of Rap1 activated by GPVI-mediated signaling.
Although GPVI-mediated Rap1 activation relies on P2Y12signaling, a significant P2Y12-independent component of Rap1 activation exists. The P2Y12-independent pathway may well involve direct GPVI-mediated Rap1 activation without the contribution of classic secondary activation pathways. For example, GPVI signaling activates phospholipase Cγ2.41 The resulting production of inositol trisphosphate leads to Ca2+ mobilization, a process that appears critical for Rap1 activation in platelets. Indeed, one Rap1 exchange factor, CalDAG-GEFI, is activated on binding Ca2+,42 which could represent a proximal link between GPVI and Rap1. The potential use of this exchange factor is bolstered by the sensitivity of Rap1 activation to BAPTA-amtreatment. Because P2Y1/Gαq signaling is not a critical component of Rap1 activation, BAPTA-am inhibition of both CVX- and ADP-induced Rap1 activation suggests that basal levels of Ca2+ in platelets are necessary for Rap1 activation.
We also found that this P2Y12-independent component involves PI3K. GPVI has previously been shown to activate protein kinase B in a PI3K-dependent manner,43 which suggests that PI3K is a downstream target of GPVI that could lead to Rap1 activation. However, the positioning of PI3K in the P2Y12-independent pathway of GPVI-mediated Rap1 activation does not preclude the contribution of PI3K to other activation pathways. For example, Woulfe et al38 show that ADP and epinephrine-mediated Rap1 activation is reduced in PI3Kγ−/− murine platelets and in human platelets treated with soluble PI3K inhibitors. Taken together, these results place PI3K downstream of both the P2Y12-dependent and -independent components of GPVI signaling. Further studies are necessary to determine exactly how PI3K signaling leads to Rap1 activation.
The use of CVX to analyze GPVI-mediated Rap1 activation does not preclude a role for α2β1 in collagen-mediated Rap1 activation. In fact, we observed that an α2 integrin-specific blocking mAb significantly reduces collagen-mediated Rap1 activation, which suggests a potential coordination between GPVI and α2β1. This coordination would be consistent with recent findings suggesting that α2β1 activation reduces the lag time of collagen-induced platelet activation.16 Additionally, RBL-2H3 cells containing GPVI but deficient in α2β1 integrin fail to activate Rap1 in response to collagen. This suggests that collagen signaling through GPVI may require input from integrin signaling to fully activate Rap1, even though GPVI alone can mediate collagen signaling under specific conditions. Therefore, a potent agonist such as CVX, which contains several potential GPVI-binding sites44,45 is not affected by α2 integrin–blocking mAbs and is able to stimulate Rap1 activation in RBL-2H3 cells. Similarly, increasing the receptor density of GPVI on the surface of RBL-2H3 cells allows their adhesion to collagen and increases their collagen-induced Ca2+mobilization.33 Although use of agents like CVX in platelets and increasing GPVI receptor density on RBL-2H3 may bypass the need for α2β1, this integrin is clearly necessary for rapid and complete platelet responses to collagen, including Rap1 activation, as shown here and in previous studies.14-16
In conclusion, we find that Rap1 is activated in response to GPVI in a manner that relies on P2Y12 ADP receptor-dependent and -independent signaling components. These findings will help elucidate the functions of Rap1 in platelets and help to clarify the contributions of GPVI in collagen-mediated platelet activation. A better understanding of how platelets coordinate collagen signaling pathways during the early events of platelet activation are necessary to better understand platelet-mediated thrombosis.
The authors wish to thank Dr Weiping Yuan and Francis DeGuzman for invaluable contributions, Dr Beverly Koller and Nicholas Foley for assistance with the P2Y1−/− mice, and Dr Lawrence Brass and Dr Donna Woulfe for critical discussion.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-05-1533.
Supported by a Howard Hughes Medical Institute Predoctoral Fellowship (M.K.L.), National Institutes of Health grants 2-P01-HL06350 and 2-P01-HL45100 (L.V.P.), the American Heart Association grant 9920376U (J.E.F.), the Buger Award (M.L.K.), and the W. W. Smith Charitable Trust (M.L.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leslie V. Parise, Department of Pharmacology, CB 7365, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: parise@med.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal