Abstract
CCAAT/enhancer binding proteins (C/EBPs) are a family of factors that regulate cell growth and differentiation. These factors, particularly C/EBPα and C/EBPε, have important roles in normal myelopoiesis. In addition, loss of C/EBP activity appears to have a role in the pathogenesis of myeloid disorders including acute myeloid leukemia (AML). Acute promyelocytic leukemia (APL) is a subtype of AML in which a role for C/EBPs has been postulated. In almost all cases of APL, a promyelocytic leukemia–retinoic acid receptor α (PML-RARα) fusion protein is expressed as a result of a t(15;17)(q22;q12) chromosomal translocation. PML-RARα inhibits expression of C/EBPε, whereas all-trans retinoic acid (tRA), a differentiating agent to which APL is particularly susceptible, induces C/EBPε expression. PML-RARα may also inhibit C/EBPα activity. Thus, the effects of PML-RARα on C/EBPs may contribute to both the development of leukemia and the unique sensitivity of APL to tRA. We tested the hypothesis that increasing the activity of C/EBPs would revert the leukemic phenotype. C/EBPα and C/EBPε were introduced into the FDC-P1 myeloid cell line and into leukemic cells from PML-RARA transgenic mice. C/EBP factors suppressed growth and induced partial differentiation in vitro. In vivo, enhanced expression of C/EBPs prolonged survival. By using a tamoxifen-responsive version of C/EBPε, we observed that C/EBPε could mimic the effect of tRA, driving neutrophilic differentiation in leukemic animals. Our results support the hypothesis that induction of C/EBP activity is a critical effect of tRA in APL. Furthermore, our findings suggest that targeted modulation of C/EBP activities could provide a new approach to therapy of AML.
Introduction
CCAAT/enhancer binding proteins (C/EBPs) are a family of proteins that play important roles in the development and differentiation of many cell types, including granulocytes. C/EBPα and C/EBPε play central roles in normal granulopoiesis. Mice that lack C/EBPα do not produce neutrophils and eosinophils,1 whereas mice that lack C/EBPε generate neutrophils and eosinophils that exhibit abnormalities in morphology, gene expression, and function.2-5
Given the part that C/EBPα and C/EBPε play in myelopoiesis, we anticipated that their function might be disrupted in human diseases, including myeloid neoplasms. Point mutations in C/EBPα have been identified in acute myeloid leukemias (AMLs)6,7; the AML with maturation-associated translocation fusion protein AML1-ETO inhibits C/EBPα expression8 and activity9; and the chronic myelogenous leukemia (CML) fusion protein BCR-ABL inhibits C/EBPα expression.10 Although C/EBPε mutations were not identified in patients with AML,11 such mutations have been observed in human patients with neutrophil-specific granule deficiency.12,13 We have suggested that altered function of C/EBPα and C/EBPε might also be important in the pathogenesis of acute promyelocytic leukemia (APL).14 15
APL represents approximately 10% of human AMLs.16 This leukemia is now defined by the presence of a t(15;17)(q22;q12) chromosomal translocation, creating a PML-RARA fusion gene (or rare variants that also result in fusions toRARA).17 In 1987, all-trans retinoic acid (tRA) was discovered to be able to induce remissions in APL18 by causing the leukemic cells to differentiate into mature neutrophils.19 Subsequently, the combination of tRA with chemotherapy has led to a marked increase in long-term survival for patients with APL; tRA treatment represents a paradigm for molecularly targeted therapy that restores normal behavior to malignant cells.20
The promyelocytic leukemia–retinoic acid receptor α (PML-RARα) fusion protein is believed to contribute to APL pathogenesis by disrupting the function of PML and by repressing transcription, including genes regulated by RARs.21 The ability of the PML protein to function in apoptosis and growth suppression is blocked by PML-RARα, which disrupts the nuclear bodies of which PML is a part. At physiologic levels of retinoids, PML-RARα represses transcription at retinoic acid response elements (RAREs) by enhancing association with nuclear corepressors. A recent study suggested that recruitment of DNA methyltransferases can also be a mechanism by which PML-RARα inhibits gene expression.22In addition to inhibiting PML and RARs, PML-RARα affects other proteins, including C/EBPs. PML-RARα blocks C/EBPα activity14 and can inhibit expression of C/EBPε.15 These observations raise the possibility that suppression of C/EBP activity has a role in the pathogenesis of APL.
Treatment of APL cells with pharmacologic doses of tRA results in the restoration of PML nuclear bodies and transcriptional activation of retinoic acid (RA)–responsive genes. Of these effects, transcriptional activation appears central because differentiation takes place even when PML nuclear bodies are not reassembled.23 C/EBPs might be important targets of tRA. Consistent with this idea, C/EBPs can arrest cell growth and induce differentiation of a variety of cell types, including myeloid cell lines.8,24-28 Furthermore, PML-RARα actually enhances C/EBPε induction in response to pharmacologic levels of tRA,15 tRA reverses the ability of PML-RARα to inhibit C/EBPα,14 and tRA induces expression of another C/EBP family member, C/EBPβ.29Together, these observations indicate that induction of C/EBP activity may underlie the unique responsiveness of APL to tRA therapy.
We assessed whether either C/EBPα or C/EBPε could reverse the leukemic phenotype of a murine model of APL. In vitro, C/EBPs caused partial differentiation of the leukemic cells. In vivo, C/EBPs repressed the leukemias regardless of whether these leukemias were responsive or resistant to tRA. Use of a tamoxifen-responsive version of C/EBPε demonstrated that C/EBPε can, like tRA, induce differentiation of leukemic cells in an animal. These results are consistent with the idea that activation of C/EBPs is central to the tRA response and demonstrate that C/EBPs themselves exhibit antileukemic activity in vivo. Thus, developing therapeutic means to enhance C/EBP activity should permit differentiation therapy to be extended to non-APL subtypes of myeloid leukemia.
Materials and methods
Plasmids
A human C/EBPα cDNA6 and a human cDNA encoding 32-kDa C/EBPε15 were inserted into the mouse stem cell virus–internal ribosomal entry site–green fluorescent protein MSCV-IRES-GFP; [MIG]) retroviral vector30-32with standard molecular cloning techniques. MIG containing rat C/EBPα with the wild-type upstream open reading frame (rC/EBPα-WT) and rat C/EBPα with deletion of the upstream open reading frame and spacer (rC/EBPα-ΔuORF) have been described previously.10 A tamoxifen-responsive C/EBPε was created by fusing a human C/EBPε cDNA (gift of K. G. Xanthopoulos, designated hCEBPε-ERTM) to the hormone-binding domain of a tamoxifen-responsive mouse estrogen receptor33; this construct, designated hCEBPε-ERTM, was also inserted into MIG.
Cell culture
FDC-P1 cells34 were grown in Dulbecco modified Eagle medium with 10% fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mM l-glutamine, and 5% X63Ag8–mouse interleukin-3 (mIL-3)–conditioned media.35 Freshly harvested cells from bone marrow and spleens of leukemic mice were cultured in Myelocult M5300 (StemCell Technologies, Vancouver, BC, Canada) with 100 U/mL penicillin G, 100 μg/mL streptomycin, 5% X63Ag8–mIL-3–conditioned media, and 5% Sp2–IL-6–conditioned media.36
Retroviral transduction
BOSC23 cells were transfected with retroviral constructs as previously described.37 Retroviral supernatants were filtered through 0.45-μm filters and stored at −80°C. FDC-P1 cells were plated at 50 000 cells per well in 24-well tissue culture plates. On each of 2 consecutive days, the cells were transduced by incubation with 2 mL viral-containing supernatant with 4 μg/mL polybrene and centrifugation at 1100 g for 1.5 hours at room temperature. Transduction of leukemic cells was similarly performed, except that leukemic cells were plated at 2 × 106 cells per well.
Western blot analysis
Whole-cell lysates were prepared by lysing 2 × 107 cells in 600 μL 2 × sample buffer, heating at 90°C to 95°C for 5 minutes, and shearing through a 20-gauge needle. Western blot analysis was performed as previously described38 with rabbit polyclonal antibodies to C/EBPα or C/EBPε (Santa Cruz Biotechnology, Santa Cruz, CA).
Analysis of growth of FDC-P1 cells
After 2 rounds of infection, transduced cells were pooled and washed once with buffered saline, and GFP+ cells were sorted using the FACSVantage cell sorter (Becton Dickinson, San Jose, CA). Culture of sorted cells started at 30 000 cells. At 48-hour intervals, the medium was changed, live cells were counted with trypan blue exclusion, and the percentage of GFP+ cells was assessed by flow cytometry.
Analysis of differentiation
For flow immunophenotyping, anti–Gr-1–phycoerythrin and anti–Mac-1–phycoerythrin (BD Pharmingen, San Diego, CA) were used. Cells were resuspended in 100 μL buffered saline and incubated with indicated antibodies for 20 to 30 minutes on ice in the dark, then washed and resuspended in buffered saline. Stained cells were analyzed on a FACScan, and at least 10 000 events were collected for each sample. Fluorescence-activated cell sorter (FACS) data were analyzed with CELLQUEST (Becton Dickinson). Differential cell counts of Wright Giemsa–stained bone marrow smears were performed according to published guidelines.39 The guidelines for the differential counts were modified for FDC-P1 cells as described in the legend to Table 1. The guidelines were also modified for leukemic cells cultured in vitro because an atypical cell type was present; myeloid cells with atypical nuclear segmentation and basophilic cytoplasm were enumerated separately from the intermediate forms and neutrophils of normal appearance.
Differential counts of FDC-P1 cells transduced with control, hC/EBPα, or hC/EBPɛ retroviral constructs
| . | Immature/blasts . | Intermediate . | Segmented . |
|---|---|---|---|
| Control | 96.0 ± 1.2 | 4.0 ± 1.2 | 0.1 ± 0.2 |
| hC/EBPα | 63.8 ± 1.3 | 24.9 ± 0.8 | 11.3 ± 2.1 |
| hC/EBPɛ | 87.0 ± 4.4 | 12.8 ± 4.0 | 0.3 ± 0.6 |
| . | Immature/blasts . | Intermediate . | Segmented . |
|---|---|---|---|
| Control | 96.0 ± 1.2 | 4.0 ± 1.2 | 0.1 ± 0.2 |
| hC/EBPα | 63.8 ± 1.3 | 24.9 ± 0.8 | 11.3 ± 2.1 |
| hC/EBPɛ | 87.0 ± 4.4 | 12.8 ± 4.0 | 0.3 ± 0.6 |
At day 4 (6 days after the first of 2 rounds of transduction), GFP+ cells were sorted and cytospins were prepared and stained with Wright Giemsa. A total of 300 cells were counted for each cytospin. Results shown are means ± SDs of an experiment performed in triplicate. Differential counts of hC/EBPα samples are significantly different from control (immature/blasts, P = .001; intermediate, P = .001; segmented,P = .01). Differential counts of hC/EBPɛ samples trend toward significance compared with control (immature/blasts,P = .06; intermediate, P = .06; segmented,P = not significant).
Immature/blasts indicates immature cells with high nuclear to cytoplasmic ratios and round nuclei; intermediate, forms including ring forms, bands, and binucleated or trinucleated cells; segmented, cells with nuclear segmentation.
Mice
Mice were bred and maintained at the University of California at San Francisco, and their care was in accordance with University of California at San Francisco guidelines. Leukemias from MRP8 PML-RARA40 and MRP8 PML-RARAm441 transgenic mice were maintained by serial transplantation. Female FVB/N mice, 6 to 8 weeks old, received sublethal irradiation (4.5 Gy) and intravenous injection into the lateral tail vein with either leukemic cells (1 × 106cells per animal for serial passaging) or sorted GFP+- transduced leukemic cells (25 000 or 50 000 cells per animal).
Tamoxifen treatment
As previously described,42 4-hydroxytamoxifen (4-HT; Sigma, St Louis, MO) was dissolved in ethanol at 100 mg/mL, then diluted in autoclaved sunflower seed oil (Sigma) at 10 mg/mL, sonicated for approximately 20 minutes, and stored at −20°C. One milligram 4-HT per mouse was injected intraperitoneally on consecutive days.
Statistical analysis
Statistical analyses were performed with Excel 2000 using the Student t test, one-tailed distribution, and unequal variance.
Results
Expression of C/EBPα or C/EBPε represses growth and induces partial differentiation of FDC-P1 cells
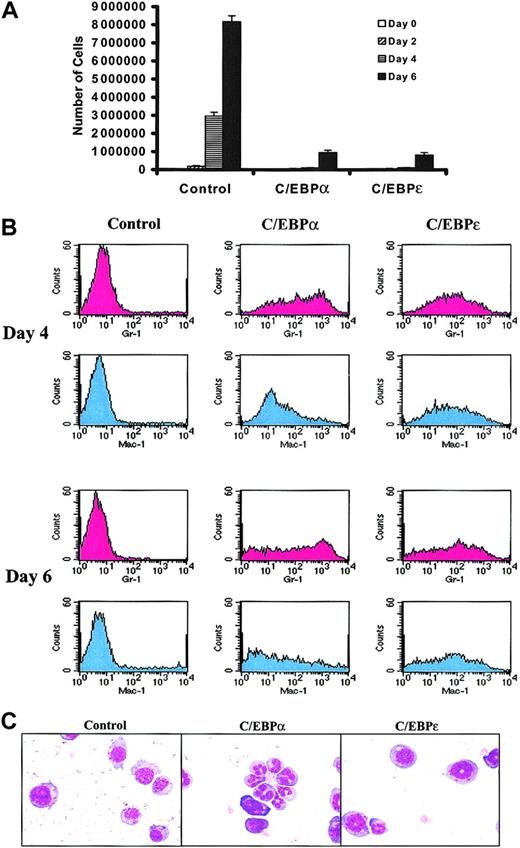
CCAAT/enhancer binding proteins, including C/EBPα and C/EBPε, have important roles in myeloid differentiation. We wished to assess whether increasing the activity of either C/EBPα or C/EBPε could suppress the leukemic phenotype of murine AML cells. We initially examined whether the C/EBPs would suppress growth and/or induce differentiation of the FDC-P1 cell line, a nonleukemic factor–dependent line of immature myeloid cells. FDC-P1 cells were transduced with retroviruses designed to express only GFP (the MIG control virus), hC/EBPα, or hC/EBPε (Figure1A). Immunoblots of FDC-P1 whole-cell lysates indicated that these retroviruses drove expression of the C/EBP proteins (Figure 1B). Compared with control, overexpression of C/EBPs in FDC-P1 cells repressed proliferation (Figure2A). In addition, C/EBPα and C/EBPε increased expression of both Ly-6G (Gr-1) and CD11b (Mac-1), which are surface markers of myeloid differentiation (Figure 2B). Although C/EBPα and C/EBPε both induced FDC-P1 cells to partially differentiate, C/EBPα-transduced cells showed greater morphologic change, including the appearance of cells with nuclear segmentation (Figure 2C; Table 1). The growth suppression and partial differentiation observed in transduced FDC-P1 cells showed that C/EBPα and C/EBPε proteins were expressed and functional.
C/EBP retroviral constructs.
(A) Expression vectors containing a bicistronic system expressing the gene of interest and GFP under the control of the MSCV promoter. MSCV-IRES-GFP (MIG) is an expression vector with a multiple cloning site (MCS), at which either a human C/EBPα (42 kDa) or human C/EBPε (32 kDa) cDNA was inserted. The human C/EBPα includes an open reading frame (uORF) upstream to the start codon for C/EBPα. rC/EBPα-WT is similar to the hC/EBPα construct, but contains rat C/EBPα. rC/EBPα-ΔuORF is rat C/EBPα without the upstream open reading frame (uORF). The hC/EBPε-ERTM retroviral construct contains human C/EBPε fused at the C-terminus to a tamoxifen-responsive mouse estrogen receptor hormone-binding domain. (B) Western blots of whole-cell lysates of transduced FDC-P1 cells. Lysates were prepared at 24 hours after the second round of transduction. (i) FDC-P1 cells were transduced with retroviruses expressing MIG (control), hC/EBPα, rC/EBPα-WT, and rC/EBPα-ΔuORF. Lysates were blotted with rabbit polyclonal antibodies raised to an internal region of rat C/EBPα. Arrow indicates the position of C/EBPα. (ii) Lysates of MIG-, hC/EBPε-, and hC/EBPε-ERTM–transduced FDC-P1 cells were blotted with rabbit polyclonal antibodies raised to the carboxy terminus of rat C/EBPε. Arrows indicate the positions of hC/EBPε (32 kDa) and hC/EBPε-ERTM (70 kDa). The identities of the cross-reacting bands in the hC/EBPε lane are uncertain. The bands in the hC/EBPε-ERTM lane below the full-length hC/EBPε-ERTM may represent smaller hC/EBPε-ER translation products initiated from downstream AUG codons.43
C/EBP retroviral constructs.
(A) Expression vectors containing a bicistronic system expressing the gene of interest and GFP under the control of the MSCV promoter. MSCV-IRES-GFP (MIG) is an expression vector with a multiple cloning site (MCS), at which either a human C/EBPα (42 kDa) or human C/EBPε (32 kDa) cDNA was inserted. The human C/EBPα includes an open reading frame (uORF) upstream to the start codon for C/EBPα. rC/EBPα-WT is similar to the hC/EBPα construct, but contains rat C/EBPα. rC/EBPα-ΔuORF is rat C/EBPα without the upstream open reading frame (uORF). The hC/EBPε-ERTM retroviral construct contains human C/EBPε fused at the C-terminus to a tamoxifen-responsive mouse estrogen receptor hormone-binding domain. (B) Western blots of whole-cell lysates of transduced FDC-P1 cells. Lysates were prepared at 24 hours after the second round of transduction. (i) FDC-P1 cells were transduced with retroviruses expressing MIG (control), hC/EBPα, rC/EBPα-WT, and rC/EBPα-ΔuORF. Lysates were blotted with rabbit polyclonal antibodies raised to an internal region of rat C/EBPα. Arrow indicates the position of C/EBPα. (ii) Lysates of MIG-, hC/EBPε-, and hC/EBPε-ERTM–transduced FDC-P1 cells were blotted with rabbit polyclonal antibodies raised to the carboxy terminus of rat C/EBPε. Arrows indicate the positions of hC/EBPε (32 kDa) and hC/EBPε-ERTM (70 kDa). The identities of the cross-reacting bands in the hC/EBPε lane are uncertain. The bands in the hC/EBPε-ERTM lane below the full-length hC/EBPε-ERTM may represent smaller hC/EBPε-ER translation products initiated from downstream AUG codons.43
C/EBPs suppress growth and induce partial differentiation of FDC-P1 cells.
FDC-P1 cells grown continuously in IL-3 were transduced on 2 sequential days with control, hC/EBPα, or hC/EBPε retroviruses. For these experiments, day 0 is considered to begin 24 hours after the second round of retroviral transduction. (A) The growth of C/EBPα- and C/EBPε-infected FDC-P1 cells was suppressed. Equal numbers of cells were plated on day 0. The growth curves represent the number of transduced FDC-P1 cells at days 0, 2, 4, and 6. At each time point, cells were counted and the percentage of cells with GFP expression was assessed. The number of GFP+ cells is shown on this graph. Results shown are means ± SDs from 3 independent experiments. (B) Both C/EBPα and C/EBPε induced expression of myeloid markers by FDC-P1 cells. Transduced FDC-P1 cells were stained for Gr-1 (Ly-6G) and Mac-1 (CD11b). Results shown in histograms are of live (based on forward scatter versus side scatter) GFP+ cell populations. These data are representative of 2 independent experiments. (C) C/EBP-transduced FDC-P1 cells were partially differentiated toward more mature forms. GFP+FDC-P1 cells were sorted at day 4 (6 days after the first round of transduction) with the FACSVantage cell sorter. Cytospins of 25 000 sorted cells of each transduction were stained with Wright Giemsa. Images were captured with × 40 objectives.
C/EBPs suppress growth and induce partial differentiation of FDC-P1 cells.
FDC-P1 cells grown continuously in IL-3 were transduced on 2 sequential days with control, hC/EBPα, or hC/EBPε retroviruses. For these experiments, day 0 is considered to begin 24 hours after the second round of retroviral transduction. (A) The growth of C/EBPα- and C/EBPε-infected FDC-P1 cells was suppressed. Equal numbers of cells were plated on day 0. The growth curves represent the number of transduced FDC-P1 cells at days 0, 2, 4, and 6. At each time point, cells were counted and the percentage of cells with GFP expression was assessed. The number of GFP+ cells is shown on this graph. Results shown are means ± SDs from 3 independent experiments. (B) Both C/EBPα and C/EBPε induced expression of myeloid markers by FDC-P1 cells. Transduced FDC-P1 cells were stained for Gr-1 (Ly-6G) and Mac-1 (CD11b). Results shown in histograms are of live (based on forward scatter versus side scatter) GFP+ cell populations. These data are representative of 2 independent experiments. (C) C/EBP-transduced FDC-P1 cells were partially differentiated toward more mature forms. GFP+FDC-P1 cells were sorted at day 4 (6 days after the first round of transduction) with the FACSVantage cell sorter. Cytospins of 25 000 sorted cells of each transduction were stained with Wright Giemsa. Images were captured with × 40 objectives.
Expression of C/EBPα or C/EBPε induces differentiation of RA-sensitive and RA-resistant murine leukemias
We next examined the ability of C/EBPα and C/EBPε to induce differentiation of leukemias derived from transgenic mice that expressed either PML-RARα (RA-sensitive leukemia no. 1111) or PML-RARαm4 (RA-resistant leukemia no. 4048.2). Freshly harvested leukemic cells were transduced with control, hC/EBPα-, or hC/EBPε-containing retroviruses. In vitro, the morphology of transduced leukemia cells was assessed by differential counts of Wright Giemsa–stained cytospins. Overexpression of C/EBP proteins induced partial morphologic differentiation of both RA-sensitive and RA-resistant leukemic cells (Figure 3). Certain aspects of these results should be noted. First, in these experiments, very few mature neutrophils were induced by the C/EBPs. Instead, the C/EBPs led to the appearance in culture of partially differentiated cells, including many with nuclear segmentation. Second, a greater number of differentiated cells was seen with the PML-RARαm4 leukemia than with the PML-RARα leukemia, including the control transduced cells. This finding is consistent with our previous observation that leukemias induced by an MRP8 PML-RARAm4transgene showed somewhat more differentiation in vivo than leukemias induced by MRP8 PML-RARA.41
C/EBPs induce partial morphologic differentiation of murine APL cells.
Freshly harvested bone marrow and spleen cells from leukemic mice were transduced on 2 consecutive days with the retroviral constructs. GFP+ cells were sorted 5 days after the first transduction. Cytospins of sorted cells were stained with Wright Giemsa, and differential counts were performed (100-300 cells). (A) RA-responsive leukemia no. 1111. Results of 3 independent experiments expressed as means ± SDs are shown. Compared with MIG-infected control, C/EBPα-infected cells showed significantly fewer immature forms or blasts (P = .03) and significantly more segmented forms (P = .01). C/EBPε-infected cells showed significantly more intermediate-stage cells without segmentation (P = .04), with the changes in immature forms or blasts and segmented forms nearing statistical significance (P = .06). (B) RA-resistant leukemia no. 4048.2. Results of 2 independent experiments expressed as means ± SDs are shown. Decreases in immature forms or blasts and increases in intermediate forms and segmented forms are apparent. Because some variation occurred between the 2 experiments, only the increase in segmented forms induced by C/EBPε reached statistical significance (P = .05). Myeloid cells with atypical nuclear segmentation and basophilic cytoplasm were enumerated separately from intermediate forms and from neutrophils of normal appearance.
C/EBPs induce partial morphologic differentiation of murine APL cells.
Freshly harvested bone marrow and spleen cells from leukemic mice were transduced on 2 consecutive days with the retroviral constructs. GFP+ cells were sorted 5 days after the first transduction. Cytospins of sorted cells were stained with Wright Giemsa, and differential counts were performed (100-300 cells). (A) RA-responsive leukemia no. 1111. Results of 3 independent experiments expressed as means ± SDs are shown. Compared with MIG-infected control, C/EBPα-infected cells showed significantly fewer immature forms or blasts (P = .03) and significantly more segmented forms (P = .01). C/EBPε-infected cells showed significantly more intermediate-stage cells without segmentation (P = .04), with the changes in immature forms or blasts and segmented forms nearing statistical significance (P = .06). (B) RA-resistant leukemia no. 4048.2. Results of 2 independent experiments expressed as means ± SDs are shown. Decreases in immature forms or blasts and increases in intermediate forms and segmented forms are apparent. Because some variation occurred between the 2 experiments, only the increase in segmented forms induced by C/EBPε reached statistical significance (P = .05). Myeloid cells with atypical nuclear segmentation and basophilic cytoplasm were enumerated separately from intermediate forms and from neutrophils of normal appearance.
C/EBPs suppress the leukemic phenotype of PML-RARα and PML-RARαm4 leukemias
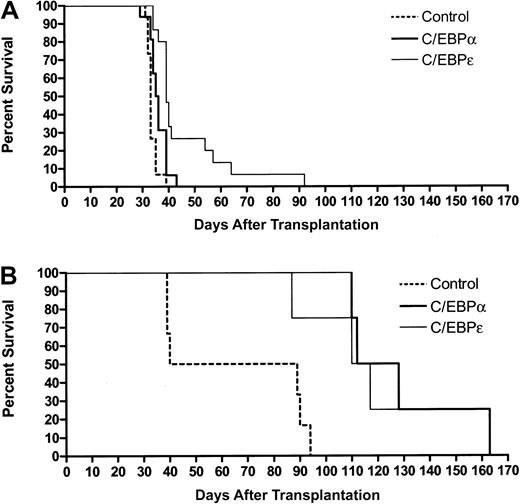
On the basis of the results with FDC-P1 cells and mouse leukemic cells in vitro, we proceeded to use retroviral transduction of 3 independent leukemias to test the ability of C/EBPα and C/EBPε to limit leukemic cell growth in vivo. In addition to the RA-sensitive and RA-resistant leukemias described in the previous paragraph, a second RA-responsive PML-RARα leukemia was studied, no. 935. Leukemic cells were harvested and then transduced on 2 consecutive days with MIG, hC/EBPα, or h/CEBPε retroviruses. GFP+ cells were sorted, and 25 000 or 50 000 cells were injected intravenously into sublethally irradiated histocompatible recipient animals. Recipients of PML-RARα leukemias transduced with C/EBP survived longer than recipients of leukemias transduced with the MIG control (Figure4A). C/EBPα modestly prolonged survival (mean increase in survival of 3 days, median 2.5 days, maximum 10 days;P = .01), whereas C/EBPε had a more substantial impact on survival (mean increase in survival of 13 days, median 6 days, maximum 59 days; P = .004). Results in Figure 4A are pooled data from 3 independent experiments. The results of the individual experiments are shown in a supplemental figure on theBlood website; see the Supplemental Figure link at the top of the online article. Of note, RA treatment of RA-sensitive leukemias prolonged survival more than did transduction by C/EBPs (mean increase in survival of 40 days [median 42 days] with a 5-mg 21-day tRA pellet; mean increase in survival of 74 days [median 41 days] with a 10-mg 21-day tRA pellet).41 44 The impact of C/EBP transduction was, however, likely to be attenuated by the presence of untransduced cells, loss of retroviral expression, and/or down-regulation of protein expression (see below). Despite these limitations, 2 recipients of cells transduced with C/EBPε had their survival prolonged to a degree comparable to that of RA treatment.
C/EBPs suppress growth of murine myeloid leukemia in vivo.
Bone marrow and spleen cells of leukemic mice were harvested and transduced with retroviruses expressing MIG (control), hC/EBPα, or hC/EBPε. At 24 hours after the second round of infection, GFP+ cells were sorted and injected intravenously into sublethally irradiated (4.5 Gy) healthy FVB/N females. (A) PML-RARα leukemias no. 935 and no. 1111, with 25 000 to 50 000 cells per animal. Survival curves include combined data from 3 independent experiments. See data from individual experiments by clicking on the Supplemental Data Set link at the top of the online article on the Blood website. Control mice, n = 15; C/EBPα mice, n = 16; C/EBPε mice, n = 15. (B) PML-RARαm4 leukemia no. 4048.2, with 25 000 cells per animal. Control mice, n = 6; C/EBPα mice, n = 4; C/EBPε mice, n = 4.
C/EBPs suppress growth of murine myeloid leukemia in vivo.
Bone marrow and spleen cells of leukemic mice were harvested and transduced with retroviruses expressing MIG (control), hC/EBPα, or hC/EBPε. At 24 hours after the second round of infection, GFP+ cells were sorted and injected intravenously into sublethally irradiated (4.5 Gy) healthy FVB/N females. (A) PML-RARα leukemias no. 935 and no. 1111, with 25 000 to 50 000 cells per animal. Survival curves include combined data from 3 independent experiments. See data from individual experiments by clicking on the Supplemental Data Set link at the top of the online article on the Blood website. Control mice, n = 15; C/EBPα mice, n = 16; C/EBPε mice, n = 15. (B) PML-RARαm4 leukemia no. 4048.2, with 25 000 cells per animal. Control mice, n = 6; C/EBPα mice, n = 4; C/EBPε mice, n = 4.
Consistent with previous observations, recipients of a PML-RARαm4 leukemia survived longer than recipients of PML-RARα leukemias, and both C/EBPα and C/EBPε substantially lengthened the lives of PML-RARαm4 recipient mice (Figure 4B). For C/EBPα, mean increase in survival was 63 days, median was 56 days, and maximum was 98 days (P = .003); for C/EBPε, mean increase in survival was 54 days, median was 49 days, and maximum was 98 days (P = .002). These results in RA-sensitive and RA-resistant leukemias show that expression of retroviruses containingCEBPA or CEBPE cDNAs can suppress the leukemic phenotype of AML.
When possible, leukemic cells were harvested from moribund recipients, and the cells were assessed for the expression of GFP (Table2A). There appeared to be selection against cells that expressed the MSCV-hC/EBPε-IRES-GFP retrovirus: In the 2 experiments in which 50 000 cells were used, GFP expression was essentially absent in leukemic cells analyzed (suggesting that untransduced cells present in the sorted population may have given rise to the leukemias). In the 2 experiments in which 25 000 cells were used, GFP expression from the C/EBPε retrovirus was seen in some animals; persistent expression of GFP was observed in mice that survived longest. Compared with MIG-transduced leukemias, no selection against persistent expression of the MSCV-hC/EBPα-IRES-GFP retrovirus was apparent.
Expression of GFP in recipients of transduced leukemias
| . | Control . | hC/EBPα . | hC/EBPɛ* . | rC/EBPα-WT† . | rC/EBPα-ΔuORF† . |
|---|---|---|---|---|---|
| PML-RARα leukemia | 67 ± 16 | 60 ± 8 | 4 ± 2 | NA | NA |
| no. 1111, 50 000 cells | (2 of 5) | (4 of 5) | (2 of 4) | ||
| PML-RARα leukemia | 88 ± 4 | 72 ± 9 | ‡0.2, 70, 80 | NA | NA |
| no. 1111, 25 000 cells | (3 of 4) | (4 of 5) | (3 of 5) | ||
| PML-RARα leukemia | 56 ± 12 | 59 ± 5 | 0.6 ± 0.3 | NA | NA |
| no. 935, 50 000 cells | (5 of 6) | (5 of 6) | (3 of 6) | ||
| PML-RARαm4 leukemia | 87 ± 7 | 92 ± 1 | ‡0.3, 32, 93 | NA | NA |
| no. 4048.2, 25 000 cells | (5 of 6) | (2 of 4) | (3 of 4) | ||
| PML-RARα leukemia | 80 ± 17 | NA | NA | 53 ± 25 | 17 ± 17 |
| no. 935, 25 000 cells | (4 of 4) | (6 of 6) | (6 of 6) |
| . | Control . | hC/EBPα . | hC/EBPɛ* . | rC/EBPα-WT† . | rC/EBPα-ΔuORF† . |
|---|---|---|---|---|---|
| PML-RARα leukemia | 67 ± 16 | 60 ± 8 | 4 ± 2 | NA | NA |
| no. 1111, 50 000 cells | (2 of 5) | (4 of 5) | (2 of 4) | ||
| PML-RARα leukemia | 88 ± 4 | 72 ± 9 | ‡0.2, 70, 80 | NA | NA |
| no. 1111, 25 000 cells | (3 of 4) | (4 of 5) | (3 of 5) | ||
| PML-RARα leukemia | 56 ± 12 | 59 ± 5 | 0.6 ± 0.3 | NA | NA |
| no. 935, 50 000 cells | (5 of 6) | (5 of 6) | (3 of 6) | ||
| PML-RARαm4 leukemia | 87 ± 7 | 92 ± 1 | ‡0.3, 32, 93 | NA | NA |
| no. 4048.2, 25 000 cells | (5 of 6) | (2 of 4) | (3 of 4) | ||
| PML-RARα leukemia | 80 ± 17 | NA | NA | 53 ± 25 | 17 ± 17 |
| no. 935, 25 000 cells | (4 of 4) | (6 of 6) | (6 of 6) |
Recipients of transduced leukemic cells were killed when visibly ill. Percentages of cells expressing GFP are shown. For most groups, values are means ± SDs. Values in parentheses indicate the number of mice in which GFP expression was assessed compared with total mice in the group.
NA indicates not applicable.
Some leukemias in hC/EBPɛ recipients do not express GFP.
The C/EBPα uORF tolerizes leukemic cells to the presence of C/EBPα-IRES-GFP retroviral transcript (rC/EBPα-WT versus rC/EBPα-ΔuORF; P = .01).
Values for individual mice are shown because of marked variations among mice.
PML-RARα leukemia tolerates expression of a C/EBPα retrovirus only when an upstream open reading frame is present
Although GFP was expressed by a high percentage of leukemic cells in most C/EBPα recipients and in some C/EBPε recipients, it was important to assess whether C/EBP proteins were in fact present in the leukemic cells. Neither C/EBPα nor C/EBPε proteins were detected by Western blot analysis (data not shown). Because the C/EBPs and GFP are encoded by a bi-cistronic transcript, the lack of C/EBP protein expression might be due to decreased translation. In fact, initiation of C/EBPα translation can be inhibited by a short open reading frame and spacer (uORF) upstream of the initiation codon for C/EBPα. We hypothesized that deletion of the uORF might create additional selective pressure against the C/EBPα retrovirus. Constructs expressing rat C/EBPα either with or without the uORF (rC/EBPα-WT and rC/EBPα-ΔuORF; Figure 1A) were used to test this idea. Both constructs were able to produce C/EBPα protein in FDC-P1 cells (Figure 1B). PML-RARα leukemic cells (no. 935) were transduced with control, rC/EBPα-WT, and rC/EBPα-ΔuORF retroviruses, and 25 000 GFP+ cells were injected into sublethally irradiated recipients. Although deletion of the uORF did not significantly influence survival (control, n = 4, mean survival 37 days; rC/EBPα-WT, n = 6, mean survival 40 days; rC/EBPα-ΔuORF, n = 6, mean survival 38 days), deletion of the uORF led to markedly diminished GFP expression in the leukemias that arose (Table2).
A tamoxifen-inducible version of C/EBPε mimics retinoic acid in a mouse model of APL
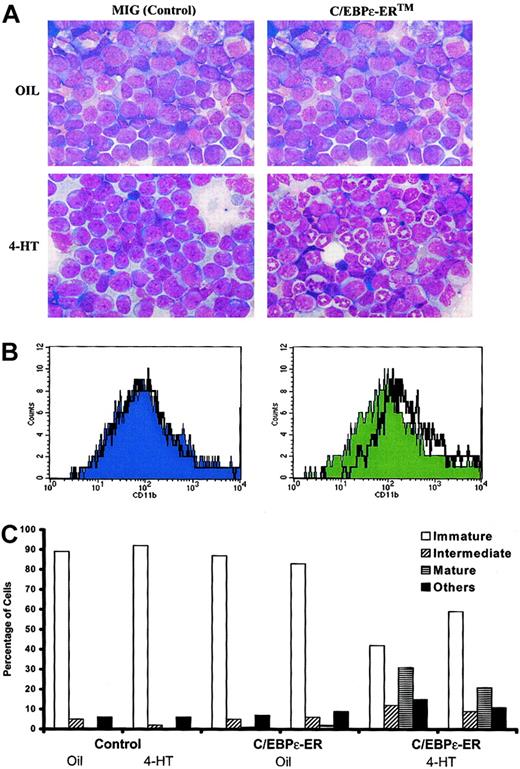
Induction of C/EBPε has been proposed to be a central mechanism by which tRA causes the differentiation of APL cells. To address whether C/EBP can induce neutrophilic maturation of leukemic cells in vivo, we used a tamoxifen-inducible form of C/EBPε, hC/EBPε-ERTM. PML-RARα leukemic cells (no. 1111) were transduced with control and C/EBPε-ERTM retroviruses, then transplanted into sublethally irradiated histocompatible mice. Leukemias were allowed to develop in recipients of either control or C/EBPε-ERTM–transduced leukemic cells. Leukemic mice were then treated with either vehicle or 4-HT. In contrast to findings in the other mice, bone marrow examination revealed that 4-HT caused neutrophilic differentiation of the leukemic cells that had been transduced with C/EBPε-ER. Morphologically, only the 4-HT–treated C/EBPε-ERTM samples showed numerous mature neutrophils (Figure 5A). In addition, the cells exhibited increased CD11b (Mac-1) expression (Figure 5B). Differential counts of bone marrow smears are presented in Figure 5C. 4-HT induced substantial neutrophilic differentiation and allowed nonmyeloid hematopoietic cells (erythroid cells and lymphocytes) to repopulate the bone marrow. These findings parallel the effects of RA on this mouse model of human APL.40
Effect of C/EBPε-ER on differentiation of myeloid leukemia in vivo.
PML-RARα leukemia no. 1111 was transduced with retroviruses expressing only GFP (MIG) or hC/EBPε-ERTM, and 25 000 sorted GFP+ cells were injected intravenously into sublethally irradiated FVB/N mice. The leukemic cells were allowed to engraft and expand. Beginning on day 27, 4-HT or vehicle was administered daily by intraperitoneal injection. The MIG recipient animals became moribund and were killed on day 30. The hC/EBPε-ERTM recipient animals were killed on day 31. (A) Bone marrow smears of recipients of MIG- and hC/EBPε-ERTM–transduced cells either with or without 4-HT treatment. Wright Giemsa stain was used, and images were captured with × 40 objectives. (B) Surface-marker expression of CD11b (Mac-1) in MIG or C/EBPε-ERTM recipients. Histograms were gated on live (based on FSC and SSC) GFP+ cells. The solid graph represents vehicle treatment, and the black line represents 4-HT treatment. (C) Differential counts (200 cells) of bone marrow smears of individual recipients of control or C/EBPε-ERTM–transduced cells, treated as indicated. Immature indicates immature forms or blasts; intermediate, intermediate forms; mature, mature neutrophilic cells; other, erythroid cells, lymphocytes, and eosinophils.
Effect of C/EBPε-ER on differentiation of myeloid leukemia in vivo.
PML-RARα leukemia no. 1111 was transduced with retroviruses expressing only GFP (MIG) or hC/EBPε-ERTM, and 25 000 sorted GFP+ cells were injected intravenously into sublethally irradiated FVB/N mice. The leukemic cells were allowed to engraft and expand. Beginning on day 27, 4-HT or vehicle was administered daily by intraperitoneal injection. The MIG recipient animals became moribund and were killed on day 30. The hC/EBPε-ERTM recipient animals were killed on day 31. (A) Bone marrow smears of recipients of MIG- and hC/EBPε-ERTM–transduced cells either with or without 4-HT treatment. Wright Giemsa stain was used, and images were captured with × 40 objectives. (B) Surface-marker expression of CD11b (Mac-1) in MIG or C/EBPε-ERTM recipients. Histograms were gated on live (based on FSC and SSC) GFP+ cells. The solid graph represents vehicle treatment, and the black line represents 4-HT treatment. (C) Differential counts (200 cells) of bone marrow smears of individual recipients of control or C/EBPε-ERTM–transduced cells, treated as indicated. Immature indicates immature forms or blasts; intermediate, intermediate forms; mature, mature neutrophilic cells; other, erythroid cells, lymphocytes, and eosinophils.
Discussion
Differentiation therapy of APL with tRA has transformed this leukemia into a highly curable illness. This remarkable impact of differentiation therapy on one subtype of AML has created hope that molecularly-targeted therapies can be developed for other types of AML, including those with complex karyotypic abnormalities and poor prognosis. C/EBPs are central mediators of neutrophil maturation. Our results show that C/EBPs induce differentiation of retinoic acid responsive and resistant leukemias. Moreover, in an animal model of AML, C/EBPs suppressed the leukemic phenotype. Thus, developing therapeutics that stimulate C/EBP expression and activity should extend the range of leukemias that can be treated with differentiation therapy.
In U937 and 32Dcl3 cell lines, overexpression of either C/EBPα or C/EBPε can block cell growth and induce differentiation.8 25-28 Similarly, we observed that C/EBPα and C/EBPε impaired growth and induced differentiation of FDC-P1 cells and mouse leukemias. However, we saw differences between our hC/EBPα and hC/EBPε constructs. hC/EBPα caused a greater degree of morphologic differentiation of FDC-P1 cells than did hC/EBPε. In contrast, the hC/EBPε vector seemed to have a greater impact on overall survival than did C/EBPα. Therefore, expression ofCEBPA and CEBPE can cause different effects. These dissimilarities could reflect intrinsic differences in the biologic activities of C/EBPα and C/EBPε or instead could be a result of differences in expression from the retroviral vector, posttranscriptional regulation of translation, or posttranslational modification.
Perrotti et al10 have shown that BCR-ABL inhibits C/EBPα expression by inducing hnRNP E2 to bind the uORF/spacer region of C/EBPα transcripts in 32Dcl3 BCR-ABL cells and in cells from human patients with chronic myelogenous leukemia in blast phase. Our observation that the presence of the uORF/spacer tolerizes a PML-RARα leukemia to the presence of C/EBPα mRNA extends the cell types in which this mechanism of posttranscriptional regulation has been seen. Posttranscriptional regulation of C/EBPα might be important in both normal and leukemic hematopoiesis. As a consequence, the factors regulating translation could represent an additional point for therapeutic intervention: inhibition of hnRNP E2 may be possible, resulting in the stimulation of C/EBPα activity, growth arrest, and differentiation.
The relationships among C/EBPs, RARs, mutations that contribute to AML, and the therapeutic effects of retinoids are complex. In APL, PML-RARα is able to inhibit RARs and may inhibit C/EBPα activity and C/EBPε expression. Activation of transcription from RAREs and induction of C/EBP activity, including α, β, and ε, may combine to underlie the therapeutic effects of tRA. C/EBPs and RARs can both induce granulocytic differentiation. Therefore, the particular sensitivity of APL to tRA may in fact be due to the ability of pharmacologic levels of tRA to reverse PML-RARα inhibition of both RARα and C/EBPs and thence to activate these factors.
The ability of C/EBPs to suppress the leukemic phenotype indicates that C/EBP induction may be critical to the tRA response. However, overexpression of these proteins may provide a more powerful stimulus to growth suppression and differentiation than the effect of pharmacologic tRA on C/EBPs. In other words, induction of C/EBP activity may be central to the response of APL to tRA, but may not represent the only important effect of tRA: it may be that the combination of C/EBP induction along with additional effects causes differentiation. Overexpression of C/EBPs could obviate the requirement for these additional effects of tRA by causing supraphysiologic effects on C/EBP targets.
In some settings, C/EBP activity appears sufficient to induce complete neutrophil differentiation whereas in other contexts C/EBPs induce only partial differentiation. Expression of C/EBPα, β, δ, or ε in 32Dcl3 cells largely recapitulates the effect of granulocyte colony-stimulating factor (G-CSF) on this cell line.26-28 45 Expression of C/EBPα and C/EBPε in FDC-P1 cells inhibited growth and induced expression of myeloid antigens, however these factors were insufficient to induce the production of mature neutrophils from this cell line. Likewise, C/EBPα and C/EBPε induced partial morphologic differentiation of leukemias from MRP8 PML-RARA and MRP8 PML-RARAm4transgenic mice in vitro, but again few mature neutrophils were generated in these cultures. The character of the cells as well as the context in which these experiments were performed, in vitro culture in the presence of IL-3 (and, for the leukemic cells, IL-6), might have limited the ability of the cells to differentiate fully. Remarkably, in vivo, activation of C/EBPε-ERTM resulted in the generation of fully differentiated neutrophils. These observations support the concept that neutrophil differentiation is the result of integrating multiple external and internal signals.
C/EBPs likely suppress the leukemic phenotype through a combination of induction of transcriptional targets and direct inhibition of cell cycle progression. Target genes of C/EBPα include G-CSF receptor, myeloperoxidase, and PU.1; targets of C/EBPε include specific granule proteins such as lactoferrin.1,4,26,46-48 Induction of these targets contributes to the phenotype of neutrophilic cells. In addition, C/EBPα is able to block growth by enhancing the activity of the cyclin-dependent kinase inhibitor p21, inhibiting E2F transactivation, and blocking cdk2 and cdk4 activity.49-56C/EBPα and C/EBPε are probably part of a self-reinforcing pathway that causes myeloid cells to become postmitotic and to assume the characteristics of mature neutrophils. New therapeutics that stimulate C/EBP activity or that mimic their dual effects should extend the reach of differentiation therapy in AML.
We thank H. Jeffrey Lawrence and Nancy Berliner for helpful discussions and Adam Olshen for advice on statistical analyses. We also acknowledge the continuing support of J. Michael Bishop, Frank McCormick, Kevin Shannon, and Daphne Haas-Kogan.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-05-1374.
Supported by the 32nd Edward Mallinckrodt Junior Scholar award (S.C.K.) and by a Burroughs Wellcome Fund Career Award (S.C.K.). Additional support was provided by grants K08-CA75986, U01-CA84221, and R01-CA88046 from the National Institutes of Health and by the Parker Hughes Trust Fund.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Scott C. Kogan, University of California San Francisco Comprehensive Cancer Center, 2340 Sutter St, Room N-361, Box 0128, San Francisco, CA 94143-0128; e-mail:skogan@cc.ucsf.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal