The fundamental hypotheses behind fetal gene therapy are that it may be possible (1) to achieve immune tolerance of transgene product and, perhaps, vector; (2) to target cells and tissues that are inaccessible in adult life; (3) to transduce a high percentage of rapidly proliferating cells, and in particular stem cells, with relatively low absolute virus doses leading to clonal transgene amplification by integrating vectors; and (4) to prevent early disease manifestation of genetic diseases. This study provides evidence vindicating the first hypothesis; namely, that intravascular prenatal administration of an adenoviral vector carrying the human factor IX (hFIX) transgene can induce immune tolerance of the transgenic protein. Following repeated hFIX protein injection into adult mice, after prenatal vector injection, we found persistence of blood hFIX and absence of hFIX antibodies in 5 of 9 mice. Furthermore, there was substantial hFIX expression after each of 2 reinjections of vector without detection of hFIX antibodies. In contrast, all adult mice that had not been treated prenatally showed a rapid loss of the injected hFIX and the development of high hFIX antibody levels, both clear manifestations of a strong immune reaction.
Introduction
Factor IX (FIX), a crucial component of the blood-clotting cascade, is dysfunctional in 1 in 25 000 males suffering from X-linked hemophilia B. Because of its high cost, recombinant human factor IX (hFIX) is given to these patients only prophylactically before surgery or palliatively during spontaneous bleeding episodes into joints or internal organs. In some patients, the development of inhibitory antibodies against the recombinant protein severely compromises therapy; neutralizing antibodies appear in 3% to 4% of treated patients.1 In roughly half of those patients who develop inhibitors, anaphylaxis or severe allergic reactions occur on infusion of any type of factor IX–containing product.2 Because the development of inhibitors can be associated with dramatically increased morbidity and mortality3 and tremendous treatment costs,4novel therapeutic strategies such as gene therapy have to address this dominant problem in the treatment of hemophiliacs.
Restitution of the factor IX gene by gene therapy promises to abrogate or ameliorate the symptoms of disease. In addition, it may provide a substantially cheaper therapeutic alternative because a single injection may provide prolonged, and perhaps permanent, factor IX production.
Several investigators have shown successful delivery and expression of adenovirus and adenoassociated virus (AAV) vectors expressing a factor IX cDNA in animal models. These studies include the intravenous delivery of adenovirus encoding hFIX to achieve sustained expression in immunosuppressed dogs.5 Application of AAV and adenovirus, respectively, by intramuscular injection to certain mouse strains has also permitted sustained expression.6,7 In particular, it has been shown that C57/BL6 mice, unlike several other strains, fail to generate an immune response to adenovirus carrying the hFIX cDNA (AdhFIX) when this vector is administered intravenously8and that adenovirus vector delivery to these mice induces tolerance of hFIX in preimmunized mice.7 However, in most other mouse strains only transient expression has been observed.9 10
Sustained FIX expression has also been observed after intramuscular injection of AAV vectors expressing canine factor IX to adult hemophiliac dogs11-14 and hFIX to adult C57/BL6 hemophiliac mice by intraportal and intravenous routes.13These investigations have led to the first clinical trial in humans that shows promising results, although only relatively low levels of hFIX have been reported so far.15
The long-term substitution of hFIX at therapeutic levels without immune response remains, therefore, one of the major tasks in hemophilia gene therapy. Advancing gene therapy from the adult to the fetus may provide higher levels of transgene expression and prevent immune responses to transgenic protein and vector as the exogenous gene products should be recognized as self and, thus, avoid immune elimination.16
A detailed study on the immune reaction to transgene expression in the fetus was performed by Lipshutz et al.17 They applied adenovirus expressing the luciferase gene by intraperitoneal injection into fetal CD-1 mice at 15 days after conception. A group of these mice were killed periodically, and hepatic luciferase activity was observed for a month. A second vector injection at 3 months, following the loss of activity, caused a temporary renewal of luciferase expression. However, a third injection of vector at 6 months precipitated substantial antibody production against luciferase and adenovirus with no renewal of luciferase expression. In contrast, prenatal intraperitoneal injection of adenoassociated virus carrying the luciferase gene resulted in sustained expression without development of antibodies against transgenic protein or vector, but no postnatal vector or protein challenge was given.18
Compared with marker genes, hFIX gene transfer is particularly suited for studies on tolerance after gene therapy. Blood sampling permits continuous longitudinal in vivo monitoring of gene expression and antibody levels, and clinical-grade hFIX is readily available for use during immune challenge.
Walter et al19 showed that a single intravenous injection of AdhFIX into adult CD-1 mice resulted in significant hFIX expression, whereas an ensuing injection led to no further expression but caused a substantial increase in adenovirus antibodies. In contrast, a primary injection in neonatal mice, which still possess an immature immune system, induced hFIX synthesis of a similar magnitude and duration yet did not preclude substantially increased hFIX production after the second injection. Furthermore, production of adenovirus antibodies was blunted. Notably, no hFIX antibody response was detected in any group. However, unfortunately, tolerance was not tested through further exposure to hFIX or adenovirus.
Previously, we found that injection of AdhFIX into the amniotic fluid of fetal MF1 mice resulted in short-term, high-level transient hFIX expression possibly curtailed by shedding of infected keratinocytes, shutdown of the cytomegalovirus (CMV) promoter, or immunity against vector or transgene.20 More recently we saw prolonged expression of hFIX at low levels after intramuscular and intraperitoneal injection of an AAVhFIX vector into fetal C57BL/6J mice.21 No immune reactions were observed, but this mouse strain may obscure the immunologic situation.
We used MF1 mice in these experiments as they have been used for several other studies on prenatal gene therapy in our laboratory.20-22 They are fecund and easily tolerate complicated in utero manipulation. Furthermore, as they are an outbred strain, they are less likely to show immunologic vagaries as observed in other strains.7 8
We demonstrate here that in utero gene transfer induces long-term postnatal tolerance of hFIX after repeated application of purified protein in 5 of 9 adult immune-competent mice.
Materials and methods
Vector preparation
E1/E3-deleted adenoviral vectors AdRSVLacZ and AdTG9397 (AdhFIX) (Transgene, Strasbourg, France) containing the nuclear-targeting bacterial β-galactosidase reporter gene and the human coagulation FIX gene, respectively, were prepared as previously described.20 Virus concentrations were determined by plaque assays on 293 cells. Each vector was tested for the presence of helper virus. Expression of the bacterial β-galactosidase reporter gene of AdRSVβgal is driven by the Rous sarcoma virus (RSV) promoter. AdTG9397 contains the CMV promoter driving the expression of the FIX gene, an adenoviral tripartite leader sequence, and the SV40 polyadenylation site.
Administration of vector
Pregnant female MF1 mice (B&K Universal, United Kingdom) at 15 and 17 days after conception were used in this study. The procedure of intravascular injection to fetuses was similar to that described by Schachtner et al.23 Under isoflurane anesthesia, the uterus was exposed through a full-depth midline laparotomy. A 34-gauge needle (Hamilton, United Kingdom) was used to perform a transuterine injection of 30 μL solution into a peripheral yolk sac vessel. Up to 10 fetuses were injected per dam. The remainder was injected subcutaneously with colloidal carbon to act as a negative marker of vector administration. The laparotomy was closed in 2 stages, using interrupted stitches of 6-0 silk suture, and the mouse was permitted to recover in a warm cage. Some fetuses were injected into the yolk sac vessel with colloidal carbon. Vector was administered as a 1-mL volume to adult mice by tail-vein injection.
For prenatal analysis, dams were killed by cervical dislocation and fetuses were decapitated. For postnatal end point analysis, mice were anesthetized with isoflurane and exsanguinated by cardiac puncture. Blood was collected into 0.109 M sodium citrate buffer (5:1 vol/vol) and centrifuged at 2500g for 5 minutes. For plasma collection, in mice older than 2 weeks, blood was also allowed to clot for serum analysis of antibodies. Fetuses and mice less than a month old were placed in 100% ethanol for 2 hours, injected with ethanol, and halved sagittally. One half was processed for immunohistochemistry and the other for X-gal staining. From older mice, viscera were dissected and fixed separately. Repeated blood sampling was performed by puncture of the lateral tail vein. For study of transgene clearance and immunity, some mice were given additional intraperitoneal injections of hFIX protein (Hematologic Technologies, USA) and bled 4 hours later.
Histochemistry
Tissue was fixed in 70% ethanol for 2 hours, then washed in phosphate-buffered saline (PBS) for 2 hours before overnight staining in X-gal solution containing potassium ferricyanide (5 mM), potassium ferrocyanide (5 mM), magnesium chloride (2 mM), and X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 1 mg/mL dissolved in dimethylsulphoxide, 1 mL). Stained tissue was fixed in 10% formaldehyde for 2 hours and then transferred to 70% ethanol for paraffin embedding. Sections (4 μm) were counterstained with neutral red.
Detection of factor IX and antibodies
Plasma concentrations of hFIX protein were determined by using the Asserachrom FIX ELISA (enzyme-linked immunosorbent assay) kit. Pooled normal human plasma assumed to contain 5 μg/mL was used as assay standard.21
Antibodies against hFIX were detected by ELISA; 96-well plates were coated at 4°C overnight with 2.5 μg/mL hFIX, washed with PBS, and then incubated with 3% bovine serum albumin for 1 hour at 37°C. Serum samples diluted 1/10 000 or 1/1 000 000 with assay buffer (Asserachrom FIX ELISA kit) were added to the wells and left standing for 90 minutes at room temperature. Some samples were also diluted 1/20 and 1/100. After washing with wash buffer (Asserachrom FIX ELISA kit), horseradish peroxidase–conjugated rabbit antimouse antibody (DAKO, Cambridge, United Kingdom) was applied for 90 minutes at room temperature. This antibody detects immunoglobulin G1 (IgG1), IgG2a, IgG2b, IgG3, IgA, and IgM. After washing, o-phenylenediamine dihydrochloride/urea peroxide substrate solution was added for exactly 3 minutes, and the reaction was stopped. Absorbances were measured at 492 nm.
Concentrations of adenovirus antibody were assessed analogously with a 96-well plate coated with 1 × 109 pfu AdRSVβgal. In both assays, sample values were expressed as a ratio of sample absorbance versus absorbance of hyperimmune serum that was generated by repeated intramuscular administration of AdhFIX and hFIX protein. A standard curve constructed from dilutions of hyperimmune serum was used to establish the range of sensitivity. At a dilution of 1/10 000, hyperimmune serum had an approximate optical density (OD) of 0.5.
Coagulation inhibition assay
Coagulation inhibition was examined using a partial thromboplastin time (PTT)–based one-stage factor IX clotting assay. All samples were diluted in Tris (tris(hydroxymethyl)aminomethane)–buffered saline with 1 mg/mL human albumin (clinical grade 20% albumin; Bio Products Laboratory, Elstree, United Kingdom). Samples of mouse plasma (50 μL, diluted × 10) were incubated with 50 μL normal pooled human plasma for 2 hours at 37°C to allow inactivation of hFIX by murine antibodies (if present). Residual hFIX was measured in a one-stage assay. hFIX-deficient human plasma (100 μL; Diagnostic Reagents, Thame, United Kingdom) and 100 μL sample from the previous incubation (further diluted × 10 or × 100) were mixed with 100 mL phospholipid/activator (cephalin/silica; Instrumentation Laboratory, Warrington, United Kingdom) and incubated for 5 minutes at 37°C. Then 100 mL 25 mM CaCl2 was added, and the clotting time was measured by using a Coag-a-Mate coagulometer (Organon Teknika, Cambridge, United Kingdom). Normal pooled human plasma diluted × 2 with Tris buffer/albumin was further diluted × 10 to × 1000 to provide a reference curve.
Results
Intravascular administration to the fetus results in strong transgene expression in the liver and cardiac muscle
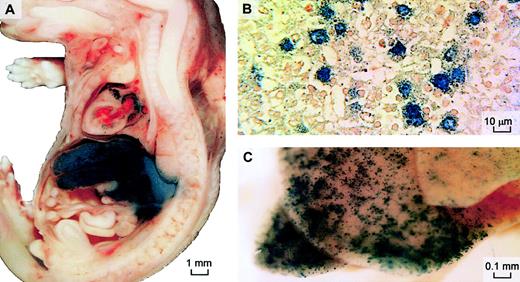
To evaluate the efficacy and location of intravascular adenoviral gene expression in our fetal mouse model, AdLacZ vector (8 × 1010 pfu/mouse) was injected via the yolk sac vessel into 6 fetal mice 16 days after conception. β-Galactosidase expression was found in the liver and muscle of the cardiac atria 2 and 9 days after vector injection (Figure 1); 28 days after injection (24 days after birth) only a few β-galactosidase–positive cells could be seen.
β-Galactosidase expression after prenatal intravenous delivery of adenoviral vector.
AdRSVLacZ was injected intravenously into prenatal mice 16 days after conception to examine distribution of β-galactosidase expression by this route. X-gal staining was performed on fetal sagittal sections 2 days after injection (A) or on whole neonatal liver 9 days after injection (B). Cellular expression in hepatocytes is confirmed on wax-embedded histologic sections of the stained whole liver 2 days after injection (original magnification, × 400) (C). Neutral red was used as counterstain.
β-Galactosidase expression after prenatal intravenous delivery of adenoviral vector.
AdRSVLacZ was injected intravenously into prenatal mice 16 days after conception to examine distribution of β-galactosidase expression by this route. X-gal staining was performed on fetal sagittal sections 2 days after injection (A) or on whole neonatal liver 9 days after injection (B). Cellular expression in hepatocytes is confirmed on wax-embedded histologic sections of the stained whole liver 2 days after injection (original magnification, × 400) (C). Neutral red was used as counterstain.
Longitudinal analysis of hFIX expression and persistence
To investigate the duration of prenatal transgene expression and its effect on the induction of postnatal immune reactions, we applied an adenovirus vector expressing hFIX via the yolk sac vessel (Figures2-4). The time course of this experiment was divided into 3 phases.
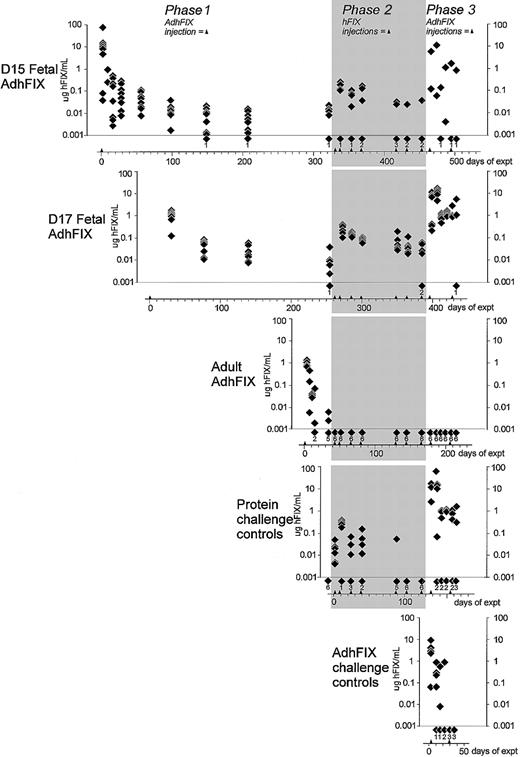
Persistence of expression and tolerance of exogenous and expressed hFIX.
Prenatal and adult mice were given an initial intravenous injection of AdhFIX and were then repeatedly rechallenged, as adults, with intraperitoneal hFIX protein then intravenous AdhFIX while hFIX concentrations were measured. The y-axis shows blood hFIX concentrations (microgram per milliliter) after in utero or adult injection of AdhFIX (phase 1), after repeated injection of hFIX protein to the adult mice (phase 2, shaded), and after repeated injection of AdhFIX to the adult mice (phase 3). The x-axis shows the experimental time course in days after first the injection (day 0). Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX group are mice initially injected in utero with AdhFIX at days 15 or 17 after conception, respectively. The adult AdhFIX group contains mice initially injected intravenously with AdhFIX as adults. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. The D15 and D17 fetal AdhFIX groups, the adult AdhFIX group, and the protein challenge control group received their first hFIX protein injection at 66, 56, 16 to 18, and 30 to 35 weeks of age, respectively. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable hFIX at each time point is depicted as a diamond and number, both lying beneath the lower dotted line. The first 2 time points (days 2 and 9) are mice from the D15 fetal AdhFIX group killed for analysis; the ensuing points are from continuous sampling of mice in all groups. The reduction of animals over the length of time was predominantly age-related death.
Persistence of expression and tolerance of exogenous and expressed hFIX.
Prenatal and adult mice were given an initial intravenous injection of AdhFIX and were then repeatedly rechallenged, as adults, with intraperitoneal hFIX protein then intravenous AdhFIX while hFIX concentrations were measured. The y-axis shows blood hFIX concentrations (microgram per milliliter) after in utero or adult injection of AdhFIX (phase 1), after repeated injection of hFIX protein to the adult mice (phase 2, shaded), and after repeated injection of AdhFIX to the adult mice (phase 3). The x-axis shows the experimental time course in days after first the injection (day 0). Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX group are mice initially injected in utero with AdhFIX at days 15 or 17 after conception, respectively. The adult AdhFIX group contains mice initially injected intravenously with AdhFIX as adults. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. The D15 and D17 fetal AdhFIX groups, the adult AdhFIX group, and the protein challenge control group received their first hFIX protein injection at 66, 56, 16 to 18, and 30 to 35 weeks of age, respectively. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable hFIX at each time point is depicted as a diamond and number, both lying beneath the lower dotted line. The first 2 time points (days 2 and 9) are mice from the D15 fetal AdhFIX group killed for analysis; the ensuing points are from continuous sampling of mice in all groups. The reduction of animals over the length of time was predominantly age-related death.
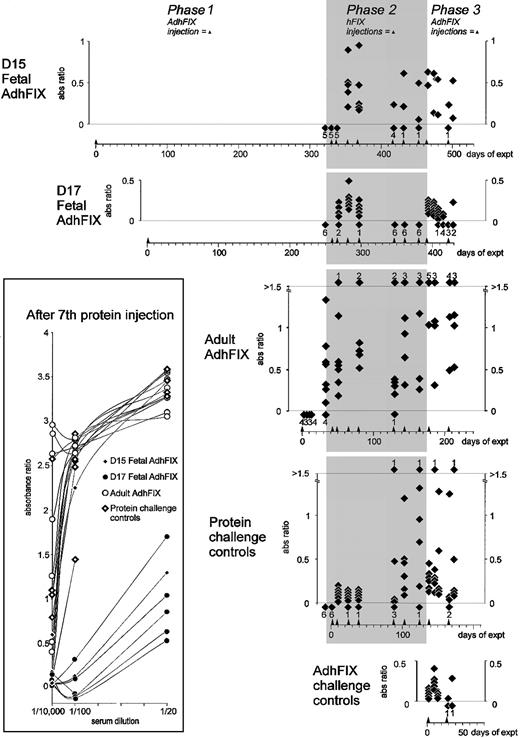
Immunity toward exogenous and expressed hFIX.
Mice were injected in utero or as adults with AdhFIX then repeatedly rechallenged with hFIX protein and AdhFIX as described in Figure 2 while hFIX antibody concentrations were measured. The y-axis shows blood hFIX antibody concentrations (ratio versus control hyperimmune serum, 1/10 000 dilution) after in utero or adult injection of AdhFIX (phase 1), repeated injection of hFIX protein to the adult mice (phase 2, shaded), and repeated injection of AdhFIX to the adult mice (phase 3). The x-axis shows the experimental time course in days after first injection. Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX groups are mice initially injected in utero with AdhFIX at days 15 and 17 after conception, respectively. The adult AdhFIX group contains mice initially injected as adults with AdhFIX. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable antibodies at each time point is depicted as a diamond and number, both lying beneath the lower dotted line. The number of samples exceeding an absorbance ratio of 1.5 at each time point is depicted as a diamond and number, both lying above the upper dotted line. The inset shows values of ratio versus control hyperimmune serum using 3 dilutions (1/20, 1/100, and 1/10 000) for individual mice after the seventh injection of hFIX protein.
Immunity toward exogenous and expressed hFIX.
Mice were injected in utero or as adults with AdhFIX then repeatedly rechallenged with hFIX protein and AdhFIX as described in Figure 2 while hFIX antibody concentrations were measured. The y-axis shows blood hFIX antibody concentrations (ratio versus control hyperimmune serum, 1/10 000 dilution) after in utero or adult injection of AdhFIX (phase 1), repeated injection of hFIX protein to the adult mice (phase 2, shaded), and repeated injection of AdhFIX to the adult mice (phase 3). The x-axis shows the experimental time course in days after first injection. Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX groups are mice initially injected in utero with AdhFIX at days 15 and 17 after conception, respectively. The adult AdhFIX group contains mice initially injected as adults with AdhFIX. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable antibodies at each time point is depicted as a diamond and number, both lying beneath the lower dotted line. The number of samples exceeding an absorbance ratio of 1.5 at each time point is depicted as a diamond and number, both lying above the upper dotted line. The inset shows values of ratio versus control hyperimmune serum using 3 dilutions (1/20, 1/100, and 1/10 000) for individual mice after the seventh injection of hFIX protein.
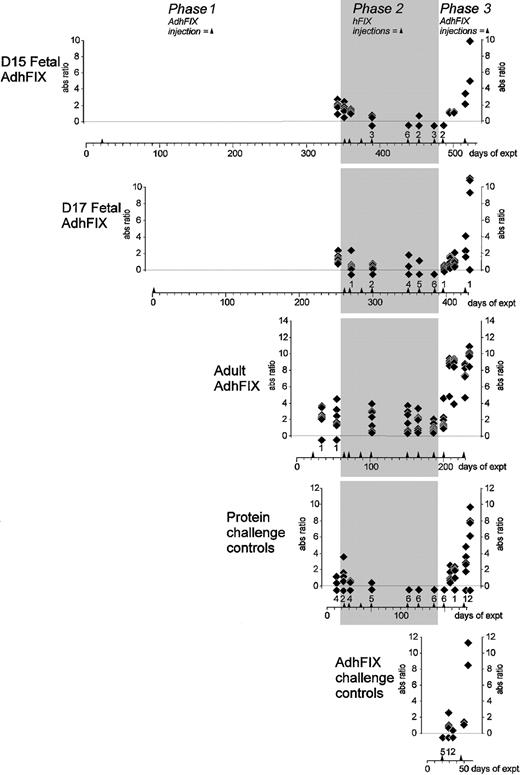
Immunity toward adenoviral vector.
Mice were injected in utero or as adults with AdhFIX then repeatedly rechallenged with hFIX protein and AdhFIX while adenovirus antibody concentrations were measured. The y-axis shows blood adenovirus antibody concentrations (ratio versus control hyperimmune serum, 1/10 000 dilution) after in utero or adult injection of AdhFIX (phase 1), repeated injection of hFIX protein to the adult mice (Phase 2, shaded), and repeated injection of AdhFIX to the adult mice (Phase 3). The x-axis shows the experimental time course in days after the first injection. Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX groups are mice initially injected in utero with AdhFIX at days 15 and 17 after conception, respectively. The adult AdhFIX group contains mice initially injected as adult with AdhFIX. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable antibodies at each time point is depicted as a diamond and number, both lying beneath the lower dotted line.
Immunity toward adenoviral vector.
Mice were injected in utero or as adults with AdhFIX then repeatedly rechallenged with hFIX protein and AdhFIX while adenovirus antibody concentrations were measured. The y-axis shows blood adenovirus antibody concentrations (ratio versus control hyperimmune serum, 1/10 000 dilution) after in utero or adult injection of AdhFIX (phase 1), repeated injection of hFIX protein to the adult mice (Phase 2, shaded), and repeated injection of AdhFIX to the adult mice (Phase 3). The x-axis shows the experimental time course in days after the first injection. Arrowheads indicate injection points. The D15 and D17 fetal AdhFIX groups are mice initially injected in utero with AdhFIX at days 15 and 17 after conception, respectively. The adult AdhFIX group contains mice initially injected as adult with AdhFIX. The protein challenge control group did not receive prior injection of AdhFIX. The AdhFIX challenge group received neither prior injections of AdhFIX nor hFIX protein. Diamonds show the analysis of individual mice at a given time point. The number of samples with no detectable antibodies at each time point is depicted as a diamond and number, both lying beneath the lower dotted line.
Phase 1, prolonged hFIX expression after in utero injection of AdhFIX.
First, the strength and persistence of hFIX expression after prenatal AdhFIX application was examined (Figure2). Eighteen fetuses were injected with AdhFIX at 15 days (D15 fetal AdhFIX group) and 7 fetuses at 17 days (D17 fetal AdhFIX group) after conception. Blood was taken regularly for measurement of hFIX (Figure 2) and hFIX antibody (Figure3) concentrations as well as adenoviral antibody concentrations (Figure 4). From the D15 fetal AdhFIX group, the earliest blood samples at 2 and 9 days after injection were collected by exsanguinations (8 and 2 mice, respectively). All later samples were collected by venipuncture. The first samples were collected from the D17 fetal AdhFIX group at 28 days after injection. As a control, 7 adult mice (12-14 weeks of age) were injected with the AdhFIX vector (adult AdhFIX group). Mice in all 3 groups received the same virus dose (2 × 1011pfu/mouse).
In most of the mice in these 3 groups, hFIX reached therapeutic concentrations after vector administration. However, although all mice in the adult AdhFIX group lost hFIX expression before 40 days after injection, expression remained high in the D15 and D17 fetal AdhFIX groups at 28 days, only falling below a considered therapeutic level of 50 ng/mL at around 55 days after injection. Expression in the D15 and D17 fetal AdhFIX groups persisted at least up to 8 months after infection and was significantly higher than in a group of noninjected controls (controls, undetectable; D15 fetal AdhFIX at 320 days, 7.8 ± 2.2 ng/mL, P = .002; D17 fetal AdhFIX at 251 days, 8.6 ± 2.9 ng/mL, P = .008; n = 6 per group). The lower detection limit of the assay was at 5 ng/mL.
Phase 2, prenatally treated mice are tolerant of repeated postnatal injection of hFIX.
To determine whether an immune response to hFIX had been affected by the prenatal expression of hFIX, we applied purified hFIX protein repeatedly to the D15 and D17 fetal AdhFIX groups and to the adult AdhFIX group (47, 37, and 16-18 weeks of age, respectively). The human protein was injected intraperitoneally on 7 occasions separated by 9, 14, 15, 49, 14, and 21 days (8.6, 43, 53, 53, 60, 60, and 60 μg/mouse, respectively). Exact amounts were determined by commercial availability of hFIX protein. These high doses were chosen to allow reliable detection despite the short half-life of the protein. Four hours after each injection (beginning with the second injection), we measured the blood concentrations of hFIX. As an additional control, 6 adult mice, which had never received AdhFIX, were also given the same protein injections (protein challenge control group; 18-24 weeks of age). In the D15 and D17 fetal AdhFIX groups, hFIX concentrations were still higher than 20 ng/mL in 5 of 9 mice after the seventh injection. In the protein challenge control group, circulating hFIX fell to undetectable levels in all 6 mice after the fifth and sixth protein injections. No hFIX was ever detected in the 7 adult AdhFIX group mice following protein injection. We consider the point in our experiment at which loss of tolerance occurs in individual mice as being defined as the first of 2 consecutive protein injections after which no circulating hFIX could be detected.
A Mann-Whitney U test was used to compare the D15 and D17 fetal AdhFIX groups with the protein challenge control group. This comparison demonstrated significantly increased tolerance toward this human protein after the sixth and seventh (P = .006) protein injections in the prenatally treated mice.
Phase 3, mice treated in utero show high and persistent hFIX expression even after repeated adenoviral application in adulthood.
In an effort to break the presumed prenatally induced tolerance against hFIX, all groups were injected intravenously with a first AdhFIX challenge (2 × 1011 pfu/mouse) 11 days after the last protein injection. Blood was collected at 2, 10, 16, and 23 days following the injection. A second identical challenge was then given, and blood was collected after 2, 8, and 37 days. The D15 and D17 fetal AdhFIX groups, the adult AdhFIX group, and the protein challenge control group received these injections at 66, 56, 16-18, and 30-35 weeks of age, respectively. The adenovirus vector was also injected into 5 adult control mice (12-14 weeks of age), which had previously received neither AdhFIX vector nor hFIX protein (AdhFIX challenge control group). After the first AdhFIX challenge, 7 of 8 surviving mice from the D15 and D17 fetal AdhFIX groups and 3 of 6 mice from the adult AdhFIX control group were generating hFIX (> 5 ng/mL). No hFIX was detected in the adult AdhFIX group at any time point, and no hFIX was detected after the second challenge in the AdhFIX challenge group at any point. The low numbers of mice surviving after the second AdhFIX challenge precluded further useful comparisons.
Kinetics of hFIX antibody development
Assuming that the described pattern of hFIX levels is strongly influenced by the development of immune responses, we also measured the concentrations of hFIX (Figure 3) antibodies during the 3 phases of our study.
Phase 1, lack of hFIX antibody development in mice treated in utero.
In the D15 and D17 fetal AdhFIX groups, despite persistent low-level hFIX expression, no hFIX antibodies could be detected in any of the mice in the final blood sample prior to injection of hFIX protein in phase 2. In contrast, hFIX antibodies were detected 32 days after injection of AdhFIX in all mice of the adult AdhFIX group.
Phase 2, tolerance induced in utero to transgenic protein persists after repeated protein injection in adult life.
hFIX injections in mice from the D15 and D17 fetal AdhFIX groups led to transient hFIX antibodies after the second, third, and fourth protein administration in all 11 mice. However, after the seventh protein injection, 7 of the remaining 9 mice had undetectable antibody levels. In contrast, high and increasing antibody concentrations were already measured after the first protein injection in the mice from the adult AdhFIX group. In the protein challenge control group, low levels of hFIX antibodies were detected until the fifth injection when 2 of 6 mice developed high antibody levels. By the sixth injection, all mice developed high antibody levels coincident with the loss of detectable hFIX. To compare more rigorously the degree of immunity to hFIX in the D15 and D17 fetal AdhFIX groups with the protein challenge control group, hFIX antibodies in sera taken after the seventh protein injection were analyzed at 1/20 and 1/100 dilutions in addition to our standard 1/10 000 dilution (Figure 3 inset). At a dilution of 1/100, 6 of 7 mice in the D15 and D17 fetal AdhFIX groups had absorbance ratios less than 2, whereas none of the mice in the adult AdhFIX group and 1 of 6 mice in the protein challenge control group had absorbance ratios less than 2; the same pattern was seen at a dilution of 1/20. Complete data are missing from 2 mice of the D15 and D17 fetal AdhFIX groups because of insufficient material. The high level of antibodies in 2 of 3 mice in the D15 fetal AdhFIX group are difficult to explain but may be due to poor injection technique in these early in utero injections that may have failed to deliver sufficient hFIX adenovirus to the fetal liver. However, the level of antibody production in most of the prenatally injected mice suggests that tolerance can been achieved in this group.
To determine whether these anti-hFIX antibodies could inhibit hFIX procoagulant activity, an assay to assess inhibition of coagulation was performed on samples taken after the sixth protein injection, using a standard one-stage FIX clotting assay to assess residual hFIX activity after treatment with murine plasma samples. Compared with a 100% buffer control, treatment with plasma samples from the protein challenge control group reduced the residual FIX activity (mean residual FIX, 70%; range, 46%-110%) more than plasma from the D17 fetal AdhFIX group (mean residual, 100%; range, 92%-113%;P = .04) or from the D15 fetal AdhFIX group (mean residual FIX, 84%; range, 67%-118%; not significant). However plasma from the adult AdhFIX group inhibited hFIX significantly more than all the other 3 groups (mean residual FIX, 33%; range, 16%-64%;P < .01 all groups). One mouse each in the protein challenge control group and the D15 fetal AdhFIX group, which had very high hFIX antibodies by ELISA, also showed the greatest anticoagulant activity in their group.
Phase 3, tolerance induced in utero to transgenic protein persists after repeated AdhFIX injection in adult life.
After the protein challenge, 1 of the 2 surviving mice from the D15 fetal AdhFIX group developed hFIX antibodies exceeding an absorbance ratio of 0.5 after the 2 postnatal adenovirus injections. A small increase, never exceeding this value, was also seen in the D17 fetal AdhFIX group. In contrast, 5 of 6 mice from the adult AdhFIX group developed an antibody value more than 0.5, concordant with the previously described total absence of hFIX expression. Two of 5 mice from the protein challenge control group developed antibodies exceeding a 0.5 ratio. A low level of hFIX antibodies was detected in the AdhFIX challenge group.
Kinetics of antibody development
Adenovirus antibodies were detected in all mice from the D15 fetal AdhFIX, D17 fetal AdhFIX, and adult AdhFIX groups. Antibodies appeared in the adult AdhFIX group as early as 12 days after injection. No analyses of antibodies were performed in the D15 and D17 fetal AdhFIX groups early in phase 1. Adenovirus antibody concentrations fell gradually in all mice from the D15 and D17 fetal AdhFIX groups and the adult AdhFIX group but remained considerably higher in the adult AdhFIX group than in D15 and D17 fetal AdhFIX groups. As expected, no adenovirus antibodies were observed above background in the protein challenge control group. Mice from the D15 and D17 fetal AdhFIX, protein challenge control, and AdhFIX challenge groups showed similar adenovirus antibody profiles in that the first AdhFIX challenge failed to elicit an antibody level greater than an absorbance ratio of 3. Following the second AdhFIX challenge, all mice except 1 in D17 fetal AdhFIX group and 1 in protein challenge control group showed antibody levels greater than this value. In contrast, mice from the adult AdhFIX group showed a rapid increase in antibodies greater than an absorbance ratio of 3 after the first AdhFIX challenge.
Morbidity and mortality
In phase 1, 3 mice from the D15 fetal AdhFIX group and 1 from the D17 fetal AdhFIX group died of unknown causes. The second injection of AdhFIX in phase 3 of the experiment led 2 days later to the acute demise of 2 mice in the D15 fetal AdhFIX group. Specific analysis for IgE in the sera of one of these mice revealed no detectable antibody titer (results not shown). One of 5 and 2 of 6 mice in the D15 and D17 fetal AdhFIX groups, respectively, developed large, solid abdominal masses approximately 420 days after birth. At this time, postmortem examination in 2 mice revealed uterus-associated tumors, which were found to be teratomas on histologic examination. Concentrations of hFIX and antibodies were comparable with those of the remaining mice in the groups as seen in Figures 2 to 4.
One mouse in the protein challenge control group died suddenly in phase 3 of a large intrathoracic hemorrhage 9 days after the second AdhFIX injection. The hFIX antibody concentration was very high (4.8 absorbance ratio) despite an initial absence of antibodies 7 days earlier.
Discussion
Prenatal injection
Injection into the yolk sac vessel provides a highly effective route for gene delivery to the fetal mouse circulation. The blood flows from the yolk sac vessel through the umbilical vein to the fetus where the blood stream is divided between the liver and via the ductus venosus to the heart. This circulation system may explain the relatively high infection in these organs. However, expression in the cardiac muscle was not restricted to tissue adjacent to the chambers and was absent in the endothelium, suggesting that infection took place via the coronary arteries rather than directly from the chamber walls. Therefore, this restricted expression pattern may not be due to physiologic constraints but instead to vector tropism or promoter selectivity.
With respect to the expression of hFIX, the yolk sac vessel route of delivery into the fetal mouse is a highly appropriate model for delivery into the umbilical vein of larger animals and the human fetus because by both routes the first organ of passage is the liver.24 In contrast, vector injected intravenously into the neonatal mouse passes through the pulmonary circuit before entering the systemic blood circulation. Differences in induction of tolerance in neonatal rather than prenatal mice have yet to be examined.
High and prolonged expression in the absence of hFIX antibodies
It is difficult to give a single reason for the approximately 10-fold higher concentration of hFIX in the D15 and, by extrapolation, the D17 fetal AdhFIX groups compared with the adult AdhFIX group in the first week after injection, as we do not know if equal numbers of cells were infected in the fetal and adult mice. However, by far the most likely reason is that as both adults and fetuses were given identical doses of AdhFIX yet the adult mouse has 30 times the mass of a D17 fetal mouse, this difference reflects the dilution factor of the secreted hFIX. Other minor factors could include a more effective rapid elimination of adenovirus in the adult by a mature innate immune mechanism and possibly different tropisms and expression efficiencies in fetal and adult tissues. Analyses of viral copy numbers could clarify differences in infectability and expression between fetal and adult mice.
The most interesting observation in this study is the prolonged expression of hFIX, without development of significant levels of hFIX antibodies, following prenatal transgene delivery and expression. This finding corroborates previous observations of prolonged erythropoietin,25 ornithine transcarbamylase,26 and hFIX expression19after injection of appropriate adenovirus into the neonatal mouse. However, in contrast with the latter study that showed no difference in the duration of gene expression and lack of hFIX antibodies between neonatal and adult CD-1 mice after intravenous AdhFIX application, we did observe a remarkable difference between prenatal and adult treatment. As different mouse strains appear to react differently with respect to mounting an immune reaction even against the same protein,8 it appears essential to choose a strain that does show a clear immune reaction in adulthood. A crucial question in the interpretation of our results is whether the intravenous injection of AdhFIX into the prenatal mouse induces the immune system to develop tolerance against the transgenic protein or whether the fetal immune system is simply too immature to mount a response. By including specific immune challenges to the prenatally treated adult mice (intraperitoneal injection of hFIX protein in phase 2 and intravenous injection of AdhFIX in phase 3), we designed our study to provide increasingly vigorous tests for this putative tolerance. Protein challenge at the end of phase 2 failed to stimulate immune clearance of hFIX in 5 of 9 of the mice of the D15 and D17 fetal AdhFIX groups, whereas all mice in the adult AdhFIX group developed immunity to hFIX. Significantly, the protein challenge control group began to clear injected hFIX and develop hFIX antibodies toward the end of phase 2. This finding indicates that some mice from the D15 and D17 fetal AdhFIX groups were indeed tolerant of hFIX protein, yet those in the protein challenge group were not. This finding is endorsed by the fact that several mice in the former groups failed to develop any significant hFIX antibody responses, even in phase 3, after challenge with additional AdhFIX injections. These results are in contrast to a previous report by Lipshutz et al17 who gave CD-1 mice an intrahepatic injection of adenovirus expressing the luciferase gene in utero and an intravenous injection as adults. These mice developed high luciferase antibody concentrations following a subsequent injection of a luciferase-expressing plasmid. Besides likely differences in immunogenicity between hFIX and luciferase, it is possible that the secretion of hFIX into the circulation as well as the protein delivery in phase 2 of our experiment have acted to reinforce tolerance toward hFIX prior to readministration of adenovirus. We also found that plasma from mice in the adult AdhFIX and protein challenge control groups extended the clotting time for human blood compared with the mice in the D15 and D17 fetal AdhFIX groups that had nearly normal clotting times. This inhibitory effect confirms the specificity of these antibodies and mirrors the adverse effect of antibody development seen in some humans during hFIX protein therapy.
Expression of hFIX mRNA could not be detected by reverse transcription–polymerase chain reaction (RT-PCR) 500 days after the first AdhFIX injection in 3 mice analyzed from the adult AdhFIX group (S.M.K.B., unpublished data, May 15, 2002). Therefore, although this could just be due to loss of vector or promoter shutdown, the possibility that cellular immunity may also play a significant role in elimination of hFIX expression cannot be discounted.
An interesting corollary to this work is the observation that repeated injection of AdhFIX to adult mice (phase 3, AdhFIX challenge group) resulted in very rapid loss of hFIX expression and concomitant antibody production. However, intraperitoneal injection of hFIX protein prior to AdhFIX administration (phase 2 and phase 3) led in 3 of 6 mice to high hFIX concentrations (protein challenge control group). This finding suggests that an altered responsiveness resulting from protein injection manifests itself in high hFIX levels despite the presence of high antibodies. As yet we have no explanation for these observations.
Adenovirus antibodies in all groups
Despite tolerance of hFIX in the D15 and D17 fetal AdhFIX groups, all mice developed high levels of adenovirus antibodies after the second injection in phase 3. This antibody response would most likely prevent successful gene delivery by a third AdhFIX reinjection by elimination of the virus. This is evidenced by the AdhFIX challenge group, which lost hFIX expression yet raised only a negligible hFIX antibody response, but showed a strong adenovirus antibody response in phase 3. The failure to induce tolerance of adenovirus, which agrees with the aforementioned report on fetal luciferase delivery,17 is interesting. Possibly, the highly immunogenic nature of adenoviral proteins and innate immunity stifles acquisition of tolerance.
Mechanisms of tolerance
Following expression of transgenic hFIX in the fetal and neonatal period, T cells reactive toward this antigen may become deleted or inactivated. Alternatively, a population of regulatory suppressor T cells may be induced or expanded, capable of inhibiting the hFIX immune response. Supporting this second possibility is the transient antibody production that we observed in the D15 and D17 fetal AdhFIX groups after the first 4 protein injections in phase 2. This may represent the short-term activation of hFIX-reactive T cells prior to the ascendancy of a suppressor population. Crucial to this mode of tolerance is the continuous presence of antigen, in the D15 and D17 fetal AdhFIX groups, hFIX persisted throughout the whole of phase 1. In contrast, the dramatic elevation of adenovirus antibodies in these groups may be due to absence of a continuous tolerizing antigen presence in the blood stream. Furthermore, whereas adenoviral antigens are quite foreign to the murine immune system, there is substantial homology between hFIX and mouse factor IX. In utero delivery to factor IX knockout mice would thus provide a sterner test for tolerance against hFIX. Use of AAV, oncoretroviral, or lentiviral vectors would be expected to result in higher and more persistent levels of antigen and could, therefore, be a better candidate for induction of tolerance from in utero intervention. However, even a low but continuous level of transgenic hFIX from a prenatally delivered vector may be sufficient to facilitate postnatal tolerance and vindicate a fetal gene therapy approach even if gene expression was subtherapeutic.
Morbidity
Factors preceding the induction of massive adenovirus antibody levels after the second injection of AdhFIX in phase 3 may have led to the demise of 2 mice in the D15 fetal AdhFIX group. However, other mice developed very high antibody levels without signs of morbidity. Of more concern is the development of teratomas. Although the incidence in this strain is undocumented (Dr Michael Festing, Leicester, United Kingdom, personal oral communication, October 12, 2001), of approximately 20 untreated female mice reaching a similar age in our labs, none have developed such tumors. These observations militate a strict and meticulous observation in all such experiments for evidence of such iatrogenic effects.
We would like to thank Prof Ted Tuddenham for his valuable advice.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-03-0779.
Supported by an MRC program grant (C.C.) and by a grant from the Katherine Dormandy Charity for Haemophilia and Allied Disorders (Reg Charity no. 1089911) (S.N.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Simon N. Waddington, Gene Therapy, Section of Cell and Molecular Biology, Imperial College Road, Imperial College School of Science, Technology and Medicine, London, SW7 2AZ, United Kingdom; e-mail: s.waddington@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal