Abstract
The signals that mediate T-cell infiltration during T-cell autoimmune diseases are poorly understood. The chemokine CCL21 (originally isolated by us and others as Exodus-2/6Ckine/SLC/TCA4) is highly potent and highly specific for stimulating T-cell migration. However, it is thought to be expressed only in secondary lymphoid organs, directing naive T cells to areas of antigen presentation. It is not thought to play a role in T-cell effector function during a normal immune response. In this study we tested the expression of T-cell chemokines and their receptors during T-cell autoimmune infiltrative skin diseases. By using immunohistology it was found that the expression of CCL21 but not CCL19 or 20 was highly induced in endothelial cells of T-cell autoimmune diseases. The receptor for CCL21, CCR7, was also found to be highly expressed on the infiltrating T cells, most of which expressed the memory CD45Ro phenotype. These data imply that the usual loss of CCL21 responsiveness in the normal development of memory T-cell effector function does not hold for autoimmune skin diseases.
Introduction
Chemokines are a family of structurally related proteins that are the major mediators of all leukocyte migration.1-4 There are many reports that chemokines or their receptors are important in human disease.1-4 They play crucial roles in many diseases that have inflammatory tissue destruction, such as adult respiratory distress syndrome, myocardial infarction, rheumatoid arthritis, and atherosclerosis.1-7Chemokines are also potent inhibitors of hematopoiesis.8-10 We and others isolated a novel CC chemokine termed CCL21 (originally Exodus-2/SLC/6Ckine/TCA4) that is the most powerful chemoattractant for T cells yet defined.10-12 CCL21 also attracts B cells and natural killer (NK) cells, although not to as great an extent as T cells.10-12 It does not chemoattract monocytes or granulocytes.10-13
CCL21 also plays an important role in T-cell adhesion. T-cell trafficking from the vasculature to tissue sites of inflammation or disease is mediated by a defined series of steps.14,15First, T cells initially tether and roll along the endothelium via L-selectin. Three chemokines, CXCL12, CCL19, and CCL21, can then mediate a firm adherence to endothelial intercellular adhesion molecule-1 (ICAM-1) by stimulating the T-cell integrin lymphocyte function antigen-1 (LFA-1). Adherent cells then follow a chemotactic gradient into inflamed tissue. Because CCL21 is strongly expressed in high endothelial venules of lymph nodes, it was thought that it might mediate T-cell trafficking though secondary lymphoid organs.14 15
This hypothesis was later found to be true. Homozygous deletion of the receptor for CCL21, CCR7, found that mice without this receptor were unable to both organize secondary lymphoid organs and mount a normal immune response.13 Other studies have found that effector memory T cells infiltrating inflamed/infected tissues in a normal immune response lacked expression of CCR7.16,17 Thus, it has been hypothesized that the CCL21/CCR7 axis is essential for appropriate naive T-cell migration to secondary lymphoid organs for antigen presentation, but that this axis is not required for effector memory T cells (postantigen exposure) to migrate toward and respond to the inflammatory stimuli.16 17
It has been thought that these effector memory T cells lacking CCR7 are the major agents of T-cell tissue infiltration during inflammation.16 17 In this study we investigated whether the expression of CCL21 implicated it in the pathogenesis of T-cell autoimmune diseases of the skin. Contrary to prevailing thought, we found that CCL21 is highly induced in the endothelium of T-cell autoimmune diseases but not in normal tissue. The receptor for CCL21, CCR7, was also highly expressed on these infiltrating T cells, most of which were of the CD45Ro memory phenotype. Thus, the migration of the effector memory T cells in these autoimmune diseases was likely regulated by CCL21.
Patients, materials, and methods
The skin diseases studied were the autoimmune diseases atopic dermatitis and lichen planus, and the related T-cell infiltrative skin disease graft-versus-host disease (GVHD). Skin biopsies were obtained after Indiana University institutional review board–approved informed consent on patients with these T-cell infiltrative diseases of the skin. Immunohistology was chosen as an assay of protein expression because it allows morphologic identification of the cell type expressing the protein being investigated. Biotinylated antibodies against human CCL19 (previously ELC/MIP-3beta/Exodus-3), CCL20 (previously LARC/MIP-3alpha/Exodus-1), CCL21, CCR7, and CD45Ra were purchased from R&D Systems (Minneapolis, MN) or from BD Pharmingen (San Diego, CA). Nonbiotinylated antibodies against CD45Ro and appropriate secondary biotinylated antibodies were purchased from BD Pharmingen. Immunohistology of paraffin-imbedded specimens was performed essentially as we previously described,18 with the exception that the strep-avidin peroxidase reaction was performed directly on the primary antibodies for those antibodies that were biotinylated. Biotin-avidin/peroxidase/diaminobenzidine (DAB) staining was used to develop the signal (Vectastain Elite ABC Kit and DAB Substrate Kit; Vector Labs, Burlingame, CA). Slides were counterstained with Hematoxylin Nuclear Counterstain (Vector Labs). Three patient skin samples were tested for each disease, and 3 individuals for healthy skin served as controls. All samples showed identical results to those shown in the photomicrographs here.
Results
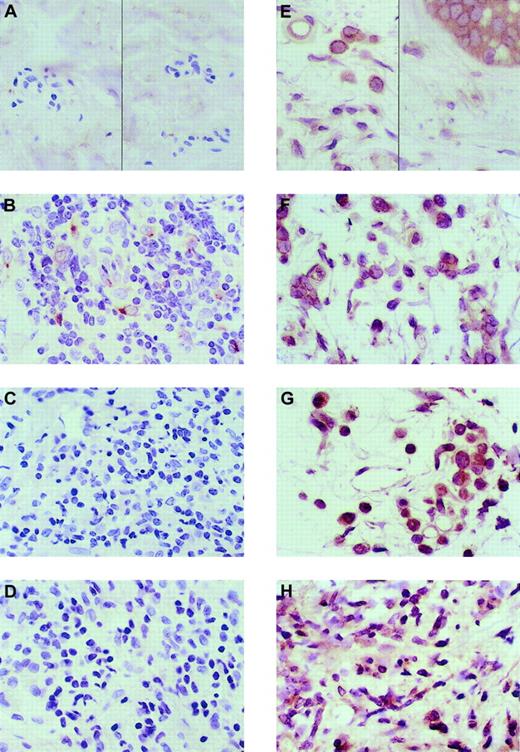
Immunohistology was used to analyze the expression pattern of potent and specific T-cell chemokines in immunologic skin diseases in which T-cell infiltration mediates the pathology. Healthy skin was used as a control. Immunohistologic analysis found that healthy skin did not express CCL19 protein in any cell type (Figure1A). None of the cases of GVHD and lichen planus expressed CCL19 in any cell type (Figure 1C-D). However, one case of atopic dermatitis of the 3 analyzed expressed CCL19 in macrophages and dendritic cells (Figure 1B). CCL20 was constitutively expressed in basal keratinocytes and macrophages of healthy and diseased skin (Figure 1E-H). Most lymphocytes in healthy or diseased skin did not express CCL20. Thus, the expression of CCL20 did not change in any of the skin diseases as compared with healthy skin.
Immunohistologic analysis of the expression of CCL19 and CCL20 in healthy and T-cell infiltrative skin diseases.
The antibody-specific peroxidase reaction is stained brown; the hematoxylin nuclear counterstain is blue. All photomicrographs are at × 50 original magnification. (A) CCL19 expression in healthy skin. (B) CCL19 in atopic dermatitis. (C) CCL19 in lichen planus. (D) CCL19 in GVHD. One of the 3 skin samples of atopic dermatitis had expression of CCL19 in dendritic cells, which is shown in panel B. (E) CCL20 expression in healthy skin keratinocytes (right) and macrophages (left). (F) CCL20 expression in atopic dermatitis, (G) CCL20 expression in lichen planus, and (H) CCL20 expression in GVHD. There was no distinction in CCL20 expression between healthy and diseased skin. Three separate patient samples were tested for healthy and 3 for diseased skin.
Immunohistologic analysis of the expression of CCL19 and CCL20 in healthy and T-cell infiltrative skin diseases.
The antibody-specific peroxidase reaction is stained brown; the hematoxylin nuclear counterstain is blue. All photomicrographs are at × 50 original magnification. (A) CCL19 expression in healthy skin. (B) CCL19 in atopic dermatitis. (C) CCL19 in lichen planus. (D) CCL19 in GVHD. One of the 3 skin samples of atopic dermatitis had expression of CCL19 in dendritic cells, which is shown in panel B. (E) CCL20 expression in healthy skin keratinocytes (right) and macrophages (left). (F) CCL20 expression in atopic dermatitis, (G) CCL20 expression in lichen planus, and (H) CCL20 expression in GVHD. There was no distinction in CCL20 expression between healthy and diseased skin. Three separate patient samples were tested for healthy and 3 for diseased skin.
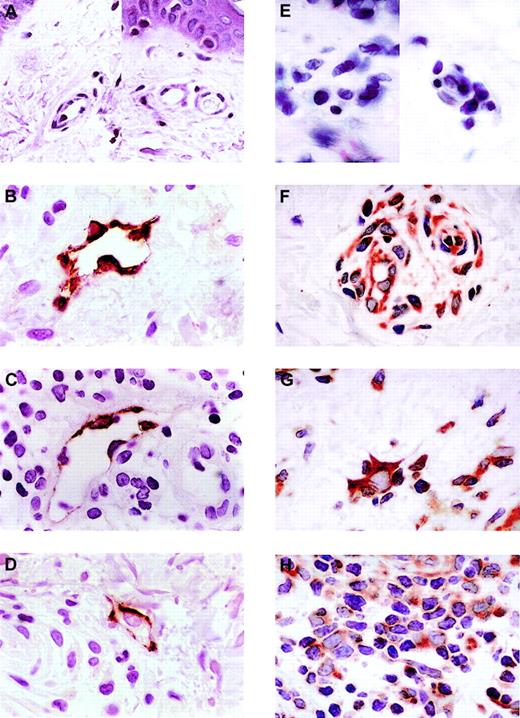
Healthy skin did not express any CCL21 protein (Figure2A). However, the expression of CCL21 was induced in dermal endothelial cells in all 3 T-cell skin diseases, atopic dermatitis, lichen planus, and GVHD (Figure 2B-D). The endothelial cells expressing CCL21 protein in all of the photomicrographs shown were in venules, as no smooth muscle cells are seen in these vessels. In addition, the expression of CCL21 was not just in a few selective venules but was in most of the venules seen in all biopsies. Note the lymphocyte adhering to the endothelium of the venule shown in GVHD (Figure 2D). When a venule expressed CCL21, that expression was generally circumferential and ubiquitous in all endothelial cells seen in that venule.
Immunohistologic analysis of the expression of CCL21 and CCR7 in healthy skin and T-cell autoimmune skin disease.
CCL21 is not expressed in samples of healthy skin (A) but is expressed in venule endothelial cells of atopic dermatitis (B), lichen planus (C), and graft-versus-host disease by immunohistology (D). (E) CCR7 expression in healthy skin. (F) CCR7 expression in atopic dermatitis. (G) CCR7 expression in lichen planus. (H) CCR7 expression in GVHD. Three distinct patient skin samples were tested for each disease and 3 for healthy skin. All showed results identical to those shown here. Lower power views of the autoimmune samples found that all venule endothelial cells express CCL21. The photomicrographs shown here are representative of all samples. All slides were photographed at × 50 original magnification.
Immunohistologic analysis of the expression of CCL21 and CCR7 in healthy skin and T-cell autoimmune skin disease.
CCL21 is not expressed in samples of healthy skin (A) but is expressed in venule endothelial cells of atopic dermatitis (B), lichen planus (C), and graft-versus-host disease by immunohistology (D). (E) CCR7 expression in healthy skin. (F) CCR7 expression in atopic dermatitis. (G) CCR7 expression in lichen planus. (H) CCR7 expression in GVHD. Three distinct patient skin samples were tested for each disease and 3 for healthy skin. All showed results identical to those shown here. Lower power views of the autoimmune samples found that all venule endothelial cells express CCL21. The photomicrographs shown here are representative of all samples. All slides were photographed at × 50 original magnification.
Next, the expression of CCR7, the receptor for CCL21, on lymphocytes in healthy and autoimmune skin diseases was assessed. CCR7 was expressed in only a few lymphocytes in healthy skin biopsies (Figure 2E). However, as seen in Figure 2F-H, most of the lymphocytes in all 3 autoimmune skin diseases, atopic dermatitis, lichen planus, and GVHD, highly express CCR7. Interestingly, there are also dendritic cells that express CCR7 that are present in the diseased skin.
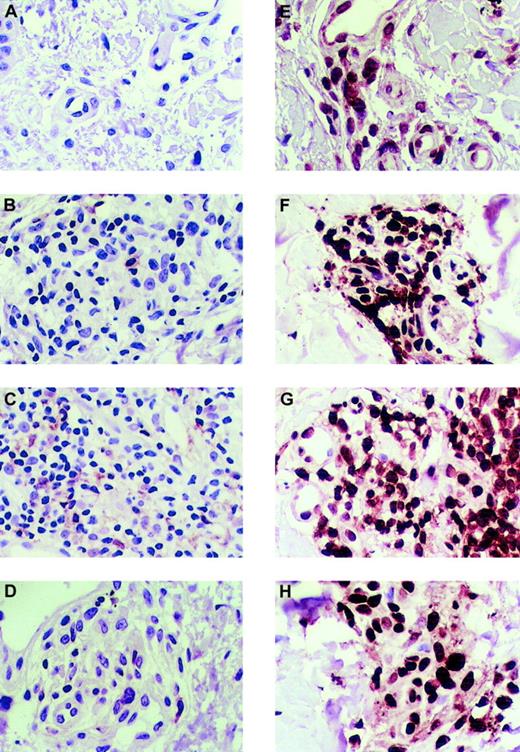
Given the previously described hypothesis of T-cell function in an immune response, in which the effector memory T cells have lost CCR7 expression and CCL21 responsiveness, this raised the question of whether the T cells infiltrating the skin of these autoimmune diseases had a naive (preantigen exposure) or memory (postantigen) phenotype. CD45Ra is a surface marker of naive T cells and CD45Ro is a marker of memory T cells. The expression of these markers on the skin-infiltrating T cells was tested by using immunohistology as before. As shown in Figure 3A-D, T cells in healthy or autoimmune skin expressed CD45Ra, the marker for naive T cells, in rare lymphocytes. In contrast, however, the marker for memory T cells, CD45Ro, was highly expressed in most of the T cells in both healthy skin and the autoimmune-diseased skin (Figure 3E-H).
Immunohistologic analysis of the expression of CD45Ra and CD45Ro in healthy skin and autoimmune skin disease.
(A) CD45Ra is poorly expressed in cells of healthy skin. CD45Ra is also poorly expressed in rare lymphocytes in skin samples from patients with T-cell autoimmune diseases: atopic dermatitis (B), lichen planus (C), and graft-versus-host disease (D). However, most of the skin-infiltrating lymphocytes in both healthy and diseased skin are CD45Ra+. (E) CD45Ro is significantly expressed in samples of healthy skin. Also, CD45Ro is highly expressed in skin samples from patients with T-cell autoimmune diseases: atopic dermatitis (F), lichen planus (G), and GVHD (H). The same 3 distinct patient skin samples were tested for each disease, and the same 3 for healthy skin as in the other figures. There was no difference between the samples displayed here and those not shown. All slides were photographed at × 50 original magnification.
Immunohistologic analysis of the expression of CD45Ra and CD45Ro in healthy skin and autoimmune skin disease.
(A) CD45Ra is poorly expressed in cells of healthy skin. CD45Ra is also poorly expressed in rare lymphocytes in skin samples from patients with T-cell autoimmune diseases: atopic dermatitis (B), lichen planus (C), and graft-versus-host disease (D). However, most of the skin-infiltrating lymphocytes in both healthy and diseased skin are CD45Ra+. (E) CD45Ro is significantly expressed in samples of healthy skin. Also, CD45Ro is highly expressed in skin samples from patients with T-cell autoimmune diseases: atopic dermatitis (F), lichen planus (G), and GVHD (H). The same 3 distinct patient skin samples were tested for each disease, and the same 3 for healthy skin as in the other figures. There was no difference between the samples displayed here and those not shown. All slides were photographed at × 50 original magnification.
Discussion
The T-cell chemokine CCL19 is rarely expressed in any cells in either healthy or diseased skin. One case of 3 of atopic dermatitis had induced expression of CCL19 in macrophages and dendritic cells. However, because that induction was not consistently seen in all cases of that skin disease, it is difficult to ascribe significance to it. Further investigation will be needed to identify whether CCL19 expression in those T-cell–infiltrative skin diseases is a prognostic factor. It is possible that CCL19 macrophage expression changes the biology of the disease, and those individual cases have distinct clinical behaviors.
CCL20 is expressed constitutively in keratinocytes and macrophages in both healthy and diseased skin. Because CCL20 expression did not change with the initiation of clinical T-cell–infiltrating skin disease, it is less likely that it plays a role in initiating or maintaining the disease pathology.
In contrast, CCL21 expression is consistently induced in the endothelial cells of all T-cell infiltrative autoimmune diseases of the skin that were tested. No other T-cell chemokine was consistently induced in T-cell–infiltrative skin diseases. The endothelial expression of CCl21 was specifically induced in those T-cell skin diseases; it is not expressed in the endothelial cells of healthy skin. In addition, most lymphocytes present in those autoimmune skin samples were CCR7+. This finding shows that the CCL21-CCR7 axis is highly induced in these T-cell infiltrative skin diseases. Because CCL21 is the most potent and specific T-cell chemokine, this raises the intriguing question of whether its induction mediates the aberrant T-cell infiltration of the skin in these diseases.
Although the studies here clearly indicate that CCL21 is overexpressed in these T-cell–mediated skin diseases, it is not clear whether such overexpression is important in initiating or rather maintaining the skin pathology. Is CCL21 expressed as a result of the disease, or does its expression produce the disease? It is clinically difficult to obtain biopsies just prior to a disease flare. All of the specimens here were obtained during clinically obvious skin pathology. All biopsies were obtained, however, at the initial time of diagnosis. Therefore, this question may require animal models to answer. It is also quite possible that CCL21 is important in both initiation (via early recruitment of self-presenting dendritic cells) and maintenance (via T-cell chemotaxis) of these diseases. No matter what the time course is, such abnormal expression of CCL21 will still result in increased T-cell adhesion and chemotaxis at sites of its expression, the pathologic hallmark of these diseases.
Most of the T cells in both healthy and autoimmune skin did not express CD45Ra, a marker of naive T cells, but highly expressed CD45Ro, a marker of memory T cells. Thus, a model can be hypothesized from these data here. In these T-cell diseases, CCL21 is induced in the endothelium of the target organ, the skin, stimulating the transmigration of CCR7+/CD45Ro+ T cells that are reactive to autologous antigens. These findings raise 2 possible steps that may be required for the skin pathology in these T-cell diseases. First, the induction of endothelial CCL21 may be important to stimulate responsive T-cell transmigration. Second, the maintenance of CCR7 in these autoreactive memory CD45Ro+ T cells would be required for this abnormal migratory response. These 2 abnormalities may underlie the pathology of this abnormal immune response.
This marked induction of CCL21 in the venule endothelial cells and the abundant presence of CCR7+/CD45Ro+ T cells in T-cell infiltrative skin diseases appears contradictory to the recent model describing effector memory T cells as lacking CCR7 and being unresponsive to CCL21.16 17 Those reports investigated models of the normal T-cell immune response, whereas the diseases studied here are autoimmune in origin and do not represent a normal T-cell immune response. Therefore, it is still possible that in the normal immune response to injury or infection CCL21 plays a little role in the tissue infiltration of effector memory T cells. Indeed, as mentioned earlier, the abnormal induction of CCL21 and the maintenance of CCR7 may lie at the heart of the pathology of the autoimmunity in the diseases studied here. However, the data here clearly indicate that the memory T cells producing this aberrant immune effect express CCR7 and, therefore, could respond to the CCL21 induced on the endothelial cells with transmigration into the target tissues.
Macrophages and dendritic cells were also found to express CCR7 in biopsy specimens of the T-cell autoimmune skin diseases studied here. This finding implies that the abnormal endothelial expression of CCL21 in these autoimmune diseases recruited antigen-presenting cells to inflamed skin. Figure 2C shows a dendritic cell with a large number of lymphocytes adhering to it, providing evidence that at least physical association between antigen-presenting cells and potential lymphocyte responders occurs in the diseased skin.
There are a number of other autoimmune diseases whose pathology relies on aberrant T-cell infiltration, such as rheumatoid arthritis and inflammatory bowel disease. It is possible that the aberrant expression of CCL21/CCR7 may also be present in these diseases. This raises the possibility that the endothelial regulation of T-cell migration could be targeted for therapy of these and other T-cell infiltrative diseases. Agents that interfere with the abnormal recruitment of T cells from the circulation to sites of pathologic inflammation by endothelial cells expressing CCL21 may be effective in treating these diseases. Blocking T-cell transmigration from the blood to the target tissues may at least decrease the pathologic damage of these tissues by cytotoxic T cells. Therefore, this study also implies that CCL21 may be an important pharmacologic target in T-cell autoimmune diseases.
Basal keratinocytes constitutively produce CCL27, a CC chemokine that we and others isolated, that chemoattracts CLA+ T cells to the skin.19,20 This chemokine also plays a role in stimulating T-cell infiltration of the skin in allergen-mediated skin diseases, as antibody neutralization of CCL27 suppresses lymphocyte recruitment to the skin in a mouse model of allergic dermatitis.20 The constitutive expression of CCL27 is probably important for T-cell infiltration of the skin in human autoimmune skin diseases. However, because CCL27 is also expressed in healthy skin and is not induced in the disease state like CCL21, it may not be the initiating step in the abnormal T-cell infiltration seen in these autoimmune diseases.
There are 2 other studies that lend credence to our hypothesis that the CCL21/CCR7 axis is critical for autoimmune T-cell skin infiltration. First, ectopic expression of CCL21 in the pancreas was sufficient to recruit abundant lymphocytes to this nonlymphoid tissue.21Thus, CCL21 expression alone can mediate abnormal T-cell tissue infiltration. Secondly, it has been reported that CCL21 is essential for the homeostatic proliferation of CD4+ T cells in lymphopenic hosts.22 When there is a decreased number of CD4+ T cells and CCL21 is not expressed, T cells cannot proliferate to homeostatically increase T-cell number. More germane to the data here, that study found that transferring antigen-specific CD4+ T cells to antigen-expressing hosts that had pancreatic expression of CCL21 induced an autoimmune disease in that organ.22 Thus, that study indicates that the aberrant induction of CCL21, such as is seen here, could induce tissue-specific autoimmune T-cell infiltration, given the appropriate antigenic circumstance.
In summary, the abnormal induction of endothelial CCL21 and the recruitment of CCR7+/CD45Ro+ T cells to target tissues may be a novel and significant pathway in tissue damage in some autoimmune diseases. The mechanism of this abnormal induction of CCL21 and the maintenance of CCR7 on CD45Ro T cells to enable them to respond to this abnormal CCL21 expression may lie at the center of the molecular pathology of these diseases. It also indicates that a modification of the effector memory hypothesis of T-cell trafficking and function needs to be made for autoimmune diseases. Finally, these data imply that CCL21/CCR7 may be a novel and beneficial target of therapy in these diseases.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-05-1586.
Supported by grant RO1 HL66308 (R.A.H.) and RO1 HL629906 (J.B.T.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert A. Hromas, Division of Hematology/Oncology and Walther Oncology Center, R4-202, Indiana University Medical Center, 1044 W Walnut St, Indianapolis, IN 46202; e-mail: rhromas@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal