P2Y1 and thromboxane-prostanoid–α (TPα) receptors on platelets belong to the G-protein–coupled 7–transmembrane domain family. They transmit signals for shape change, mobilization of calcium, and platelet aggregation. Immunogold labeling with a monoclonal antibody (MoAb) to the amino-terminal domain of P2Y1 and a polyclonal antibody to the C-terminal domain of TPα revealed that while present at the platelet surface, both receptors were abundantly represented inside the platelet. Specifically, receptors were found in membranes of α-granules and elements of the open-canalicular system. A similar organization was found in mature megakaryocytes. Activation of platelets by adenosine diphosphate (ADP) and the thromboxane A2(TXA2) analog, I-BOP [1S-(1 α,2 β(5Z),3 α-(1E,3S)4 α)-7-(3-(3- hydroxy-4-(p-iodophenoxy)-1-butenyl)-7-oxabicyclo(2.2.1)hept-2-yl)-5-heptenoic acid], increased the labeling of both P2Y1 and TPα at the surface and in intracellular pools, suggesting that activation resulted in greater antibody accessibility to the receptor. A return to a platelet discoid shape and to basal values of labeling accompanied receptor desensitization. Platelets lacking the P2Y12 ADP receptor normally expressed P2Y1 and TPα, both before and after activation. Studies with the anti–ligand-induced binding site (anti-LIBS) MoAb, AP-6, confirmed that stored fibrinogen associated with internal pools of αIIbβ3 at the start of secretion in a microenvironment containing agonist receptors. Pharmacologic antagonism of ADP or TXA2 receptors in antithrombotic therapy may need to take into account blockade of internal receptor pools.
Introduction
Receptors for primary agonists in platelets, including protease-activated receptors (PARs), belong mostly to the 7-transmembrane domain G-protein–coupled receptor (GPCR) family.1 Adenosine diphosphate (ADP), important for both hemostasis and thrombosis, possesses 2 platelet receptors belonging to this family. P2Y1 is coupled to Gq and is responsible for shape change, Ca2+ mobilization, and the initiation of aggregation.2,3 The more recently cloned P2Y12 is coupled to Gαi2 and is necessary for the formation and stabilization of large aggregates.4,5 Apart from platelets, P2Y1 is present in endothelial cells, skeletal muscle cells, and placenta. Thromboxane prostanoid (TP) constitutes the major thromboxane-prostanoid receptor on platelets.6 TP is responsible for shape change, Ca2+ mobilization, and platelet aggregation. It is associated not only with Gq protein,7 but also with Gα12 and Gα13,8 through which it acts as a modulator of the Na+/H+ exchanger.9 TP is present in thymus, lung, kidney, spleen, and placenta.10,11 TPα is the form that has been preferentially identified in platelets.12-14 Although mRNA from the β-isoform derived from an alternative splicing at the third exon of the TP gene has been reported in platelets,12,13 doubts remain about the presence of TPβ protein.14 Both P2Y1 and TP initiate platelet activation through the phospholipase C-β (PLCβ) pathway.15 ADP- and thromboxane A2 (TXA2)–dependent activation pathways are major targets for antithrombotic therapy.16 Both agonists can be released during platelet activation; TXA2 is produced during arachidonic acid metabolism, whereas ADP is stored in a secretable pool in dense granules.
Many receptors in platelets are found in both surface and intracellular membranes, an example being the αIIbβ3integrin.17 For GPIb, an adhesion receptor, platelet activation is followed by its transient internalization.17The PAR-1 receptor for thrombin, a GPCR, has been shown to traffic during platelet activation.18,19 Desensitization of GPCRs has been suggested to involve sequestration and/or uncoupling of the receptor from G proteins.20,21 Little is known about the localization of ADP and TXA2 receptors on platelets. In this study, we have combined immunogold labeling on ultrathin cryosections with electron microscopy to evaluate the cellular distribution of P2Y1 and TPα. Similarities were found in the distribution of both receptors: (1) labeling was greater inside the platelets than on the plasma membrane; (2) pools were present in membranes of α-granules and those of the open canalicular system (OCS); (3) increased labeling was seen after platelet activation with ADP and TXA2; and (4) for each receptor, prolonged exposure to agonist returned the labeling density to baseline. By using the monoclonal antibody (MoAb) AP-6, which recognizes a ligand-induced binding site (LIBS) on the integrin,17 we were able to show integrin activation early in secretion. Secreted ADP or newly formed TXA2 would be likely to come into contact with these receptors prior to the external pool.
Materials and methods
Platelet preparation
Peripheral blood was obtained by clean venipuncture from adult human volunteers who had had no medication for at least a week. The initial 3 mL blood was discarded. Blood was collected into acid-citrate-dextrose National Institutes of Health (NIH) formula A (ACD-A) (1 vol anticoagulant to 6 vol blood).5Platelet-rich plasma (PRP) was prepared by centrifugation at 120g for 10 minutes at room temperature. To prepare washed platelets, 0.05 U/mL apyrase grade VII (Sigma Chemical, Saint-Quentin-Fallavier, France) and ACD-A (1 vol ACD-A to 9 vol PRP) were added immediately to the PRP. Platelets were sedimented by centrifugation at 1200g for 15 minutes and resuspended in 137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, 5 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.1% (wt/vol) bovine serum albumin (BSA) (Sigma), and 0.05 U/mL apyrase, pH 7.4 (Hepes-buffered modified tyrode [HBMT]). Studies were also performed on platelets from one patient (ML) with an inherited disease recently linked to the absence of the second platelet ADP receptor, P2Y12.4 Platelets from this patient show a reduced and rapidly reversible aggregation to high doses of ADP.5 They also show a reduced response to thromboxane analogs.22 Patient ML gave informed consent to participate in the study and to allow the investigators to use identifying information in publications.
For platelet activation, unstirred suspensions of washed platelets at 2.5 × 108/mL were incubated at 37°C for the stated times with 10 μM ADP (Sigma) or 10 nM I-BOP [1S-(1 α,2 β(5Z),3 α-(1E,3S)4 α)-7-(3-(3-hydroxy-4-(p-iodophenoxy)-1-butenyl-7-oxabicyclo(2.2.1)hept-2-yl)-5-heptenoic acid], (Caiman Chemical, Ann Arbor, MI) in the presence or absence of 400 μg/mL purified human fibrinogen (Fg) (a gift from Dr K. Boulimez, Biochemistry Department, Hôpital Cardiologique, Pessac, France) (the Fg was greater than 95% pure as assessed by sodium dodecyl sulfate [SDS]–polyacrylamide gel electrophoresis). Desensitization was achieved by incubating platelet suspensions with 1 mM ADPβS (Sigma), a stable analog of ADP, for 1 hour at 37°C as described by Baurand et al,23 or with 10 nM I-BOP for 1 hour at 37°C, again in the absence of stirring.
Megakaryocytes
Bone marrow was taken by sternal puncture performed during a cardiovascular intervention in a hematologically normal patient. Informed consent was obtained. A volume (0.5 mL) of bone marrow was delicately added to 5 mL phosphate-buffered saline (PBS) without disruption of the marrow structure.24 The material was fixed in 1.25% (vol/vol) glutaraldehyde (Fluka, Buchs, Switzerland) in PBS and processed according to our standard procedures,24 and as described for platelets in the next section.
Electron microscopy and immunogold labeling
Antibodies.
A MoAb recognizing the P2Y1 receptor was obtained by immunizing mice against a 16–amino acid (16aa) peptide corresponding to the N-terminal domain of the receptor coupled to keyhole limpet hemocyanin.25 It was used as purified immunoglobulin G (IgG). The polyclonal antibody to TPα was raised in rabbits against a peptide composed of 15 aa's (327-341) located at the end of the carboxyl-terminal tail, which corresponds to a specific sequence of TPα that differs from that of TPβ.26 The immunoglobulin fraction was isolated by means of the E-Z-SEPkit (Amersham Pharmacia, Les Ulis, France) and its specificity shown.26 AP-6 is an IgM MoAb prepared against the 204-to-227 amino acid sequence of β3. It is an anti-LIBS, binding to its epitope only after Fg has bound to activated αIIbβ3.17 27 It was generously provided by Dr T. Kunicki (Scripps Research Institute, La Jolla, CA).
Sample preparation.
Platelets were fixed in 1.25% (vol/vol) glutaraldehyde diluted in 0.1 M phosphate buffer (pH 7.2) for 1 hour at room temperature.27 After washing, pellets were infused with 2.3 M sucrose (Fluka) before being frozen in propane and then in liquid nitrogen with a Reichert KF 80 freezing system (Leica, Vienna, Austria).27 Ultrathin sections of approximately 80 nm were cut at −120°C with the Ultracut E ultramicrotome equipped with an FC 4E cryokit attachment (Leica) and placed on collodion-coated nickel grids. Then, the grids were incubated for 10 minutes on drops of washing buffer consisting of PBS supplemented with 0.5% or 1% BSA before being incubated with antibodies.
Immunolabeling procedures.
An amplification procedure was used for localizing P2Y1. The grids were first placed on drops containing 10 μg/mL of the anti-P2Y1 MoAb for 45 minutes at room temperature, then on drops containing a 1/100 dilution of fluorescein isothiocyanate (FITC)–conjugated affinity-purified F(ab′)2 fragments of sheep antimouse IgG (Amrad; Eurobio, Paris, France) for 30 minutes. This was followed by incubation with a rabbit antibody to FITC (Dako, Trappes, France) at a 1/1000 dilution in PBS–0.5% albumin (alb). Finally, the sections were incubated for 30 minutes with a goat antirabbit antibody adsorbed onto 10-nm gold particles (1/100 dilution of AuroProbe EM G10; Amersham). For TPα, the sections were incubated directly with a 1/100 dilution of the polyclonal antibody to TPα in PBS–1% alb and then with a goat antirabbit antibody adsorbed onto 10-nm gold particles as described. The anti-LIBS MoAb AP-6 was used at a dilution of 1/10 000 and its binding assessed with the use of an anti-IgM antibody associated with gold particles (1/100 dilution of AuroProbe EM GAM IgM G10; Amersham). In double-staining, the anti–P-selectin used corresponded to a mixture of 3 MoAbs (VH10, 2.5 μg/mL; S12, 2.5 μg/mL; and AK6, 1 μg/mL) as described previously.27 P-selectin was detected by means of AuroProbe EM GAM G5.
Controls included the absence of primary antibody or its replacement with an irrelevant IgG or IgM of the same species and at the same concentration. The MoAb anti-CD56 (Dako) was used instead of P2Y1 at the same concentration, and isolated IgG of a rabbit antibody directed against S100 protein (Dako) was used instead of the anti-TPα. We also performed blocking experiments by preincubating the P2Y1 MoAb with 200 μg/mL of the peptide used for the immunization and likewise the rabbit antibody with 50 μg/mL of the peptide used for immunization as described by Habib et al26 The mixtures were then incubated with the sections on the grids as described.
Electron microscopy and quantitative analyses.
The grids were floated several times on PBS and then on water. The cryosections were stained by uranyl acetate and osmium according to our standard procedures and embedded in a thin film of methylcellulose prior to observation with a Jeol JEM-1010 transmission electron microscope (Jeol, Croissy-sur-Seine, France) at 80 kV.24For quantitative analyses of immunogold labeling, the mean surface area of each platelet section was calculated for at least 50 sections by means of Metamorph software (Universal Imaging, Paris, France) and a Pentium III computer.24 The gold particles were counted visually for each platelet section. The results are expressed as mean values ± standard deviation (SD) for a minimum of 50 sections. Statistical analysis was performed by means of the Studentt test.
Flow cytometry
Unstimulated platelets or those activated with 10 μM ADP for 10 minutes were fixed in 1% (wt/vol) paraformaldehyde (PFA) as described previously.27 To permit access to the internal compartment, platelets were treated with 0.1% (vol/vol) Triton X-100 for 30 minutes, washed, and then incubated overnight at 4°C with the anti-P2Y1 MoAb (10 μg/mL). After further washing, platelets were incubated with phycoerythrin (PE)–labeled F(ab′)2 fragments of a sheep antimouse IgG (Silenus Laboratories, Hawthorn, Australia). Negative controls were performed in the presence of 10 μg/mL of a MoAb to CD56 instead of P2Y1. Samples were analyzed by FACScan (Becton Dickinson, Le Pont de Claix, France). Gating to select the majority of platelets was based on preliminary determinations of forward and wide-angle light scatter. Fluorescence was measured after passage through a 530-nm-long pass interference filter. Histograms were generated from measurements of 10 000 cells, and data were analyzed by means of the LYSYS II software of the FACScan system.
Results
Immunolocalization of P2Y1
Resting platelets.
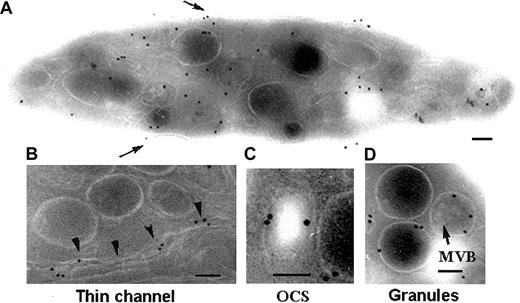
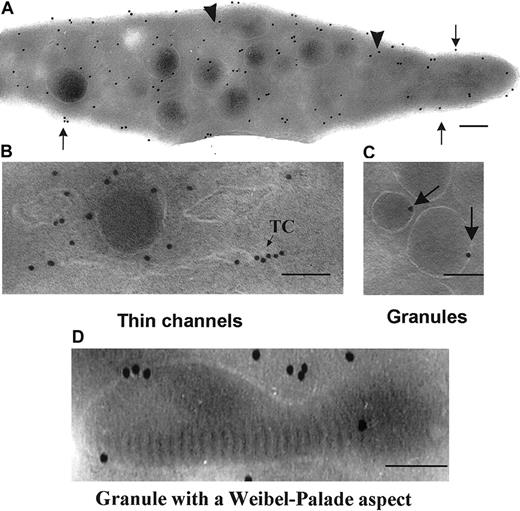
We first examined the distribution of P2Y1 within the membrane systems of unstimulated platelets. With the use of a single MoAb, an amplification procedure involving successive incubations with an FITC-labeled antimouse IgG and a polyclonal antibody to FITC proved necessary for optimal visualization of the receptor. Labeling of ultrathin sections showed a majority of gold particles localized to membrane systems within the interior of the cells although surface labeling was observed (Figure 1A). Note the typical discoid shape of this unstimulated platelet. Details of intracellular structures containing P2Y1 are illustrated in Figure 1. Gold particles were associated with thin channels (Figure 1B). Previously, we reported that these can link the platelet surface to the granules and may represent routes of trafficking for proteins and receptors (Nurden et al27 and “Discussion”). Labeling was also observed in more dilated elements of the OCS as shown in Figure 1C. Finally, there was occasional labeling of the membranes of α-granules. There was no labeling of multivesicular bodies that are sometimes observed in α-granules28 and are clearly visible in the granule to the right of Figure 1D.
Detection of P2Y1 in unstimulated platelets by immunogold labeling of frozen ultrathin sections using a MoAb directed against the amino-terminal domain.
(A) A typical distribution of the labeling within the different membrane systems of the platelet. Surface labeling is highlighted (arrows). (B-D) Higher-power magnification of labeled intracellular structures: thin channels of the OCS (arrowheads; B), a more dilated element of the OCS (C), and the delimiting membranes of α-granules (arrow) (D). The presence of multivesicular bodies (MVBs) inside the granule is indicated. Bars = 0.1 μm.
Detection of P2Y1 in unstimulated platelets by immunogold labeling of frozen ultrathin sections using a MoAb directed against the amino-terminal domain.
(A) A typical distribution of the labeling within the different membrane systems of the platelet. Surface labeling is highlighted (arrows). (B-D) Higher-power magnification of labeled intracellular structures: thin channels of the OCS (arrowheads; B), a more dilated element of the OCS (C), and the delimiting membranes of α-granules (arrow) (D). The presence of multivesicular bodies (MVBs) inside the granule is indicated. Bars = 0.1 μm.
Control experiments performed with an equivalent amount of an irrelevant mouse IgG resulted in virtually no labeling (Table1 footnote). Also, preincubation of the MoAb to P2Y1 with blocking amounts of the peptide used for immunization resulted in minimal background labeling and none of the features highlighted in the previous paragraph (not shown).
Semiquantitative analyses of P2Y1 in the membrane systems of platelets before and after stimulation with ADP or desensitization with ADPβS
| Platelets . | Gold particles per platelet section, no.* . | Mean surface area per platelet section, μm2 . | ||
|---|---|---|---|---|
| Surface . | Internal membranes . | Total . | ||
| Controls | ||||
| Unstimulated | 9.48 ± 7.31 | 53.30 ± 25.43 | 62.77 ± 29.54 | 2.36 ± 0.92 |
| ADP stimulated | 17.37 ± 12.91 | 62.8 ± 31.10 | 80.20 ± 37.34 | 2.77 ± 0.91 |
| Desensitized | 9.49 ± 7.38 | 48.81 ± .14 | 58.30 ± 31.96 | 2.22 ± 1.1 |
| Patient ML | ||||
| Unstimulated | 11.22 ± 7.35 | 44.59 ± 16.30 | 55.81 ± 19.39 | 2.58 ± 0.93 |
| ADP stimulated | 14.85 ± 11.13 | 59.02 ± 32.36 | 73.87 ± 40.07 | 2.8 ± 1.12 |
| Platelets . | Gold particles per platelet section, no.* . | Mean surface area per platelet section, μm2 . | ||
|---|---|---|---|---|
| Surface . | Internal membranes . | Total . | ||
| Controls | ||||
| Unstimulated | 9.48 ± 7.31 | 53.30 ± 25.43 | 62.77 ± 29.54 | 2.36 ± 0.92 |
| ADP stimulated | 17.37 ± 12.91 | 62.8 ± 31.10 | 80.20 ± 37.34 | 2.77 ± 0.91 |
| Desensitized | 9.49 ± 7.38 | 48.81 ± .14 | 58.30 ± 31.96 | 2.22 ± 1.1 |
| Patient ML | ||||
| Unstimulated | 11.22 ± 7.35 | 44.59 ± 16.30 | 55.81 ± 19.39 | 2.58 ± 0.93 |
| ADP stimulated | 14.85 ± 11.13 | 59.02 ± 32.36 | 73.87 ± 40.07 | 2.8 ± 1.12 |
Immunogold labeling using a MoAb to the amino terminal of P2Y1 was as described in “Materials and methods.” Gold particles were counted visually on the sections. Control values for irrelevant antibody were fewer than 3 particles per 100 sections. Surface area was calculated by computer analysis. Results are ± SD.
Effects of platelet activation and receptor desensitization.
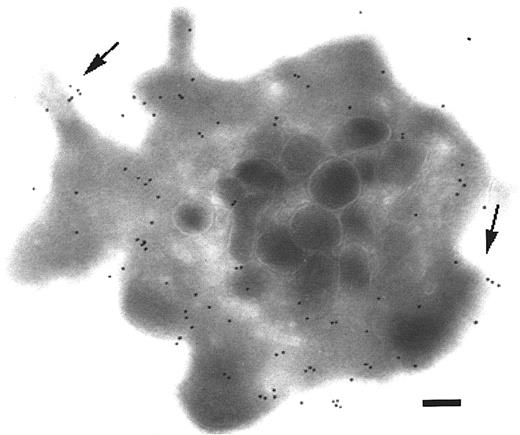
The distribution of P2Y1 was next examined on platelets activated by ADP. Washed platelets were resuspended at 37°C and incubated with 10 μM ADP for 10 minutes without stirring (Figure2). Typical activated platelets are illustrated in Figure 2A-B; note their more spherical shape and the presence of pseudopods (PSs). The granules are also centralized. Globally, the labeling was increased after activation. Pseudopods were often labeled with gold particles, showing that P2Y1 was present. Thin channels were mostly identified by lines of gold particles and often appeared oriented toward the surface. In Figure 2C, gold particles were clearly present in a channel surrounding the centralized granules. This channel has a localization resembling that of the microtubular ring, leaving open the possibility of an association between these structures.
Immunolocalization of P2Y1 in platelets activated with ADP.
Unstirred suspensions of washed platelets were incubated at 37°C for 10 minutes with 10 μM ADP in the presence of 400 μg/mL Fg. (A-B) Surface labeling for P2Y1, now includes pseudopods (PSs). Intracellular labeling remains; lines of gold particles (arrows) can be seen. Also recognized by the MoAb is a clear zone in continuity with a thin channel (arrowheads). (C) P2Y1 within a thin channel (arrows) circulating around the centralized granules. Bars = 0.2 μm.
Immunolocalization of P2Y1 in platelets activated with ADP.
Unstirred suspensions of washed platelets were incubated at 37°C for 10 minutes with 10 μM ADP in the presence of 400 μg/mL Fg. (A-B) Surface labeling for P2Y1, now includes pseudopods (PSs). Intracellular labeling remains; lines of gold particles (arrows) can be seen. Also recognized by the MoAb is a clear zone in continuity with a thin channel (arrowheads). (C) P2Y1 within a thin channel (arrows) circulating around the centralized granules. Bars = 0.2 μm.
Homologous desensitization experiments were performed at 37°C by incubating platelets without stirring with 1 mM ADPβS, chosen because of its stability (Baurand et al23). Platelet function testing showed that platelets initially aggregated with ADPβS when stirred, whereas electron microscopy and immunogold labeling showed changes at 10 minutes of unstirred incubation similar to changes seen with native ADP (not illustrated). However, after incubation with ADPβS for 1 hour, the platelets were unable to aggregate even to freshly added 10 μM ADP and were desensitized. Immunogold labeling of ultrathin sections showed that these platelets now had a discoid shape and that the distribution of P2Y1 within the different membrane systems was similar to that of unstimulated platelets (Figure3).
Detection of P2Y1 in ADP-desensitized platelets.
Desensitization reverses the changes seen during ADP-induced platelet activation. Platelets were incubated at 37°C with the stable ADP analog ADPβS (1 mM) for 1 hour without stirring in the presence of Fg. Immunolocalization on ultrathin sections of the now discoid platelets showed that the P2Y1 distribution resembled that seen on unstimulated platelets with labeling (arrows) of both surface and internal membrane systems. Platelets are illustrated for 2 healthy donors (A-B). Bar = 0.2 μm.
Detection of P2Y1 in ADP-desensitized platelets.
Desensitization reverses the changes seen during ADP-induced platelet activation. Platelets were incubated at 37°C with the stable ADP analog ADPβS (1 mM) for 1 hour without stirring in the presence of Fg. Immunolocalization on ultrathin sections of the now discoid platelets showed that the P2Y1 distribution resembled that seen on unstimulated platelets with labeling (arrows) of both surface and internal membrane systems. Platelets are illustrated for 2 healthy donors (A-B). Bar = 0.2 μm.
Semiquantitative analyses.
Table 1 shows the values of a semiquantitative analysis performed by counting gold particles on a minimum of 100 sections of unstimulated platelets from a pool of 6 control donors. Results confirmed that approximately 5-fold more gold particles were associated with membrane pools inside the platelet than with the platelet surface. On sections of platelets activated by ADP, the density of gold particles increased both at the platelet surface (P < .001) and in the internal membrane systems (P < .02). At the same time, there was an increase in surface area (P < .02). The values for desensitized platelets were close to those obtained for unstimulated platelets for both the surface and the internal compartment. The surface area also returned to values close to those of unstimulated platelets.
Platelets lacking P2Y12.
Platelets of patient ML lack P2Y12; therefore only P2Y1 can assure their activation by ADP. Semiquantitative analysis showed that P2Y1 was normally distributed in the patient's platelets and that total particle counts were not significantly different from the values for normal platelets (P > .05). Interestingly, after ADP activation, the increase in platelet surface area was no longer significant (P > .05). Immunogold labeling of P2Y1 in unstimulated and stimulated platelets from the patient was unchanged from that of the control platelets, as illustrated in Figures 1-2. After a 10-minute incubation with 10 μM ADP, shape change occurred and granules centralized. Labeling concerned the plasma membranes, the membranes of α-granules, and thin channels, showing that the deficiency of P2Y12 has no consequences on the distribution of P2Y1 (not illustrated).
Flow cytometry analysis of P2Y1
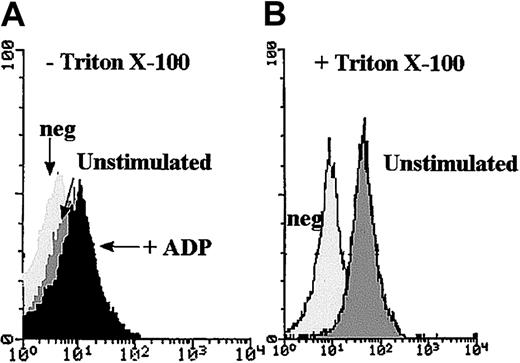
Flow cytometry was used as a second approach to confirm the presence of internal pools of P2Y1. Histograms corresponding to the binding of the anti-P2Y1 MoAb to the surface of PFA-fixed unstimulated platelets showed weak labeling (Figure 4A), confirming previous results.25 A slight increase in mean fluorescence intensity (MFI) was observed after ADP stimulation. A clearly increased MFI for permeabilized platelets with Triton X-100 showed an internal pool, thus agreeing with the results found by electron microscopy and the semiquantitative analysis (Figure 4B).
Flow cytometric analysis of the binding of an anti-P2Y1 MoAb to normal platelets.
(A) Experiments were performed with the use of unstimulated PFA-fixed platelets and platelets incubated for 10 minutes with 10 μM ADP before fixation. There was no permeabilization step. (B) The histograms were obtained after permeabilization of PFA-fixed platelets with Triton X-100 and show the intensity of the internal pool of P2Y1in the internal compartment of unstimulated platelets. The control histograms (neg) were obtained in the presence of irrelevant antibody instead of P2Y1.
Flow cytometric analysis of the binding of an anti-P2Y1 MoAb to normal platelets.
(A) Experiments were performed with the use of unstimulated PFA-fixed platelets and platelets incubated for 10 minutes with 10 μM ADP before fixation. There was no permeabilization step. (B) The histograms were obtained after permeabilization of PFA-fixed platelets with Triton X-100 and show the intensity of the internal pool of P2Y1in the internal compartment of unstimulated platelets. The control histograms (neg) were obtained in the presence of irrelevant antibody instead of P2Y1.
Immunolocalization of TPα
Immunolocalization in resting, activated, and desensitized platelets.
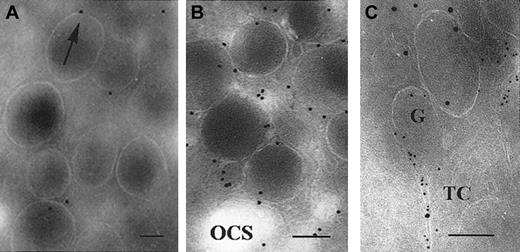
Preliminary experiments showed that when the polyclonal antibody specific to the TPα receptor was used, amplification steps in the labeling procedure were not necessary. The labeling of unstimulated control platelets is shown in Figure 5. Figure 5A shows a typical discoid unstimulated platelet. While gold particles were present at the surface, labeling was mostly inside the platelet. Because the antibody recognized the cytoplasmic part of the receptor, labeling often appeared to be closely associated with the membrane or even just below it. Higher-magnification illustrations showing details of labeled intracellular structures are shown in Figure 5B-D. Thin channels within the OCS can be distinguished and are seen in Figure 5B. Some particles were in lines. Labeling of α-granule membranes is shown in Figure 5C. In Figure 5D is shown an α-granule with a Weibel-Palade–like structure.
Detection of TPα in the membrane systems of unstimulated platelets using a rabbit polyclonal antibody to the carboxyl-terminal domain.
(A) A typical platelet section. Surface labeling is highlighted (arrows) as is the labeling of thin channels within the OCS (arrowheads). (B-C) Higher-power magnifications showing abundant labeling within the thin channels of the OCS and labeling of the membranes of α-granules (arrows). (D) A labeled α-granule containing a Weibel-Palade–like structure. Bars = 0.1 μm.
Detection of TPα in the membrane systems of unstimulated platelets using a rabbit polyclonal antibody to the carboxyl-terminal domain.
(A) A typical platelet section. Surface labeling is highlighted (arrows) as is the labeling of thin channels within the OCS (arrowheads). (B-C) Higher-power magnifications showing abundant labeling within the thin channels of the OCS and labeling of the membranes of α-granules (arrows). (D) A labeled α-granule containing a Weibel-Palade–like structure. Bars = 0.1 μm.
Platelets were also incubated with I-BOP, a stable analog of TXA2, for 10 minutes without stirring. The morphology of the now activated platelet resembled that of ADP-treated platelets, with a rounded shape, centralization of granules, and the presence of pseudopods. Labeling again not only was increased on the surface, but was also greater in the internal pools (Figure6).
Detection of TPα in the membrane systems of I-BOP–activated platelets.
Platelets were incubated with I-BOP for 10 minutes at 37°C without stirring. The illustrated platelet shows both surface (arrows) and intracellular labeling. Pseudopods are present and express TPα. Bars = 0.2 μm.
Detection of TPα in the membrane systems of I-BOP–activated platelets.
Platelets were incubated with I-BOP for 10 minutes at 37°C without stirring. The illustrated platelet shows both surface (arrows) and intracellular labeling. Pseudopods are present and express TPα. Bars = 0.2 μm.
After desensitization by incubation for 1 hour with I-BOP, platelet shape returned to a discoid form (not illustrated), and labeling values close to those of unstimulated platelets were obtained (see “Semiquantitative analyses”). Control experiments in which the polyclonal antibody was replaced by an equivalent amount of an irrelevant rabbit antibody showed a much decreased labeling (see Table2 footnote). Furthermore, preincubation of the antibody to TPα with blocking amounts of the peptide used for immunization also resulted in a loss of the labeling (not illustrated).
Number of gold particles per platelet section after labeling with the anti-TPα receptor
| Platelets . | Gold particles per platelet section, no.* . | Mean surface area per platelet section, μm2 . | ||
|---|---|---|---|---|
| Surface . | Internal membranes . | Total . | ||
| Controls | ||||
| Unstimulated | 16.60 ± 7.94 | 51.71 ± 18.67 | 68.32 ± 22.92 | 2.36 ± 0.92 |
| I-BOP stimulated | 32.84 ± 16.90 | 85.69 ± 49.93 | 118.53 ± 61.36 | 3.19 ± 1.27 |
| Desensitized | 15.89 ± 6.16 | 59.17 ± 22.71 | 75.06 ± 23.54 | 2.52 ± 1.12 |
| Patient ML | ||||
| Unstimulated | 18.53 ± 11.16 | 51.85 ± 29.78 | 70.38 ± 37.84 | 2.58 ± 0.93 |
| I-BOP stimulated | 32.67 ± 11.18 | 103.92 ± 50.46 | 136.59 ± 58.68 | 2.8 ± 1.12 |
| Platelets . | Gold particles per platelet section, no.* . | Mean surface area per platelet section, μm2 . | ||
|---|---|---|---|---|
| Surface . | Internal membranes . | Total . | ||
| Controls | ||||
| Unstimulated | 16.60 ± 7.94 | 51.71 ± 18.67 | 68.32 ± 22.92 | 2.36 ± 0.92 |
| I-BOP stimulated | 32.84 ± 16.90 | 85.69 ± 49.93 | 118.53 ± 61.36 | 3.19 ± 1.27 |
| Desensitized | 15.89 ± 6.16 | 59.17 ± 22.71 | 75.06 ± 23.54 | 2.52 ± 1.12 |
| Patient ML | ||||
| Unstimulated | 18.53 ± 11.16 | 51.85 ± 29.78 | 70.38 ± 37.84 | 2.58 ± 0.93 |
| I-BOP stimulated | 32.67 ± 11.18 | 103.92 ± 50.46 | 136.59 ± 58.68 | 2.8 ± 1.12 |
Experimental procedures were as for Table I. Results are ± SD. For controls done with an irrelevant rabbit antibody, the number of gold particles evaluated for 100-platelet sections constituted, respectively. 0.74% (unstimulated) and 0.48% (stimulated) of the total number of gold particles seen with the anti-TPα antibody.
Semiquantitative analyses.
Results in Table 2 show that for this antibody between 3- and 4-fold more gold particles were associated with the internal membrane pools than with the platelet surface. The total number of gold particles increased significantly for both the surface membrane (P < .001) and the internal pool (P < .001) after I-BOP treatment. The surface area of the platelets also increased significantly (P < .001). All values returned to initial levels after desensitization. For patient ML, I-BOP stimulation also induced a significantly greater antibody labeling (P < .001) for both the surface and the internal compartment. In contrast, the increase in surface area was not significant (P > .05).
Intraplatelet activation of αIIbβ3associated with secretion.
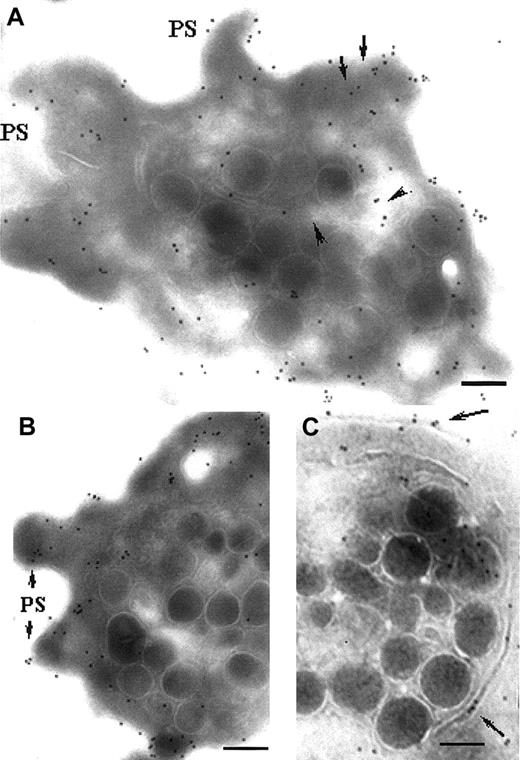
Because receptors for 2 primary agonists have been revealed to be associated with thin channels and α-granule membranes, we looked closely at the activation of internal pools of αIIbβ3 using a MoAb, AP-6, recognizing this integrin after Fg has bound. In agreement with our initial report,27 with unstimulated platelets, occasional labeling with AP-6 is mostly confined to the α-granule membrane (Figure7A). After incubating platelets for 10 minutes with 10 μM ADP, labeling with AP-6 increased on the granule membrane and also in closely associated thin channels (Figure 7B). In Figure 7C, double-labeling performed with AP-6 (10-nm gold particles) and P-selectin (5-nm gold particles) showed their concomitant presence in a thin channel apparently connecting with an α-granule, showing that trafficking of P-selectin and the integrin-bound Fg were occurring simultaneously, thereby ruling out visualization of Fg uptake. This was also confirmed by performing experiments in the absence of added Fg (not shown).
Detection of ligand-bound αIIbβ3 by the anti-LIBS MoAb, AP-6.
(A) Unstimulated, washed platelets. Only a few gold particles were present per platelet, and these were located in the vicinity of α-granule membranes (arrow). (B) A similar illustration, but for unstirred platelets incubated with 10 μM ADP for 10 minutes. Note the increased labeling in the vicinity of the α-granule membrane. (C) Double-staining has been performed for platelets incubated with ADP as in panel B; an initiation of secretion is shown by the concomitant labeling with P-selectin (5-nm gold particles) and AP-6 (10-nm gold particles) in a thin channel extending from an α-granule. Bars = 0.1 μm.
Detection of ligand-bound αIIbβ3 by the anti-LIBS MoAb, AP-6.
(A) Unstimulated, washed platelets. Only a few gold particles were present per platelet, and these were located in the vicinity of α-granule membranes (arrow). (B) A similar illustration, but for unstirred platelets incubated with 10 μM ADP for 10 minutes. Note the increased labeling in the vicinity of the α-granule membrane. (C) Double-staining has been performed for platelets incubated with ADP as in panel B; an initiation of secretion is shown by the concomitant labeling with P-selectin (5-nm gold particles) and AP-6 (10-nm gold particles) in a thin channel extending from an α-granule. Bars = 0.1 μm.
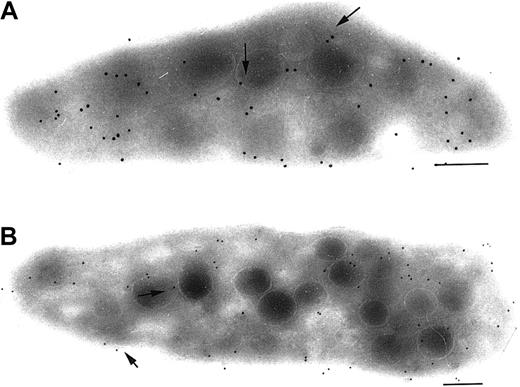
Immunogold labeling of P2Y1 and TPα in megakaryocytes.
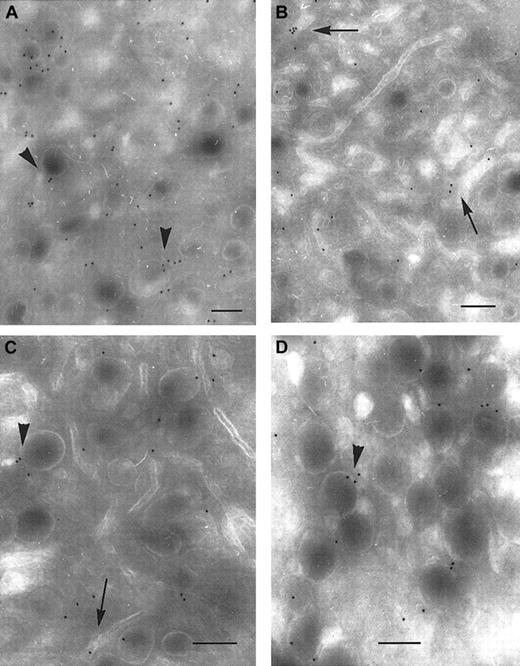
We finally looked to see how P2Y1 and TPα were distributed in mature human megakaryocytes (MKs) obtained from a control donor. MKs were identified in a marrow aspirate by their typical morphology and the presence of a polylobular nucleus.24 Labeling was detected on surface membranes, but was relatively sparse (not illustrated). Significantly, both P2Y1 and TPα were present along the demarcation membrane system (DMS). Both receptors were also detected in membranes of α-granules (Figure 7). Thus, both P2Y1 and TPα are expressed during megakaryocyte maturation and are therefore likely to be present in the different membrane systems of platelets at the time of their release (Figure 8).
Immunolocalization of P2Y1 and TPα receptors in the membrane systems of mature human megakaryocytes.
(A-B) Immunolocalization of P2Y1. In panel A, gold particles are seen associated with α-granule membranes (arrowheads); in panel B, they are present in the DMS (arrows). (C-D) Immunolocalization of TPα receptors. The labeling observed with an anti-TPα is also associated with α-granule membranes and the DMS. Bars = 0.2 μm.
Immunolocalization of P2Y1 and TPα receptors in the membrane systems of mature human megakaryocytes.
(A-B) Immunolocalization of P2Y1. In panel A, gold particles are seen associated with α-granule membranes (arrowheads); in panel B, they are present in the DMS (arrows). (C-D) Immunolocalization of TPα receptors. The labeling observed with an anti-TPα is also associated with α-granule membranes and the DMS. Bars = 0.2 μm.
Discussion
We have compared the distribution within the platelet of 2 receptors, P2Y1 and TPα, belonging to the GPCR family. Although many members of this family have been identified in platelets, apart from PAR-1, a receptor for thrombin,1,18,19 little is known about their distribution within the different membrane systems. As well as having a plasma membrane, platelets have a well-developed OCS and several types of storage organelles. Major changes are seen in the distribution of these membranes during platelet activation and secretion (reviewed in Nurden17). Interestingly, although P2Y1 and TPα were found as expected in the plasma membrane, the bulk of the immunogold labeling on ultrathin sections concerned internal membrane pools. These included the membranes of α-granules and those of the OCS, including an important network of thin channels that ramify from the surface into the interior of the platelet. The presence of major intracellular pools of these receptors for primary agonists in platelets can have important implications for platelet physiology.
P2Y1 and TPα are both associated with Gα proteins and generate signals inducing platelet activation.3,7-9,15 ADP is the specific ligand for P2Y1 in platelets. ADP also has a second receptor, P2Y12, only recently cloned.4,29 The sparse labeling observed at the platelet surface with the anti-P2Y1 MoAb is in accordance with the low binding previously observed with the same antibody in flow cytometry25 and also confirmed here. ADP receptors have been quantified with the use of radiolabeled 2-methyl-thiol-ADP (2MeS-ADP), which binds to between 500 and 1000 sites per platelet.5,30-32 This stable ADP analog binds to both P2Y1 and P2Y12. Evaluation of the specific contribution of P2Y1 is allowed by binding studies performed under conditions in which one receptor is blocked or absent. In the 2 well-characterized patients lacking P2Y12, the number of 2MeS-ADP–binding sites fell to about 30 for one patient (ML)5 and to about 170 for a second patient.30 This low range of values explains why an amplification procedure was required to detect P2Y1 with the use of the single MoAb available to us. Because it is unlikely that 2MeS-ADP can reach all of the internal receptor pools identified by us, the number of sites found with the use of radiolabeled ligand almost certainly underestimates the total number of copies per platelet. Intriguingly, in the SP1999 mouse model in which P2Y12 was deleted, no specific binding with 2MeS-ADP could be measured.29 Given that our results for patient ML show a relatively normal distribution of P2Y1 between the surface and internal pools, the hypothesis of a down-regulation of P2Y1 in the absence of P2Y12 can be excluded, at least in humans. Surprisingly, the surface area of platelets increased less for the patient than for the controls during ADP activation, suggesting that although shape change is present and pseudopods form, an associated volume change is absent.
When a rabbit antibody to the carboxyl-domain of TPα was used, sufficient labeling was obtained for a classic detection procedure to be used. Yet labeling of the surface membrane again remained sparse. Once more, greater numbers of gold particles were seen inside platelets, with the membranes of α-granules and those of the thin channels of the OCS again labeled. Previous binding studies with radiolabeled I-BOP showed close to 1500 sites of TPα per platelet.33,34 I-BOP binds to both high- and low-affinity sites. With doubts being expressed over the presence of TPβ, it was suggested that TPα can represent the 2 affinity states recognized by TXA2 mimetics in platelets.26 It is probable that our MoAb recognizes both forms. As P2Y1and TPα show a similar distribution in the different membrane systems of the platelet, it will be interesting to extend these studies to other GPCR receptors, particularly to P2Y12, to see if this is a common finding. Platelets from patient ML have a much decreased aggregation response to I-BOP.22 This was interpreted as showing that ADP (via P2Y12) was a major cofactor in this response. The normal distribution of TPα in the platelets of one patient (ML), and the normal response to activation and desensitization with I-BOP, would be in line with this conclusion.
After platelet activation, the labeling of P2Y1 and TPα significantly increased both at the platelet surface and inside the cell, although the latter increase was lower. Because the capacity for protein synthesis in platelets is very low, an explanation is that their reactivity and/or accessibility to the antibodies on the platelet sections is increased. Trafficking of receptors from the internal pool to the periphery of the cell may also contribute. Interestingly, desensitization was accompanied by a return to a discoid shape and basal surface area of platelets and reactivity with the antibodies on platelet sections, which is in favor of a reversible change in receptor accessibility and/or conformation associated with activation. Another GPCR, the β2-adrenergic receptor, is known to modify its conformation after stimulation through removal of a constraint imposed by an ionic lock located in internal cytoplasmic domains.35 Events such as this could potentially influence the binding of antibodies.
Baurand et al23 observed a decreased number of 2MeS-ADP–binding sites on desensitized platelets and concluded that internalization was responsible although it was not excluded that the receptors remained refractory to further contact with ligand. Internalization of P2Y1 was shown in transfected Jurkat cells on incubation with ADP, but this model is very different from platelets, where a large proportion of receptors were already present in the internal compartment. Even if internalization is frequently associated with desensitization of GPCRs,36 there may be large differences in the responses of different cell types. In our results, the receptor partition between the internal and surface pools after desensitization was similar to that on resting platelets, and we found no evidence of coated pits, endosomes, or receptor accumulation in lysosomal granules. The presence of both receptors in mature MKs resembled that in unstimulated platelets, a fact that argues against down-regulation during platelet isolation.
A major question relates to the possible functional significance of the internal pools, present not only on the membranes of the OCS and thin channels, but also in the membrane of the α-granules. Platelets possess a storage pool of ADP within the dense granules. This pool is secreted into the channels of the OCS before being released to the external medium.37 TXA2is produced in platelets following arachidonic acid release and activation of the prostaglandin synthesis pathway.38Liberation of arachidonic acid from membrane phospholipids can be induced by many agonists, including ADP and collagen.38,39It can be hypothesized that during the activation that follows platelet attachment to subendothelial constituents such as collagen or von Willebrand factor (VWF), the internal pools of receptors for primary agonist would be in contact with TXA2 before their homologs present at the platelet surface. Similarly, ADP released from the granules can have access to P2Y1receptors inside the platelet before the external pool. Using a mathematical model, Fogelson and Wang,40 have shown that following diffusion of ADP through the channels, the near-surface concentration is very low compared with the internal concentration. In δ-storage pool disease (SPD), where ADP is absent or severely decreased in dense granules,41 or in aspirin-treated platelets where TXA2 cannot be formed,38 no second wave of aggregation is seen after activation by ADP, whereas collagen-induced platelet activation is also much reduced. The ability of ADP to induce secretion from platelets is controversial42 and may depend on the presence or absence of Ca2+ in the medium. Notwithstanding this, incubation of platelets with ADP in our studies resulted in an increased amount of ligand-bound αIIbβ3 on α-granule membranes and in associated thin channels; these presumably represented initiation of secretory pathways, for they contained P-selectin. The extent of the changes was nonetheless very different from the maximal secretion that occurred after activation of platelets with thrombin, where Fg associates rapidly with internal pools of αIIbβ3 prior to being translocated to the exterior.27
Definitive proof of a functional role of the intracellular receptors is not easy to obtain. Pharmacologic inhibitors of ADP receptors such as adenosine 3′,5′-diphosphate (A3P5P; P2Y1) and N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene ATP (AR-C69931MX; P2Y12) or ADP-eliminating enzymes such as creatine phosphate/creatine phosphokinase (CP/CPK), while blocking surface receptors and removing extracellular ADP, would enter the OCS to an unknown extent. Thrombus buildup on collagen under flow was markedly affected by a combination of A3P5P and AR-C69931MX, with both adhesion and platelet-to-platelet interactions affected.43 Yet the addition of CP/CPK with total ADP removal was more complete, leaving open a partial inhibition of receptors in the OCS by the antagonists. Similarly, we have shown that a combination of A3P5P, AR-C69931MX, and CP/CPK leads to a more effective inhibition of thrombin receptor-activated peptide (TRAP)–induced platelet aggregation than occurs with the individual antagonists alone or in pairs, perhaps suggesting that total inhibition of the receptor pools is difficult to achieve (P.N., unpublished data, January, 2002).
In summary, we have observed that platelets, in addition to the plasma membrane pool of receptors for primary agonists ADP and TXA2, possess an internal pool present in membranes of α-granules and in a network of channels connected to the surface membrane, in proximity to integrin effectors and ligands. These constitute a microenvironment that becomes interlinked when exocytosis occurs. A subcellular localization of α-subunits of trimeric G-proteins to α-granule membranes has been previously shown,44 so another important part of the signaling machinery is also in place for agonists that could act alone or in synergy.45 Our results therefore raise the question of the role of this internal compartment of agonist receptors and its contribution in platelet activation. At the present time, ADP receptors are a major target for antithrombotic therapy, as is TPα.3,16,38 Although thienopyridines, such as clopidogrel, target P2Y12,46 whose platelet distribution has yet to be established, recent results for P2Y1 null mice47 suggest that it too is an appropriate target for antithrombotic therapy. Future antithrombotic strategies may well need to take into account less accessible internal pools.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-02-0642.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paquita Nurden, UMR 5533 CNRS, Hôpital Cardiologique, 33604 Pessac, France; e-mail:paquita.nurden@cnrshl.u-bordeaux2.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal