Abstract
Cyclic adenosine monophosphate (cAMP) is a negative regulator of T-cell activation. However, the effects of cAMP on signaling pathways that regulate cytokine production and cell cycle progression remain unclear. Here, using primary human T lymphocytes in which endogenous cAMP was increased by the use of forskolin and 3-isobutyl-1-methylxanthine (IBMX), we show that increase of cAMP resulted in inhibition of T-cell receptor (TCR)/CD3 plus CD28–mediated T-cell activation and cytokine production and blockade of cell cycle progression at the G1 phase. Increase of cAMP inhibited Ras activation and phosphorylation of mitogen-induced extracellular kinase (MEK) downstream targets extracellular signal–related kinase 1/2 (ERK1/2) and phosphatidylinositol-3-kinase (PI3K) downstream target protein kinase B (PKB; c-Akt). These functional and biochemical events were secondary to the impaired activation of ZAP-70 and phosphorylation of LAT and did not occur when cells were stimulated with phorbol ester, which bypasses the TCR proximal signaling events and activates Ras. Increase of cAMP also inhibited activation of Rap1 mediated by TCR/CD3 plus CD28. Importantly, inhibition of Rap1 activation by cAMP was also observed when cells were stimulated with phorbol ester, although under these conditions Ras was activated and cells progressed into the cell cycle. Thus, TCR plus CD28–mediated activation of ERK1/2 and PKB, cytokine production, and cell cycle progression, all of which are inhibited by cAMP, require activation of Ras but not Rap1. These results indicate that signals that regulate cAMP levels after encounter of T cells by antigen will likely determine the functional fate toward clonal expansion or repression of primary T-cell responses.
Introduction
Cyclic adenosine monophosphate (cAMP) is a second messenger that regulates a wide variety of cellular functions. Intracellular levels of cAMP are controlled by 2 distinct enzyme superfamilies: the adenyl cyclases, which use adenosine triphosphate (ATP) as a substrate to synthesize cAMP and the cAMP-specific phosphodiesterases (PDEs), which hydrolyze cAMP to biologically inactive adenosine 5′-monophosphate.1,2 cAMP stimulates the proliferation of epithelial cells, hepatocytes, keratinocytes, pancreatic islet β cells, Schwann cells, and Swiss 3T3 cells.3 In contrast, cAMP inhibits proliferation of fibroblasts, smooth muscle, lymphoid, neuronal, and glial cells.4,5 Specifically, in T lymphocytes cAMP is a negative regulator of activation.6,7 It has been shown that increasing the cAMP levels by cAMP analogues, cAMP-elevating drugs, or PDE inhibitors results in inhibition of T-cell proliferation and production of Th1 cytokines but stimulation of Th2 gene expression.8
With few exceptions, the actions of cAMP are mediated through cAMP-dependent protein kinase A type I (PKAI).9 This isoenzyme is thought to play a dominant role in cAMP-mediated effects in T lymphocytes because it redistributes and colocalizes with the T-cell receptor (TCR) during T-cell activation.10Interestingly, intracellular cAMP is elevated in T cells from patients infected with HIV and PKAI-selective antagonists restore their impaired proliferation in response to mitogenic stimulation.11,12Similarly, increased levels of cAMP have been observed in T cells from patients with common variable immunodeficiency (CVI). As in HIV-infected individuals, addition of PKAI-selective antagonists markedly improves the T-cell responsiveness of patients with CVI to CD3-mediated stimulation.13
We have recently observed that increase of intracellular cAMP is detected in anergic T-cell clones, has a causative role in the elevation of endogenous p27kip1, and correlates with inhibition of antigen-specific proliferation and interleukin 2 (IL-2) production.14 CREB, a member of the cAMP response element–binding proteins, binds to the IL-2 promoter and is mandatory for IL-2 transcription. Mice expressing a dominant-negative form of CREB have defective thymocyte proliferation and IL-2 production.15 However, a complex of the cAMP response element–binding proteins, CREB and CREM (cAMP response element–binding protein), may function as transcriptional inhibitor and contribute to the defective IL-2 production in anergic cells.16 Stimulation of normal T lymphocytes via their TTCR leads to the production of cAMP, suggesting that cAMP-mediated repression of T-cell activation following TCR/CD3 ligation may represent a physiologic negative feedback control mechanism.17 Understanding the effects of cAMP on signaling pathways that regulate the T-cell activation may have biologic and therapeutic implications in clinical conditions related to altered levels of intracellular cAMP. Pharmacologic increase of intracellular cAMP may suppress unwanted T-cell responses in autoimmune diseases and organ transplantation. These observations prompted us to examine the effects of cAMP on signaling pathways critical for T-cell activation, cytokine production, and cell cycle progression.
In the present studies, we show that increase of intracellular cAMP resulted in inhibition of TCR/CD3 plus CD28–mediated T-cell proliferation, production of cytokines, and cell cycle progression. Increase of cAMP led to inhibition of Ras activation and blocked phosphorylation of mitogen-induced extracellular kinase (MEK) downstream targets extracellular signal–related kinase 1/2 (ERK1/2), as well as the phosphorylation of phosphatidylinositol-3-kinase (PI3K) target protein kinase B (PKB). These events were associated with impaired activation of ZAP-70 and phosphorylation of LAT. In contrast, increased cAMP did not inhibit cell cycle progression, activation of Ras, and phosphorylation of ERK1/2 and PKB on stimulation with phorbol ester, which bypasses the TCR proximal signaling events and directly activates Ras. Strikingly, increase of cAMP inhibited activation of Rap1 mediated by TCR/CD3 plus CD28 but also by phorbol ester. Thus, cAMP-induced inhibition of Ras activation is mediated on TCR proximal substrates, whereas inhibition of Rap1 activation is mediated on downstream targets. These results provide evidence that activation of Ras but not Rap1 is required for TCR plus CD28–mediated activation of ERK1/2 and PKB, cytokine production, and cell cycle progression, all of which are inhibited by cAMP. Moreover, these results indicate that activation of Ras and Rap1 is regulated by distinct mechanisms in primary human T lymphocytes.
Materials and methods
Isolation of primary peripheral blood T cells
Leukopacks were obtained from the blood banks of the Dana-Farber Cancer Institute and Brigham and Women's Hospital. Mononuclear cells were isolated by Ficoll/Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation for 20 minutes at 2000 rpm at room temperature. T cells were enriched by depletion of plastic-adherent mononuclear cells and positive selection by E-rosetting using sheep red blood cells (Biowhittaker, Walkersville, MD). The cells were then washed extensively to deplete contaminating platelets and rested overnight at 37°C in RPMI/10% fetal calf serum (FCS). CD14+, natural killer (NK), and B cells were removed by the use of the appropriate monoclonal antibodies (mAbs) and antimouse Ig–coated magnetic beads. Such cell preparations were more than 95% CD3+ cells, with the remaining being CD16+/CD56+ NK cells and less than 1% CD14+ and CD20+ as assessed by flow cytometry.
Cell culture
Cells were maintained in RPMI (Cellgro/Mediatech, Herndon, VA) medium supplemented with 10% (vol/vol) fetal bovine serum (FBS; Harlan, Indianapolis, IN), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 10 mM, Cellgro/Mediatech), minimum essential medium (MEM) sodium pyruvate (1 mM, Gibco/Invitrogen, Carlsbad, CA), and penicillin (50 IU/mL) and streptomycin (50 μg/mL) (Cellgro/Mediatech) in a 5% CO2 humidified atmosphere at 37°C.
Agents and antibodies
Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma (St Louis, MO). The T-cell–activating anti-CD3 (CLB-T3/4.E, 1XE; IgE) and anti-CD28 (CLB-CD28/1, 15E8; IgG1) mAbs were from Research Diagnostics (Franklin Lakes, NJ). Forskolin and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma and were initially used at a range of concentrations for titration. Subsequently, in all other experiments forskolin was used at 3 μM and IBMX was used at 62 μM. T cells were incubated with these factors for 30 minutes at 4°C prior to the initiation of culture or in vitro activation.
Proliferation (DNA synthesis) assay
Cells were seeded in triplicate at a concentration of 1 × 105 cells/well in RPMI/10% FCS in 96-well flat-bottom plates (Costar, Corning, Corning, NY) with the indicated factors. Cells were pulsed with 0.5 μCi (0.0185 MBq)/well [3H]-thymidine (TdR) for the last 6 hours of a 72-hour incubation period and harvested onto membranes; the incorporated [3H]TdR was measured in a liquid scintillation counter (Wallac Trilux, Turku, Finland).
Assessment of viability by flow cytometry
T cells were seeded at a concentration of 2 × 106cells/well in 4 mL RPMI/10% FBS in 6-well plates (Costar) and left untreated or treated as indicated. Quantitative determination of viability was performed using an annexin V–based apoptosis detection kit and the manufacturer's protocol (R & D Systems, Flanders, NJ). Briefly, cells were suspended in the appropriate binding buffer, stained with fluorescein isothiocyanate (FITC)–conjugated annexin V and propidium iodide at room temperature for 15 minutes, and subsequently analyzed by flow cytometry.
T-cell activation and Western blotting
For T-cell stimulation by TCR/CD3 cross-linking, T cells (107) were seeded in Eppendorf tubes in 1 mL serum-free RPMI medium and left untreated or treated with anti-CD3 (1 μg/mL) or anti-CD28 (1 μg/mL) or both for 30 minutes on ice. Cells were washed twice with plain medium and then stimulated by cross-linking with rabbit antimouse immunoglobulins (20 μg/mL; Dako, Carpinteria, CA) in 1 mL prewarmed medium for the indicated times. For mitogenic stimulation that bypasses the TCR, T cells were first incubated with anti-CD28 mAb and during the cross-linking step with rabbit antimouse immunoglobulins at 37°C, PMA (50 ng/mL) was added. Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer (0.5% NP-40, 50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8.0, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium orthovanadate, 10 μg/mL leupeptin, and 10 mg/mL aprotinin) for 20 to 30 minutes on ice. Protein concentration was determined with Bio-Rad DC Protein Assay. The lysates were mixed 1:2 with Laemmli sample buffer (Bio-Rad, Hercules, CA), heated for 5 minutes at 95°C, and proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membranes (Millipore, Bedford, MA), and incubated with blocking buffer (Tris-buffered saline/Tween 20 [TBST]/0.5% bovine serum albumin [BSA] or TBST/3% nonfat dry milk) overnight at 4°C. Immunoblot was done with the indicated primary antibody followed by the appropriate horseradish peroxidase (HRP)–conjugated goat antimouse (1:5000) or goat antirabbit (1:5000) IgG and immunodetection with enhanced chemiluminescence (ECL; NEN Life Science Products, Boston, MA). Stripping and reprobing of the blots was performed as described.18 Preparation of nuclear extracts was done according to described protocol.14 Antibodies specific for c-Jun, phospho-c-Jun, and c-Fos were from Santa Cruz Biotechnology (Santa Cruz, CA) and for nuclear factor of activated T cells (NFATp) from Transduction Laboratories (Lexington, KY). Cyclin D2, cyclin D3, cyclin E, cyclin A, cyclin dependent kinase 4 (cdk4), cdk6, and cdk2 antibodies were from Santa Cruz. The p27kip1 mAb was from Transduction Laboratories and pRb antibody from Pharmingen (Palo Alto, CA).
Detection of Ras and Rap1 activation
To study activation of Ras, the guanosine triphosphate (GTP)–bound form of Ras was affinity purified by the use of Raf1-RBD-GST complexed with glutathione beads as described.19 Complexes were analyzed by SDS-PAGE and immunoblot with Ras-specific antibody. For the detection of the GTP-bound form of Rap1 RalGDSRBD-GST complexed with glutathione beads was used. After SDS-PAGE the presence of activated Rap1 was determined by immunoblot with Rap1-specific antibody.
Jurkat cell transfection and luciferase assays
For assessment of IL-2 transcription, Jurkat T cells were transiently transfected with 20 μg reporter constructs of luciferase driven by either the 2-kb IL-2 promoter/enhancer, AP-1, NFAT (Clontech, Palo Alto, CA), or nuclear factor κB (NF-κB).20 Forty hours after transfection 5 × 105 cells were aliquoted and cultured for 6 hours as indicated in each experiment and luciferase activity was measured. Transfection efficiency was normalized by cotransfection with pEF-lacZ and assay for β-galactosidase.
Cytokine ELISA
Culture supernatants were harvested at 24, 48, and 72 hours of culture and analyzed for cytokine production according to the manufacturer's protocol by enzyme-linked immunosorbent assay (ELISA) for IL-2 (Endogen, Cambridge, MA), interferon γ (IFN-γ), IL-4, and IL-10 (R & D Systems, Minneapolis, MN). Endogenous cAMP after incubation with forskolin and IBMX was also determined by ELISA (R & D Systems).
Results
Increase of intracellular cAMP in primary human T cells inhibits proliferation in response to TCR/CD3 plus CD28 but not in response to phorbol ester plus CD28
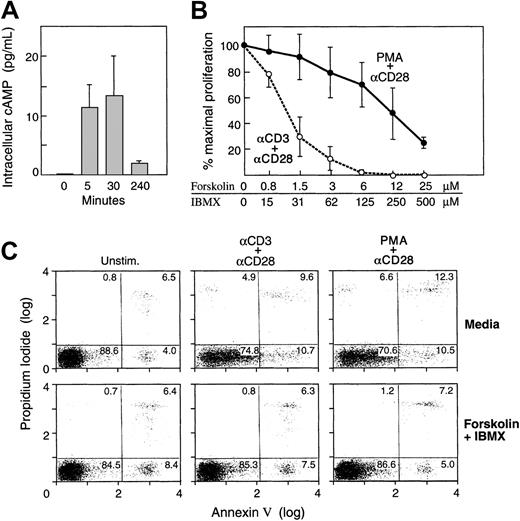
To avoid the use of artificial cAMP analogues, we used forskolin and IBMX to increase levels of endogenous cAMP. Forskolin is an activator of adenyl cyclase and IBMX is a xanthin-derivative that inhibits PDEs. The combination of both agents leads to increased production and decreased degradation of cAMP. We first examined the efficiency and the kinetics of cAMP induction by forskolin and IBMX. As shown in Figure 1A, increase of endogenous cAMP was detectable after 5 minutes, peaked at 30 minutes, and returned to baseline by 4 hours of culture. Based on these observations, for all subsequent experiments cells were preincubated with forskolin and IBMX for 30 minutes prior to initiation of stimulation. These concentrations were comparable to those previously observed in an antigen-specific clonal system after antigenic stimulation.14
Increase of intracellular cAMP in primary human T cells inhibits proliferation in response to TCR/CD3 plus CD28 but not in response to phorbol ester plus CD28.
(A) Kinetics of intracellular increase of cAMP. Purified primary human T cells were cultured for the indicated time intervals with forskolin and IBMX as described in “Materials and methods” and levels of endogenous intracellular cAMP were examined. Results are representative of 3 independent experiments. (B) Purified primary human T cells were cultured with either anti-CD3 and anti-CD28 mAbs or with phorbol ester and anti-CD28 mAb as described in “Materials and methods,” either in media or with the indicated concentrations of forskolin and IBMX. Incorporation of 3H-thymidine was examined at the final 6 hours of a total 72-hour culture interval. Results are representative of 5 independent experiments. Although there was variability in the responses among different donors, the same trend was observed in all tested samples. Error bars indicate range of responses. (C) Increase of intracellular cAMP does not induce apoptosis. Primary human T cells were cultured under the indicated conditions in the presence or absence of forskolin and IBMX and the percentage of apoptotic cells was examined by annexin and propidium iodide. Cells were analyzed at various time intervals (24, 48, 72 hours after culture), and results representing the 72 hours of culture are shown. The results are representative of 2 experiments.
Increase of intracellular cAMP in primary human T cells inhibits proliferation in response to TCR/CD3 plus CD28 but not in response to phorbol ester plus CD28.
(A) Kinetics of intracellular increase of cAMP. Purified primary human T cells were cultured for the indicated time intervals with forskolin and IBMX as described in “Materials and methods” and levels of endogenous intracellular cAMP were examined. Results are representative of 3 independent experiments. (B) Purified primary human T cells were cultured with either anti-CD3 and anti-CD28 mAbs or with phorbol ester and anti-CD28 mAb as described in “Materials and methods,” either in media or with the indicated concentrations of forskolin and IBMX. Incorporation of 3H-thymidine was examined at the final 6 hours of a total 72-hour culture interval. Results are representative of 5 independent experiments. Although there was variability in the responses among different donors, the same trend was observed in all tested samples. Error bars indicate range of responses. (C) Increase of intracellular cAMP does not induce apoptosis. Primary human T cells were cultured under the indicated conditions in the presence or absence of forskolin and IBMX and the percentage of apoptotic cells was examined by annexin and propidium iodide. Cells were analyzed at various time intervals (24, 48, 72 hours after culture), and results representing the 72 hours of culture are shown. The results are representative of 2 experiments.
T cells were stimulated with anti-CD3 and anti-CD28 in media or in the presence of increasing concentrations of forskolin and IBMX and DNA synthesis was assessed. Culture with anti-CD3 and anti-CD28 mAbs resulted in significant proliferation, consistent with previous observations, and this response was inhibited by forskolin and IBMX (Figure 1B). Pretreatment with various concentrations of these factors showed that they inhibited proliferation in a concentration-dependent manner. To bypass TCR proximal signaling events, T cells were incubated with phorbol ester (PMA) plus anti-CD28 mAb in media or with forskolin and IBMX. Although cAMP-elevating agents induced a dose-dependent inhibition on PMA plus CD28–mediated T-cell proliferation, the inhibitory effect of these factors was significantly reduced as compared to their effect on TCR/CD3 plus CD28–mediated T-cell proliferation (Figure 1B). These results suggest that cAMP inhibited T-cell expansion by mediating its effect on pathways proximal to TCR but upstream of protein kinase C (PKC) and the Ca2+-diacylglycerol-dependent guanidine nucleotide exchange factor II (CD-GEFII) specific for Ras,21 22 both of which are activated directly by PMA.
A critical question regarding the effect of the cAMP-elevating agents is whether these substances may induce apoptosis, thereby preventing proliferation. To address this question, we examined the presence of apoptotic cells, by annexin and propidium iodide, in cultures established in the presence or in the absence of forskolin and IBMX. Increase of cAMP not only did not induce apoptosis, but resulted in decreased percentage of apoptotic cells as compared to identical cultures in the absence of cAMP-elevating agents (Figure 1C). These results indicate that increased cell death is not responsible for the cAMP-mediated inhibition of T-cell proliferation.
T cells express well-defined markers in response to activation. Because cAMP prevented T-cell proliferation, we examined whether cAMP had any effect on the ability of these cells to up-regulate activation markers. Expression of CD69 and CD71 as well as expression of the IL-2 receptor α, β, and γ chains were diminished in T cells stimulated with TCR/CD3 plus CD28 in the presence of forskolin and IBMX (data not shown). Consistent with the less prominent inhibitory effect of these cAMP-elevating factors on PMA plus CD28–mediated proliferation, expression of these markers was almost unaffected in T cells stimulated with PMA plus CD28 in the presence of forskolin and IBMX (data not shown).
Increase of intracellular cAMP in primary human T cells allows TCR/CD3 plus CD28–mediated G1 phase entry but inhibits cell cycle progression
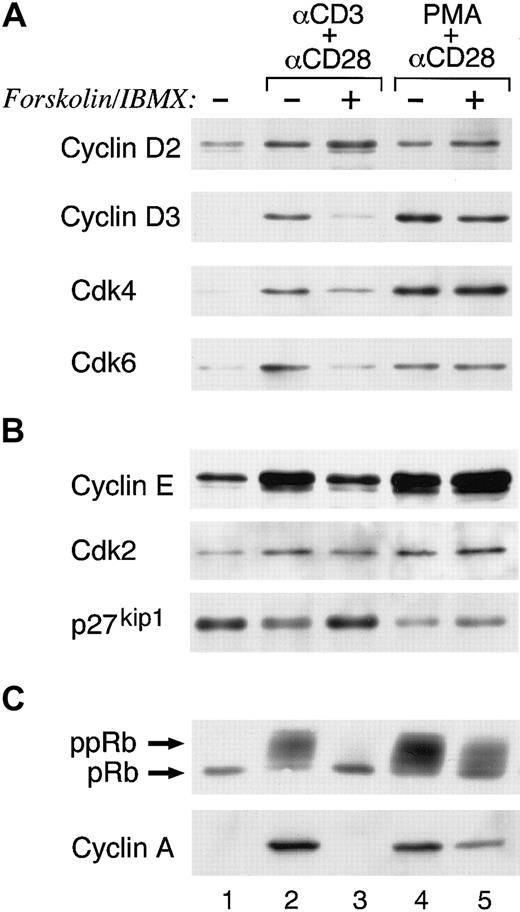
Previous studies have shown that TCR/CD3 plus CD28 costimulation regulates T-cell entry into the cell cycle and progression through the G1 phase, resulting in down-regulation of p27kip1 cdk inhibitor and activation of cyclin D2–associated cdk4/cdk6 and cyclin E–associated cdk2. These events led to hyperphosphorylation of the retinoblastoma (Rb) gene product, synthesis of S-phase genes, and cell cycle progression.23Because increase of cAMP inhibited cell cycle progression and DNA synthesis, we examined its effects on the molecular mechanisms that control entry and progression through the cell cycle. T cells were stimulated via TCR/CD3 plus anti-CD28 in media or with forskolin and IBMX and the expression of cell cycle regulatory molecules was examined at the indicated time intervals. Stimulation via TCR/CD3 plus CD28 resulted in entry to the G1 phase of the cell cycle as determined by induction of cyclin D2, the expression of which was not affected by increase of cAMP. The cyclin D2–associated cdk4 and cdk6 were also induced albeit at lower levels as compared to the culture in the absence of forskolin and IBMX (Figure2A, compare lane 1 to lanes 2 and 3). However, progression through the G1 restriction point to the late G1 and S phase was impaired by forskolin and IBMX. Cyclin D3, which is synthesized in the late G1 phase and cyclin E, which is synthesized at the G1 restriction point were barely induced (Figure 2A-B, compare lane 1 to lanes 2 and 3). Moreover, cyclin A, which is synthesized at the S phase, was not detected (Figure 2C, compare lane 1 to lanes 2 and 3). In contrast, in cultures of T cells stimulated in the presence of forskolin and IBMX, p27kip1 was elevated as compared to cells stimulated via TCR/CD3 plus CD28 alone but also as compared to cells cultured with media alone (Figure 2B, compare lane 1 to lanes 2 and 3). Under these conditions hyperphosphorylation of Rb was inhibited (Figure2C).
Increase of intracellular cAMP in primary human T cells allows TCR/CD3 plus CD28–mediated G1 phase entry but inhibits cell cycle progression.
(A-B) T cells were cultured with TCR/CD3 plus anti-CD28 or with phorbol ester plus anti-CD28 either in media or with forskolin and IBMX. At 48 hours of culture, whole cell lysates were prepared and expression of the indicated cell cycle regulatory molecules was examined by 10% SDS-PAGE analysis followed by Western blot. (C) For detection of Rb phosphorylation the same cell lysates were analyzed by 6% SDS-PAGE followed by Western blot with Rb-specific antibody. Cyclin A was examined by stripping and reprobing the immunoblots used in panels A and B. Results are representative of 3 experiments.
Increase of intracellular cAMP in primary human T cells allows TCR/CD3 plus CD28–mediated G1 phase entry but inhibits cell cycle progression.
(A-B) T cells were cultured with TCR/CD3 plus anti-CD28 or with phorbol ester plus anti-CD28 either in media or with forskolin and IBMX. At 48 hours of culture, whole cell lysates were prepared and expression of the indicated cell cycle regulatory molecules was examined by 10% SDS-PAGE analysis followed by Western blot. (C) For detection of Rb phosphorylation the same cell lysates were analyzed by 6% SDS-PAGE followed by Western blot with Rb-specific antibody. Cyclin A was examined by stripping and reprobing the immunoblots used in panels A and B. Results are representative of 3 experiments.
In contrast to these results, addition of cAMP-elevating agents did not inhibit PMA plus CD28–mediated induction of cdk's and cyclins except for a slight reduction of cyclin A (Figure 2A-C, compare lane 1 to lanes 4 and 5). Likewise, significant hyperphosphorylation of Rb was observed (Figure 2C). These results indicate that the proliferative response of T cells to PMA plus CD28 was only partially impaired by forskolin and IBMX (Figure 1B), because under these conditions most of the molecular events that regulate cell cycle progression remained unaltered (Figure 2A-C).
Increase of intracellular cAMP in primary human T cells inhibits production of both Th1- and Th2-type cytokines
It is well documented that increased cAMP inhibits IL-2 production.6,9 However, several studies have suggested that cAMP mediates transcription and secretion of Th2-type cytokines.8 24 Therefore, we examined the effect of endogenous cAMP increase on Th1- and Th2-type cytokine production in our system. Cultures were established as shown in Figure 1B using concentrations of forskolin and IBMX that completely inhibited TCR/CD3 plus CD28–mediated proliferation, but only marginally affected PMA plus CD28–mediated proliferative responses. As shown in Figure3, increase of endogenous cAMP abrogated production of IL-2, IFN-γ, IL-4, and IL-10 mediated by TCR/CD3 plus CD28. In contrast, when TCR proximal signals were bypassed by using PMA instead of anti-CD3, increase of intracellular cAMP inhibited IL-2 and IFN-γ production by 50%. Under these conditions, elevation of cAMP had different effects on the Th2-type cytokines tested. Although IL-10 production was abrogated, the levels of IL-4 remained unaltered (Figure3). Thus, elevation of cAMP has a global inhibitory effect on TCR/CD3 plus CD28–mediated production of Th1- and Th2-type cytokines. In contrast, when TCR proximal events are bypassed by the use of phorbol ester, elevation of cAMP has a significantly diminished inhibitory effect on the production of Th1-type cytokines and has no inhibitory effect on IL-4 production.
Increase of intracellular cAMP in primary human T cells inhibits production of both Th1- and Th2-type cytokines.
Cultures of primary human T lymphocytes were established as described in the legend to Figure 1B using concentrations of forskolin and IBMX, which completely inhibited TCR/CD3 plus CD28–mediated proliferation, but only slightly inhibited PMA plus CD28–mediated proliferative responses. Supernatants were collected at 24, 48, and 72 hours of culture and the levels of the indicated cytokines were examined by ELISA. The time interval at which the maximum production was detected in anti-CD3 plus anti-CD28 culture is shown for each cytokine; that interval was 24 hours for IL-2, 48 hours for IL-4, and 72 hours for IFN-γ and IL-4. Results are representative of 4 experiments. Error bars indicate range of responses generated in these experiments.
Increase of intracellular cAMP in primary human T cells inhibits production of both Th1- and Th2-type cytokines.
Cultures of primary human T lymphocytes were established as described in the legend to Figure 1B using concentrations of forskolin and IBMX, which completely inhibited TCR/CD3 plus CD28–mediated proliferation, but only slightly inhibited PMA plus CD28–mediated proliferative responses. Supernatants were collected at 24, 48, and 72 hours of culture and the levels of the indicated cytokines were examined by ELISA. The time interval at which the maximum production was detected in anti-CD3 plus anti-CD28 culture is shown for each cytokine; that interval was 24 hours for IL-2, 48 hours for IL-4, and 72 hours for IFN-γ and IL-4. Results are representative of 4 experiments. Error bars indicate range of responses generated in these experiments.
Mechanism of inhibition of IL-2 transcription by cAMP in primary human T cells
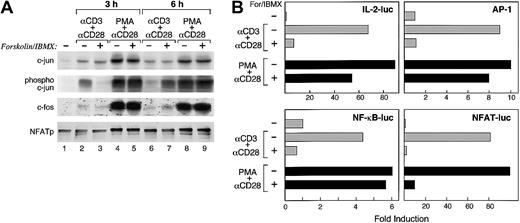
Production of IL-2 is a prominent indicator of T-cell activation. Impaired IL-2 production and defective transactivation of AP-1 and NFAT promoter elements are hallmarks of anergic T cells, in which cAMP is elevated.14,25 26 Therefore, we examined the mechanism via which increase of endogenous cAMP resulted in defective IL-2 production in our system. T cells were stimulated with either anti-CD3 plus anti-CD28 or PMA plus anti-CD28 in the presence of media or with forskolin and IBMX. After 2 different time intervals of culture (3 and 6 hours), expression and phosphorylation of c-Jun, expression of c-Fos, and dephosphorylation of NFATp were examined by Western blot. Increase of cAMP during TCR/CD3 plus CD28–mediated stimulation did not affect induction of c-Jun expression (Figure 4A first panel, lanes 1-3, 6, and 7). However, it resulted in delayed phosphorylation of c-Jun (Figure 4A second panel, lanes 1-3, 6, and 7), slightly delayed induction of c-Fos (Figure 4A third panel, lanes 1-3, 6, and 7), and delayed dephosphorylation of NFATp (Figure 4A fourth panel, lanes 1-3, 6, and 7). Surprisingly, increase of cAMP with forskolin and IBMX did not affect the expression or the phosphorylation status of AP-1 or NFAT transcription factors when T cells were stimulated by PMA plus anti-CD28 mAb (Figure 4A all panels, lanes 1, 4, 5, 8, and 9).
Mechanism of inhibition of IL-2 transcription by cAMP in primary human T cells.
(A) T cells were stimulated with either anti-CD3 plus anti-CD28 or PMA plus anti-CD28 in the presence of either media alone or with forskolin and IBMX. At the indicated time intervals (3 and 6 hours) of culture whole cell extracts were prepared and expression of transcription factors was determined by SDS-PAGE and Western blot, using antibodies specific for c-Jun, phospho–c-Jun, c-Fos, and NFATp. Results are representative of 2 experiments. (B) Jurkat T cells were transiently transfected with a reporter construct driven by the 2-kb IL-2 promoter/enhancer or with reporter constructs of luciferase driven by either AP-1, NF-κB, or NFAT. Forty hours after transfection 5 × 105 cells were aliquoted and cultured with either media, a combination of anti-CD3 plus anti-CD28 mAb, or with phorbol ester and anti-CD28 mAb either alone or with forskolin and IBMX. After 6 hours of culture luciferase activity was measured. Results are representative of 3 experiments.
Mechanism of inhibition of IL-2 transcription by cAMP in primary human T cells.
(A) T cells were stimulated with either anti-CD3 plus anti-CD28 or PMA plus anti-CD28 in the presence of either media alone or with forskolin and IBMX. At the indicated time intervals (3 and 6 hours) of culture whole cell extracts were prepared and expression of transcription factors was determined by SDS-PAGE and Western blot, using antibodies specific for c-Jun, phospho–c-Jun, c-Fos, and NFATp. Results are representative of 2 experiments. (B) Jurkat T cells were transiently transfected with a reporter construct driven by the 2-kb IL-2 promoter/enhancer or with reporter constructs of luciferase driven by either AP-1, NF-κB, or NFAT. Forty hours after transfection 5 × 105 cells were aliquoted and cultured with either media, a combination of anti-CD3 plus anti-CD28 mAb, or with phorbol ester and anti-CD28 mAb either alone or with forskolin and IBMX. After 6 hours of culture luciferase activity was measured. Results are representative of 3 experiments.
To examine the consequence of these findings on IL-2 transcription and transactivation of IL-2 transcription factors, we transfected a luciferase-linked reporter driven by the 2-kb IL-2 promoter/enhancer into Jurkat T cells. Forskolin and IBMX inhibited TCR/CD3 plus CD28–mediated IL-2 transcription by 89% (Figure 4B). In contrast, forskolin and IBMX inhibited PMA plus CD28–mediated IL-2 transcription by 40%. Moreover, forskolin and IMBX abrogated TCR/CD3 plus CD28–mediated AP-1-luc, NF-κB-luc, and NFAT-luc reporter activity (Figure 4B). In contrast, forkolin and IBMX inhibited PMA plus anti-CD28–mediated AP-1-luc reporter activity only by 20% and NF-κB-luc reporter activity by 10% but inhibited NFAT-luc reporter activity by 90%. Thus, increased cAMP results in a global defect on TCR/CD3 plus CD28–mediated expression, phosphorylation, and transactivation of AP-1 and NFAT as well as transactivation of NF-κB transcription factors. In contrast, increased intracellular cAMP does not affect the expression or the phosphorylation status of AP-1 and NFAT transcription factors mediated by PMA plus anti-CD28. However, increased intracellular cAMP significantly inhibited transactivation of NFAT, which will likely account for the reduced production of IL-2 as compared to culture with PMA plus anti-CD28. Notably, under these conditions, cAMP-mediated defective NFAT transactivation was not due to defective dephosphorylation of this transcription factor, suggesting that other mechanisms, which regulate NFAT transactivation, may be targets of cAMP in primary human T cells.
Increase of cAMP in primary human T cells inhibits CD3 plus CD28–mediated activation of PKB and ERK1/2
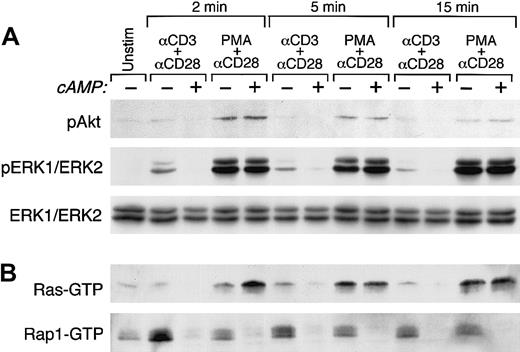
We have recently shown that the PI3K/PKB and the MEK/ERK1/2 pathways have mandatory roles for TCR/CD3 plus CD28–mediated cytokine production and cell cycle progression of primary human T lymphocytes. Moreover, PKB has a direct role in these events because direct inactivation of the PKB downstream target GSK-3 by LiCl can substitute for CD28 costimulatory signals and mediate cell cycle progression in the presence of TCR/CD3 ligation.18 Based on these observations, we examined the effect of increased cAMP on activation of PKB and ERK1/2. Purified primary human T cells were cultured with media or with anti-CD3 plus anti-CD28 mAbs followed by cross-linking with rabbit antimouse immunoglobulin for the indicated time intervals. Pretreatment with forskolin and IBMX inhibited TCR/CD3 plus CD28–mediated activation of PKB (Figure5A top panel, lanes 1-3, 6. 7, 10, and 11) but did not affect PMA plus CD28–mediated activation of PKB (Figure 5A top panel, lanes 1, 4, 5, 8, 9, 12, and 13). Similarly, forskolin plus IBMX inhibited TCR/CD3 plus CD28–mediated but not PMA plus CD28–mediated activation of MEK1/2 as determined by lack of phosphorylation of its downstream targets ERK1 and ERK2 (Figure5A middle panel). These results indicate that PMA plus anti-CD28–mediated activation of PBK and ERK1/2, which are both required for cell cycle progression, are not inhibited by increased intracellular cAMP and provide an explanation for our observations, shown in Figure 2, that forskolin and IBMX did not affect the cell cycle regulatory molecular events, which are induced by PMA plus anti-CD28.
Activation of PKB, ERK1/2, Ras, and Rap1.
(A) Increase of cAMP inhibits TCR/CD3 plus CD28–mediated but not phorbol ester–mediated activation of PKB and ERK1/2. Purified primary human T cells were cultured either with media, with anti-CD3 plus anti-CD28 mAbs, or with phorbol ester plus anti-CD28 mAb followed by cross-linking with rabbit antimouse immunoglobulin for the indicated time intervals. Where indicated, cells were pretreated with forskolin and IBMX. At the indicated time intervals cell lysates were prepared and activation of PKB and ERK1/2 was examined by SDS-PAGE and Western blot with phospho-specific antibodies. Immunoblots were stripped and reprobed with ERK1/2-specific antibody, which recognizes total nonphosphorylated ERK1/2 to confirm equal loading and ERK1/2 protein expression. (B) Increase of cAMP inhibits TCR/CD3 plus CD28–mediated but not phorbol ester–mediated activation of Ras but inhibits both TCR/CD3 plus CD28– and phorbol ester–mediated activation of Rap1. In the same cell lysates activation of Ras and Rap1 was examined by pull-down assays using glutathione beads coated with Raf1RBD-GST for Ras activation and RalGDSRBD-GST for Rap1 activation. Incubation of cells with forskolin and IBMX alone did not result in activation of either Ras or Rap1 (data not shown). Results are representative of 3 experiments.
Activation of PKB, ERK1/2, Ras, and Rap1.
(A) Increase of cAMP inhibits TCR/CD3 plus CD28–mediated but not phorbol ester–mediated activation of PKB and ERK1/2. Purified primary human T cells were cultured either with media, with anti-CD3 plus anti-CD28 mAbs, or with phorbol ester plus anti-CD28 mAb followed by cross-linking with rabbit antimouse immunoglobulin for the indicated time intervals. Where indicated, cells were pretreated with forskolin and IBMX. At the indicated time intervals cell lysates were prepared and activation of PKB and ERK1/2 was examined by SDS-PAGE and Western blot with phospho-specific antibodies. Immunoblots were stripped and reprobed with ERK1/2-specific antibody, which recognizes total nonphosphorylated ERK1/2 to confirm equal loading and ERK1/2 protein expression. (B) Increase of cAMP inhibits TCR/CD3 plus CD28–mediated but not phorbol ester–mediated activation of Ras but inhibits both TCR/CD3 plus CD28– and phorbol ester–mediated activation of Rap1. In the same cell lysates activation of Ras and Rap1 was examined by pull-down assays using glutathione beads coated with Raf1RBD-GST for Ras activation and RalGDSRBD-GST for Rap1 activation. Incubation of cells with forskolin and IBMX alone did not result in activation of either Ras or Rap1 (data not shown). Results are representative of 3 experiments.
Increase of cAMP in primary human T cells inhibits activation of Ras mediated by TCR/CD3 plus CD28 but not by PMA plus CD28 and inhibits activation of Rap1 mediated by TCR/CD3 plus CD28 and by PMA plus CD28
The growth-inhibitory effect of cAMP is proposed to be mediated through cAMP-dependent inactivation of ERK1/2.27 ERK1 and ERK2 are phosphorylated and activated by MEK1 and 2, which are phosphorylated and activated by Raf1 protein kinase.28 The activities of Raf1 are regulated by the Ras family of small GTP-binding proteins, Ras and Rap1.29 Three different mechanisms have been suggested to be involved in the inhibition of ERK by cAMP: (1) phosphorylation of Raf1 on Ser43 by PKA inhibits Raf1 activity thereby preventing MEK/ERK1/2 activation30; (2) cAMP-activated PKA phosphorylates Rap1, which regulates formation of Rap1-GTP31,32; and (3) cAMP activates EPAC (exchange proteins directly activated by cyclic AMP), a GEF exchange factor for Rap1 resulting in generation of Rap1-GTP.33 It is has been shown that Rap1-GTP competes with Ras for binding of Raf1 thereby blocking Ras-induced Raf1/MEK/ERK1/2 activation.29
First, we examined whether in our system, the inhibitory effect of cAMP was exerted directly on Raf1. If this is the mechanism of cAMP-mediated inhibition of ERK1/2, activation of Ras, which is upstream of Raf1 should not be affected by cAMP. T cells were cultured and activation of Ras was examined. As shown in Figure 5B (top panel, lanes 1-3, 6, 7, 10, and 11), cAMP inhibited activation of Ras induced by TCR/CD3 plus CD28. This observation indicated that for inhibition of ERK1/2 activation by cAMP, inhibition of Raf1 was not required because inhibition of Ras, which is upstream of Raf1, was readily detectable. Interestingly, when TCR-proximal signals were bypassed by the use of PMA, which directly activates Ras via PKC and CD-GEFII, increase of cAMP did not inhibit activation of Ras (Figure 5B top panel, lanes 1, 4, 5, 8, 9, 12, and 13). These results provide evidence that TCR-proximal substrates upstream of Ras are the dominant targets of cAMP in primary human T lymphocytes.
To determine the potential involvement of Rap1 in ERK1/2 inhibition by increase of cAMP, we examined the status of Rap1 activation in the same samples. Strikingly, increase of cAMP inhibited activation of Rap1 induced not only by TCR/CD3 plus CD28 but also by PMA plus CD28 (Figure5B bottom panel). The observation that cAMP inhibited PMA plus CD28–mediated activation of Rap1 but not Ras suggests that cAMP-sensitive signals downstream of CD-GEFs, which are directly activated by phorbol ester,22,34 are involved in the activation of Rap1. Moreover, these observations indicate that Rap1 is not activated in a Ras-dependent manner as has been proposed in other systems.35 Instead, distinct mechanisms appear regulate activation of Ras and Rap1 in primary human T cells.
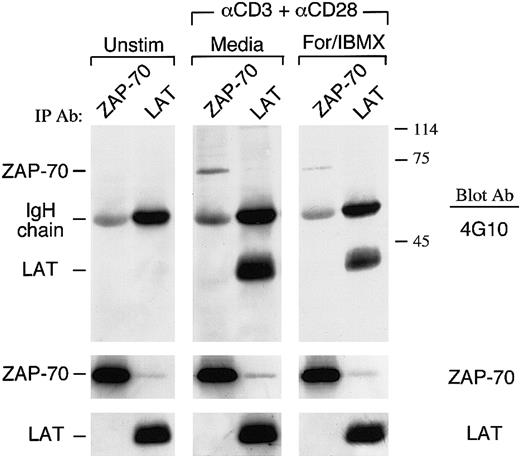
Increase of cAMP in primary human T cells inhibits activation of ZAP-70 and phosphorylation of LAT mediated by TCR/CD3 plus CD28
Two different mechanisms, one protein tyrosine kinase (PTK) independent and one PTK dependent, activate Ras. Following TCR ligation, active PLCγ1 hydrolyses phosphatidylinositol-4, 5-bisphosphate (PIP2) to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C (PKC), resulting in suppression of Ras-GAP activity,36,37 and also binds to and activates CD-GEFII, which directly activates Ras.21,22In the second, PTK-dependent mechanism, Ras activation after TCR/CD3 engagement occurs from recruitment to the plasma membrane of the adaptor protein growth factor receptor-binding protein (Grb2) complexed to son of sevenless (Sos), a GEF for Ras.38 The Grb2/Sos complex is recruited to the membrane by phosphorylated LAT.39 The active, GTP-bound Ras associates with and translocates the serine/threonine kinase Raf-1 from the cytosol to the membrane, exposing Raf1 to activating kinases localized at the plasma membrane.40,41 Activated Raf1, in turn, phosphorylates and activates MEK that phosphorylates and activates the mitogen-activated protein kinases (MAPKs) ERK1 and ERK2, resulting in activation of AP-1.28 42
Because our studies showed that cAMP inhibited upstream events leading to activation of Ras by TCR plus CD28, whereas activation of Ras by PMA plus C28 remained intact, we examined whether the PTK-dependent pathway of Ras activation was a target of cAMP. We examined the phosphorylation status of LAT, which recruits the Grb2/Sos complex to the plasma membrane to activate Ras. T cells were stimulated via TCR/CD3 plus CD28 in media or with forskolin and IBMX and immunoprecipitations were performed with LAT-specific antibody, followed by immunoblot with antiphosphotyrosine antibody. As shown in Figure6, increase of intracellular cAMP by forskolin and IBMX resulted in defective phosphorylation of LAT.
Increase of cAMP inhibits TCR/CD3 plus CD28–mediated activation of ZAP-70 and phosphorylation of LAT.
Purified T cells were cultured either with media or with anti-CD3 plus anti-CD28 mAbs followed by cross-linking with rabbit antimouse immunoglobulin for 3 minutes. Where indicated, cells were pretreated with forskolin and IBMX. Cell lysates were prepared and immunoprecipitations were performed with ZAP-70–specific or LAT-specific antibody, followed by immunoblot with antiphosphotyrosine antibody. Immunoblots were stripped and sequentially reprobed with an antibody specific for ZAP-70.
Increase of cAMP inhibits TCR/CD3 plus CD28–mediated activation of ZAP-70 and phosphorylation of LAT.
Purified T cells were cultured either with media or with anti-CD3 plus anti-CD28 mAbs followed by cross-linking with rabbit antimouse immunoglobulin for 3 minutes. Where indicated, cells were pretreated with forskolin and IBMX. Cell lysates were prepared and immunoprecipitations were performed with ZAP-70–specific or LAT-specific antibody, followed by immunoblot with antiphosphotyrosine antibody. Immunoblots were stripped and sequentially reprobed with an antibody specific for ZAP-70.
LAT is a substrate of ZAP-70, which is directly activated by TCR/CD3 ligation.39 Therefore, the diminished LAT phosphorylation by increased cAMP might be the consequence of defective activation of ZAP-70. Immunoprecipitation of lysates from the same cultures with ZAP-70–specific antibody showed reduced activation of ZAP-70 as determined by antiphosphotyrosine immunoblot in cells treated with forskolin and IBMX (Figure 6). Attempts to determine whether cAMP altered the activation status of fyn and lck did not lead to conclusive results (data not shown). Thus, inhibition of Ras activation by cAMP was associated with inhibition of ZAP-70, the TCR-proximal protein kinase, which is indispensable for T-cell activation.
Discussion
cAMP is a negative regulator of T-cell activation. Most studies have focused on the role of PKAI, which colocalizes with the TCR/CD3 complex, in mediating the effects of cAMP in T lymphocytes.10 Studies have suggested that the inhibitory effects of PKAI are mediated via activation of Csk1, which negatively regulates lck activity.43 Other studies have reported that PKA interferes with Ras/MAPK signaling by mediating Ser43 phosphorylation of Raf1, which inhibits its activity.30 In contrast, some reports suggested that the effects of cAMP in T cells are mediated in a PKA-independent manner.44
In the present studies we did not address the role of PKAI, but we focused on the effects of cAMP on PI3K/PKB and MEK/ERK1/2 signaling pathways, which as previously shown, are indispensable for cytokine production and cell cycle progression of primary human T lymphocytes.18 We also examined the effect of cAMP on the activation of Ras and Rap1. Our results showed that increased cAMP inhibited TCR/CD3 plus CD28–mediated activation of PI3K/PKB and MEK/ERK1/2 pathways, resulting in impaired cytokine secretion and cell cycle progression. These events were secondary to defective TCR/CD3 plus CD28–mediated activation of ZAP-70, phosphorylation of LAT, and activation of Ras and were not observed when proximal TCR signals were bypassed by the use of phorbol ester, which activates Ras.
Surprisingly, cAMP inhibited activation of Rap1 mediated both by TCR/CD3 plus CD28 and by PMA plus CD28. These observations suggest that in primary human T lymphocytes, Ras and Rap1 are activated by different mechanisms and have distinct roles. Our studies clearly showed that although cAMP inhibited activation of Rap1 mediated by PMA plus CD28, it did not prevent expression of cyclin D3 and cyclin A and DNA synthesis. Under these conditions, Ras was activated, ERK1/2 and PKB were phosphorylated, and cells were capable of producing cytokines and progressing into the cell cycle. Thus, in contrast to Ras, activation of Rap1 is not obligatory for cytokine production and cell cycle progression in primary human T lymphocytes.
Two different mechanisms, one PTK independent and one PTK dependent, activate Ras after TCR/CD3 ligation. It was previously thought that DAG-mediated activation of PKC activates Ras by suppressing the activity of Ras-GAP.36,37 However, the recent identification of the CD-GEFs, which bind to DAG, suggest a new mechanism for Ras activation.21,22,34 CD-GEFII (also known as RasGRP) binds not only to DAG but also to phorbol ester, suggesting a direct activation of Ras by phorbol esters. The second PTK-dependent mechanism for Ras activation occurs from recruitment of the Grb2/Sos complex to the plasma membrane by phosphorylated LAT.39Active Ras associates with and translocates Raf-1 from the cytosol to the membrane, resulting in its activation and initiation of the Raf1/MEK/ERK1/2 cascade.40-42
In contrast to these detailed studies on Ras activation, little is known about events related to Rap1 activation. One mechanism of Rap1 activation involves a cbl/crkL-dependent activation of C3G, Rap1-GEF.45 EPACs and CD-GEFs consist of new families of Rap1-specific GEFs. EPACs are activated by direct binding of cAMP,33,46 whereas CD-GEFI and CD-GEFIII mediate Ca2+- and DAG-dependent activation of Rap1.22,34 An additional newly proposed mechanism that may regulate Rap1 activity involves PLC-ε, which is a new Rap1-GEF candidate and is activated in a Ras-dependent manner.35Ras activation may occur via the Grb2/Sos complex or by CD-GEFII. Intriguingly, CD-GEFII can be activated be either TCR or phorbol ester–mediated signaling,21 suggesting that both these conditions may result in concomitant Ras-dependent activation of Rap1.
Our observation that cAMP inhibits activation of Rap1 mediated by PMA plus CD28 while Ras activity remains intact suggests that such a Ras-dependent activation of Rap1, secondary to the activation of GD-GEFII by phorbol ester, does not appear to be operative in primary human T cells. Moreover, the observation that cAMP not only did not activate but rather inhibited Rap1 activity induced by either TCR or phorbol ester suggests that EPACs may not be directly involved in Rap1 activation in primary human T cells. However, these studies cannot exclude a different role of EPAC or other cAMP-dependent GEFs on different downstream effectors in primary human T cells. It is also possible that EPACs may not have a role in primary human T lymphocytes but rather in activated T cells or cell lines. In fact, we previously observed that forced expression of EPAC inhibited IL-2 transcription in Jurkat T cells.14
The role of Rap1 on Ras activation has been a matter of ongoing study and debate. It has been proposed that activated Rap1 inhibits Ras activation or that activated Rap1 blocks Ras downstream events because it competes with Ras for common effectors.29,47 We have previously shown that in human T lymphocytes both Ras and Rap1 are activated by TCR/CD3 plus CD28–mediated signals and the ratio of Ras-GTP to Rap1-GTP determines the decision toward anergic or productive immune response and IL-2 production.48 Our present studies confirm and extend our previous observations indicating that in primary human T lymphocytes, TCR/CD3 plus CD28 ligation mediates simultaneous activation of Ras and Rap1 and these events are not mutually exclusive. In addition, our present studies show that Rap1 activation is not obligatory for cytokine production and cell cycle progression. As determined using cells treated with PMA plus CD28, increase of cAMP abrogated Rap1 activation but did not prevent production of cytokines, expression of cyclin D3 and cyclin A, and DNA synthesis.
Another issue of debate is the role of cAMP on Rap1 activation and its consequences on MAPK and PKB activation. Rap1 is differentially activated by cAMP in various cell types and regulates Raf1/MEK/ERK1/2 and PI3K/PKB downstream signaling.27,29,32,49,50 In PC12 cells cAMP mediates growth and differentiation via Rap1 activation, which results in MAPK activation in a B-Raf–dependent manner.50 In fibroblasts, cAMP activates Rap1 but inhibits ERK activity and cell growth.51 cAMP and trophic hormones in which cAMP is the second messenger activate PI3K, PKB, and p70S6 kinase in thyroid and ovarian granulosa cells, mediating cAMP-induced growth.52,53 In these systems, cAMP activates PI3K/PKB and this is suggested to be secondary to cAMP-induced activation of Rap1.54,55 In contrast, in rat astrocytes and glioma cells, cAMP inactivates ERK and PKB and inhibits growth and these effects are thought to be secondary to cAMP-mediated inhibition of Rap1.27 Inhibition of PI3K/PKB by cAMP has also been reported in Swiss 3T3 cells, HEK293, COS, and Rat2 cells, although the mechanism is largely unknown.56 Our studies on primary human T lymphocytes show that cAMP not only did not activate Rap1 but rather inhibited its activation that was induced by either TCR/CD3 plus CD28 or by PMA plus CD28 stimulation. Moreover, activations of Rap1 and PKB were not linked because increased cAMP inhibited Rap1 activation in TCR/CD3 plus CD28–stimulated cells in which PKB activation was inhibited, but also in PMA plus CD28–stimulated cells in which PKB activity remained intact.
In studying the molecular mechanisms that regulate cell cycle progression, we found that increase of cAMP during TCR/CD3 plus CD28–mediated stimulation resulted in a profile that was also observed when the PI3K inhibitor LY294002 or the MEK inhibitor UO126 were used.18 T cells were able to enter G1 phase and synthesize cyclin D2, cdk4, and cdk6 but neither cyclin D3 and cyclin A expression nor hyperphosphorylation of Rb was detected. Moreover, p27kip1 was increased as compared to identical cultures without cAMP-elevating agents and to cultures with media alone. Taken together our data show that increase of intracellular cAMP in primary human T cells leads to inhibition of primary T-cell responses. Thus, signals that regulate cAMP levels after encounter of a T cell by antigen will likely determine its fate toward clonal expansion or repression. Therefore, pharmacologic regulation of intracellular cAMP levels may be a therapeutic target for the modulation of the immune response.
We thank Alla Berezovskaya for technical support, Dr Leonard Appleman for helpful discussions and suggestions, and Dr Johannes Bos for providing the Raf1RBD-GST and RalGDSRBD-GST constructs.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-06-1665.
Supported by National Institutes of Health grants AI 43552 and AI 46548.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vassiliki A. Boussiotis, Dana-Farber Cancer Institute, Mayer 547, 44 Binney St, Boston, MA 02115; e-mail:vassiliki_boussiotis@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal