Vascular endothelial growth factor (VEGF) receptor 3 (VEGFR-3), a receptor for VEGF-C, was shown to be essential for angiogenesis as well as for lymphangiogenesis. Targeted disruption of theVEGFR-3 gene in mice and our previous study using an antagonistic monoclonal antibody (MoAb) for VEGFR-3 suggested that VEGF-C/VEGFR-3 signals might be involved in the maintenance of vascular integrity. In this study we used an in vitro embryonic stem (ES) cell culture system to maintain the VEGFR-3+ endothelial cell (EC) and investigated the role of VEGFR-3 signals at the cellular level. In this system packed clusters of ECs were formed. Whereas addition of exogenous VEGF-A induced EC dispersion, VEGF-C, which can also stimulate VEGFR-2, promoted EC growth without disturbing the EC clusters. Moreover, addition of AFL4, an antagonistic MoAb for VEGFR-3, resulted in EC dispersion. Cytological analysis showed that VEGF-A– and AFL4-treated ECs were indistinguishable in many aspects but were distinct from the cytological profile induced by antagonistic MoAb for VE-cadherin (VECD-1). As AFL4- induced EC dispersion requires VEGF-A stimulation, it is likely that VEGFR-3 signals negatively modulate VEGFR-2. This result provides new insights into the involvement of VEGFR-3 signals in the maintenance of vascular integrity through modulation of VEGFR-2 signals. Moreover, our findings suggest that the mechanisms underlying AFL4-induced EC dispersion are distinct from those underlying VECD-1–induced dispersion for maintenance of EC integrity.
Introduction
Vascular endothelial growth factor receptor 3 (VEGFR-3, encoded by Flt-4),1 VEGFR-1 (encoded by Flt-1),2 and VEGFR-2 (encoded by KDR/Flk-1)3constitute an endothelial cell (EC) receptor tyrosine kinase family that in concert regulates the process of blood vessel formation during embryogenesis and also in pathological conditions.4-8Concerning VEGFR-3 signal, VEGF-C and VEGF-D are the known ligands, both of which also activate VEGFR-2.9-12
While the role of VEGFR-3 and its ligands in lymphangiogenesis is a rapidly emerging issue,13-17 previous targeted inactivation of the VEGFR-3 gene in mice demonstrated clearly its role in vascular remodeling during blood vessel development.18 As the VEGFR-3 signal is involved in angiogenesis in both embryos and adults,19 20 how VEGFR-3 regulates the process of vascular remodeling remains unresolved, as does its role in lymphangiogenesis.
In previous studies, we found that blockade of VEGFR-3 signal during tumor-induced angiogenesis caused disruption of vascular integrity at the postcapillary venule and precapillary arteriole levels.21 Based on these histological observations, we hypothesized that VEGFR-3 signal may play a role in the maintenance of the integrity of the EC sheet.
In order to evaluate this hypothesis, analysis of the role of VEGFR-3 signals on vascular endothelial cells at the cellular level is required, but obtaining and maintaining pure VEGFR-3+ ECs have been difficult obstacles to overcome. We have developed an experimental system by which vascular ECs can be induced from mouse embryonic stem (ES) cell cultures.
In this study, we purified vascular endothelial cadherin+(VE-cadherin+)22 ECs from this culture system and examined the role of VEGFR-3 signal on thus-prepared ECs by using antagonistic monoclonal antibody (MoAb) for VEGFR-3 (AFL4) in combination with VEGF-family ligands such as VEGF-A and VEGF-C. Our results demonstrate that VEGFR-3 signal modulates sensitivity to VEGFR-2, thereby suppressing overactivation of VEGFR-2. These results provide new insight into the molecular mechanisms underlying the maintenance of vascular integrity during neovascularization when ECs are exposed to excitatory signals.
Materials and methods
Cell lines
EB5, a subline derived from E14tg2a ES cell line, was a kind gift from Dr H. Niwa (Department of Nutrition and Physiological Chemistry, Osaka University School of Medicine, Osaka, Japan). In the EB5 cell line, Aspergillus blastcidin S deamininas gene was knocked into a single allele of the Oct-3/4 gene, a regulator of pluripotency, to remove differentiated cells in the presence of leukemia inhibitory factor (LIF; R&D Systems, Minneapolis, MN) and blastcidin S hydrochloride (Blast S; Funakoshi, Tokyo, Japan).23 Therefore, the EB5 cell line can be grown and maintained in a multipotent undifferentiated state providing they are exposed to LIF and Blast S. The EB5 cell line was maintained in culture dishes coated with gelatin (type A from porcine skin; Sigma, St Louis, MO) using Glasgow minimum essential medium (G-MEM; Gibco BRL, Grand Island, NY) supplemented with 10% knockout serum (Gibco), 1% fetal calf serum (FCS; Whittaker Bioproducts, Walkersville, MD), 100 μM 2-mercaptoethanol (2-ME; Merck, Darmstadt, Germany), 10 mM nonessential amino acids (Gibco), 1 mM sodium pyruvate (Sigma), 20 μg/mL Blast S, and 1000 U/mL LIF. CCE ES cell line,24 a kind gift from Dr M. Evans (Wellcome/CRC Institute, Cambridge, United Kingdom), was maintained by previously described procedures.25 OP9 stromal cell line was maintained in MEM-α medium (Gibco) supplemented with 20% FCS (HyClone Laboratories, Logan, UT).26
Monoclonal antibodies (MoAbs), cell staining, and sorting
MoAbs for murine VEGFR-3 (AFL4), VEGFR-2 (AVAS12), and VE-cadherin (VECD-1) have been described previously.21,27,28 These MoAbs were prepared and labeled in our laboratory. Unconjugated and fluorescein isothiocyanate (FITC)–conjugated MoAbs for murine platelet–endothelial cell adhesion molecule 1 (PECAM-1; Mec13.3) were purchased from Pharmingen (San Diego, CA). Mouse MoAb for ZO1 was kindly provided by Dr Tsukita and Dr Furuse (Department of Cell Biology, Faculty of Medicine, Kyoto University, Kyoto, Japan).29 Mouse MoAb for β-catenin was purchased from Zymed (San Francisco, CA); mouse MoAb for γ-catenin was purchased from Transduction Laboratories (Lexington, KY). MoAb for VE-cadherin (clone 11D4.1 from Pharmingen) was dialyzed against phosphate-buffered saline (PBS) to remove sodium azide. MoAb for interleukin 7 (IL-7) receptor α (A7R34) was previously described.30 Purified rat immunoglobulin G (IgG) was purchased from Chemicon (Temecula, CA). TO-PRO-3 iodide, which was used for the staining of the nuclei, was purchased from Molecular Probes (Eugene, OR).
For flow cytometry, cultured cells were harvested in dissociation buffer (Gibco), blocked in mouse serum for 20 minutes on ice, and incubated with several combinations of labeled MoAbs. The biotinylated MoAb was detected by allophycocyanin (APC)–streptoavidin. Stained cells were suspended in Hanks balanced salt solution (Gibco) containing 1% bovine serum albumin (Sigma) and 5 μg/mL propidium iodide (PI; Sigma). Cells were analyzed and sorted by FACS Vantage (Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ).
Reagents
Preparation of recombinant protein of the mature form of human wild-type VEGF-C (δNδC) was kindly provided by Dr Kari Alitalo (University of Helsinki, Finland) was described previously.10,31 Recombinant human VEGF165 was purchased from R&D systems. The mFlt-1-hIgG chimeric protein was described previously.32
In vitro differentiation of ES cells
For induction of differentiation, 4 × 104 EB5 ES cells (1 × 104 CCE) were cultured for 5 days in 25-cm2 flasks containing confluent OP9 stroma and the induction medium (MEM-α supplemented with 10% FCS and 50 μmol/L 2-ME) in the absence of LIF and Blast S. For analysis of in vitro EC behavior, VE-cadherin+ PECAM-1+ cells were sorted and 500 sorted cells per well were cultured for 3 days in 24-well plates containing OP9 stromal cells in the presence or absence of various reagents.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was prepared from cultured cells using ISOGEN (Nippon Gene, Toyama, Japan). RNA purified from 1 × 105 cells was reverse transcribed with Superscript II reverse transcriptase (Gibco) and oligo (dT) 12-18 primers (Gibco) according to the manufacturer's instructions. PCR assays were performed in reaction mixtures containing 1× Ex Taq Buffer (Takara Shuzo, Osaka, Japan), 200 μmol/L deoxyribonucleoside triphosphates (dNTPs; Takara Shuzo), 25 U/mL Ex Taq DNA polymerase (Takara Shuzo), several dilutions of cDNA, and 2 μM of the following primers: VEGF-A, VEGF-C,33 angiopoietin-1 (Ang-1), Ang-2, β-actin, and VEGF-D.34 35
Immunostaining
Cytological analyses of EC cultures were performed as follows. The cultured cells were fixed in situ with 4% paraformaldehyde (PFA; Nacalai Tesque, Kyoto, Japan) in PBS for 10 minutes at 4°C for AVAS12 and Mec13.3. After washing in PBS, cells were blocked with 2% skim milk in PBS for 1 hour at room temperature. The fixed cells were incubated with MoAbs overnight at 4°C, followed by incubation with alkaline phosphatase (ALP)–conjugated antirat IgG (H+L; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. The substrate used was 4-nitro blue tetra-zolium chloride/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP; Roche Molecular Biochemicals, Basel, Switzerland). Endogenous ALP activity was blocked by 2 mmol/L Levamisol (Sigma) before incubation with MoAbs.
Immunofluorescence staining
Sorted ECs were grown on polystyrene-coated glass slides (Becton Dickinson) containing OP9 stromal cells. The cultured cells were rinsed in PBS containing 0.9 mM CaCl2 and 0.33 mM MgCl2, fixed in situ with 4% PFA, and stained with VECD-1, Mec13.3, and MoAbs for β-catenin, γ-catenin, and the MoAb for ZO1 with or without methanol permeabilization. Cells were pretreated with 5% dimethylsulfoxide (Nacalai Tesque, Kyoto, Japan) in methanol for 11D4.1 and VECD-1. Primary antibodies were detected using Alexa 488–conjugated goat antimouse IgG antibody (Ab) or Alexa 546–conjugated goat antirat IgG Ab (Molecular Probes). Biotinylated VECD-1 was detected using Alexa 546–conjugated streptavidin (Molecular Probes) and Cy3-conjugated antibiotin Ab (Sigma). FITC-Mec13.3 was detected using Alexa 488–conjugated anti-FITC Ab. Immunostained cells were visualized using Leica TCS SP2 Laser scanning microscopy and image software (Leica-Microsystems, Wetzlar, Germany). All images were scanned sequentially to minimize signal cross-interference.
Quantitative analysis of the endothelial colony
In this study, only EC colonies that were round and packed (round EC clusters) were counted. To quantify the number of round EC clusters, all VEGFR-2+ or PECAM-1+round EC clusters were counted per well of the 24-well plate. Each value represents the mean ± SD of 5 independent experiments. The Mann-Whitney U test analysis was used for statistical evaluation of the data.
Results
Expression of VEGFR-3 in nascent ECs generated from ES cells
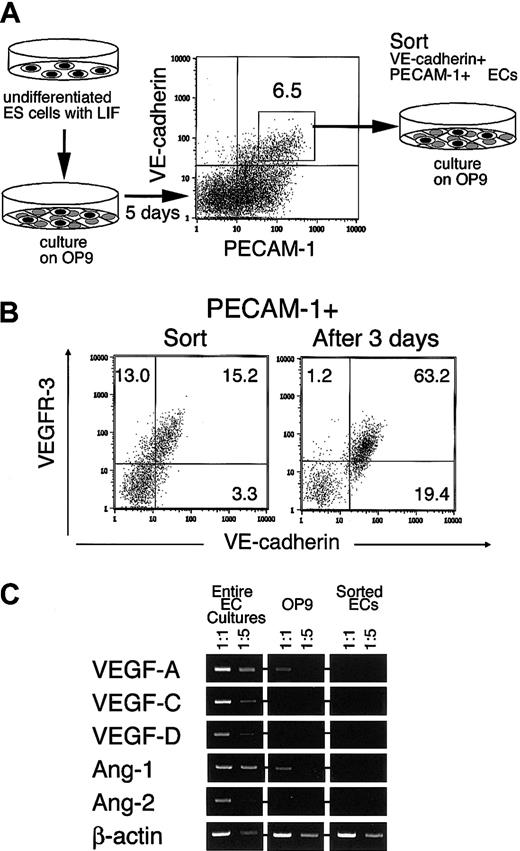
Previous studies suggested that VEGFR-3 is a molecule that is expressed in the EC inducibly rather than constitutively.13,36 This indicated that the conditions necessary for studying the role of VEGFR-3 require establishment of a method to prepare ECs that express VEGFR-3. To see whether or not ECs generated from ES cells fit this purpose, we investigated the expression of VEGFR-3 in ECs generated by the experimental protocol illustrated in Figure 1A. To obtain an EC-rich population, ES cells were cultured on an OP9 stromal cell layer for 5 days. As described in our previous paper, OP9 efficiently supported ES cell differentiation to lateral mesoderm within 3 to 4 days and subsequent differentiation to ECs in the next 24 hours.32 VE-cadherin+ ECs generated under this culture condition were further purified by fluorescent activated cell sorting (FACS) using VE-cadherin and PECAM-1 as surface markers.
Preparation of VEGFR-3+ ECs from ES cells.
(A) The experimental procedure. ES cells maintained in the presence of LIF were transferred onto OP9. Five days later VE-cadherin+PECAM-1+ ECs were sorted and recultured on OP9 for 3 days in the presence or absence of exogenous reagents. (B) Expression of VEGFR-3 in the EC. VE-cadherin+ PECAM-1+ ECs were analyzed by flow cytometry at the time of sorting (left panel) and after 3 days' culture (right panel). OP9 cells were easily distinguishable from ES cell–derived cells in forward and side scatter so that they could be excluded from the cells analyzed here. The numbers indicate the percentage of cells that appeared in each quadrant. (C) Expression of the angiogenetic factors was analyzed by RT-PCR with RNA extracted from entire EC cultures (ECs and OP9), OP9 stromal cells without cocultured ECs, and sorted VE-cadherin+ PECAM-1+ ECs cultured on OP9. PCR reactions were carried out using cDNAs obtained from 100 and 20 cells. PCR products were electrophoresed on 1% agarose gels and detected by staining with ethidium bromide.
Preparation of VEGFR-3+ ECs from ES cells.
(A) The experimental procedure. ES cells maintained in the presence of LIF were transferred onto OP9. Five days later VE-cadherin+PECAM-1+ ECs were sorted and recultured on OP9 for 3 days in the presence or absence of exogenous reagents. (B) Expression of VEGFR-3 in the EC. VE-cadherin+ PECAM-1+ ECs were analyzed by flow cytometry at the time of sorting (left panel) and after 3 days' culture (right panel). OP9 cells were easily distinguishable from ES cell–derived cells in forward and side scatter so that they could be excluded from the cells analyzed here. The numbers indicate the percentage of cells that appeared in each quadrant. (C) Expression of the angiogenetic factors was analyzed by RT-PCR with RNA extracted from entire EC cultures (ECs and OP9), OP9 stromal cells without cocultured ECs, and sorted VE-cadherin+ PECAM-1+ ECs cultured on OP9. PCR reactions were carried out using cDNAs obtained from 100 and 20 cells. PCR products were electrophoresed on 1% agarose gels and detected by staining with ethidium bromide.
As shown in Figure 1B, most VE-cadherin+PECAM-1+ cells express VEGFR-3 at the time of cell sorting. We confirmed this by RT-PCR (data not shown). To assess the behavior of these purified ECs, we used the same OP9 stromal cell layer, as it supports clonogenic growth of the EC and its progenitor.27 32 In this study, we investigated whether or not VEGFR-3 expression is maintained during culture with OP9. Approximately 80% of VE-cadherin+ cells in culture were VEGFR-3+ ECs after 3 days of culture with OP9 (Figure 1B), indicating that the OP9 feeder layer supported the maintenance of VEGFR-3+ ECs. This result and our previous studies strongly suggested that OP9 stromal cells express a set of angiogenic molecules that are sufficient for clonogenic growth of the EC. During this culture period, we could detect both VEGFR-1 and VEGFR-2 expression in ECs. Hence, all these signaling pathways are potentially available in the EC. And then approximately 80% of ECs expressed von Willebrand factor by immunohistochemistry and approximately 50% of ECs expressed E-selectin by surface staining (data not shown).
RT-PCR analysis performed to see the expression of angiogenic molecules in this culture system revealed that VEGF-A, Ang-1, and VEGF-C were expressed (Figure 1C). While VEGF-A and Ang-1 were expressed constitutively in OP9 stromal cells, expression of other angiogenic molecules was enhanced when OP9 cells were cocultured with ECs. ECs alone did not express any of those angiogenic factors.
Effect of exogenous VEGF-A and VEGF-C on EC proliferation on OP9
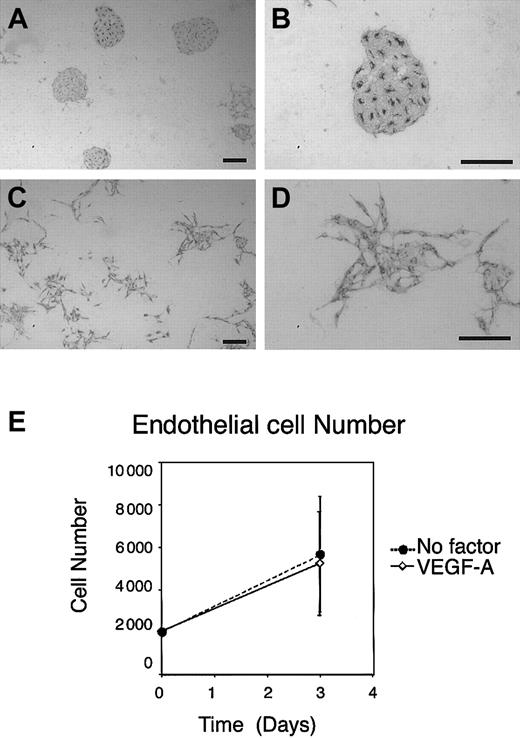
Even without addition of exogenous growth factors, sorted VE-cadherin+ ECs as well as VEGFR-2+endothelial progenitors grew to form packed and round EC clusters on OP9 in this EC culture system (Figure 2A-B). Although addition of mFlt-1-hIgG chimeric protein that sequestered VEGF-A37-39 suppressed colony formation of VEGFR-2+ VE-cadherin− EC progenitors in both size and number,32 the same treatment of VE-cadherin+ ECs resulted only in reduction of colony size (Figure 3C). This result suggested that multiple molecules are involved in clonogenic proliferation of the EC on OP9. In addition to the growth factor properties of VEGF-A, our previous study demonstrated that exogenous VEGF-A exerted additional effects on EC proliferation on OP9. As shown in Figure 2C-D, the addition of exogenous VEGF-A, in the range of 3 to 50 ng/mL, to the culture induced morphological changes from polygonal to spindlelike shapes. In parallel, cell clusters that otherwise formed packed clusters became dispersed, although cell-cell junctions were still maintained at the edges of each cell. Of note is that exogenous VEGF-A did not enhance proliferation of ECs (Figure 2E). This result indicated that OP9-derived endogenous VEGF-A already exceeds the optimum dose required for EC proliferation. As shown in our previous studies, placenta growth factor (PLGF), even at high doses, showed no effect on EC activity under this condition. Thus, VEGFR-1, although it could play a role as a competitive inhibitor of VEGFR-2 for VEGF-A40, has no role as a signal transducer.
Response of ECs to exogenous VEGF-A on OP9.
ES cell–derived VE-cadherin+ PECAM-1+ECs were induced, purified, and recultured on OP9. After 3 days of incubation, cultured ECs were stained by MoAb for VEGFR-2. (A-B) Round clusters of ECs were formed on OP9 stroma in the absence of exogenous factors. (C-D) Addition of 50 ng/mL exogenous VEGF-A resulted in dispersion of EC clusters. Scale bar, 200 μm. (E) Quantitation of the number of ECs before and after culture with or without addition of exogenous VEGF-A. To examine the recovery of sorted ECs in the presence or absence of exogenous VEGF-A, the number of ECs was calculated before and after culture using flow cytometry. Error bars indicate standard deviation of 3 independent assays.
Response of ECs to exogenous VEGF-A on OP9.
ES cell–derived VE-cadherin+ PECAM-1+ECs were induced, purified, and recultured on OP9. After 3 days of incubation, cultured ECs were stained by MoAb for VEGFR-2. (A-B) Round clusters of ECs were formed on OP9 stroma in the absence of exogenous factors. (C-D) Addition of 50 ng/mL exogenous VEGF-A resulted in dispersion of EC clusters. Scale bar, 200 μm. (E) Quantitation of the number of ECs before and after culture with or without addition of exogenous VEGF-A. To examine the recovery of sorted ECs in the presence or absence of exogenous VEGF-A, the number of ECs was calculated before and after culture using flow cytometry. Error bars indicate standard deviation of 3 independent assays.
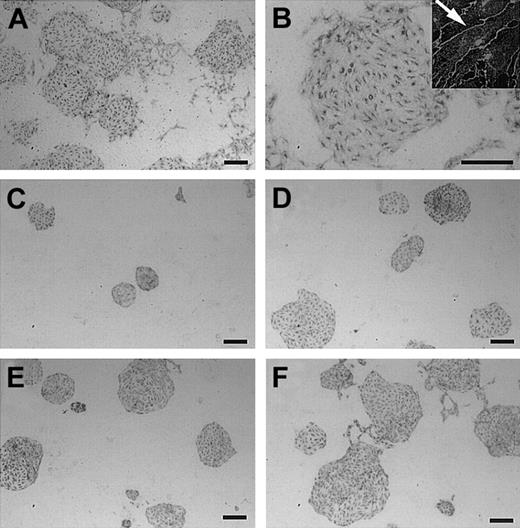
We next examined the effect of exogenous VEGF-C on the formation of EC colonies. In contrast to the strong ability of VEGF-A to induce dispersion of ECs, 100 ng/mL VEGF-C did not induce the dispersion of EC clusters, though it was active in inducing changes of EC shape to elongated spindlelike forms (Figure 3A-B). To further confirm that the preparation of VEGF-C used in this study is active, we examined whether or not VEGF-C could rescue the suppression of EC colony formation induced by mFlt-1-hIgG sequestering endogenous VEGF-A. Consistent with our previous study, mFlt-1-hIgG suppressed formation of EC colonies on OP9 (Figure 3C).32 This suppression was restored by addition of VEGF-C; the size of EC colonies increased in a dose-dependent manner (Figure 3D-F). Thus, the VEGF-C used in this study is active in stimulating VEGFR-29,10 12 but could not induce dispersion of EC colonies, which is the most representative activity of VEGF-A in our system.
Effect of VEGF-C on ECs.
(A-B) Cultures stimulated by 100 ng/mL VEGF-C. In contrast to VEGF-A, VEGF-C did not induce EC dispersion. (B, inset) Larger magnification (× 2000) shows that VEGF-C–treated ECs become spindlelike (arrow), although EC clusters are maintained. (C) In this culture, addition of mFlt-1-hIgG reduced the size of EC colonies. Addition of exogenous VEGF-C overcomes this suppression in a dose-dependent manner. Concentrations of VEGF-C were 10 ng/mL (panel D), 30 ng/mL (panel E), and 100 ng/mL (panel F). While the size of EC clusters increased, colonies are packed. Scale bar, 200 μm.
Effect of VEGF-C on ECs.
(A-B) Cultures stimulated by 100 ng/mL VEGF-C. In contrast to VEGF-A, VEGF-C did not induce EC dispersion. (B, inset) Larger magnification (× 2000) shows that VEGF-C–treated ECs become spindlelike (arrow), although EC clusters are maintained. (C) In this culture, addition of mFlt-1-hIgG reduced the size of EC colonies. Addition of exogenous VEGF-C overcomes this suppression in a dose-dependent manner. Concentrations of VEGF-C were 10 ng/mL (panel D), 30 ng/mL (panel E), and 100 ng/mL (panel F). While the size of EC clusters increased, colonies are packed. Scale bar, 200 μm.
Effect of blockade of VEGFR-3 function
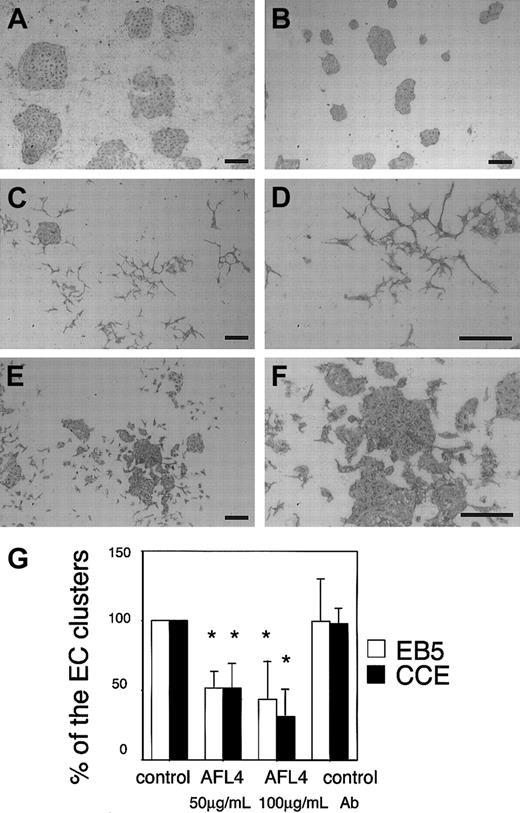
As VEGF-A binds only to VEGFR-2, whereas VEGF-C binds to both VEGFR-2 and VEGFR-3, an interpretation of differences in VEGF-A and VEGF-C actions could be that VEGFR-3 signals can suppress VEGF-A–induced dispersion of ECs under the conditions used in this experiment. To test this possibility, we blocked VEGFR-3 function in the EC culture on OP9 using AFL4, an antagonistic MoAb for VEGFR-3. EC grew on the OP9 layer and formed packed clusters in the presence of control MoAb (Figure 4A) or nonspecific rat IgG (data not shown). As both VEGF-A and VEGF-C are expressed by OP9 stromal cells, these colonies were considered to be formed in the presence of both VEGFR-2 and VEGFR-3 signals. We then blocked VEGFR-3 signals by addition of AFL4. As shown in Figure 4C-D, the addition of the AFL4 induced dispersion of EC clusters in a dose-dependent manner. This dispersion resulted in a significantly reduced number of packed EC clusters (Figure 4G). Although 2 distinct ES cell lines, EB5 and CCE, differed in the efficiency of generating VEGFR-2+ mesoderm cells, ECs derived from the 2 cell lines behaved in an almost identical manner in response to addition of AFL4 (Figure 4G). This ability of AFL4 to induce dispersion of ECs was not observed when OP9-derived VEGF-A was sequestered by addition of mFlt-1-hIgG chimeric protein (Figure 4B). AFL4 has neither a positive nor a negative effect on the mFlt-1-hIgG–induced suppression of EC growth (data not shown). This result suggested that VEGFR-3 signal has a negative effect on VEGF-A-induced dispersion of ECs.
Induction of EC dispersion by an antagonistic MoAb for VEGFR-3 (AFL4).
Sorted ECs were cultured on OP9 in the presence or absence of MoAbs, and the cultures were immunostained by Mec13.3. Addition of 100 μg/mL AFL4 induced dispersion of ECs (panels C-D) compared with the matched 100 μg/mL control MoAb (A7R34; panel A). AFL4-induced EC dispersion was not observed in the culture in which endogenous VEGF-A was sequestered by mFlt-1-hIgG (panel B). Dispersion was induced by addition of anti–VE-cadherin MoAb VECD-1 (panels E-F); no alteration in shape was observed. Scale bar, 200 μm. (G) Quantitative analysis of the number of round EC clusters. Reduction in the number of packed colonies by EC dispersion by Ab treatment is expressed as a percentage against untreated cultures. Experiments were performed using different ES cell lines: EB5 (open bars) and CCE (black bars). Each error bar represents mean ± SD of 5 independent experiments. *P < .05. P values were calculated using the Mann-Whitney U test.
Induction of EC dispersion by an antagonistic MoAb for VEGFR-3 (AFL4).
Sorted ECs were cultured on OP9 in the presence or absence of MoAbs, and the cultures were immunostained by Mec13.3. Addition of 100 μg/mL AFL4 induced dispersion of ECs (panels C-D) compared with the matched 100 μg/mL control MoAb (A7R34; panel A). AFL4-induced EC dispersion was not observed in the culture in which endogenous VEGF-A was sequestered by mFlt-1-hIgG (panel B). Dispersion was induced by addition of anti–VE-cadherin MoAb VECD-1 (panels E-F); no alteration in shape was observed. Scale bar, 200 μm. (G) Quantitative analysis of the number of round EC clusters. Reduction in the number of packed colonies by EC dispersion by Ab treatment is expressed as a percentage against untreated cultures. Experiments were performed using different ES cell lines: EB5 (open bars) and CCE (black bars). Each error bar represents mean ± SD of 5 independent experiments. *P < .05. P values were calculated using the Mann-Whitney U test.
Cytological similarity between AFL4- and VEGF-A–induced EC dispersion
In the absence of exogenous factors, ECs were polygonal and formed sheetlike clusters (Figure 5A-I). VE-cadherin (Figure 5B,E,H), ZO1 (Figure 5A), γ-catenin (Figure 5D), PECAM-1 (Figure 5G), and β-catenin (data not shown) were localized at cell-cell contact sites. Although ECs were elongated in shape and cell dispersion was induced in the AFL4-treated ECs, cell-cell junctions were still maintained within a narrow region, where VE-cadherin (Figure6H,K), γ-catenin (Figure 6G), and PECAM-1 (Figure 6J) were localized. In contrast to the stringlike cell-cell junctions observed in undisturbed EC colonies, formation of thick beltlike junctions was often found between AFL4-treated ECs (Figure 6G, arrow). As such a beltlike junction may reflect the twisted baso-lateral side of cell-cell junction, it may indicate enhanced cell motility. Interestingly, nearly identical cytological changes, including the formation of the beltlike junctions (Figure 6A, arrow), were also induced by VEGF-A.
Immunofluorescence images of ECs cultured on OP9 without exogenous factors.
(A-I) In the absence of exogenous factors, sheetlike clusters were formed. ECs were polygonal and VE-cadherin, γ-catenins, and PECAM-1 were colocalized at stringlike cell-cell junctions (ZO1–VE-cadherin [VECD-1], panels A-B; γ-catenins–VE-cadherin [VECD-1], panels D-E; PECAM-1–VE-cadherin [VECD-1], panels G-H). Distributions of adhesion molecules were compared in merged images (panels C, F, I). Scale bar, 20 μm.
Immunofluorescence images of ECs cultured on OP9 without exogenous factors.
(A-I) In the absence of exogenous factors, sheetlike clusters were formed. ECs were polygonal and VE-cadherin, γ-catenins, and PECAM-1 were colocalized at stringlike cell-cell junctions (ZO1–VE-cadherin [VECD-1], panels A-B; γ-catenins–VE-cadherin [VECD-1], panels D-E; PECAM-1–VE-cadherin [VECD-1], panels G-H). Distributions of adhesion molecules were compared in merged images (panels C, F, I). Scale bar, 20 μm.
Cytological similarity between AFL4- and VEGF-A–treated ECs.
(A-F) Addition of 50 ng/mL VEGF-A results in elongation of ECs. This shape change did not disrupt the cell-cell junctions; VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels A-B) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels D-E) were concentrated at intercellular contact sites. (G-L) In cultures treated with 100 μg/mL AFL4, ECs became spindle-shaped. VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels G-H) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels J-K) were localized at cell-cell contact sites. Unlike the thin stringlike junctions in untreated cultures (Figure 5), thicker beltlike junctions were formed in VEGF-A– and AFL4-treated cultures (panels A,G, arrows). Note that otherwise, the 2 groups are similar in all aspects examined here. Panels C, F, I, and L are merged images. Scale bar, 20 μm.
Cytological similarity between AFL4- and VEGF-A–treated ECs.
(A-F) Addition of 50 ng/mL VEGF-A results in elongation of ECs. This shape change did not disrupt the cell-cell junctions; VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels A-B) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels D-E) were concentrated at intercellular contact sites. (G-L) In cultures treated with 100 μg/mL AFL4, ECs became spindle-shaped. VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels G-H) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels J-K) were localized at cell-cell contact sites. Unlike the thin stringlike junctions in untreated cultures (Figure 5), thicker beltlike junctions were formed in VEGF-A– and AFL4-treated cultures (panels A,G, arrows). Note that otherwise, the 2 groups are similar in all aspects examined here. Panels C, F, I, and L are merged images. Scale bar, 20 μm.
The addition of VECD-1, an antagonistic MoAb for VE-cadherin, an essential component of the adherence junctions of ECs,41-43 also induced EC dispersion without affecting cell proliferation (Figures 4E-F and 7A-F). However, EC dispersion induced in this manner appeared different from that induced by AFL4 or VEGF-A in that the cell shape remained polygonal and molecules (γ-catenin [Figure 7A], β-catenin [data not shown], and VE-cadherin [Figure7B,E]) associated with the adherence junction were internalized by anti–VE-cadherin treatment. Immunostaining with secondary Ab in the VECD-1–treated ECs showed that VE-cadherin on the cell surface may be moved to the intracellular cytoplasm (Figure 7E). Moreover, internalization of VE-cadherin was confirmed by nuclear staining (data not shown) and by immunostaining without permeabilization (data not shown). Interestingly, even after internalization of molecular components of the adherence junction, ZO1 (Figure 7D) was still localized at cell-cell contact sites.
Intracellular localization of VE-cadherin in VECD-1–treated ECs.
(A-F) The addition of 20 μg/mL VECD-1 disrupted EC-EC contact, thereby allowing ECs to scatter. VE-cadherin was translocated from cell surfaces to the intracellular cytoplasm (panel E, arrow). Internalized VE-cadherin colocalized with γ-catenin (panel A, arrow) (γ-catenin–VE-cadherin [VECD-1]; panels A-B). (D-F) In contrast, ZO1 remains at the cell-cell junctions (ZO1–secondary Ab; panels D-E). Panels C and F are merged images. Scale bar, 20 μm.
Intracellular localization of VE-cadherin in VECD-1–treated ECs.
(A-F) The addition of 20 μg/mL VECD-1 disrupted EC-EC contact, thereby allowing ECs to scatter. VE-cadherin was translocated from cell surfaces to the intracellular cytoplasm (panel E, arrow). Internalized VE-cadherin colocalized with γ-catenin (panel A, arrow) (γ-catenin–VE-cadherin [VECD-1]; panels A-B). (D-F) In contrast, ZO1 remains at the cell-cell junctions (ZO1–secondary Ab; panels D-E). Panels C and F are merged images. Scale bar, 20 μm.
Discussion
Previously, we described a clonogenic culture system that supports proliferation and differentiation of a single EC and its progenitor.32 The initial purpose of establishing this culture system was to investigate the frequency of clonogenic growth of EC as well as its progenitor, and we believe that this still represents the most efficient method available. We demonstrated the potential of this system to analyze the behavior of individual EC in response to a variety of stimuli. In this study, we used this feature for analysis of the role of VEGFR-3 in the growth of ECs.
VEGFR-3, a receptor for VEGF-C, was shown to be essential for the development of the vascular system and also tumor-induced angiogenesis.18,21 Our previous histological analysis of the intratumor vascular system of mice treated with AFL4 suggested that VEGFR-3 was involved in the maintenance of the integrity of ECs.21 The purpose of the present study was to recapitulate this in vivo phenomenon in an in vitro culture system to further investigate the biological role of VEGFR-3 signal at the cellular level.
As a source of VEGFR-3+ ECs, we used VE-cadherin+ cells that were generated from ES cells in 5-day cultures on OP9 stromal cells. At the time of cell harvesting, most VE-cadherin+ cells coexpressed VEGFR-3, and this expression was maintained during their culture on OP9. Others have found that VEGFR-3 expression in ECs is inducible rather than constitutive,13,36 thus posing a difficulty in obtaining and maintaining ECs that express VEGFR-3. In this regard, ES cell–derived VEGFR-3+ ECs induced and purified by our method constitute a useful tool for studying the biological role of VEGFR-3 signals, although the number of cells generated may not be sufficient for biochemical analysis. To rule out the possibility that in vitro–induced VEGFR-3+ cells are lymphatic ECs rather than vascular ECs, we measured the expression of Prox1, an essential factor for lymphatic differentiation. The absence of Prox1 indicated that ES cell–derived ECs likely represent vascular ECs.44 45
On the OP9 feeder layer, VE-cadherin+VEGFR-3+cells undergo clonogenic growth, giving rise to packed, round EC clusters. This growth is likely dependent on angiogenic factors expressed by OP9; VEGF-A, VEGF-C, VEGF-D, Ang-1, and Ang-2 were detected in OP9 by RT-PCR. Interestingly, expression of these molecules was enhanced by the coculture of OP9 with ECs that were purified by FACS, suggesting that ECs have the capacity to act on surrounding cells to induce various consequences. In fact, mutual interaction between ECs and mural cells has been implicated in vascular remodeling.46-50 Moreover, it was recently suggested that ECs play a role in induction of pancreatic anlage.51 Such an inducer function of ECs might be a common phenomenon found in various processes of embryogenesis.
OP9 stromal cells provide a complex environment that constitutively or inducibly expresses multiple angiogenic molecules, which support the clonogenic growth of the EC. As the addition of exogenous VEGF-A did not enhance the rate of magnitude of EC proliferation, the level of VEGF-A required for EC proliferation is already present in the OP9 culture. Of note is that while this culture condition is sufficient to support the basic activities of the EC, it is also useful for assessing EC behavior in response to exogenous molecules. For instance, addition of exogenous VEGF-A induced scattering in ECs that otherwise form packed clusters. Our previous study led to a conclusion that VEGF-A has multiple roles: it functions as a growth factor at low concentrations and as an enhancer of cell motility at higher concentrations. Under the same culture conditions, however, exogenous VEGF-C had little effect in inducing scattering of ECs. This difference between VEGF-A and VEGF-C is intriguing, because it has been demonstrated that VEGF-C as well as VEGF-A can activate VEGFR-2.9 52
The addition of exogenous VEGF-C is able to overcome suppression of EC growth–mediated mFlt-1-hIgG chimeric protein that sequesters VEGF-A. Thus, VEGF-C can replace VEGF-A for VEGFR-2 activation in our culture conditions. What then is the basis for the failure of VEGF-C to induce EC dispersion? A possibility might be that VEGF-C binds VEGFR-2 at a lower efficiency, so that the dose tested in this study was insufficient for inducing EC dispersion. We think, however, that this is likely not to be the case, as 3 ng/mL of VEGF-A was as effective in inducing EC dispersion as 100 ng/mL. Of note in this context is that VEGF-C can stimulate both VEGFR-2 and VEGFR-3, whereas VEGF-A acts only on VEGFR-2. Thus, the difference between VEGF-A and VEGF-C activities is more likely to be qualitative rather than quantitative. Assuming that VEGFR-3 can modulate VEGFR-2, the balance of the 2 signals, rather than their absolute strength, might be an important factor in determining the activity of the EC. This would propose that culture conditions with the OP9 feeder layer alone or OP9 plus exogenous VEGF-C favor undisturbed EC growth, whereas the addition of exogenous VEGF-A may shift this balance toward EC scattering.
This working hypothesis would also account for the results of our experiment of blocking the function of VEGFR-3 by an antagonistic MoAb, AFL4. Although the addition of AFL4 may not affect the concentration of VEGF-A, the blockade of VEGFR-3 signal resulted in EC dispersion at a VEGF-A concentration that otherwise does not induce it. Consistent with this is the finding that the dispersion is inhibited by addition of mFlt-1-hIgG chimeric protein. Thus, blockage of the VEGFR-3 signal alone is not sufficient; the dispersion requires a VEGF-A signal.
We have shown that the integrity of the endothelial sheet is disturbed by AFL4 treatment during tumor-induced angiogenesis.21Probably in this in vivo situation, the blockade of VEGFR-3 may result in an increased scattering activity of EC in the region where neoangiogenesis is induced, eventually resulting in instability of EC-EC junctions. It should be noted that this is an entirely VEGF-A–dependent process, as the blockade of VEGFR-3 has no effect on the blood vessels of non–tumor-bearing sites.
Nonetheless, after transferring some aspects of VEGFR-3 function in the EC to the in vitro culture system, the next issue is to investigate the mechanisms underlying VEGFR-3–induced stability of ECs. The most important molecule involved in the maintenance of the integrity of EC-EC junctions is VE-cadherin, disruption of which results in multiple and extensive hemorrhages.41-43 In the same culture system, we have demonstrated that VE-cadherin is the major functional cadherin involved in the formation of adherence junctions among ECs, as an antagonistic anti–VE-cadherin MoAb alone was sufficient to disrupt the adherence junctions of EC cultured on OP9. In this study, ECs scattered upon disruption of EC-EC junctions by VECD-1. Although VE-cadherin has been implicated as a target molecule of VEGFR-2 and VEGFR-3 signals,53 the mechanisms underlying AFL4-induced EC dispersion appear to be distinct from those underlying VECD-1–induced dispersion. First, ECs in the AFL4-treated culture take on an elongated spindlelike shape, whereas those in VECD-1–treated cultures remain polygonal. Second, although ECs scatter upon addition of AFL4 treatment, adherence junctions where VE-cadherin, β-catenins, and γ-catenins are concentrated are maintained at EC-EC junction sites, although intercellular contact areas are reduced compared with EC clusters in untreated cultures. In contrast, anti–VE-cadherin treatment disrupts EC-EC junctions and results in internalization of VE-cadherin, β-catenins, and γ-catenins to the cytoplasm, although ZO1 (Figure 7D), which is a tight junction protein, is still localized at cell-cell contact sites.
Interestingly, it seems that the same cytological changes induced by AFL4 treatment were found in EC treated with exogenous VEGF-A. Indeed, the morphological features of ECs in VEGF-A– and AFL4-treated cultures are indistinguishable. These cytological observations suggest that VEGFR-3 modulates EC activity through negative regulation of VEGFR-2 signals; thus, inhibition of VEGFR-3 signals enhances the activity of VEGFR-2 to promote cell motility.
In this study, we succeeded in ascribing some aspects of the vascular defects caused by the blockade of VEGFR-3 to an outcome of cross-talk between VEGFR-2 and VEGFR-3 signals. Thus, the next questions are how VEGFR-2 signals induced such alteration of EC behavior and how VEGFR-3 affects VEGFR-2. To address these questions, biochemical analyses are required, and our experimental system is still limiting in the number of ECs that can be obtained. Because of this limitation, biochemical analyses of ECs have been carried out using cell lines. Although a number of studies have reported biochemical consequences of VEGFR signals, none of those detected in various cell lines could be correlated to the actual behavior of the EC. We believe that one solution to overcome this problem would be to increase the efficiency of EC production from the ES cell and establish a system by which more than 107 cells can be easily obtained for biochemical studies. Efforts along this line are in progress.
We thank Dr M. J. Evans for providing CCE ES cell line, Dr K. Alitalo for human wild-type VEGF-C, Dr H. Niwa for EB5 ES cell line, Dr N. Matsuyoshi for VECD-1 MoAb, and Dr Tsukita and Dr Furuse for MoAb for ZO1. We thank Dr Ruth Yu for critical reading of this paper.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-05-1329.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (No. 11182101); the Organization of Pharmaceutical Safety and Research; and the Ministry of Health, Labor and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazuyoshi Matsumura, Department of Molecular Genetics, Graduate School of Medicine, Kyoto University, 53 Kawahara-cho, shogoin, Sakyo-ku, Kyoto, 606-8507, Japan; e-mail:kazuy@kuhp.kyoto-u.ac.jp.

![Fig. 5. Immunofluorescence images of ECs cultured on OP9 without exogenous factors. / (A-I) In the absence of exogenous factors, sheetlike clusters were formed. ECs were polygonal and VE-cadherin, γ-catenins, and PECAM-1 were colocalized at stringlike cell-cell junctions (ZO1–VE-cadherin [VECD-1], panels A-B; γ-catenins–VE-cadherin [VECD-1], panels D-E; PECAM-1–VE-cadherin [VECD-1], panels G-H). Distributions of adhesion molecules were compared in merged images (panels C, F, I). Scale bar, 20 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-05-1329/3/m_h80433817005.jpeg?Expires=1769087547&Signature=tyMlqZauXShcluxy3y8HShsSZ8~JJrZR81tfalh0fLa-coemSl62jM41P6Rph3y0wgbq5Z5GdDbBzeB8E~FK91brKxN2sESYi5PbwsSgldluj5vN-SphjzTaTqxrxieCNNHF~kH8AtEhs3iKFmxxMnG7FMvDUeteVn2kqaAHPZtBCFv7ROP33Sc0aRUe1-nGjc7bdXdnRNNdiaVqDosgNF8NZJZoctl56dsX4N3zF2KHs5-ghaef2OvQ3bN-EZ9towl9uJs~~M5rHzpp1YSG51xsMW9MqjvHwkU3k3zvYhjmnNJN~JPdSMFnO3No5E3IO8QEeLhGflCMGtGMBt-5aQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Cytological similarity between AFL4- and VEGF-A–treated ECs. / (A-F) Addition of 50 ng/mL VEGF-A results in elongation of ECs. This shape change did not disrupt the cell-cell junctions; VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels A-B) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels D-E) were concentrated at intercellular contact sites. (G-L) In cultures treated with 100 μg/mL AFL4, ECs became spindle-shaped. VE-cadherin, γ-catenin (γ-catenin–VE-cadherin [VECD-1]; panels G-H) and PECAM-1 (PECAM-1–VE-cadherin [VECD-1]; panels J-K) were localized at cell-cell contact sites. Unlike the thin stringlike junctions in untreated cultures (Figure 5), thicker beltlike junctions were formed in VEGF-A– and AFL4-treated cultures (panels A,G, arrows). Note that otherwise, the 2 groups are similar in all aspects examined here. Panels C, F, I, and L are merged images. Scale bar, 20 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-05-1329/3/m_h80433817006.jpeg?Expires=1769087547&Signature=2GQ~4fRgWX0NLOxOjm4OcKi7NjbGH5uDZ1IN39BbJxAjndhN8QI0voTOlnuGdbv4Wlkvc8uEu8BfdPpVPBEu3jwkBd1yvJzha4H2ItQ0j5CJK3e4pQy8KtmmxdC1w3TaBalW-c2Y7CeQjStXNdavutGZy1OtXkvrNzSBMfjBUXB45lW~UeajQZTD~cLsX7TQkOcpT41UhcE-hGcXBWRJ~nP~1WiSlLtU-Ht2oX-lKAq9bX-NY7f0OcIqqC5G0gGGzTRkXR6OVwpqKpq~tzF0vtLKMa1Hhb0~RR5xSf86q1RlHzkp-2A2Vk96l9vz08jbwTTDwtzGE6RVJcmBNrwaAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Intracellular localization of VE-cadherin in VECD-1–treated ECs. / (A-F) The addition of 20 μg/mL VECD-1 disrupted EC-EC contact, thereby allowing ECs to scatter. VE-cadherin was translocated from cell surfaces to the intracellular cytoplasm (panel E, arrow). Internalized VE-cadherin colocalized with γ-catenin (panel A, arrow) (γ-catenin–VE-cadherin [VECD-1]; panels A-B). (D-F) In contrast, ZO1 remains at the cell-cell junctions (ZO1–secondary Ab; panels D-E). Panels C and F are merged images. Scale bar, 20 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-05-1329/3/m_h80433817007.jpeg?Expires=1769087547&Signature=NbJbZIZJpHoPmMb9rJBkV0Ij4~L3n-8vYI2VjnZAcPGCrhxEne5PKPa2JfLi5fkRebc2Wxig26Ym4p1sbVe7h5Gdk-QGHi3QfKwiCO1f6vX0wD-J4C0ryaPWgSWDAo2LHCd3yfb4UOGGV-gkw67I~CsyhtMdWn7dd~u1e7CXK9nrQF1V8EAc7swW94azIQxay26vFt2WJEvfCGEdLjKTuzRmg8BONXJmmtwwEXNARkJa3Vzk6Ru~wlOQuzADZVfppf3DynivY0~T2Xxm-a3AFYuKVi81FCIVjAQOg-VkQ9qskpYbUEVHMvFdre9SSCE8Mumgf-KfC7vq~OK-ASrErA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal