Abstract
Recent advances have made haploidentical transplantation for leukemia feasible, but the rigorous T-cell depletion used contributes to the high relapse rates observed. We have attempted to improve the graft-versus-leukemia (GVL) effect by generating allorestricted cytotoxic T lymphocytes (CTLs) directed against human CD45. Such CTLs should recognize patient hematopoietic cells including leukemia, enhancing donor cell engraftment and improving the GVL effect, but they should not recognize host nonhematopoietic tissues or donor cells from the graft. Using the T2 binding assay, 4 CD45-derived peptides were found to bind HLA-A2 molecules. These peptides were used to generate cytotoxic T-cell lines from HLA-A2− donors by sequential stimulation with peptide-pulsed HLA-A2+ stimulators, and the lines obtained were screened for peptide-specific cytotoxicity. Using one of these peptides (P1218), it was possible to generate peptide-specific, allorestricted CTLs in 3 of 7 responders. P1218-specific CTL lines show potent cytotoxicity against hematopoietic cell lines coexpressing HLA-A2 and CD45 but not CD45 loss variants. Studies with stable transfectants of 293 cells demonstrated recognition by P1218-specific CTLs of endogenously expressed CD45. Likewise P1218-specific CTLs recognized peripheral blood mononuclear cells (PBMCs) from HLA-A2+ patients with chronic myeloid leukemia (CML) and leukemic blasts in HLA-A2+ patients with acute myeloid leukemia (AML), but they were unable to lyse HLA-A2+ fibroblasts or HLA-A2− normal PBMCs. Coculture of CD34+ PBMCs and bone marrow mononuclear cells (BMMCs) with P1218-specific CTL significantly inhibited colony-forming unit–granulocyte macrophage (CFU-GM) formation in HLA-A2+healthy controls and CML patients but resulted in no significant inhibition in HLA-A2− healthy controls. These studies demonstrate that P1218-specific cytotoxic T lymphocytes (CTLs) have potent activity against leukemic progenitors and suggest that adoptive immunotherapy with allorestricted CTLs directed against CD45 epitopes may be useful in restoring the GVL effect after HLA-A2–mismatched haploidentical transplantation. Further, because P1218-specific CTLs also recognize healthy HLA-A2+ progenitors, such CTLs could also contribute to host myeloablation and enhance donor cell engraftment.
Introduction
Recent advances have made hematopoietic stem cell transplantation (HSCT) from haploidentical donors feasible in terms of reliable engraftment and acceptable rates of graft-versus-host disease (GVHD),1,2 though morbidity and mortality rates related to posttransplantation immunodeficiency remain problematic. A number of lines of evidence demonstrate that donor T lymphocytes play a critical role in eradicating residual leukemia after HSCT. These include experimental studies in vitro and in animal models,3-5 the higher relapse rate seen after T-cell–depleted HSCT,6 and the efficacy of donor lymphocyte infusions in restoring remission in patients who experience relapse after HSCT.7 However, after HLA-mismatched and haploidentical HSCT, this graft-versus-leukemia (GVL) effect is likely to be lost because of the rigorous T-cell depletion used, contributing to the high relapse rates seen in these patients. Several groups have investigated the potential use of ex vivo–generated antigen-specific cytotoxic T lymphocytes (CTLs), recognizing antigens that differ in their expression between patient and donor for adoptive immunotherapy to restore the GVL effect.4,5 8 Candidate target antigens for such an approach include tumor-specific antigens, those that are overexpressed in tumor cells, and mismatched minor histocompatibility antigens. In contrast, we have investigated the possibility of taking advantage of the disparity in HLA molecules needed to present shared antigens rather than relying on a difference in antigenic expression between host and donor. We have studied the antileukemic activity of such allorestricted CTLs in patients who have undergone HLA-mismatched HSCT.
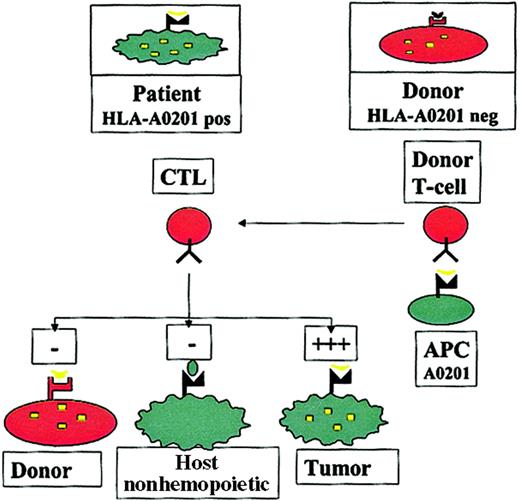
The concept of this approach has previously been described9 and is summarized in Figure1. Autologous T cells recognizing self-peptides derived from cellular proteins are usually clonally deleted, leading to self-tolerance. In contrast, in the absence of the appropriate HLA molecule, antigen-presenting cells (APCs) from an HLA-mismatched donor may not present such a peptide, so that the donor may have T cells recognizing epitopes to which the patient is tolerant. In patients who undergo HLA-mismatched or haploidentical HSCT, this could be exploited by stimulating donor T cells with APCs carrying the host HLA restriction (eg, HLA A201), pulsed with epitopes from a hematopoietic protein, to generate donor T cells that recognize hematopoietic antigens in the context of host HLA molecules. It could be predicted that such allorestricted CTLs would kill patient leukemic cells, which express the hematopoietic antigen and the appropriate HLA restriction, but they would not kill host nonhematopoietic tissues, which do not express the antigen (averting GVHD), or donor hematopoietic cells, which do not have the appropriate HLA restriction to present the antigen (averting toxicity to the graft).
The allorestricted cytotoxic T-cell concept.
Hematopoietic cells from an HLA-A0201+ patient present a hematopoietic epitope (eg, a CD45-derived peptide), represented by the yellow tick, on surface HLA-A0201 molecules. Because of tolerance, T cells recognizing this self-peptide have been clonally deleted in the patient. In contrast, in an HLA-A0201−donor who shares this antigen, the epitope cannot be presented because of the lack of HLA-A0201 on the cells; consequently T cells directed against this epitope are not deleted. Stimulation of such T cells with HLA-A0201+ APCs pulsed with the epitope may generate allorestricted CTLs that recognize leukemic and normal host hematopoietic cells, which coexpress HLA-A0201 and the hematopoietic antigen, but that do not recognize host nonhematopoietic tissues, which do not express the antigen or donor hematopoietic cells from the graft and are HLA-A0201−.
The allorestricted cytotoxic T-cell concept.
Hematopoietic cells from an HLA-A0201+ patient present a hematopoietic epitope (eg, a CD45-derived peptide), represented by the yellow tick, on surface HLA-A0201 molecules. Because of tolerance, T cells recognizing this self-peptide have been clonally deleted in the patient. In contrast, in an HLA-A0201−donor who shares this antigen, the epitope cannot be presented because of the lack of HLA-A0201 on the cells; consequently T cells directed against this epitope are not deleted. Stimulation of such T cells with HLA-A0201+ APCs pulsed with the epitope may generate allorestricted CTLs that recognize leukemic and normal host hematopoietic cells, which coexpress HLA-A0201 and the hematopoietic antigen, but that do not recognize host nonhematopoietic tissues, which do not express the antigen or donor hematopoietic cells from the graft and are HLA-A0201−.
Although such an approach could be used with any HLA mismatch, we focused on HLA-A0201 because it is expressed in approximately 50% of the white population; mismatches of this molecule were relatively common in haploidentical patient-donor pairs and in 1 antigen-mismatched patient-donor pair. We have previously demonstrated that HLA-A0201 allorestricted CTLs directed against an epitope from the Wilm tumor transcription factor (WT-1) kill leukemic cell lines and inhibit hematopoietic colony formation from progenitor cells from patients with chronic myeloid leukemia (CML).10WT-1 is overexpressed in myeloid leukemias and is thus an excellent target for such an approach in these diseases, but it may not be so useful for lymphoid malignancies that do not express WT-1 (eg, chronic lymphoid leukemia). Here we have investigated CD45 as an alternative target for allorestricted CTLs. CD45 is a 180- to 220-kDa plasma membrane-associated tyrosine phosphatase critical for T- and B-cell receptor–mediated activation.11 12 A number of features make it an excellent target for the immunotherapy of leukemias. It is abundantly expressed on most leucocytes, including leukemic blasts, so that CTLs directed against CD45 epitopes would likely be active in myeloid and lymphoid malignancies. Furthermore, allorestricted CTLs directed against CD45 should recognize normal host T cells and myelopoiesis and might thus contribute to immunosuppression and myeloablation of the host as an immunologic arm to conditioning. Finally, CD45 expression is restricted to hematopoietic cells; hence, CTLs recognizing CD45 epitopes should not target nonhematopoietic cells and would not be expected to cause GVHD. In this study we have explored the possibility of exploiting CD45 as a target molecule for allorestricted CTLs in patients with HLA-A0201 mismatch, and we have demonstrated that such CTLs show significant antileukemic activity in vitro.
Materials and methods
Cell lines, generation of primary fibroblasts, and purification of CD34+ cells
The T2 cell line is transporter-associated with antigen processing (TAP) deficient, resulting in inefficient loading of human leukocyte antigen class 1 molecules with endogenous peptides.13 As a consequence, the HLA-A0201 molecules of T2 cells can be efficiently loaded with exogenous peptides.Drosophila cells transfected with HLA-A0201 under the control of a metallothionein-inducible promoter, human β-2 microglobulin, B7.1, and intracellular adhesion molecule 1 (ICAM-1) were the kind gift of Dr M. Jackson. RMA/S-A2 is a mouse lymphoma line transfected with a human genomic HLA-A0201 clone including the endogenous promoter. C1R-A2 is an HLA-A0201–transfected Epstein-Barr virus (EBV)–transformed lymphoblastoid cell line, and WS29 cell is an EBV-transformed lymphoblastoid cell line from an HLA-A0201–negative patient. BV173 is an HLA-A0201–positive cell line derived from a patient with CML in lymphoid blast crisis that lost expression of CD45. HeLa-A2 cells were made by transfecting the human cervical epithelial cell line HeLa with a genomic HLA-A0201 clone, including the endogenous promoter. T4D7 and MCF-7 are HLA-A2− and HLA-A2+ breast cancer epithelial cell lines, respectively. U-266 is a myeloma cell line known to be biphenotypic with regard to CD45 expression.14 This cell line was sorted into CD45− and CD45+ fractions using a FACScan flow cytometer (BD Biosciences, San Jose, CA). Stable transfectants of 293 cells (a human embryonic kidney cell line) expressing CD45RO were made using a transient retroviral supernatant as described in the report by Kelly et al.15 Briefly, the pZIPLCA1 plasmid16 expressing CD45RO in pZIPNeo was cotransfected with the plasmids peq-PAM3 (containing the Moloney murine leukemia virus gag and pol genes) and pRD114-env (containing the env gene from the feline leukemia virus) into 293T cells using Fugene 6 (Roche Diagnostics, Lewes, United Kingdom). Forty-eight hours later the supernatant from these cells was used to transduce wild-type 293 cells with 8 μg/mL polybrene (Specialty Media, Philipsburg, NJ). After another 48 hours, cells were placed under selection with 750 μg/mL G418. Initial experiments with 2 constructs carrying full-length CD45RA yielded neomycin-resistant cells, but CD45 expression was not detected, suggesting this gene may be toxic in some nonhematopoietic cells. In neomycin-resistant 293 cultures stably transfected with pZIPLCA1, approximately 20% of cells expressed CD45. These CD45+ cells were isolated using a FACScan flow cytometer (BD Biosciences) and maintained expression of this molecule in culture. Primary fibroblast cultures were established by culture of fragments of a fresh 4-mm skin biopsy specimen under coverslips in Dulbecco modified Eagle medium (DMEM) supplemented with 20% fetal calf serum in 6-well plates, followed by passaging into flasks. In some experiments, fibroblasts were cultured with 250 IU/mL interferon-γ (IFN-γ) for 48 hours before cytotoxicity assays to increase the expression of HLA class 1 and 2 molecules. Fibroblasts were harvested for cytotoxicity assays using enzyme-free cell-dissociation buffer (Gibco-BRL, Paisley, United Kingdom). CD34+ peripheral blood mononuclear cells (PBMCs) or bone marrow mononuclear cells (BMMCs) were purified using the MiniMACs system according to manufacturer's instructions (Miltenyi Biotech, Bisley, United Kingdom) and had a purity of more than 90% as assessed by fluorescence-associated cell sorter (FACS) analysis.
Synthetic peptides, peptide-binding assays, and intracytoplasmic flow cytometry
Using the BIMAS computer model (http://bimas.dcrt.nih.gov/), nonameric and decameric peptides from the human CD45 sequence were ranked according to their predicted ability to bind HLA-A0201. Peptides derived from human CD45 and a control HLA-A0201 binding peptide derived from the core antigen of hepatitis B virus (residues 18-27, FLPSDFFPSV) were synthesized using fluoronylmethoxycarbonyl chemistry. The quality of the peptides was assessed by high-performance liquid chromatography analysis, and the expected molecular weight was observed using matrix-assisted laser desorption mass spectrometry. Peptides were dissolved in phosphate-buffered saline (PBS; pH 7.4) to give a concentration of 2 mM. Peptide-binding assays were performed by overnight incubation of T2 cells (105 cells/well) in 96-well plates with serial dilutions of peptides in RPMI 1640 with 10% boiled fetal calf serum (to prevent protease activity), followed by FACS analysis for surface expression of HLA-A0201. Mean fluorescence intensities at varying concentrations of peptide were compared.
Intracytoplasmic flow cytometry for IFN-γ was performed using the FastImmune intracellular cytokine detection assay (Becton Dickinson, San Jose, CA) according to manufacturer's instructions. Briefly, CTLs were stimulated with peptide-loaded T2 cells or 25 ng/mL phorbol 12-myristate 13-acetate (PMA)/1 μg/mL ionomycin for 6 hours. Then 10 μg/m brefeldin A was added for the final 4 hours of coculture. Following this, cells were washed and stained with surface antibodies to CD4 or CD8 (PerCP conjugates) and CD69 (phycoerythrin [PE] conjugate) to ensure activation. Cells were then fixed, permeabilized, and stained with an anti–IFN-γ antibody (fluorescein isothiocyanate [FITC] conjugate) and were analyzed by 3-color flow cytometry.
Generation of allo-HLA–restricted CTL lines
PBMCs were separated from buffy-coat packs using Ficoll-Hypaque density gradient centrifugation and were stained with monoclonal antibodies HB54 (anti–HLA-A2, B17) and HB117 (anti–HLA-A2, A28). A2-negative PBMCs were used as responders. T2 cells that had been loaded with 100 μM peptide for 6 hours and then irradiated (60 Gy) were used as initial stimulators. Each well of a 24-well plate received 2 × 106 responder PBMCs and 2 × 105 irradiated, peptide-loaded T2 stimulators in 2 mL RF10 (RPMI with 10% fetal calf serum) with 2.5 ng/mL recombinant human IL-7 (huIL-7) and 500 nM peptide. Drosophila A2 cells were used for the initial restimulation. These were induced in 100 mM CuSO4 for 48 hours, washed 3 times with medium, and loaded with 100 μM peptide for 4 hours. On day 6, T cells were harvested, plated at a density of 5 × 105 per well, and restimulated with 2 × 105 peptide-coatedDrosophila A2 cells with the addition of 2 × 106 autologous irradiated (30 Gy) PBMCs as feeders, in fresh medium containing 10% QS4120 culture supernatant (containing anti-CD4 antibodies), 10 U/mL recombinant huIL-2 (Roche Diagnostics, Lewes, United Kingdom), 2.5 ng/mL recombinant huIL-7, and 500 nM peptide. On day 18, T cells were harvested and restimulated in a similar fashion but using RMA/S-A2 cells that had been incubated at 25°C to induce HLA-A0201 expression, loaded with 100 μM peptide, and irradiated (80 Gy) as stimulators. On day 26, after 3 cycles of stimulation, bulk cultures were seeded in 96-well plates at densities of 1, 20, 50, and 100 cells per well; 2 × 104peptide-coated irradiated T2 cells and 2 × 105irradiated HLA-A2–negative PBMC feeders were added to each well in RF10 with 10 U/mL huIL-2 and 2.5 ng/mL huIL-7. Cultures received additional stimulator and feeder cells on day 40. Six days later, the cytotoxicity of each well was tested against T2 target cells coated with the immunizing peptide or a control HLA-A0201–binding peptide. Peptide-specific microcultures were expanded and restimulated weekly in 24-well plates by adding 2 × 106 irradiated feeders and 2 × 105 peptide-loaded, irradiated T2 cells as stimulators in medium containing 10 U/mL IL-2 and 2.5 ng/mL IL-7.
Cytotoxicity and colony-inhibition assays
Cytotoxic T-lymphocyte assays were performed as described. Briefly, 3 × 106 targets were labeled with 100 μCi (3.7 MBq) chromium Cr 51 for 2 hours, washed 3 times, and added to serial dilutions of effector cells in triplicate, round-bottomed, 96-well plates to obtain a total volume of 200 μL/well. In some experiments T2 cells were peptide loaded by preincubation with 100 μM synthetic peptide before labeling. In antibody-blocking experiments, 103 chromium-labeled target cells were preincubated with 10 μL blocking antibodies to HLA class 1 (clone W6/32; DAKO, Carpinteria, CA), HLA class 2 (clone CR3/43; DAKO), or isotype control for 30 minutes at 37°C before the addition of CTLs. In some experiments a 50-fold excess of cold K562 cells or a 20-fold excess of peptide-loaded T2 cells was added to cultures to assess specificity. Assay plates were incubated for 6 hours at 37°C, 5% CO2, and 100 μL supernatant was harvested and counted using a Wallac gamma counter (Wallac, Milton Keynes, United Kingdom). Specific lysis was calculated by the equation (experimental release − spontaneous release)/maximum release − spontaneous release) × 100%. Colony-inhibition assays were performed by culturing CD34+ PBMCs and BMMCs in the presence and absence of CTLs at an effector-target ratio of 10:1 for 6 hours. CTL-treated and mock-treated CD34+ cells were then plated in duplicate plates in methylcellulose supplemented with 100 ng/mL granulocyte–colony-stimulating factor (G-CSF), 1 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF), 5 ng/mL recombinant human IL-3 (rhIL-3), and 20 ng/mL recombinant human stem cell factor (rhSCF). Granulocyte macrophage–colony-forming units (CFU-GM) with more than 100 cells were counted after 10 to 12 days. Percentage inhibition of colony formation was determined as (no. CFU-GM in the presence of CTL/no. CFU-GM in the absence of CTL) × 100%.
Results
Identification of CD45 peptides binding HLA A201 and generation of CD45-specific CTLs
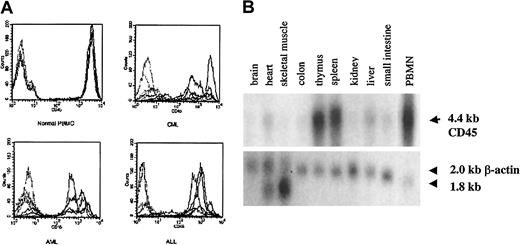
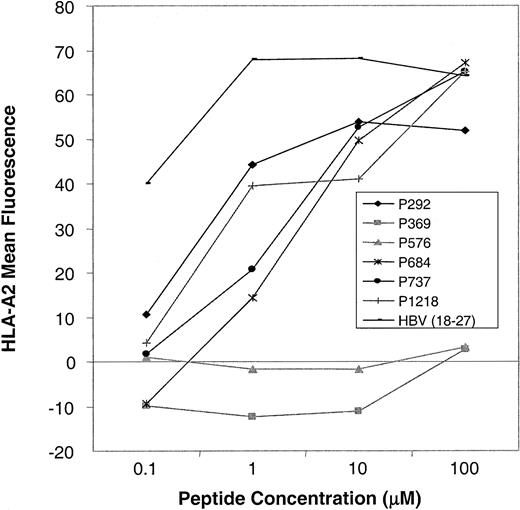
As shown in Figure 2A, we confirmed high levels of CD45 expression on leukemic blasts from patients with acute lymphoblastic leukemia (ALL) and AML and on PBMCs from patients with CML, indicating that CTLs directed against CD45 are likely to have activity against myeloid and lymphoid leukemias. The level of expression was higher in normal and CML PBMCs than in AML and ALL blasts. To determine the expression pattern of CD45 in a variety of human tissues, we performed Northern blot analysis. Figure 2B shows that the expression of CD45 is restricted to hematopoietic tissues, with high levels of mRNA expression in the thymus, spleen, and peripheral blood. The apparent low level of CD45 RNA seen in heart and liver probably resulted from the presence of contaminating hematopoietic cells. To identify CTL epitopes in CD45, we synthesized 16 peptides that were predicted by the BIMAS computer model to bind to HLA-A0201. We investigated the relative ability of these 16 peptides to bind HLA-A0201 by assaying the ability of exogenously pulsed peptide to up-regulate HLA-A0201 expression on the cell surface in the TAP-deficient cell line T2. Figure 3shows that 4 peptides (P292, P684, P737, P1218) showed significant binding to HLA-A0201, whereas the remaining peptides did not bind.
CD45 is expressed on leukemic blasts and is hematopoiesis specific.
(A) FACS analysis showing surface CD45 expression in PBMCs from 5 healthy controls compared with 5 patients with CML and BMMCs from 5 patients with AML or ALL. Each sample was stained with isotype control (dotted lines) and anti-CD45 (solid lines) antibodies, and the corresponding histograms are shown. (B) Northern blot analysis of CD45 RNA expression in various tissues.
CD45 is expressed on leukemic blasts and is hematopoiesis specific.
(A) FACS analysis showing surface CD45 expression in PBMCs from 5 healthy controls compared with 5 patients with CML and BMMCs from 5 patients with AML or ALL. Each sample was stained with isotype control (dotted lines) and anti-CD45 (solid lines) antibodies, and the corresponding histograms are shown. (B) Northern blot analysis of CD45 RNA expression in various tissues.
Peptide-binding assays.
Up-regulation of HLA-A0201 expression on T2 cells after preincubation with CD45 peptides (P292-P1218) and a positive control peptide (HBV 18-27) was determined by FACS analysis. Mean fluorescence intensity at varying concentrations of peptide in a representative experiment is shown. In total, 16 CD45-derived peptides were tested. Four peptides showed HLA-A0201 binding (this figure), and 12 peptides showed no binding (this figure and additional data not shown).
Peptide-binding assays.
Up-regulation of HLA-A0201 expression on T2 cells after preincubation with CD45 peptides (P292-P1218) and a positive control peptide (HBV 18-27) was determined by FACS analysis. Mean fluorescence intensity at varying concentrations of peptide in a representative experiment is shown. In total, 16 CD45-derived peptides were tested. Four peptides showed HLA-A0201 binding (this figure), and 12 peptides showed no binding (this figure and additional data not shown).
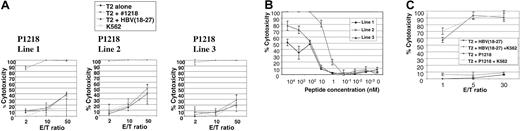
We then attempted to generate allorestricted CTLs with 4 of these peptides by stimulation of PBMCs from healthy HLA A0201–negative responders with a variety of HLA A0201+ APCs exogenously pulsed with peptide, followed by limiting-dilution cultures to isolate peptide-specific CTL lines. No peptide-specific lines were generated using P292, P684, or P737. However, peptide-specific CTL lines directed against P1218 were generated from 3 of 7 donors. This peptide (sequence FLYDVIAST) is located in the second phosphotyrosine phosphatase domain of the cytoplasmic tail of CD45 and was the best binder to HLA A0201 in peptide-binding assays. As illustrated in Figure4A, all 3 of these CTL lines showed exquisite peptide specificity in cytotoxicity assays, with almost total killing of T2 targets pulsed with P1218 at low effector-target ratios and little cytotoxicity against T2 cells alone or pulsed with a control peptide known to bind HLA A0201. Little major histocompatibility complex (MHC)–unrestricted cytotoxicity against K562 targets was observed, and cytotoxicity against T2 cells pulsed with P1218 was not abrogated by a 50-fold excess of cold K562 cells (Figure 4C), demonstrating the observed cytotoxicity was specific. All 3 CTL lines were initially 60% to 70% CD8+ and 30% to 40% CD4+, but with prolonged culture CD8+ cells increased to 90% to 95% and the CD4+ population decreased to 3% to 5%. To test the avidity of our T-cell lines, we performed cytotoxicity assays against T2 targets titrating the concentration of exogenously pulsed P1218. As shown in Figure 4B, line 2 showed the highest avidity with significant cytotoxicity at nanomolar levels of peptide. Line 2 was therefore selected for further experiments.
Peptide specificity of P1218-specific CTLs.
(A) Representative experiment showing cytotoxicity of P1218-specific CTL lines 1 to 3 against T2 cells alone, pulsed with P1218 or with a control HLA-A0201 binding peptide. Results are shown as the means ± SDs of triplicate wells. (B) Peptide titration of P1218-specific CTLs. Cytolytic activity of lines 1 to 3 against T2 cells pulsed with varying concentrations of P1218 is shown. Results are shown as the means ± SDs of triplicate wells. (C) Cold target inhibition of cytotoxic activity of P1218-specific CTLs. Cytotoxic activity of CTL line 2 against T2 cells pulsed with P1218 or control peptide in the presence or absence of a 50-fold excess of unlabeled K562 cells. Results are shown as the means ± SDs of triplicate wells.
Peptide specificity of P1218-specific CTLs.
(A) Representative experiment showing cytotoxicity of P1218-specific CTL lines 1 to 3 against T2 cells alone, pulsed with P1218 or with a control HLA-A0201 binding peptide. Results are shown as the means ± SDs of triplicate wells. (B) Peptide titration of P1218-specific CTLs. Cytolytic activity of lines 1 to 3 against T2 cells pulsed with varying concentrations of P1218 is shown. Results are shown as the means ± SDs of triplicate wells. (C) Cold target inhibition of cytotoxic activity of P1218-specific CTLs. Cytotoxic activity of CTL line 2 against T2 cells pulsed with P1218 or control peptide in the presence or absence of a 50-fold excess of unlabeled K562 cells. Results are shown as the means ± SDs of triplicate wells.
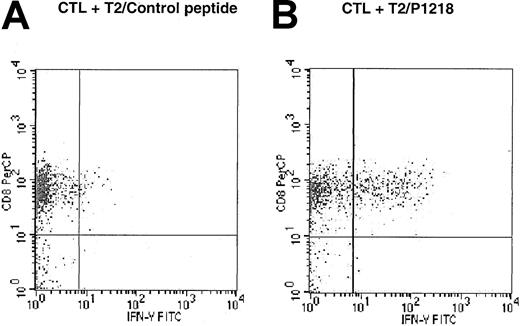
To determine whether the response against P1218 peptide-pulsed T2 cells was mediated by CD4 or CD8+ cells, intracytoplasmic flow cytometry was used to study the expression of IFN-γ after the exposure of P1218-specific CTLs to peptide-pulsed T2 cells. As illustrated in Figure 5, 28.4% of CD8+ cells from line 2 expressed IFN-γ in response to T2 cells pulsed with P1218, but only 2.5% expressed IFN-γ in response to T2 cells pulsed with control peptide. This demonstrates peptide-specific secretion of IFN-γ by the CD8+population within the CTL line. In contrast, there was no secretion of IFN-γ in response to either peptide by CD4+ cells (data not shown).
Peptide specificity of CD45-specific CTLs is mediated by CD8+ cells.
CTL line 2 was stimulated for 6 hours in the presence of brefeldin A, and T2 cells were pulsed with (A) control HLA-A0201 binding peptide HBV core (18-27) or with (B) P1218. Intracytoplasmic flow cytometry was used to demonstrate expression of IFN-γ in response to P1218 in CD8+ cells but not CD4+ cells.
Peptide specificity of CD45-specific CTLs is mediated by CD8+ cells.
CTL line 2 was stimulated for 6 hours in the presence of brefeldin A, and T2 cells were pulsed with (A) control HLA-A0201 binding peptide HBV core (18-27) or with (B) P1218. Intracytoplasmic flow cytometry was used to demonstrate expression of IFN-γ in response to P1218 in CD8+ cells but not CD4+ cells.
CD45-specific CTLs kill leukemic cell lines
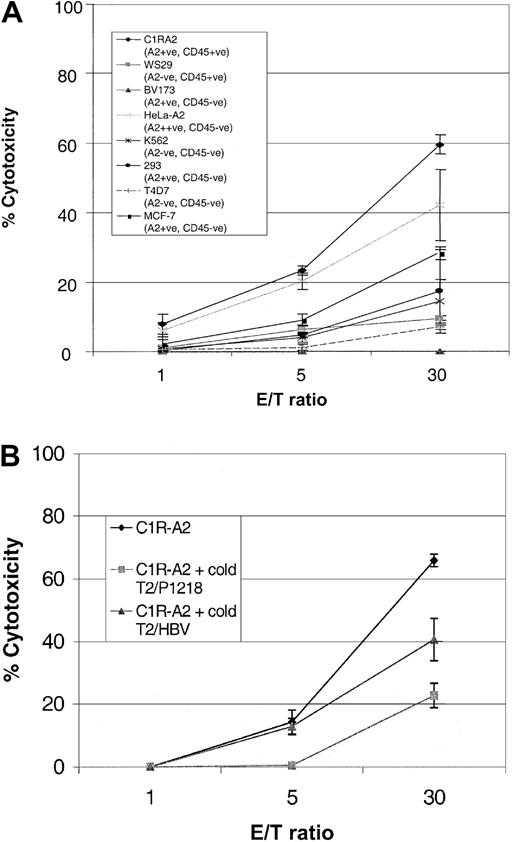
To determine whether P1218-specific CTLs were able to recognize endogenously processed CD45, we analyzed cytotoxicity against a panel of leukemic cell lines. As can be seen in Figure6, P1218-specific CTLs were able to efficiently lyse the B-lymphoblastoid cell line, CIR-A2, which expresses CD45 and HLA-A0201, but they did not kill a similar lymphoblastoid cell line, WS29, which expresses CD45 but is HLA-A0201−. Likewise there was no significant cytotoxicity against the breast cancer epithelial cell line T4D7, which is HLA-A0201− and CD45− , or against HLA-A0201− K562 cells, a target for natural killer (NK) cells. These results indicate that the observed cytotoxicity was HLA-A0201 restricted and independent of NK activity. Cytotoxicity against C1R-A2 cells was partially abrogated in blocking experiments with an anti-HLA class 1 antibody but not with an anti-HLA class 2 antibody (data not shown). P1218-specific CTLs were unable to lyse BV173, a leukemia cell line that expresses HLA-A0201 but has lost expression of CD45 or 293 cells, an embryonic kidney cell line expressing HLA-A0201. These results demonstrate that HLA-A0201 expression alone is insufficient for recognition by this CTL line and suggest that coexpression of CD45 is required. Surprisingly, at high effector-target ratios, 2 nonhematopoietic targets, MCF-7, an HLA-A0201+ breast cancer cell line, and HeLa-A2, a cervical epithelial cell line transfected with HLA-A0201, were also killed by P1218-specific CTLs (though less well than CIR-A2 targets), despite the fact that neither of these cell lines expressed detectable CD45 by FACS staining. The cytotoxicity of P1218-specific CTL against both these targets was only partially inhibited by a 50-fold excess of cold K562 cells (data not shown), indicating that recognition of these HLA-A0201+, CD45− targets is not primarily caused by NK activity.
Cytolytic activity of P1218-specific CTLs against cell line targets.
(A) Cytotoxicity against cell lines that do or do not express HLA-A0201 and CD45. The figure shows the means ± SDs of 3 experiments performed in duplicate or triplicate. (B) Cytotoxicity against the HLA-A0201+, CD45+ cell line C1R-A2 in the presence of a 20-fold excess of cold T2 targets loaded with either P1218 or the control peptide HBV (18-27). Mean cytotoxicity ± SD is shown in a representative experiment performed in triplicate.
Cytolytic activity of P1218-specific CTLs against cell line targets.
(A) Cytotoxicity against cell lines that do or do not express HLA-A0201 and CD45. The figure shows the means ± SDs of 3 experiments performed in duplicate or triplicate. (B) Cytotoxicity against the HLA-A0201+, CD45+ cell line C1R-A2 in the presence of a 20-fold excess of cold T2 targets loaded with either P1218 or the control peptide HBV (18-27). Mean cytotoxicity ± SD is shown in a representative experiment performed in triplicate.
The observed cytotoxicity against CD45− targets could be attributed to cross-recognition of other HLA-A0201/peptide complexes by CD45-specific CTLs or to the presence of a different A0201-alloreactive clone in the CTL line. To address this, the killing of HLA-A0201+, CD45+ targets was analyzed in the presence of an excess of cold T2 target cells loaded with the P1218 peptide or of an irrelevant HLA-A0201–binding peptide derived from hepatitis B virus. Figure 6B shows that at a low effector-target ratio (5:1), the P1218-loaded cold targets completely inhibited killing of the HLA-A0201+, CD45+ targets, whereas inhibition was incomplete at high (30:1) CTL numbers. Conversely, cold T2 targets presenting the irrelevant hepatitis B virus (HBV) peptide did not inhibit killing at a low effector-target ratio but showed some inhibition at high CTL numbers. These data suggest that the CD45-specific CTL line contains a small number of CTLs recognizing HLA-A0201 molecules presenting irrelevant peptide epitopes. This is consistent with the observation that subcloning of the CTL line gave raise to short-term CTL clones that displayed P1218 specificity and killed C1R-A2 cells, but not the nonhematopoietic HeLa-A2 cells (not shown). We were unable to expand and maintain these subclones, which probably reflects an important function of CD4 T cells in promoting long-term CTL growth, as has previously been shown in vivo.17 18
P1218-specific CTLs recognize endogenously presented CD45 in malignant hematopoietic cell lines
To determine whether P1218-specific CTLs are capable of recognizing endogenously processed CD45 in malignant hematopoietic cells, we took advantage of the observation that the myeloma cell line U-266 is naturally biphenotypic with respect to CD45 expression. This cell line was FACS sorted into CD45− and CD45+ fractions (Figure 7Aii,iv), and these were used as targets in cytotoxicity assays. As shown in Figure 7Av, P1218-specific CTLs were able to lyse CD45+ U-266 cells efficiently, whereas the CD45− U-266 cells were killed poorly. These results demonstrate that endogenously expressed CD45 was sufficient for recognition of U-266 cells by the CTLs. The low-level cytotoxicity observed against the CD45− U-266 fraction may reflect lysis of contaminating CD45+ cells because a minority of cells show phenotypic conversion to CD45+ after prolonged culture (Figure 7Ai-iv).
To further show that P1218-specific CTLs recognize endogenously processed CD45, we generated a stable transfectant of the HLA-A0201+ kidney cell line, 293, expressing CD45RO (Figure 7Bi-iv). As shown in Figure 7Bv, wild-type 293 cells were killed poorly, whereas after the introduction of CD45 this same cell line was efficiently lysed by P1218-specific CTLs, demonstrating that these CTLs recognized endogenously expressed CD45.
CD45-specific CTLs are cytotoxic to primary normal hematopoietic and leukemic cells
To analyze the activity of P1218-specific CTLs against primary leukemic targets, cytotoxicity assays were performed using CML PBMCs and leukemic blasts from HLA-A0201–positive patients with AML. As illustrated in Table 1, P1218-specific CTLs showed significant cytotoxicity against PBMCs from 4 of 4 patients with CML and against leukemic blasts from 4 of 4 patients with AML tested. No killing of HLA-A0201− normal or CML PBMCs was observed, demonstrating that the observed cytotoxicity is HLA-A2 restricted. Leukemic blasts from 2 HLA-A0201+ patients with ALL, both of which expressed CD45, were not killed (data not shown).
Cytotoxic activity of P1218-specific CTLs against primary targets
| . | Donor 1 . | Donor 2 . | Donor 3 . | Donor 4 . | Mean ± SD . |
|---|---|---|---|---|---|
| Effector-target ratio, 30:1 | |||||
| HLA A2+ AML blasts | 19.2 | 37.8 | 28.8 | 10 | 24.0 ± 12.0 |
| HLA A2+ CML PBMCs | 11.3 | 14.4 | 16 | 15.2 | 14.2 ± 2.0 |
| HLA A2− normal PBMCs | 0 | 0.2 | 2.2 | 0.2 | 0.7 ± 1.0 |
| HLA A2+fibroblasts | 0 | 3 | 1.9 | 0 | 1.2 ± 1.5 |
| HLA A2+fibroblasts+P1218 | 28.2 | 34.1 | 38.1 | 12.6 | 28.3 ± 11.2 |
| Effector-target ratio, 5:1 | |||||
| HLA A2+ AML blasts | 10 | 21.4 | 24.6 | 8.9 | 16.2 ± 7.9 |
| HLA A2+ CML PBMCs | 6.9 | 5.3 | 3.9 | 1.9 | 4.5 ± 2.1 |
| HLA A2− normal PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2+fibroblasts | 0 | 7.2 | 0 | 0 | 1.8 ± 3.6 |
| HLA A2+ fibroblasts+P1218 | 20.1 | 30.8 | 31.5 | 9.2 | 22.9 ± 10.5 |
| Effector-target ratio, 1:1 | |||||
| HLA A2+ AML blasts | 0 | 17 | 13.6 | 0.9 | 7.9 ± 8.7 |
| HLA A2+ CML PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2− normal PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2+ fibroblasts | 0 | 3.1 | 1.2 | 0 | 1.1 ± 1.5 |
| HLA A2+fibroblasts+P1218 | 14.4 | 23.2 | 31.6 | 9.7 | 19.7 ± 9.7 |
| . | Donor 1 . | Donor 2 . | Donor 3 . | Donor 4 . | Mean ± SD . |
|---|---|---|---|---|---|
| Effector-target ratio, 30:1 | |||||
| HLA A2+ AML blasts | 19.2 | 37.8 | 28.8 | 10 | 24.0 ± 12.0 |
| HLA A2+ CML PBMCs | 11.3 | 14.4 | 16 | 15.2 | 14.2 ± 2.0 |
| HLA A2− normal PBMCs | 0 | 0.2 | 2.2 | 0.2 | 0.7 ± 1.0 |
| HLA A2+fibroblasts | 0 | 3 | 1.9 | 0 | 1.2 ± 1.5 |
| HLA A2+fibroblasts+P1218 | 28.2 | 34.1 | 38.1 | 12.6 | 28.3 ± 11.2 |
| Effector-target ratio, 5:1 | |||||
| HLA A2+ AML blasts | 10 | 21.4 | 24.6 | 8.9 | 16.2 ± 7.9 |
| HLA A2+ CML PBMCs | 6.9 | 5.3 | 3.9 | 1.9 | 4.5 ± 2.1 |
| HLA A2− normal PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2+fibroblasts | 0 | 7.2 | 0 | 0 | 1.8 ± 3.6 |
| HLA A2+ fibroblasts+P1218 | 20.1 | 30.8 | 31.5 | 9.2 | 22.9 ± 10.5 |
| Effector-target ratio, 1:1 | |||||
| HLA A2+ AML blasts | 0 | 17 | 13.6 | 0.9 | 7.9 ± 8.7 |
| HLA A2+ CML PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2− normal PBMCs | 0 | 0 | 0 | 0 | 0 ± 0 |
| HLA A2+ fibroblasts | 0 | 3.1 | 1.2 | 0 | 1.1 ± 1.5 |
| HLA A2+fibroblasts+P1218 | 14.4 | 23.2 | 31.6 | 9.7 | 19.7 ± 9.7 |
Cytotoxicity was determined in 51Cr release assays against circulating or bone marrow blasts from 4 HLA-A2+ patients with AML, PBMCs from 4 HLA A2+ healthy controls and 4 patients with CML, and primary fibroblasts from 4 HLA-A2+ healthy controls. Fibroblasts were pretreated with IFN-γ to induce expression of HLA class 1 molecules and, in some experiments, were pulsed with 100 μM P1218 peptide for 1 hour before labeling with 51Cr. Results are the means of triplicate wells.
To determine tissue specificity of P1218-specific CTLs against primary targets, we performed cytotoxicity assays using primary fibroblasts from HLA-A0201+ healthy donors as targets. After induction with IFN-γ, fibroblasts showed comparable levels of HLA class 1 expression to PBMCs, as assessed by FACS analysis (data not shown). As can be seen in Table 1, P1218-specific CTLs showed no cytotoxicity against HLA-A2+ fibroblasts, suggesting that P1218-specific CTLs kill in a hematopoiesis-specific manner. In contrast, fibroblasts pulsed with exogenous P1218 were lysed, demonstrating that the lack of cytotoxicity against unpulsed fibroblasts was caused by the absence of the cognate antigen.
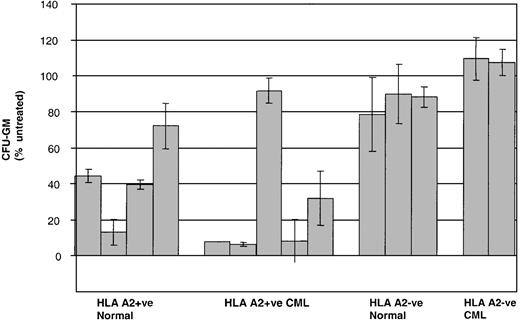
Given that CML PBMCs are a relatively heterogeneous population of primitive and more mature leukemic cells, we then went on to test the activity of P1218-specific CTLs against earlier normal and leukemic clonogenic progenitors in colony-forming assays. As shown in Figure8, preincubation of CD34+BMMCs with P1218-specific CTLs significantly inhibited CFU-GM formation in 4 of 4 healthy HLA-A2+ controls. Similarly, strong inhibition of CFU-GM formation was observed in 4 of 5 HLA-A2+ patients with CML. The HLA-A2+ patient in whom no inhibition was observed was only serologically typed and might have had a subtype other than A0201. No inhibition of colony formation was observed in HLA-A2− healthy controls or HLA-A2− CML patients.
P1218-specific CTLs recognize endogenously presented CD45.
(A) Cytotoxicity against the myeloma cell line U-266. (Panels Ai-iv) FACS analysis of expression of HLA-A2 (i,iii) and CD45 (ii,iv) in sorted CD45− (i-ii) and CD45+ (iii-iv) fractions. (Panel Av) Cytotoxicity of P1218-specific CTL line 2 against the sorted CD45− and CD45+ fractions. Results are shown as the means ± SDs of 3 experiments performed in triplicate. (B) Cytotoxicity against 293 cells stably transfected with CD45. (Panels Bi-iv) FACS analysis of expression of HLA-A2 (i, iii) and CD45 (ii, iv) in wild-type 293 cells (i-ii) and 293 cells stably transfected with CD45 (iii-iv). (Panel Bv) Cytotoxicity of P1218-specific CTL line 2 against these targets. Results are shown as the means ± SDs of triplicate wells in a representative experiment.
P1218-specific CTLs recognize endogenously presented CD45.
(A) Cytotoxicity against the myeloma cell line U-266. (Panels Ai-iv) FACS analysis of expression of HLA-A2 (i,iii) and CD45 (ii,iv) in sorted CD45− (i-ii) and CD45+ (iii-iv) fractions. (Panel Av) Cytotoxicity of P1218-specific CTL line 2 against the sorted CD45− and CD45+ fractions. Results are shown as the means ± SDs of 3 experiments performed in triplicate. (B) Cytotoxicity against 293 cells stably transfected with CD45. (Panels Bi-iv) FACS analysis of expression of HLA-A2 (i, iii) and CD45 (ii, iv) in wild-type 293 cells (i-ii) and 293 cells stably transfected with CD45 (iii-iv). (Panel Bv) Cytotoxicity of P1218-specific CTL line 2 against these targets. Results are shown as the means ± SDs of triplicate wells in a representative experiment.
Inhibition of CFU-GM formation by P1218-specific CTLs.
CD34+ BMMC/PBMC derived from healthy controls and from patients with CML were cultured in the presence or absence of P1218-specific CTLs at an effector-target ratio of 10:1 before plating in methylcellulose. Means ± SDs of the percentage of CFU-GM after treatment with CTLs are compared with mock-treated control in duplicate plates for 4 HLA A2+ and 3 HLA-A2− healthy controls and 5 HLA-A2+ patients and 2 HLA-A2−patients with CML.
Inhibition of CFU-GM formation by P1218-specific CTLs.
CD34+ BMMC/PBMC derived from healthy controls and from patients with CML were cultured in the presence or absence of P1218-specific CTLs at an effector-target ratio of 10:1 before plating in methylcellulose. Means ± SDs of the percentage of CFU-GM after treatment with CTLs are compared with mock-treated control in duplicate plates for 4 HLA A2+ and 3 HLA-A2− healthy controls and 5 HLA-A2+ patients and 2 HLA-A2−patients with CML.
Discussion
We have investigated a novel approach to adoptive immunotherapy to restore the GVL effect following haploidentical HSCT. Rather than relying on a disparity in antigenic expression between host and donor, we have exploited an HLA mismatch to generate CTLs directed against a hematopoietic antigen common to host and donor, recognized only in the context of host HLA molecules. Because it circumvents immunologic tolerance, this allorestricted approach is suitable for raising CTLs against any hematopoietic antigen. CD45 was chosen as a candidate because of its hematopoietic-restricted expression and because it is abundantly expressed in myeloid and lymphoid leukemia. The described P1218 epitope is located on the C-terminal second phosphotyrosine phosphatase domain and is not known to be polymorphic or to function as a minor histocompatibility antigen in patients with HLA match. It is anticipated that autologous and HLA-matched CTLs are tolerant of this epitope. The allorestricted CTL lines generated in this study contained predominantly CD8+ cells, particularly after prolonged culture, though some CD4+ cells persisted in all lines established. In previous experiments with allorestricted CTL lines directed against WT-1, CD4+ cells were also present and showed no proliferative response to peptide or T2 cells but did show strong interleukin-2 (IL-2)–dependent proliferation in the absence of antigen (H.J.S., unpublished data, 2000). Subcloning of these CTL lines yielded CD8+ CTL clones that showed the killing specificity displayed by the line, but the life span of these clones was limited to 6 to 8 weeks in the absence of CD4+ T cells. Similarly, we were unable to expand and maintain subclones of the P1218-specific CTL line. This in vitro observation is similar to what has been observed in clinical trials of adoptive immunotherapy with antiviral CTLs, suggesting that that the presence of helper T cells may increase the persistence of infused CTLs.17 18
Our observations that lysis of cell line targets, including U-266, by P1218-specific CTL requires coexpression of HLA-A0201 and CD45, together with our data from stable transfectants of 293 cells, show that this CTL line is capable of recognizing endogenously expressed CD45. Formal demonstration of endogenous presentation of the P1218 epitope, however, will require peptide elution from HLA-A0201+ hematopoietic cells. The activity of P1218-specific CTLs against the HLA-A0201+ nonhematopoietic cell lines HeLa-A2 and MCF-7 in the absence of CD45 has been explored in cold-target inhibition experiments. Results suggest that the unexpected killing activity is not caused by MHC-unrestricted NK activity but rather is most likely caused by the presence of a small number of alloreactive CTLs capable of recognizing A0201 molecules presenting irrelevant peptide epitopes. It is interesting to note that though our CTL line showed significant killing of TAP-deficient T2 cells without peptide pulsing (Figure 2A), and of HeLa-A2 and MCF-7 cells (Figure 6A), little killing was observed against other A0201-positive targets such as BV173, 293, and IFN-γ–induced primary fibroblasts (Figure 6A, Table 1). This suggests that alloreactivity is not directed against HLA-A0201 molecules per se but involves a peptide, or a set of peptides, presented by T2, HeLA-A2, and MCF-7. Interestingly, using a T2 stimulation protocol similar to the one used here, Moris et al19 have recently demonstrated the presence of alloreactive CTL capable of recognizing a limited set of peptides presented by HLA-A0201.
Allorestricted CTLs directed against P1218 show significant cytotoxicity against primary leukemic targets from HLA-A0201+ patients with AML and CML. Leukemic blasts from 2 HLA-A0201+ patients with ALL were not lysed, and further studies will be needed to determine whether this is generally true in ALL. In this regard it is interesting to note that ALL blasts are also less susceptible to lysis by alloreactive NK clones, perhaps because of their lack of LFA-1 expression,20 and they also appear resistant to anti-CD45 antibody-mediated lysis (M.K.B., personal communication, June 2002). Additionally, P1218-specific CTLs strongly inhibit colony formation from CD34+ CML progenitors in an HLA-A0201–restricted fashion. These results suggest that P1218-specific CTLs may have significant antileukemic activity in the context of an HLA-A0201+ patient undergoing transplantation from an HLA-A0201− donor. However, the extent to which our in vitro data will correlate with in vivo antileukemic activity is unknown. In this regard, it will be necessary to determine whether P1218-specific CTLs can eliminate leukemic stem cells in the context of SCID/NOD mice, as has been shown with CTLs directed against minor histocompatibility antigens.21 The lack of cytotoxicity of P1218-specific CTLs against HLA 0201+ fibroblasts suggests that, as expected, such CTLs do not recognize primary nonhematopoietic targets and, therefore, are unlikely to cause GVHD, provided that CTL therapy is used in patients with high levels of donor chimerism who undergo transplantation. Recent murine studies have clearly indicated that antigen presentation by host APCs plays a key role in triggering GVHD.22 Similarly, the absence of activity of P1218-specific CTLs against HLA-A2−normal PBMCs suggests that, by virtue of their HLA restriction, such CTLs would not adversely affect donor-derived cells from the graft. These data suggest that allorestricted CTLs directed against CD45 epitopes may provide a useful novel reagent in restoring the GVL effect after haploidentical HSCT. Further, in contrast to allorestricted CTLs directed against WT-1,10 our colony-inhibition assays demonstrate that P1218-specific CTLs also recognize healthy HLA-A0201+ progenitors. Such CTLs potentially contribute to myeloablation as an immunologic conditioning agent.
Sufficient numbers of P1218-specific CTLs can be generated for clinical protocols using our current methodology; once the CTL lines are established, they are easily expanded. Clearly, however, if such an approach is to be adapted to the clinical setting, our protocol for the initial generation of allorestricted CTLs will have to be simplified greatly. Selection of P1218-specific CTLs from bulk cultures with HLA-A0201 tetramers loaded with P1218 peptide may allow rapid isolation of peptide-specific CTLs without resorting to cloning. Alternatively, T-cell receptor gene transfer from existing CTL lines could be used to redirect the specificity of circulating T cells, obviating the need to generate allorestricted CTLs for each patient.23
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-02-0525.
Supported by the Medical Research Council of the United Kingdom and the Leukemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Persis J. Amrolia, Department of Bone Marrow Transplantation, Great Ormond St Children's Hospital, Great Ormond St, London WC1, United Kingdom; e-mail:amrolp1@gosh.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal