Introduction
The hemoglobin molecule is a tetramer consisting of 2 pairs of globin chains, each of which contains a heme group. During fetal development, the major hemoglobin is Hb F (α2γ2). In a normal adult, the major hemoglobin is Hb A (α2β2). The α-globin gene cluster is located on chromosome 16pter-p13.3 and is made up of one embryonic ζ-globin and 2 α-globin genes in tandem (in cis): 5′-ζ2-α2-α1-3′.1 Because each person has 2 chromosomes 16, most people have 4 α-globin genes. The β-globin gene cluster, 5′-ε-Gγ-Aγ-δ-β-3′ is located on chromosome 11p15.5.
α-Thalassemia is caused by mutations or deletions affecting either one or more α-globin genes, leading to decreased or absent α-globin chain production from the affected gene(s).2 The deletion or inactivation of only one α-globin gene usually results in insignificant hematologic findings. When 2 α-globin genes are deleted or inactivated, either both on the same chromosome 16 (in cis) or one on each of the 2 chromosomes 16 (in trans), the affected person is well but has borderline anemia, as well as microcytic and hypochromic red blood cells.3
When 3 α-globin genes become inactive because of deletions with or without concomitant nondeletional mutations, the affected individual would have only one functional α-globin gene. These people usually have moderate anemia and marked microcytosis and hypochromia. In affected adults, there is an excess of β-globin chains within their erythrocytes that will form β4tetramer, also known as Hb H. This hereditary disorder is known as Hb H disease.3
The most severe form of α-thalassemia is that of fetuses lacking all α-globin genes. Some succumb early in gestation. Most develop hydrops fetalis syndrome and die in utero during the second or the third trimester of pregnancy, or shortly after birth.3-5
α-Thalassemia mutations
Deletional mutations
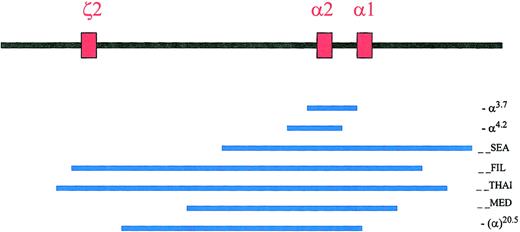
Deletions removing one α-globin gene, such as the rightward (-α3.7) or leftward (-α4.2) single α-globin gene deletions, are results of misalignment crossovers during meiosis (Figure 1). These deletions are very common and are found in 30% of African Americans and up to 60% to 80% of people living in parts of Saudi Arabia, India, Thailand, Papua New Guinea, and Melanesia.6,7 These single α-globin gene deletions and other point mutations involving a single α-globin gene (see “Nondeletional mutations”) are known as the α+-thalassemia mutations.2
Common deletions in the ζ-α-globin gene cluster.
The 3 red boxes represent the 3 active globin genes, the one embryonic ζ2-globin gene, and the α2- and α1-globin genes. They span approximately 26 Kb in length from ζ2 to α1 globin genes. The blue lines represent the common α-thalassemia deletions. Figure adapted with permission from Cambridge University Press2 andBlood.5
Common deletions in the ζ-α-globin gene cluster.
The 3 red boxes represent the 3 active globin genes, the one embryonic ζ2-globin gene, and the α2- and α1-globin genes. They span approximately 26 Kb in length from ζ2 to α1 globin genes. The blue lines represent the common α-thalassemia deletions. Figure adapted with permission from Cambridge University Press2 andBlood.5
There are more than 20 known natural deletions that remove both α-globin genes on the same chromosome 16 (in cis) or the complete ζ-α-globin gene cluster, and they are known as the α0-thalassemia mutations.2 Some deletions might measure 100 to 300 kb or more in length. In addition, there are rare deletions that silence α-globin gene expression by removing the HS-40 regulatory sequences upstream of the ζ-α-globin gene cluster.2
The (--SEA) type of α0-thalassemia deletion of approximately 19.3 kb in length removing both α-globin genes in cis but sparing the embryonic ζ-globin gene is common in Southeast Asia. It is found in 14% of people living in Northern Thailand, for example.5 This mutation is the most common cause for Hb H disease (Table 1) and hydrops fetalis syndrome in that part of the world. In addition, the (--FIL), (--MED), and −(α)20.5 deletions are relatively common in the Philippines and in the Mediterranean region, respectively (Figure 1).
| Hb H disease . | No. of patients . | % . |
|---|---|---|
| Deletional | 266 | 83 |
| (--SEA/-α3.7) | 175 | 55 |
| (--SEA/-α4.2) | 37 | 12 |
| (--FIL/-α3.7) | 36 | 11 |
| (--MED/-α3.7) | 8 | 2 |
| (--THAI/-α3.7) | 2 | < 1 |
| (--BRIT/-α3.7) | 1 | < 1 |
| (--SA/-α3.7) | 1 | < 1 |
| (--de novo/-α3.7) | 1 | < 1 |
| (--SEA/-α4.2 Q-Thailand [Codon 74 GAC>CAC or Asp→His]) | 4 | 1 |
| (--FIL/-α4.2 Q-Thailand [Codon 74 GAC>CAC or Asp→His]) | 1 | < 1 |
| Nondeletional | 53 | 17 |
| (--SEA/αConstant Spring [Codon 142 TAA>CAA or Ter→Gin]α) | 31 | 10 |
| (--SEA/αQuong Sze [Codon 125 CTG>CCG or Leu→Pro]α) | 7 | 2 |
| (--SEA/αCodon 30 deletion of GAGα) | 3 | < 1 |
| (--TOT/αCodon 30 deletion of GAGα) | 1 | < 1 |
| (--SEA/αInitiation codon ATG>AGα) | 1 | < 1 |
| (--THAI/αInitiation codon ATG>AGα) | 1 | < 1 |
| (--SEA/αCodon 31 AGG>AAG or Arg→Lysα) | 2 | < 1 |
| (--FIL/αCodon 35 TCC>CCC or Ser→Proα) | 1 | < 1 |
| (--MED/αIVS I deletion of TGAGGα) | 1 | < 1 |
| (--SEA/αCodon 59 GGC>GAC or Gly→Aspα) | 1 | < 1 |
| (--SEA/αPakse [Codon 142 TAA>TAT or Ter→Tyr)α) | 2 | < 1 |
| (--THAI/αConstant Spring [Codon 142 TAA>CAA or Ter→Gln]α) | 1 | < 1 |
| (αHb Sallanches [Codon 104 TGC>TAC or Cys→Tyr] α/αHb Sallanchesα) | 1 | < 1 |
| Hb H disease . | No. of patients . | % . |
|---|---|---|
| Deletional | 266 | 83 |
| (--SEA/-α3.7) | 175 | 55 |
| (--SEA/-α4.2) | 37 | 12 |
| (--FIL/-α3.7) | 36 | 11 |
| (--MED/-α3.7) | 8 | 2 |
| (--THAI/-α3.7) | 2 | < 1 |
| (--BRIT/-α3.7) | 1 | < 1 |
| (--SA/-α3.7) | 1 | < 1 |
| (--de novo/-α3.7) | 1 | < 1 |
| (--SEA/-α4.2 Q-Thailand [Codon 74 GAC>CAC or Asp→His]) | 4 | 1 |
| (--FIL/-α4.2 Q-Thailand [Codon 74 GAC>CAC or Asp→His]) | 1 | < 1 |
| Nondeletional | 53 | 17 |
| (--SEA/αConstant Spring [Codon 142 TAA>CAA or Ter→Gin]α) | 31 | 10 |
| (--SEA/αQuong Sze [Codon 125 CTG>CCG or Leu→Pro]α) | 7 | 2 |
| (--SEA/αCodon 30 deletion of GAGα) | 3 | < 1 |
| (--TOT/αCodon 30 deletion of GAGα) | 1 | < 1 |
| (--SEA/αInitiation codon ATG>AGα) | 1 | < 1 |
| (--THAI/αInitiation codon ATG>AGα) | 1 | < 1 |
| (--SEA/αCodon 31 AGG>AAG or Arg→Lysα) | 2 | < 1 |
| (--FIL/αCodon 35 TCC>CCC or Ser→Proα) | 1 | < 1 |
| (--MED/αIVS I deletion of TGAGGα) | 1 | < 1 |
| (--SEA/αCodon 59 GGC>GAC or Gly→Aspα) | 1 | < 1 |
| (--SEA/αPakse [Codon 142 TAA>TAT or Ter→Tyr)α) | 2 | < 1 |
| (--THAI/αConstant Spring [Codon 142 TAA>CAA or Ter→Gln]α) | 1 | < 1 |
| (αHb Sallanches [Codon 104 TGC>TAC or Cys→Tyr] α/αHb Sallanchesα) | 1 | < 1 |
Nondeletional mutations
In contrast to β-thalassemia, nondeletional α+-thalassemia mutations are relatively uncommon. There is an inherent difficulty in deciphering these mutations, because nucleotide sequencing of the α-globin genes with their high GC content is not an easy task. In recent years, increasing numbers of nondeletional α+-thalassemia mutations have been described. More than 30 of these mutations are tabulated in the human globin gene mutation database on the World Wide Web (http://globin.cse.psu.edu). The α2-globin gene normally accounts for 2 to 3 times more α-globin mRNA and α-globin chain production than the α1-globin gene.8 Therefore, α-thalassemia point mutations of the α2-globin gene generally cause more severe anemia than the same mutations involving the α1-globin gene.
Most of these mutations affect the transcriptional or translational processes of α-globin chain production. Others, notably Hb Constant Spring (α2 codon 142 TAA>CAA or Ter→Gln) lead to markedly decreased and abnormally elongated α-globin chains.2Recently, it was reported that as many as 15% of patients previously thought to be carriers of Hb Constant Spring based on hemoglobin analysis were in fact carriers of Hb Pakse (α2 codon 142 TAA>TAT or Ter→Tyr).9,10,141 Other mutations produce highly unstable α-globin chains or hemoglobins, such as Hb Agrinio (α2 codon 29 CTG>CCG or Leu→Pro), Hb Suan-Dok (α2 codon 109 CTG>CGG or Leu→Arg), Hb Quong Sze (α2 codon 125 CTG>CCG or Leu→Pro), and Hb Pak Num Po (α1 codon 131/132 +T), and result in an α-thalassemia phenotype.11-14
Genotypes of Hb H disease
Deletional and nondeletional Hb H disease
Hb H disease is commonly caused by a deletion removing both α-globin genes on one chromosome 16, plus a deletion removing only a single α-globin gene on the other chromosome 16 such as the (-α3.7) or (-α4.2) deletions. These are known as “deletional Hb H disease.”11 15-33
In a smaller proportion of patients, Hb H disease is caused by a deletion removing both α-globin genes on one chromosome 16, plus an α+-thalassemia point mutation, or small insertion/deletion, involving either the α2- or α1-globin gene on the other chromosome 16. These are collectively labeled as the “nondeletional Hb H disease.” Patients with nondeletional Hb H disease usually are more anemic, more symptomatic, more prone to have significant hepatosplenomegaly, and more likely to require transfusions.11,18,20,24,25,31 32
Rarely, homozygosity or compound heterozygosity of nondeletional α+-thalassemia mutation(s) involving α2-globin gene on each of the 2 chromosomes 16, can lead to a phenotype similar to Hb H disease. They include initiation codon mutation (ATG>ACG), Hb Sallanches (codon 104 TGC>TAC or Cys→Tyr), or polyadenylation signal mutation (AATAAA>AATAAG).25,29 34-37 Even in this small subset of patients, their phenotypes can vary from relatively mild to very severe, requiring regular transfusions.
Population genetics
Hb H disease is found in many parts of the world, including Southeast Asian, Middle Eastern, and Mediterranean populations. It is particularly prevalent in Southeast Asia and in southern China, because of high carrier frequencies of the (--SEA), and to a lesser extent, the (--FIL) types of α-thalassemia deletions there.5,38,39 In Thailand with a population of 62 million people, it is estimated that 7000 infants with Hb H disease are born annually, and that there are 420 000 patients with Hb H disease in that country.40
The genotypes of 319 patients with Hb H disease from California, Hong Kong, and Ontario were reported during the past 2 years.31,33 41 These genotypes are summarized in Table1 and serve to illustrate the heterogeneity of Hb H disease genotypes, especially in many multicultural communities in North America and elsewhere. Of those patients, 266 patients or 83% (95% confidence interval [CI], 79%-87%) have deletional Hb H disease. The most common genotype is (--SEA/-α3.7) found in 175 patients (55%), followed by (--SEA/-α4.2) 37 patients (12%), and (--FIL/ -α3.7) 36 patients (11%).
Fifty-three patients or 17% (95% CI, 13%-21%) have nondeletional Hb H disease. The most prevalent genotype among this subgroup is (--SEA/αConstant Spring α) found in 31 patients (10%). In Thailand, nondeletional Hb H disease with αConstant Spring is even more common, reportedly found in 40% to 50% of patients with Hb H disease.18
Among the 638 chromosomes examined in these 319 patients with Hb H disease, (--SEA) is found in 263 chromosomes (41%), (-α3.7) in 224 chromosomes (35%), (-α4.2) in 42 chromosomes (7%), (--FIL) in 38 chromosomes (6%), and (αConstant Spring α) in 32 chromosomes (5%). The remaining 14 other mutations are found in 39 chromosomes (6%).
In the Mediterranean region, the most common deletion removing both α-globin genes in cis is the (--MED) deletion. Among 78 Cypriot patients with Hb H disease, 79% had the (--MED) deletion and 17% had the −(α)20.5deletion.28
Pathophysiology
In Hb H disease, there is a deficiency of α-globin mRNA and α-globin chains. These are reflected in the α/β globin mRNA ratio being below 0.5, and the α/β globin chain synthetic ratios ranging from 0.2 to 0.7.3,11,15 18 During fetal development, the excess γ-globin chains form γ4 tetramers (Hb Bart). In adults, the excess β-globin chains form β4 tetramers (Hb H). These homotetramers have high oxygen affinity, lack heme-heme interaction, and deliver only insignificant amounts of oxygen to tissues.
Hb H (β4) is relatively unstable and can be oxidized to form intracellular precipitates. If present within developing erythroblasts, these precipitates are thought to cause intramedullary early erythroid cell death and ineffective erythropoiesis. More often, these precipitates are formed in circulating erythrocytes with time and become attached to cell membrane. They cause local oxidative damage, membrane dysfunction, and shortened red cell survival.42Hb H disease erythrocytes are rigid, and their membrane is more stable than normal.43 The membrane associated with Hb Constant Spring is even more rigid.44 These properties retard the passage of red blood cells through microvasculature and can promote erythrophagocytosis.45
There is evidence that pyrexia can enhance formation of intraerythrocytic Hb H inclusion bodies that might account for the acute hemolytic crisis and precipitous drop in hemoglobin associated with infections in some patients.11,46-48 Loss of normal membrane phospholipid asymmetry, particularly the exposure of phosphatidylserine on thalassemic cell surface, likely mediates intramedullary erythroblast apoptosis and engulfment of abnormal or aging circulating erythrocytes by macrophages.49-52Erythrocyte membrane–associated immunoglobulin G (IgG) and complement components may also have similar roles in promoting erythrophagocytosis.45
It is generally accepted that hemolysis is the major cause of anemia in Hb H disease, although ineffective erythropoiesis also plays a pathogenetic role in this syndrome.15,51 53-56 In contrast, ineffective erythropoiesis is the dominant cause for the anemia in β-thalassemia intermedia and major.
Nondeletional Hb H disease is generally more severe.11,18,20,24,25,31,32 Given that α2-globin gene produces 2.5 times more α-globin mRNA than the α1-globin gene, the combined α-globin mRNA production from both α-globin genes on a normal chromosome 16 could be assigned 3.5 “arbitrary units” for this discussion.8,45 In an α-globin gene cluster harboring a point mutation inactivating α2-globin gene, the α-globin mRNA production by the remaining and normal α1-globin gene is merely 1.0 “unit.” However, with the single α-globin gene deletion of the (-α3.7) type as a result of a misalignment crossover event during meiosis, the α-globin mRNA production by the remaining single hybrid α-globin gene is 2.0 “units.”45 This consideration provides at least partial explanation for the observation that patients with nondeletional Hb H disease such as (--SEA/αConstant Springα) are more severely affected than those with deletional Hb H disease such as (--SEA/-α3.7).31 45
In some cases, the nondeletional α-globin gene mutation leads to highly unstable α-globin chains or variant hemoglobin, such as Hb Quong Sze (α2 codon 125 CTG>CCG or Leu→Pro).13 In addition to the more severe α-globin chain deficiency, the intracellular aggregates of hyperunstable variant α-globin chains or hemoglobins might also cause additional membrane damage and dysfunction. In one report, patients with nondeletional Hb H disease were found to have higher malonyldialdehyde levels, which is a secondary product of lipid peroxidation, and lower levels of vitamin E.57
Clinical and laboratory findings
Hb H disease is generally thought to be a mild disorder. However, there is a marked phenotypic variability, ranging from asymptomatic, to need for periodic transfusions, to severe anemia with hemolysis and hepatosplenomegaly, and even to fatal hydrops fetalis syndrome in utero. Patients with identical α-globin genotypes can have different phenotypes,30 suggesting that there are other yet undefined genetic and/or environmental factors that can affect phenotypic expression of Hb H disease.58 59
Anemia
The hemoglobin level, mean erythrocyte corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) of patients with Hb H disease are tabulated in Table 2. There are significant variations in these values between individuals. For example, in one report, hemoglobin levels ranged between 70 and 129 g/L among 10 male patients and between 70 and 114 g/L among 41 female patients, all with deletional Hb H disease.33 MCV ranged from 51 to 73 fL. There are elevated reticulocyte counts (5%-10%), polychromasia, and moderate anisopoikilocytosis.60
Hemoglobin, MCV and MCH in patients with Hb H disease
| Hb H disease . | Hemoglobin, g/L (n) . | MCV, fL (n) . | MCH, pg (n) . | Reference . | |||
|---|---|---|---|---|---|---|---|
| Male . | Female . | Male . | Female . | Male . | Female . | ||
| Deletional | 111 ± 11 (28) | 94 ± 12 (59) | 65 ± 7 (121) | 19 ± 2 (120) | 3 | ||
| 105 ± 10 (40) | 88 ± 11 (47) | 63 ± 5 (40) | 64 ± 6 (47) | 19 ± 1 (40) | 19 ± 2 (47) | 31 | |
| 106 ± 13 (10) | 91 ± 9 (41) | 62 ± 4 (10) | 62 ± 5 (41) | 19 ± 2 (10) | 19 ± 2 (41) | 33 | |
| 100 ± 12 (67) | 67 ± 7 (56) | 19 ± 2 (56) | 18 | ||||
| Nondeletional | 105 ± 10 (6) | 85 ± 7 (5) | 68 ± 6 (14) | 19 ± 2 (14) | 3 | ||
| 86 ± 19 (11) | 83 ± 20 (14) | 73 ± 7 (11) | 72 ± 7 (14) | 21 ± 2 (11) | 20 ± 2 (14) | 31 | |
| 96 ± 11 (64) | 77 ± 5 (47) | 21 ± 2 (47) | 18 | ||||
| Hb H disease . | Hemoglobin, g/L (n) . | MCV, fL (n) . | MCH, pg (n) . | Reference . | |||
|---|---|---|---|---|---|---|---|
| Male . | Female . | Male . | Female . | Male . | Female . | ||
| Deletional | 111 ± 11 (28) | 94 ± 12 (59) | 65 ± 7 (121) | 19 ± 2 (120) | 3 | ||
| 105 ± 10 (40) | 88 ± 11 (47) | 63 ± 5 (40) | 64 ± 6 (47) | 19 ± 1 (40) | 19 ± 2 (47) | 31 | |
| 106 ± 13 (10) | 91 ± 9 (41) | 62 ± 4 (10) | 62 ± 5 (41) | 19 ± 2 (10) | 19 ± 2 (41) | 33 | |
| 100 ± 12 (67) | 67 ± 7 (56) | 19 ± 2 (56) | 18 | ||||
| Nondeletional | 105 ± 10 (6) | 85 ± 7 (5) | 68 ± 6 (14) | 19 ± 2 (14) | 3 | ||
| 86 ± 19 (11) | 83 ± 20 (14) | 73 ± 7 (11) | 72 ± 7 (14) | 21 ± 2 (11) | 20 ± 2 (14) | 31 | |
| 96 ± 11 (64) | 77 ± 5 (47) | 21 ± 2 (47) | 18 | ||||
The MCV in nondeletional Hb H disease is higher than in deletional Hb H disease (Table 2). Anemia is more pronounced in nondeletional Hb H disease and so is reticulocytosis. This finding may partially account for the higher MCV. α-Thalassemic erythrocytes are hyperhydrated, especially those inherited with Hb Constant Spring.43 In 15 patients with nondeletional Hb H disease/Hb Constant Spring, their Hb was 82 ± 19 g/L, and MCV was 76 ± 7 fL. In 5 other patients with nondeletional Hb H disease/Hb Quong Sze, their Hb was 86 ± 10 g/L, and MCV was 71 ± 7 fL. (V.C., unpublished observations, August 2002). It is postulated, although unproven, that the early closing of the K-Cl cotransporter in α-thalassemic erythrocytes, in contrast to β-thalassemic erythrocytes, prevents loss of K-Cl and water and leads to erythrocyte hyperhydration and higher MCV.45
There are occasions, such as increased hemolysis secondary to infections, fever, ingestion of oxidative compounds or drugs, aregenerative anemia as a result of Parvovirus B19 and other infections, hypersplenism, and pregnancy, when severe anemia may ensue and transfusions become necessary. In Thailand, 29% of 221 patients with deletional Hb H disease and 50% of 136 patients with nondeletional Hb H disease had been transfused.18 In Hong Kong, 46% of 114 patients with Hb H disease had been transfused, but only very few patients required regular transfusions.31
The microcytic and hypochromic anemia found in Hb H disease might lead to the erroneous diagnosis of iron deficiency anemia. On occasions, inappropriate iron supplement treatment or invasive investigations to rule out possible gastrointestinal bleeding are prescribed.
Iron overload
Iron overload as manifested by markedly elevated serum ferritin levels is present in 70% to 75% of adult patients with Hb H disease.61-63 Raised serum ferritin levels are correlated with increasing age but are unrelated to previous history of transfusions, iron supplement, or herbal medicine treatment.22,31 It is likely that iron absorption is increased in Hb H disease, secondary to enhanced erythropoiesis as a result of hemolysis and anemia.64 Excessive alcohol consumption is an additional risk factor.62
In Hong Kong, 60 patients underwent computed tomographic (CT) scan of liver, and 51 of them (85%) had a signal-intensity ratio of less than 1, indicative of iron overload.31,65 In others, liver biopsy revealed increased liver iron content and hepatic fibrosis and/or cirrhosis, without serologic evidence of either hepatitis B or C.22,31 In addition, 25 asymptomatic adult patients had cardiac echocardiography done, and all showed abnormal left ventricular diastolic function.31 There was a trend of increasing cardiac abnormality with increasing serum ferritin levels. Three patients had heart failure as a result of cardiac iron overload. Another patient developed hemosiderosis and diabetes mellitus.66 These findings underscore the importance of monitoring adult patients with Hb H disease for iron overload and to initiate iron chelation therapy when indicated.
There are reports from the Mediterranean region that iron overload in Hb H disease is uncommon.11,30,67 The apparent discrepancy might be due to differences in other genetic or environmental factors. In Sardinia, some adult patients had serum ferritin levels at or close to 1000 μg/L.25 Long-term follow-up on those apparently unaffected patients should be informative.
Hepatosplenomegaly
Hepatomegaly was present in 70% of 502 patients in Thailand, 60% of 153 patients in Sardinia, and 14% of 88 patients in Taiwan.15,22,25 Jaundice was found in 25% of patients in Thailand, 20% to 30% in Sardinia, and 43% in Taiwan.18,22,25 Splenomegaly is also prevalent in Hb H disease, found in 79% of patients in Thailand, 60% in Sardinia, and 47% in Taiwan.15,22,25 In Hong Kong, a third of 27 patients with nondeletional Hb H disease had undergone splenectomy. In contrast, only 5% of 87 patients with deletional Hb H disease were splenectomized.31
Cholelithiasis
Seventy-seven adult patients in Hong Kong had abdominal ultrasonography done. Twenty-six patients (34%) were found to have cholelithiasis, and 5 of them underwent cholecystectomy.31Nineteen percent of 88 patients in Taiwan and 15% of 81 patients in Thailand had cholelithiasis.22,68 A promoter polymorphism in uridine diphosphate (UDP)–glucuronosyl-transferase, as in Gilbert disease, is a risk factor for cholelithiasis in hereditary spherocytosis, β-thalassemia, and sickle cell disease.69-73 Whether this correlation also holds true for Hb H disease awaits confirmation.
Growth and development in children
Thirteen percent of children with Hb H disease in Hong Kong had a growth rate below the third percentile.31 In another report, 2 infants with Hb H disease were followed from birth to 6 months of age.74 At birth, the total hemoglobin was approximately 155 g/L, but only 110 to 120 g/L represented functional hemoglobins (Hb F and Hb A). The other 30 to 40 g/L were Hb Bart (γ4) and some Hb H (β4), which have markedly impaired ability to deliver oxygen to tissues. At 1 to 2 months of age, the functional hemoglobin fractions fell to 70 g/L. By 3 months, these fractions had returned to 85 to 95 g/L. These anecdotal reports underscore the importance of a systematic investigation of the natural history of Hb H disease during early infancy and childhood.37
Pregnancy
There is usually an increasing severity of anemia during pregnancy, possibly related to expansion of blood volume. Hb level may sometimes fall to 60 g/L or even less, necessitating the need for blood transfusion to maintain the health of the mother and the developing fetus.15,25,75,76 However, other women go through pregnancies without the need for transfusions.77
In 34 pregnancies among 29 Thai women, Hb fell to 71 ± 20 g/L during the second and third trimesters.75 Preeclampsia occurred in 18% of the cases, and 9% developed congestive heart failure. There was one case each of miscarriage and perinatal death and 3 cases of premature births. Galanello et al25 reported that there were 7 miscarriages (12%) among 58 pregnancies in 24 Italian women with Hb H disease. It is vitally important to carry out prospective and in-depth studies of pregnancies in women with Hb H disease to define the risks and criteria for treatment.
Another important issue is related to testing the woman's partner and genetic counseling. This issue is discussed in more detail in “Genetic counseling.”
Hb H hydrops fetalis syndrome
This devastating syndrome represents the most severe form of Hb H disease and was first described in detail in 1985.78 It is the subject of a recent review.79 The α-globin genotypes of the affected fetuses consist of deletion of both α-globin genesin cis or the complete ζ-α-globin gene cluster on one chromosome 16 and a nondeletional mutation involving α2-globin gene on the other chromosome 16.79-81 These fetuses suffer from severe anemia and hypoxia in utero, with its sequelae such as edema, pericardial effusion, congenital anomalies, and even death.
The pathophysiology of this serious inherited disorder is not well understood. One hypothesis is that the nondeletional α-globin gene mutations, such as α2 codon 35 TCC>CCC or Ser→Pro, codon 59 GGC>GAC or Gly→Asp, or codon 66 CTG>CCG or Leu→Pro, result in highly unstable hemoglobin and severe anemia, akin to the dominant β-thalassemia syndrome.79-82
With the mutation α2 codon 30 ΔGAG, hydrops fetalis occurs only with the coinheritance of a deletion removing the complete ζ-α-globin gene cluster.80 Other individuals who have inherited the same ΔGAG mutation together with the Southeast Asian type of deletion removing both α-globin genes in cis, but sparing the embryonic ζ-globin gene, survive to adulthood, albeit with moderate to severe anemia (Hb, 72-105 g/L).31,80 83
Hb H disease in combination with other hemoglobinopathies
Patients with both Hb H disease and heterozygosity for β-globin variants such as Hb S, Hb C, or Hb E have hemoglobin levels similar to most patients with Hb H disease.85-89 The proportions of the variant hemoglobins are low (20%-25%), because of the lower affinity of the α-globin chains for the variant β-globin chains as compared with the normal β-globin chains.90There are exceptions, such as βJ-Iran (codon 77 CAC>GAC or His→Asp) that is a negatively charged variant β-globin chain.91 With Hb H disease, the proportion of this variant Hb increases to 65%.91 These patients with variant β-globin chains as well as those with β-thalassemia trait have low Hb H levels and scanty Hb H inclusion bodies.
Hb E is very common in Southeast Asia. In Thailand, interactions of Hb H disease with Hb E and/or β-thalassemia give rise to many different complex genotypes. They are broadly known as AE-Bart disease (Hb H disease and heterozygosity for Hb E), and EF-Bart disease (Hb H disease and homozygosity for Hb E or Hb E/β-thalassemia).88 89They present with thalassemia intermedia phenotype with moderate to severe anemia and with decreased or no Hb H and inclusion bodies detected.
Patients who have Hb H disease together with heterozygosity for βNew York (codon 113 GTG>GAG or Val→Glu) have severe anemia (Hb between 34 and 68 g/L) and hepatosplenomegaly.92 The variant βNew Yorkhas higher affinity for the α-globin chains to form Hb New York that is an unstable hemoglobin. This worsens the deficiency of α-globin chains already present in Hb H disease and causes even more severe anemia. Coinheritance of Hb H disease and several other uncommon variant β-globin chains has been described, such as Hb J Bangkok (codon 56 GGC>GAC or Gly→Asp), Hb Pyrgos (codon 83 GGC>GAC or Gly→Asp), and Hb Hope (codon 136 GGT>GAT or Gly→Asp).93-95
Coinheritance of Hb H disease and β-thalassemia heterozygosity leads to anemia usually more severe than β-thalassemia heterozygote alone, but less than common Hb H disease.96-98 However, there are exceptions and some patients require transfusions.28,98They have marked microcytosis and hypochromia, but low or absent Hb H and Hb H inclusion bodies. Hb A2 levels are elevated, as in most other β-thalassemia heterozygotes.96-98
Other findings
The following findings associated with Hb H disease have been reported: dysmorphic facial features, skeletal changes, retinopathy, extramedullary hematopoietic tumors, risk of thromboembolic disorders, and leg ulcers.3 99-102
Erythrocyte glucose-6-phosphate dehydrogenase (G6PD) deficiency is prevalent in the same populations in whom thalassemias are prevalent. Therefore, coinheritance of both Hb H disease and G6PD deficiency is to be expected.47 103 Recently in Hong Kong, a newborn was found to have Hb H disease (--SEA/αConstant Springα) and G6PD deficiency (0.1 U/g Hb in cord blood). At birth, the infant was anemic with Hb of 125 g/L and had hepatosplenomegaly. At 1 year of age, his Hb fell to 42 g/L soon after an episode of infection and fever (E. S. K. Ma, unpublished observations, August 2002).
Alpha-thalassemia/mental retardation (ATR) syndromes
ATR-16 syndrome
The affected children have chromosomal rearrangements that delete as much as 1 to 2 Mb of chromosome 16 short-arm (16p) telomere, including the ζ-α-globin gene complex, resulting in monosomy for the 16p telomere, and α-thalassemia phenotype.104 In some, the chromosomal abnormalities can be visualized by cytogenetic analysis or fluorescent in situ hybridization (FISH). In most cases, one parent carried a balanced translocation that the child inherited in an unbalanced fashion. In others, the abnormality arose as de novo events. If the affected child also inherited a common single α-globin gene deletion from the other parent, the child would have Hb H disease.105
It is thought that mental retardation and other congenital anomalies observed in this syndrome are caused by the deletion of dosage-sensitive genes on one 16p telomere and other concomitant chromosomal abnormalities. Children with deletions of up to 350 kb in length from one 16p telomere, including the complete ζ-α-globin gene complex, have α-thalassemia trait but are developmentally normal.106 It is critical for understanding the genetic basis of mental retardation to identify these genes on 16p telomere that can affect severe cognitive and developmental abnormalities when only one copy is deleted.105 107
ATR-X syndrome
This syndrome is an X-linked disorder, caused by mutations of theATRX gene located on chromosome Xq13.3.108 It is more common than the ATR-16 syndrome. The affected males usually have very severe intellectual and physical handicaps and many other congenital anomalies. They often have characteristic facial features. Genital abnormalities, such as ambiguous genitalia, and skeletal deformities are present in 90% of these patients.109 They present with an α-thalassemia phenotype but with considerable variations with Hb H inclusion bodies found in none to up to 32% of circulating erythrocytes.105
The functions of ATRX protein in vivo are not yet understood and are under active investigation.110 It contains motifs that have been implicated in the regulation of transcription mediated by chromatin modification, protein-protein interaction, adenosine triphosphatase (ATPase), and helicase activities and that affect genomic methylation pattern.111,112 The highly variable phenotypes in patients suggest that ATRX interacts with other genetic factors to bring about normal expression of many genes of developmental importance, including the α- but not the β-globin genes.105
Acquired Hb H disease in myeloproliferative disorders
This syndrome has been described mostly in elderly men who have primary marrow disorders such as erythroleukemia, myelodysplasia, refractory anemia with excessive blasts, acute leukemia, and so forth. Its molecular pathology has not been defined, although it is conceivable that the down-regulation of α-globin gene expression in these patients is the result of somatic mutation(s) in transcription factor(s).
Diagnosis
Hb H disease is characterized by moderate hypochromic and microcytic anemia (see “Anemia”). Formation of Hb H (β4) precipitates within red cells (Hb H inclusion bodies) can be induced by incubation with mild oxidants such as Brilliant Cresyl Blue.113 These inclusion bodies are numerous and can easily be seen by light microscopy in most circulating erythrocytes. In addition, Hb H ranging between 5% and 30% can be detected by hemoglobin electrophoresis, isoelectric focusing (IEF), or high-performance liquid chromatography (HPLC).60 Hb H levels are usually higher in nondeletional Hb H disease than in deletional Hb H disease. It should be noted that some commercial electrophoretic methods are incapable of detecting Hb H.114 Hb A2 level is usually down in the 1.0% to 2.0% range.60
Patients who have Hb H disease and concomitant heterozygous β-hemoglobinopathies, such as Hb S, Hb C, Hb E, or β-thalassemia, have low or absent Hb H (β4) and many fewer Hb H inclusion bodies.85-89 96-98 These hematologic findings may confound the diagnosis of the underlying Hb H disease.
Correct DNA-based genotyping is necessary for genetic counseling and prenatal diagnosis. Southern blotting is often used for diagnosing deletional mutations. More recently, Gap-polymerase chain reaction (PCR) methods have been developed for many of the common deletions.115-117 In large deletions that remove the complete ζ-α-globin gene cluster, the diagnosis is more difficult and often depends on family studies and Southern blotting analysis using probes distal to the deletional breakpoints.118 119
For point mutations, or small deletion/insertion mutations, the diagnosis is often based on direct nucleotide sequencing of the PCR-amplified product of either α2- or α1-globin gene.79-81,83,84 Reverse dot-blot by hybridization with allele specific oligonucleotide probes and multiplex ARMS (PCR-based amplification refractory mutation system) assays have been developed for rapid diagnosis of several α+-thalassemia point mutations.120 121
Hb Bart (γ4) is a fast-moving hemoglobin that can be determined by electrophoresis, IEF, or HPLC. Newborns who have inherited Hb H disease have 20% to 40% of Hb Bart at birth.60 Universal newborn screening for Hb H disease was implemented in 1996 in California, based on an elevated level of a fast-moving peak on HPLC of hemolysates eluted from newborn blood spots impregnated on filter papers.41 Of 101 newborns found to have 25% or more of this peak, 89 were confirmed by DNA diagnostics to have Hb H disease. Table 3shows that in California, there are many more newborns with Hb H disease than with other commonly screened for inherited metabolic disorders such as phenylketonuria and galactosemia.
Number of newborns diagnosed annually through the universal newborn screening program in California41
| Disorders . | No. of newborns . | % . |
|---|---|---|
| Congenital hypothyroidism | 190 | 53.4 |
| Sickling disorders (Hb SS, SC, S/β-thalassemia) | 112 | 31.5 |
| Hb H disease | 36 | 10.1 |
| Phenylketonuria | 13 | 3.6 |
| Galactosemia | 5 | 1.4 |
| Disorders . | No. of newborns . | % . |
|---|---|---|
| Congenital hypothyroidism | 190 | 53.4 |
| Sickling disorders (Hb SS, SC, S/β-thalassemia) | 112 | 31.5 |
| Hb H disease | 36 | 10.1 |
| Phenylketonuria | 13 | 3.6 |
| Galactosemia | 5 | 1.4 |
The (--SEA) type of α0-thalassemia deletion is very common in Southeast Asia. An enzyme-linked immunosorbent assay (ELISA) to detect embryonic ζ-globin chains in peripheral blood erythrocytes was reported to be a simple, rapid, and reliable screening test to identify adult carriers of the (--SEA) α0-thalassemia deletion.122-125This test should help to identify couples at risk of conceiving fetuses with Hb H disease and also homozygous α0-thalassemia hydrops fetalis syndrome.
Treatment
The treatment for Hb H disease is primarily preventive and supportive in nature. With hemolysis and increased erythropoiesis, folic acid supplement is usually recommended. Avoidance of oxidative compounds and medications, prevention of unnecessary iron therapy unless iron deficiency is documented, prompt treatment of infections, and alertness to the possibility of either hypersplenism or aregenerative anemia are indicated. In adults, periodic monitoring for possible iron overload and related organ dysfunction is necessary.31 When indicated, iron chelation therapy may have to be instituted.
Most patients are asymptomatic despite their moderate anemia and often do not require blood transfusions. However, when anemia becomes severe, as in hemolytic crises secondary to infections, pyrexia, oxidative challenge, aregenerative anemia, hypersplenism, or during pregnancy, transfusion therapy may be indicated.
Some patients with unusually severe Hb H disease require regular transfusions and, therefore, will also need to have iron chelation therapy. Careful documentation of baseline hemoglobin levels of these patients, in the absence of other extraneous conditions such as infection, and thorough clinical assessment are critically important before committing these patients to a long-term transfusion program with its inherent and potentially harmful complications.3
Special attention for pregnant women with Hb H disease is required, as they might have anemia severe enough to adversely affect their health and that of the developing fetuses. There is a suggestion that the risk of fetal neural tube defect is increased in pregnant women who are either α- or β-thalassemia carriers, possibly because of relative folic acid deficiency secondary to increased erythropoiesis.126 For pregnant women with Hb H disease, it is especially prudent to prescribe folic acid supplement during the periconceptional period and beyond.
In those pregnant women found to have iron deficiency, iron supplement is indicated. However, it is very important to ensure that these women do not continue iron supplement therapy unnecessarily once the pregnancy is over, because as patients with Hb H disease, they are prone to develop iron overload with time.22,31 61-63
In patients with marked splenomegaly and hypersplenism, splenectomy can result in highly significant hematologic and clinical improvements.127 Postoperative complications include septicemia, deep venous thrombosis, and pulmonary embolism.128 129 Cholecystectomy may have to be done whenever surgically indicated.
Genetic counseling
When an individual near or at reproductive age is found to have Hb H disease, screening his or her partner and other family members for their α- and β-thalassemia carrier status is highly recommended. If the partner is a carrier of either a deletion or point mutation affecting one single α-globin gene, there is a 25% risk in each pregnancy of conceiving a fetus with Hb H disease (Table4).
Potential risks of fetuses with inherited α-thalassemia syndromes
| α-Globin genotypes . | Potential risks of fetuses, % . | ||
|---|---|---|---|
| One parent with Hb H disease . | α-Globin genotype of the other parent . | Hb H disease (--/-α) or (--/αThα) . | Homozygous α0-thalassemia (--/--) . |
| (--/-α) or (--/αThα) | (-α/αα) or (αThα/αα) | 25 | Nil |
| (-α/-α) or (αThα/-α) or (αThα/αThα) | 50 | Nil | |
| (--/αα) | 25 | 25 | |
| (--/-α) or (--/αThα) | 50 | 25 | |
| α-Globin genotypes . | Potential risks of fetuses, % . | ||
|---|---|---|---|
| One parent with Hb H disease . | α-Globin genotype of the other parent . | Hb H disease (--/-α) or (--/αThα) . | Homozygous α0-thalassemia (--/--) . |
| (--/-α) or (--/αThα) | (-α/αα) or (αThα/αα) | 25 | Nil |
| (-α/-α) or (αThα/-α) or (αThα/αThα) | 50 | Nil | |
| (--/αα) | 25 | 25 | |
| (--/-α) or (--/αThα) | 50 | 25 | |
Individuals who have single α-globin gene deletion or inactivation usually have normal or near normal hematologic findings, including MCV.33,130 It is necessary whenever indicated to carry out DNA-based genotypic analysis regardless of hematologic parameters.130 A recent case report is instructive in this regard.81 A woman of Asian ancestry was a carrier of the (--SEA) α0-thalassemia deletion. Her partner of Scottish-Irish ancestry had near normal hematologic findings (Hb, 140 g/L; MCV, 79 fL) and was a carrier of a novel mutation of the α2-globin gene, Hb Dartmouth (codon 66 CTG>CCG or Leu→Pro). Their twin sons inherited both parental mutations, were born with severe anemia (Hb, 50 and 57 g/L), and had transfusion-dependent Hb H disease.
If the partner is homozygous or compound heterozygous for single α-globin gene deletion or inactivation, there is a 50% risk in each pregnancy that the fetus might have Hb H disease.
If the partner is heterozygous for deletion involving both α-globin genes in cis on the same chromosome 16, there is a 25% risk that the fetus will have Hb H disease and another 25% risk that the fetus will have the devastating Hb Bart hydrops fetalis syndrome lacking all α-globin genes. It should be noted that some heterozygous carriers of β-thalassemia mutation with low MCV and elevated Hb A2 levels might also be heterozygous for α0-thalassemia deletion. Depending on the α- and β-globin genotypes of their partners, these individuals are potentially at risk of having offspring with Hb H disease, homozygous α0-thalassemia, and β-thalassemia major.5 131
If both members of the couple have Hb H disease, there is a 50% risk in each pregnancy that the fetus might have Hb H disease and another 25% risk that the fetus might have inherited the Hb Bart hydrops fetalis syndrome.
It is generally accepted that prenatal diagnosis is not warranted in pregnancies at risk of fetuses with Hb H disease alone. However, in cases at risk for possible Hb H hydrops fetalis syndrome, genetic counseling and prenatal diagnosis ought to be offered to the couple, according to accepted ethical principles and local cultural consideration.
Prenatal diagnosis is usually performed by analysis of fetal DNA obtained by chorionic villus sampling (CVS) at 9 to 12 weeks of gestation or amniocentesis at 16 to 18 weeks of gestation, respectively. The risk of miscarriage following either procedure is 0.5% to 1.0% for amniocentesis and 1% to 2% for CVS, respectively.132-134 There is no convincing evidence that the risk of limb reduction defects is increased after CVS.135 In general, DNA-based prenatal diagnostics are highly accurate and specific.
For those couples who are reluctant to undergo an invasive testing, ultrasound monitoring of hydropic changes may be offered as an alternative.136,137 In most cases, hydropic changes should be evident by the second trimester. Recent reports of using fetal DNA found in maternal plasma for prenatal diagnosis offers a novel noninvasive approach in the future.138-140
Population-wide perinatal screening and diagnosis of Hb H disease can be readily carried out, especially in jurisdictions where newborn screening for sickle cell diseases is already in place as in California.41 The early diagnosis will facilitate implementation of proper preventive health care measures to ensure the well-being of the affected infants, to ensure prompt treatment of potentially serious hemolytic crises and infections, to alleviate unnecessary parental distress, and also to help heighten awareness of other devastating α- and β-thalassemia syndromes in the communities.
Summary
Hb H disease is a form of α-thalassemia often manifested clinically as thalassemia intermedia with moderate anemia. It is commonly found in Southeast Asian, Middle Eastern, and Mediterranean populations. There is a wide spectrum of genotypes and phenotypic presentations. These range from those who appear clinically to be asymptomatic, to others who are more anemic, having significant hepatosplenomegaly and requiring occasional or even regular transfusions, to the severe Hb H hydrops fetalis syndrome that can cause death in the affected fetuses late in gestation.
This hereditary disorder is usually caused by deletions removing all but one single α-globin gene (deletional Hb H disease). A small proportion of patients have deletions removing 2 α-globin genes plus a nondeletional mutation affecting a third α-globin gene (nondeletional Hb H disease). In general, nondeletional Hb H disease has a more severe clinical course than the deletional form.
Review of recent literature suggests that Hb H disease is not as benign a disorder as previously thought. It can bring about growth retardation during childhood and iron overload in adults regardless of previous transfusion history, leading to hepatic, cardiac, and endocrine dysfunction. Significant anemia might occur during infections, fever, hypersplenism, or pregnancy that may necessitate the need for blood transfusions. An essential part of the maternal/child health care should include screening the partners of all pregnant women with Hb H disease for their thalassemia carrier status, and providing these and other couples who are at risk of conceiving offspring with Hb H disease with appropriate genetic counseling.
Prospective and systematic studies of the natural history of Hb H disease, particularly during infancy and childhood, as well as during pregnancy, have not been carried out and are much needed. Information derived from these investigations can lead to better insight to potential risk factors associated with severe disease and can help to formulate future medical interventions and treatment strategies.
We are indebted to our many clinical and laboratory colleagues for their collaboration and support throughout many years. We thank Prof Michael C. Brain143 and Dr Man-Chiu Poon, both of Calgary, and Dr Edmond S. K. Ma of Hong Kong for their critical reading of this manuscript and helpful suggestions. S. F. is a Senior Research Scholar of the Thailand Research Fund.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-07-1975.
References
Author notes
David H. K. Chui, Department of Medicine, Evans 211, Boston University School of Medicine, 88 East Newton St, Boston, MA 02118.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal