Normal immunoglobulin G for therapeutic use (intravenous immunoglobulin [IVIg]) is used in an increasing number of immune-mediated conditions, including acute and chronic/relapsing autoimmune diseases, transplantation, and systemic inflammatory disorders. Several mutually nonexclusive mechanisms of action account for the immunoregulatory effects of IVIg. Although IVIg inhibits T-cell proliferation and T-cell cytokine production, it is unclear whether these effects are directly dependent on the effects of IVIg on T cells or they are dependent through the inhibition of antigen-presenting cell activity. Here, we examined the effects of IVIg on differentiation, maturation, and function of dendritic cells (DCs). We show that IVIg inhibits the differentiation and maturation of DCs in vitro and abrogates the capacity of mature DC to secrete interleukin-12 (IL-12) on activation while enhancing IL-10 production. IVIg-induced down-regulation of costimulatory molecules associated with modulation of cytokine secretion resulted in the inhibition of autoreactive and alloreactive T-cell activation and proliferation. Modulation of DC maturation and function by IVIg is of potential relevance to its immunomodulatory effects in controlling specific immune responses in autoimmune diseases, transplantation, and other immune-mediated conditions.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that are specialized in the uptake of antigens and their transport from peripheral tissues to the lymphoid organs.1,2 Because of their capacity to stimulate naive T cells, DCs have a central role in the initiation of primary immune responses and are considered promising tools and targets for immunotherapy. To acquire naive T-cell stimulatory ability, DCs must undergo maturation, which involves the up-regulation of surface major histocompatibility complex (MHC) class 2 and of costimulatory molecules during the process of migration from periphery to T-cell areas of secondary lymphoid tissues.3 The secretion of DC-derived immunoregulatory cytokines plays a crucial role in the cascade of events that occur during the priming of naive T cells.4
In addition to substitutive treatment of patients with primary and secondary antibody deficiencies, therapeutic preparations of normal polyspecific immunoglobulin G (intravenous immunoglobulin [IVIg]) are used in a large number of immune-mediated conditions, including acute and chronic/relapsing autoimmune diseases, transplantation, and systemic inflammatory disorders.5-9 Several mutually nonexclusive mechanisms of action account for the immunoregulatory effects of IVIg.9-12 Although IVIg inhibits T-cell proliferation and T-cell cytokine production,13-16 it is unclear whether these effects are directly dependent on the effects of IVIg on T cells or they are dependent through the inhibition of antigen-presenting cell (APC) activity. In the present study, we addressed the effects of IVIg on differentiation, maturation, and function of DCs. We demonstrate that DCs are one of the targets for the immunosuppressive effects of IVIg. Thus, IVIg inhibits the maturation of DCs and modulates their activation and survival, resulting in abrogation of T-cell activation and proliferation.
Materials and methods
Antibodies and reagents
Recombinant human (rh) interleukin-4 (IL-4) was obtained from R&D Systems (Abingdon, Oxon, United Kingdom), and rh granulocyte macrophage–colony-stimulating factor (GM-CSF; Leukomax, Dardilly, France) was obtained from Schering-Plough (Dardilly, France). Phycoerythrin (PE)–conjugated goat antihuman immunoglobulin G (IgG), fluorescein isothiocyanate (FITC)–conjugated goat antimouse IgG, FITC-conjugated monoclonal antibody (mAb) to CD16, CD86, PE-conjugated mAbs to CD40, CD80, CD83, PE cyanin5-conjugated anti-CD14 mAb (RMO52), and dichlorotriazinyl aminofluorescein (DTAF)–conjugated goat antihuman IgG were obtained from Immunotech (Marseilles, France). FITC-conjugated mAbs to HLA-DR, FITC-conjugated mouse IgG1, and PE-conjugated mouse IgG1 were purchased from Becton Dickinson (Le Pont de Claix, France). FITC-conjugated mAb to CD86 and PE-conjugated mAb to CD95 were from PharMingen (Le Pont de Claix, France). FITC-conjugated mAb to CD1a (clone OKT6) was from Ortho Diagnostic Systems (Issy Les Moulineaux, France). Lipopolysaccharide (LPS) (Escherichia coli) was purchased from Sigma (St Quentin Fallavier, France). Plasma-derived human serum albumin (HSA) was obtained from Laboratoire Française de Biotechnologies (Les Ulis, France).
Therapeutic intravenous immunoglobulin
Sandoglobulin (ZLB Bioplasma, Bern, Switzerland), a therapeutic preparation of pooled normal IgG obtained from plasma of healthy donors, was used throughout the study unless otherwise mentioned. Other preparations of IVIg were Gammagard (N V Baxter SA, Lessines, Belgium), Endobuline (Immuno AG, Vienna, Austria), and Intraglobin (Biotest Pharma GmbH, Dreieich, Germany). For the present study, a stock solution of 100 mg/mL (0.6 mM) IVIg was prepared in phosphate-buffered saline (PBS) and was dialyzed twice against large volumes of PBS at 4°C before use to remove the stabilizing agents. Fragment crystallizable (Fc) fragments of IVIg were obtained from Dr M. C. Bonnet (Institut Mérieux, Lyon, France). F(ab′)2 fragments were prepared from IVIg by pepsin digestion (2% wt/wt; Sigma, St Louis, MO) in acetate buffer, pH 4.1, for 18 hours at 37°C, followed by chromatography on protein G–Sepharose (Pharmacia, Uppsala, Sweden). F(ab′)2fragments were free of intact IgG and Fc fragments, as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and enzyme-linked immunosorbent assay (ELISA). F(ab′)2 and Fc fragments tested negative for contamination with endotoxins. Sandoglobulin used in the study contained mostly monomeric IgG (96%-98%) and negligible amounts of dimers, as assessed by a calibrated Sepharose 300 HR gel filtration column connected to a fast-performance liquid chromatography (FPLC) system (Pharmacia).
Affinity purification of IgG from therapeutic intravenous immunoglobulin
Protein G coupled to activated CH Sepharose 4B (Pharmacia) was equilibrated with PBS, pH 7, before it was loaded with 10 mg IVIg/mL matrix. IVIg was allowed to interact with the matrix for 3 hours at room temperature or overnight at 4°C before the column was washed with PBS, and it was eluted using 0.1 M glycine-HCl buffer containing 0.5 M NaCl (pH 2.8). Eluted IgG was neutralized with 3 M Tris (tris(hydroxymethyl)aminomethane) and dialyzed against PBS. The affinity-purified IgG molecules thus obtained were further subjected to gel filtration, and fractions corresponding to IgG were collected and concentrated to 50 mg/mL.
Reactivity of intravenous immunoglobulin with cytokines and LPS
The reactivity of IVIg with GM-CSF, IL-4, and LPS was assessed using ELISA. Microtiter plates were coated with 50 μL cytokines or LPS (1 μg/mL) in PBS, pH 7.4, overnight at 4°C. Wells were washed with PBS/0.1% Tween-20 before saturation with PBS containing 1% bovine serum albumin (BSA). Plates were then incubated at 37°C for 1 hour with sequential dilutions of IVIg (0.008 to 25 mg/mL). The negative control consisted of myeloma IgG protein. After washing, plates were incubated with horseradish peroxidase–conjugated F(ab′)2-specific goat antihuman IgG (ICN/Cappel, West Chester, PA) at 37°C for 1 hour before the addition of revealing substrate.
Generation and culture of monocyte-derived human dendritic cells
Dendritic cells (DCs) were generated from monocytes as described.17 In brief, peripheral blood mononuclear cells were isolated from heparinized buffy coats of healthy adult donors by adherence to plastic cell culture dishes in RPMI 1640 medium supplemented with 10% human AB serum, glutamine, and antibiotics for 60 minutes. Nonadherent cells were removed by 3 gentle washings with medium. Adherent monocytes were cultured in RPMI medium and were supplemented with 10% fetal calf serum (FCS), glutamine, and antibiotics in the presence of 500 IU/mL rhIL-4 and 1000 IU/mL rhGM-CSF. Half the medium, including all supplements, was replaced every 2 days. After 5 days of culture, nonadherent and loosely adherent cells corresponding to the DC-enriched fraction were harvested, washed, and used for subsequent experiments. Flow cytometric analysis (Becton Dickinson) demonstrated that the DCs were 90% or more pure DCs.
Effect of IVIg on differentiation and maturation of dendritic cells
To investigate the effect of IVIg on the differentiation of DCs, we cultured monocytes in the presence of GM-CSF and IL-4 with or without IVIg (0.15 mM) for 5 days. Half the medium, including all the supplements, was replaced every 2 days. After 5 days, cell surface staining was performed with specifically labeled mAbs or appropriate isotypic control antibodies in 100 μL saline.
To determine the role of IVIg on the constitutive maturation of DCs, we treated immature DCs (0.5 × 106/mL) obtained after 5 days of culture of monocytes (CD1a high-positive cells) with 0.15 mM IVIg in the presence of IL-4 and GM-CSF for 48 hours. To study the effect of IVIg on LPS-mediated maturation of DCs, immature DCs were treated with equimolar amounts of IVIg (0.15 mM/0.5 × 106 cells) or HSA (irrelevant protein control) or medium alone for 12 hours, followed by incubation with LPS (1 μg/mL) for another 48 hours in the presence of all supplements, including IL-4 and GM-CSF. Ten thousand cells were analyzed for each sample, and data were processed by means of the CellQuest software (BD Biosciences). For each sample, cell-free culture supernatant was collected after 48 hours for cytokine assays.
Mixed lymphocyte reaction
Responder CD4+ T cells used for autologous and allogeneic mixed-lymphocyte reaction (MLR) assays were isolated using the MACS cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany). Five-day-old immature DCs were cultured in the presence of IVIg (0.15 mM) or in the absence of IVIg for an additional 48 hours. In another series of experiments, immature DCs were treated with IVIg for 12 hours followed by stimulation with LPS (1 μg/mL), or they were treated with LPS alone for an additional 48 hours (mature DCs). Graded doses (2000-20 000) of DCs were seeded with 1 × 105 responder T cells/well/200 μL in complete RPMI 1640 medium supplemented with 10% human AB serum. After 4 days of culture, the cells were pulsed overnight with 1 μCi (0.037 MBq) [3H]thymidine to quantify T-cell proliferation. Radioactive incorporation was measured by standard liquid scintillation counting, and results were expressed as counts per minute (mean ± SD of triplicate values).
Cytokine assays
Cytokines were quantified in cell-free culture supernatants using Quantikine sandwich immunoassay kits from Immunotech (IL-10 and tumor necrosis factor-α [TNF-α]) and R&D Systems (IL-12 p70). Detection limits were 5 pg/mL for IL-12 p70 and IL-10 and 10 pg/mL for TNF-α.
Statistical analysis
An unpaired Student t test was used to determine the statistical significance of the data. P < .05 was considered the level of statistical significance.
Results
Down-regulation of costimulatory molecules by IVIg during differentiation of dendritic cells
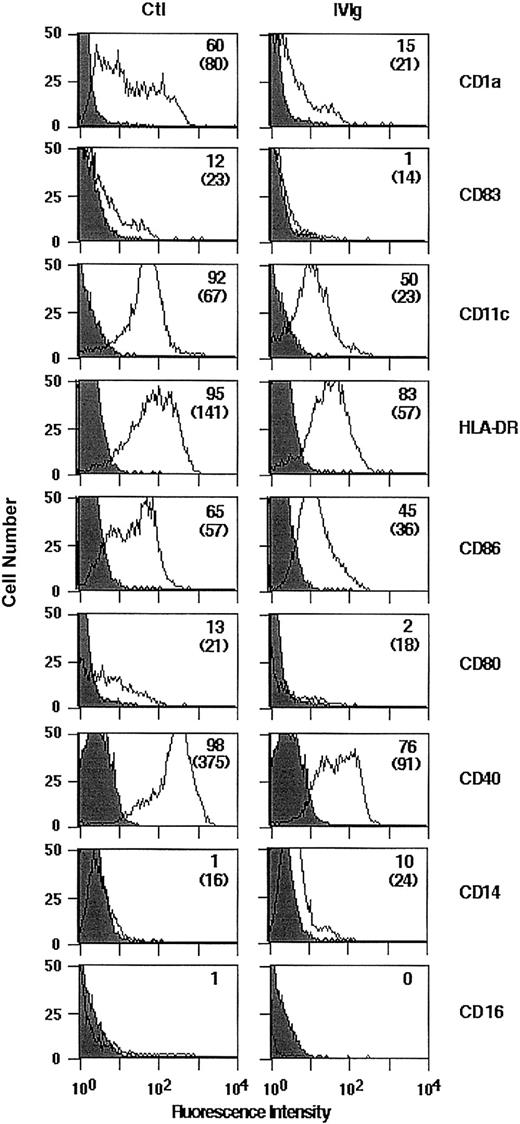
We examined whether the treatment of monocytes with IVIg would affect their differentiation into DCs on culture in the presence of GM-CSF and IL-4. The concentration of IVIg selected for these experiments (0.15 mM) was within the range of IgG concentrations reached in the plasma of patients treated with IVIg (20-35 mg/mL; 0.12-0.21 mM). Human peripheral blood monocytes of healthy donors were cultured in the presence of GM-CSF and IL-4 with or without IVIg for 5 days. After 5 days of culture, the cells exhibited morphologic and phenotypic features characteristic of DCs, including the down-regulation of the monocyte/macrophage marker CD14 and the absence of CD16 (Figure 1). The expression of CD1a, CD83, CD11c, and HLA-DR and of the costimulatory molecules CD86, CD80, and CD40 on IVIg-treated cells was decreased compared with untreated cells (Figure 1), indicating an arrest in the normal development of IVIg-treated DCs. The observed down-regulation of surface markers on IVIg-treated cells was not caused by interference with the binding of various fluorescence-labeled mAbs by IVIg (data not shown). Mean fluorescence intensities were significantly decreased on IVIg-treated cells in CD1a, CD83, HLA-DR, CD86, and CD40 (Table1).
Flow cytometric analysis of surface molecules expressed by DCs differentiated in the presence of IVIg.
Monocytes were cultured for 5 days with 1000 IU/mL rhGM-CSF and 500 IU/mL rhIL-4 in the presence of IVIg (0.15 mM; right panel) or the absence of IVIg (Ctl, left panel). Surface expression of cellular receptors was analyzed following incubation with relevant mAb or isotype control. Percentage of cells positive for the indicated markers are depicted in upper right corners, and MFI is indicated in parentheses. Results shown here are representative of 3 independent experiments.
Flow cytometric analysis of surface molecules expressed by DCs differentiated in the presence of IVIg.
Monocytes were cultured for 5 days with 1000 IU/mL rhGM-CSF and 500 IU/mL rhIL-4 in the presence of IVIg (0.15 mM; right panel) or the absence of IVIg (Ctl, left panel). Surface expression of cellular receptors was analyzed following incubation with relevant mAb or isotype control. Percentage of cells positive for the indicated markers are depicted in upper right corners, and MFI is indicated in parentheses. Results shown here are representative of 3 independent experiments.
Phenotype analysis of DCs differentiated in the presence of IVIg
| Treatment . | CD1a . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . |
|---|---|---|---|---|---|---|
| Ctl | 121 ± 27 | 21 ± 5 | 153 ± 26 | 55 ± 5 | 24 ± 4 | 282 ± 81 |
| IVIg | 31 ± 6* | 11 ± 2* | 59 ± 6* | 40 ± 5* | 21 ± 5 | 93 ± 15* |
| Treatment . | CD1a . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . |
|---|---|---|---|---|---|---|
| Ctl | 121 ± 27 | 21 ± 5 | 153 ± 26 | 55 ± 5 | 24 ± 4 | 282 ± 81 |
| IVIg | 31 ± 6* | 11 ± 2* | 59 ± 6* | 40 ± 5* | 21 ± 5 | 93 ± 15* |
Monocytes were cultured for 5 days with 1000 IU/mL rhGM-CSF and 500 IU/mL rhIL-4 in the presence of IVIg (0.15 mM; IVIg) or the absence of IVIg (Ctl). Results are expressed as MFI, calculated by subtraction of the MFI obtained with the isotype-matched control value from that obtained with the relevant mAb. Results are from 3 independent experiments from different donors. Statistical significance as determined by unpaired Student t test is indicated.
P <. 05.
IVIg blocks the constitutive maturation of immature DCs
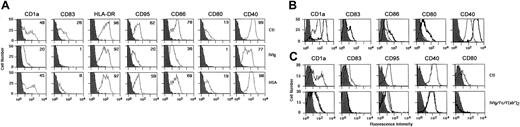
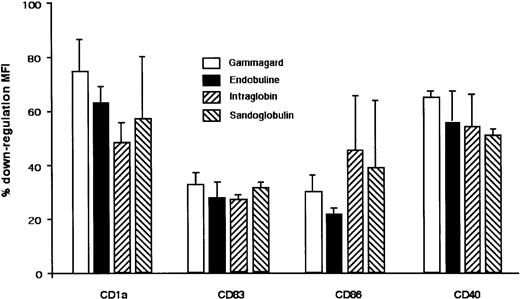
We then investigated the effect of IVIg on the phenotype of DCs undergoing constitutive maturation in vitro. Monocytes were cultured for 5 days in the presence of GM-CSF and IL-4. Cells became nonadherent and clustered, exhibiting protruding veils typical of DCs. Immature DCs thus obtained were analyzed for purity by flow cytometry. More than 90% of the cells displayed a typical DC phenotype. Cells were washed and incubated with IL-4, GM-CSF, and IVIg (0.15 mM) for 48 additional hours. We observed that IVIg blocked the spontaneous maturation of DCs. Thus, the percentage of cells expressing CD83 was reduced up to 96%, CD80 up to 92%, CD86 up to 54%, CD40 up to 22%, and CD95 up to 68% (Figure 2A). Mean fluorescence intensities were also significantly decreased in the case of the following markers: CD1a, CD83, CD86, and CD40 (Table2). Similar results were obtained when DCs were treated with IgG molecules affinity- purified from IVIg preparations (Sandoglobulin) on protein G chromatography and subsequently on FPLC, thus indicating that the observed inhibitory action of IVIg was caused by IgG molecules (Figure 2B). On the contrary, treatment with HSA used as a control had no significant impact on the phenotype of DC except in the case of CD83. Although the percentage of cells expressing CD83 was down-regulated by HSA, the mean fluorescence intensity (MFI) was within the range of control (medium alone). Statistical analysis showed no difference in MFI between HSA-treated cells and control (medium alone) (Table 2). To understand whether the inhibitory function of IVIg on DCs is Fc dependent or variable region dependent, we treated DCs obtained by 5-day culture of monocytes with equimolar concentrations of Fc or F(ab′)2fragments of IVIg for 48 hours. Both types of IVIg fragments inhibited DC maturation similarly to intact IVIg (Figure 2C). A similar pattern of inhibition on constitutive maturation of DCs was also observed with 3 other sources of IVIg, namely Gammagard, Endobuline, and Intraglobin (Figure3).
IVIg inhibits the constitutive maturation of monocyte-derived DCs.
(A) DCs were generated as described in “Materials and methods” in the presence of GM-CSF and IL-4. At day 5, immature DCs were cultured in the presence of IVIg (0.15 mM; middle row) or the absence of IVIg (Ctl, top row) for an additional 48 hours. Equimolar concentration of human serum albumin (HSA; bottom row) was used as control. At day 7, cells were harvested and analyzed for the expression of surface molecules using flow cytometry with labeled antibodies. Percentages of cells positive for the indicated markers are depicted in upper right corners. Results shown are representative of 4 independent experiments from different donors. (B) The inhibitory effect of IVIg on DC is mediated by IgG molecules. IgG molecules were affinity purified from Sandoglobulin on Protein G column followed by gel filtration. Five-day-old DCs were incubated with affinity-purified IgG (0.15mM) for 48 hours. Cells treated with medium alone (control) are denoted by thick lines, and thin lines represent IgG-treated cells. (C) DCs obtained by culturing monocytes for 5 days were incubated independently in the presence of equimolar concentrations (0.15 mM) of IVIg (thin lines) or F(ab′)2 fragments of IVIg (dashed lines) or Fc fragments (thick lines) for 48 hours (bottom row). Top row shows results of control experiment. Cells were analyzed for the expression of surface molecules using flow cytometry, and data were processed using CellQuest software.
IVIg inhibits the constitutive maturation of monocyte-derived DCs.
(A) DCs were generated as described in “Materials and methods” in the presence of GM-CSF and IL-4. At day 5, immature DCs were cultured in the presence of IVIg (0.15 mM; middle row) or the absence of IVIg (Ctl, top row) for an additional 48 hours. Equimolar concentration of human serum albumin (HSA; bottom row) was used as control. At day 7, cells were harvested and analyzed for the expression of surface molecules using flow cytometry with labeled antibodies. Percentages of cells positive for the indicated markers are depicted in upper right corners. Results shown are representative of 4 independent experiments from different donors. (B) The inhibitory effect of IVIg on DC is mediated by IgG molecules. IgG molecules were affinity purified from Sandoglobulin on Protein G column followed by gel filtration. Five-day-old DCs were incubated with affinity-purified IgG (0.15mM) for 48 hours. Cells treated with medium alone (control) are denoted by thick lines, and thin lines represent IgG-treated cells. (C) DCs obtained by culturing monocytes for 5 days were incubated independently in the presence of equimolar concentrations (0.15 mM) of IVIg (thin lines) or F(ab′)2 fragments of IVIg (dashed lines) or Fc fragments (thick lines) for 48 hours (bottom row). Top row shows results of control experiment. Cells were analyzed for the expression of surface molecules using flow cytometry, and data were processed using CellQuest software.
Phenotype analysis of DCs matured in the presence of IVIg
| Treatment . | CD1a . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . | CD95 . |
|---|---|---|---|---|---|---|---|
| Ctl | 114 ± 22 | 21 ± 2 | 169 ± 35 | 62 ± 16 | 30 ± 12 | 295 ± 53 | 40 ± 12 |
| IVIg | 34 ± 15* | 12 ± 1* | 120 ± 52 | 36 ± 5* | 20 ± 9 | 95 ± 14* | 29 ± 10 |
| HSA | 124 ± 2 | 20 ± 3 | 154 ± 7 | 59 ± 6 | 25 ± 8 | 197 ± 34 | 37 ± 9 |
| Treatment . | CD1a . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . | CD95 . |
|---|---|---|---|---|---|---|---|
| Ctl | 114 ± 22 | 21 ± 2 | 169 ± 35 | 62 ± 16 | 30 ± 12 | 295 ± 53 | 40 ± 12 |
| IVIg | 34 ± 15* | 12 ± 1* | 120 ± 52 | 36 ± 5* | 20 ± 9 | 95 ± 14* | 29 ± 10 |
| HSA | 124 ± 2 | 20 ± 3 | 154 ± 7 | 59 ± 6 | 25 ± 8 | 197 ± 34 | 37 ± 9 |
Immature DCs of 5 day were cultured in the presence of IVIg (0.15 mM; IVIg) or the absence of IVIg (Ctl) for 48 hours. Equimolar concentration of HSA was used as control. Results are expressed as MFI, calculated by subtraction of the MFI obtained with the isotype-matched control value from that obtained with the relevant mAb. Results are from 4 independent experiments from different donors. Statistical significance as determined by unpaired Student t test is indicated.
P < .05.
Inhibitory effect of IVIg from various commercial sources on constitutive maturation of DCs.
Dendritic cells were generated from healthy donors by culturing monocytes in the presence of GM-CSF and IL-4. At day 5, DCs were incubated with Gammagard, Endobuline, Intraglobin, and Sandoglobulin (0.15 mM) for an additional 48 hours. Percentage down-regulation of MFI of surface molecules on DC after IVIg treatment is shown. DCs cultured in medium alone represented 100% expression. Data are represented as means and standard deviations calculated from 3 independent experiments from different donors.
Inhibitory effect of IVIg from various commercial sources on constitutive maturation of DCs.
Dendritic cells were generated from healthy donors by culturing monocytes in the presence of GM-CSF and IL-4. At day 5, DCs were incubated with Gammagard, Endobuline, Intraglobin, and Sandoglobulin (0.15 mM) for an additional 48 hours. Percentage down-regulation of MFI of surface molecules on DC after IVIg treatment is shown. DCs cultured in medium alone represented 100% expression. Data are represented as means and standard deviations calculated from 3 independent experiments from different donors.
IVIg renders DCs refractory to LPS-induced maturation
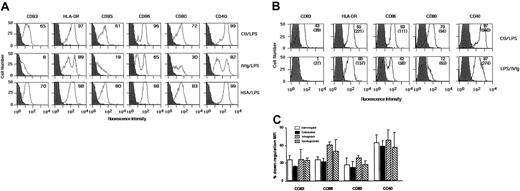
Immature DCs obtained by culturing monocytes for 5 days in the presence of IL-4 and GM-CSF were incubated with LPS (1 μg/mL) for 48 hours. As shown in Figure 4A, maturation under these conditions was associated with down-regulation of CD1a (data not shown), induction of CD83, and up-regulation of CD80, CD86, HLA-DR, CD95, and CD40 (Figure 4A). To examine the effect of IVIg on LPS-induced maturation of DCs, immature DCs were pretreated with IVIg (0.15 mM; Sandoglobulin) for 12 hours, followed by incubation with LPS for an additional 48 hours. IVIg-treated DCs were refractory to maturation induced by LPS stimulation, as assessed by phenotypic analysis. However, the inhibitory effect of IVIg on the expression of HLA-DR was marginal (Figure 4A, Table 3). Mean fluorescence intensities were significantly decreased in the case of the following markers: CD83, CD86, CD80, and CD40 (Table 3). Similar results were obtained on prior stimulation of DCs with LPS (1 μg/mL) for 3 hours followed by incubation with IVIg for 48 hours (Figure 4B). Interestingly, LPS stimulation of IVIg-treated DCs generated 2 distinct populations of DCs (Figure 4A). One population was characterized by the down-regulation of costimulatory molecules, whereas the other population responded to LPS stimulation, as indicated by the increased expression of HLA-DR, CD86, CD80, and CD40, similar to that observed with LPS-stimulated, IVIg-untreated cells. Different preparations of IVIg were evaluated for their effect on LPS-induced DC maturation. Immature DCs of 5 days were stimulated with LPS (1 μg/mL) for 3 hours, followed by incubation with 4 different preparations of IVIg for 48 hours. An inhibitory effect on LPS-induced maturation of DCs was observed with all the IVIg preparations (Figure 4C).
IVIg renders DCs refractory to LPS-mediated maturation.
(A) DCs were generated as described in “Materials and methods.” At day 5, immature DCs were treated with IVIg (0.15 mM) for 12 hours followed by stimulation with LPS (1 μg/mL) (middle row) or were treated with LPS alone (top row) for 48 hours. In control experiments, cells were treated with HSA as an irrelevant protein for 12 hours followed by stimulation with LPS (1 μg/mL; bottom row). Percentages of cells positive for the indicated markers are depicted in upper right corners. Dark histograms represent isotype control. (B) In a second set of experiments, immature DCs were stimulated with LPS (1 μg/mL) for 3 hours followed by incubation with IVIg (bottom row) or without IVIg (top row) for 48 hours. Percentages of cells positive for the indicated markers are depicted in upper right corners, and MFI is indicated in parentheses. Results shown are representative of 4 independent experiments from different donors. (C) Effect of different sources of IVIg on LPS-mediated maturation of dendritic cells. Monocyte-derived DCs were stimulated with LPS (1 μg/mL) for 3 hours followed by incubation with Gammagard, Endobuline, Intraglobin, and Sandoglobulin (0.15 mM) for 48 hours. The percentage down-regulation of MFI of surface molecules on DCs following IVIg treatment is shown. LPS-treated DCs represented 100% expression. Data are represented as means and standard deviations calculated from 2 independent experiments from different donors.
IVIg renders DCs refractory to LPS-mediated maturation.
(A) DCs were generated as described in “Materials and methods.” At day 5, immature DCs were treated with IVIg (0.15 mM) for 12 hours followed by stimulation with LPS (1 μg/mL) (middle row) or were treated with LPS alone (top row) for 48 hours. In control experiments, cells were treated with HSA as an irrelevant protein for 12 hours followed by stimulation with LPS (1 μg/mL; bottom row). Percentages of cells positive for the indicated markers are depicted in upper right corners. Dark histograms represent isotype control. (B) In a second set of experiments, immature DCs were stimulated with LPS (1 μg/mL) for 3 hours followed by incubation with IVIg (bottom row) or without IVIg (top row) for 48 hours. Percentages of cells positive for the indicated markers are depicted in upper right corners, and MFI is indicated in parentheses. Results shown are representative of 4 independent experiments from different donors. (C) Effect of different sources of IVIg on LPS-mediated maturation of dendritic cells. Monocyte-derived DCs were stimulated with LPS (1 μg/mL) for 3 hours followed by incubation with Gammagard, Endobuline, Intraglobin, and Sandoglobulin (0.15 mM) for 48 hours. The percentage down-regulation of MFI of surface molecules on DCs following IVIg treatment is shown. LPS-treated DCs represented 100% expression. Data are represented as means and standard deviations calculated from 2 independent experiments from different donors.
Phenotype analysis of DCs matured after exposure to LPS in the presence of IVIg
| Treatment . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . | CD95 . |
|---|---|---|---|---|---|---|
| Ctl-LPS | 30 ± 2 | 208 ± 32 | 84 ± 16 | 53 ± 7 | 381 ± 34 | 46 ± 9 |
| IVIg-LPS | 21 ± 43-150 | 156 ± 50 | 54 ± 103-150 | 40 ± 43-150 | 210 ± 326 | 34 ± 10 |
| HSA-LPS | 29 ± 4 | 173 ± 12 | 111 ± 4 | 56 ± 5 | 404 ± 156 | 46 ± 2 |
| Treatment . | CD83 . | HLA-DR . | CD86 . | CD80 . | CD40 . | CD95 . |
|---|---|---|---|---|---|---|
| Ctl-LPS | 30 ± 2 | 208 ± 32 | 84 ± 16 | 53 ± 7 | 381 ± 34 | 46 ± 9 |
| IVIg-LPS | 21 ± 43-150 | 156 ± 50 | 54 ± 103-150 | 40 ± 43-150 | 210 ± 326 | 34 ± 10 |
| HSA-LPS | 29 ± 4 | 173 ± 12 | 111 ± 4 | 56 ± 5 | 404 ± 156 | 46 ± 2 |
Immature DCs obtained after 5-day culture with IL-4 and GM-CSF were treated with IVIg (0.15 mM) for 12 hours followed by stimulation with LPS (1 μg/mL) (IVIg-LPS) or were treated with LPS alone (Ctl-LPS) for 48 hours. In control experiments, cells were treated with HSA, an irrelevant protein, for 12 hours followed by stimulation with LPS (HSA-LPS). Results are expressed as MFI. Data are from 4 independent experiments from different donors. Statistical significance as determined by unpaired Student t test is indicated.
P < .05.
IVIg modulates the secretion of cytokines by DCs
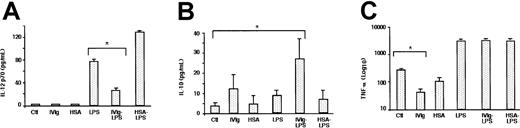
Incubation of immature DCs in the presence of IVIg or HSA did not induce immature DCs to secrete IL-12 (Figure5). In contrast, IVIg-treated immature DCs secreted increased levels of IL-10, and they decreased the levels of TNF-α 7-fold (Figure 5). Stimulation of DCs with LPS induced the production of bioactive IL-12 by the cells (77 ± 3 pg/mL). IL-12 secretion was significantly decreased when DCs were pretreated with IVIg before stimulation with LPS (27 ± 2 pg/mL;P < .05) (Figure 5). Furthermore, whereas LPS-stimulation of DC did not alter the levels of secreted IL-10, treatment of DCs with IVIg before the addition of LPS enhanced IL-10 secretion 8-fold. TNF-α production by LPS-stimulated DCs was unaffected by IVIg.
IVIg modulates cytokine production by DCs.
Immature DCs obtained from culturing monocytes for 5 days in the presence of IL-4 and GM-CSF were incubated in the presence of IVIg (0.15 mM; IVIg) or in the absence of IVIg (Ctl) for an additional 48 hours. In a second set of experiments, DCs were treated with IVIg for 12 hours followed by stimulation with LPS (1 μg/mL) (IVIg-LPS), or they were treated with LPS alone (LPS) for an additional 48 hours to obtain mature DCs. Similarly, immature DCs were treated with HSA for 48 hours (HSA) or were treated 12 hours before the addition of LPS (HSA-LPS). The cell-free culture supernatant was used to measure the secretion of (A) IL-12, (B) IL-10, and (C) TNF-α by Quantikine ELISA. Statistical significance as determined by unpaired Student t test (*P < .05) is indicated.
IVIg modulates cytokine production by DCs.
Immature DCs obtained from culturing monocytes for 5 days in the presence of IL-4 and GM-CSF were incubated in the presence of IVIg (0.15 mM; IVIg) or in the absence of IVIg (Ctl) for an additional 48 hours. In a second set of experiments, DCs were treated with IVIg for 12 hours followed by stimulation with LPS (1 μg/mL) (IVIg-LPS), or they were treated with LPS alone (LPS) for an additional 48 hours to obtain mature DCs. Similarly, immature DCs were treated with HSA for 48 hours (HSA) or were treated 12 hours before the addition of LPS (HSA-LPS). The cell-free culture supernatant was used to measure the secretion of (A) IL-12, (B) IL-10, and (C) TNF-α by Quantikine ELISA. Statistical significance as determined by unpaired Student t test (*P < .05) is indicated.
IVIg-treated DCs fail to stimulate autologous and allogeneic MLR
Stimulation of autologous and MLR by DCs serve as an in vitro model for T-cell unresponsiveness in autoimmune patients undergoing therapy and for graft rejection, respectively. Pretreatment of immature DCs with IVIg resulted in the inhibition of autologous and allogeneic MLR (Figure 6). Although the inhibition of allogeneic MLR was 2-fold (P < .05), that of autologous MLR was up to 28-fold (P < .001), indicating a stronger inhibitory effect of IVIg on the ability of DCs to stimulate autoreactive T cells. Similar results were obtained when DCs were stimulated by LPS. There was a 1.5-fold reduction of proliferation of allogeneic CD4+ T cells on the pretreatment of DCs with IVIg (P < .05). Reduction reached 33-fold for autoreactive T cells (P < .001).
Effects of IVIg treatment on DC-mediated T-cell proliferation.
IVIg treatment abrogates the capacity of DCs to stimulate autologous (A-B) and allogeneic (C-D) T cells. Immature DCs obtained by culturing monocytes for 5 days were cultured in the presence of IVIg (0.15 mM) or HSA (0.15 mM) or in the absence of IVIg (Ctl) for an additional 48 hours. Graded doses of DCs were used to stimulate autologous (A) and allogeneic (C) CD4+ T cells (1 × 105cells/well) in MLR. In a second type of experiment, immature DCs were treated for 12 hours with IVIg or HSA followed by stimulation with LPS (1 μg/mL) or treated with LPS alone for 48 hours to obtain mature DCs followed by stimulation of autologous (B) and allogeneic (D) T cells. Thymidine incorporation was measured on day 5 by a 16-hour pulse with 1 μCi (0.037 MBq) [3H]thymidine. Results are shown as means ± SDs of triplicate values. Statistical significance as determined by unpaired Student t test (*P < .05; **P < .001) is indicated.
Effects of IVIg treatment on DC-mediated T-cell proliferation.
IVIg treatment abrogates the capacity of DCs to stimulate autologous (A-B) and allogeneic (C-D) T cells. Immature DCs obtained by culturing monocytes for 5 days were cultured in the presence of IVIg (0.15 mM) or HSA (0.15 mM) or in the absence of IVIg (Ctl) for an additional 48 hours. Graded doses of DCs were used to stimulate autologous (A) and allogeneic (C) CD4+ T cells (1 × 105cells/well) in MLR. In a second type of experiment, immature DCs were treated for 12 hours with IVIg or HSA followed by stimulation with LPS (1 μg/mL) or treated with LPS alone for 48 hours to obtain mature DCs followed by stimulation of autologous (B) and allogeneic (D) T cells. Thymidine incorporation was measured on day 5 by a 16-hour pulse with 1 μCi (0.037 MBq) [3H]thymidine. Results are shown as means ± SDs of triplicate values. Statistical significance as determined by unpaired Student t test (*P < .05; **P < .001) is indicated.
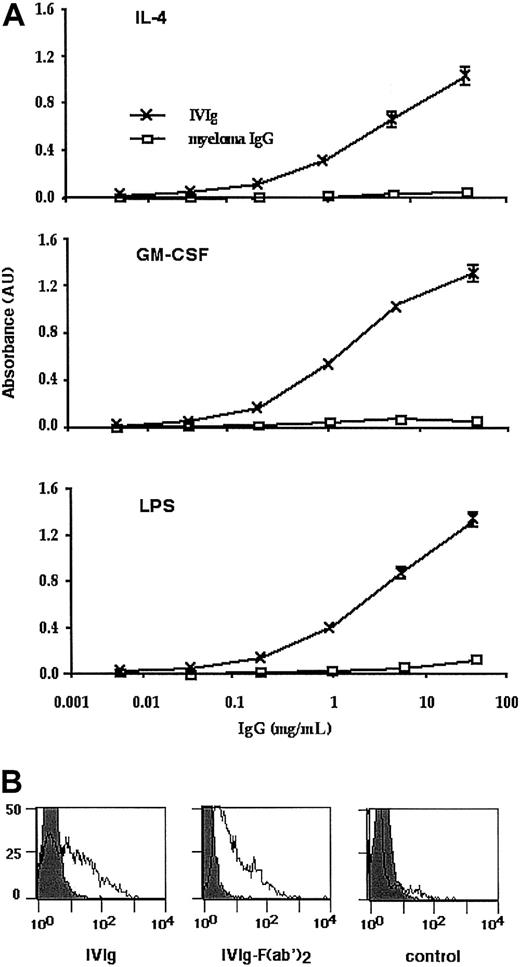
IVIg interacts with cytokines, LPS, and dendritic cells
Anticytokine (GM-CSF and IL-4) and LPS reactivity of IVIg was probed using ELISA. As shown in Figure 7, IVIg bound to GM-CSF, IL-4, and LPS in a dose-dependent manner, whereas an irrelevant myeloma IgG protein failed to recognize cytokines and LPS. Results were consistent with previous reports that pools of normal human IgG contain variable amounts of specific and high-avidity antibodies to certain cytokines.18 Further, we demonstrated that IVIg-mediated effects on DCs involves a direct interaction of IVIg with DCs. Five-day-old DCs were incubated with 0.15 mM intact IVIg or F(ab′)2 fragments of IVIg for 48 hours. Binding of IVIg/F(ab′)2 fragments was then revealed using DTAF-conjugated goat antihuman IgG. The results indicate that IVIg specifically bound to DCs, whereas an irrelevant IgG myeloma protein did not bind to the surface of DCs (Figure 7B).
IVIg interacts with cytokines, LPS, and dendritic cells.
(A) Reactivity of IVIg with cytokines and LPS. Microtiter ELISA plates were coated with 1 μg/mL IL-4 (top graph), GM-CSF (middle graph), or LPS (bottom graph), blocked, and incubated with sequential dilutions of IVIg (0.008 to 25 mg/mL) followed by goat antihuman IgG coupled to horseradish peroxidase. The negative control consisted of human myeloma IgG protein. Values shown are the differences between the absorbance of specific binding and the background for each concentration and are represented as means of triplicate wells ± SDs. (B) IVIg binds to dendritic cells. Five-day-old DCs were incubated with 0.15 mM intact IVIg (left) or F(ab′)2 fragments (middle) of IVIg for 48 hours. The negative control consisted of human myeloma IgG protein (right). Binding of IVIg or F(ab′)2 fragments was then revealed using DTAF-conjugated goat antihuman IgG.
IVIg interacts with cytokines, LPS, and dendritic cells.
(A) Reactivity of IVIg with cytokines and LPS. Microtiter ELISA plates were coated with 1 μg/mL IL-4 (top graph), GM-CSF (middle graph), or LPS (bottom graph), blocked, and incubated with sequential dilutions of IVIg (0.008 to 25 mg/mL) followed by goat antihuman IgG coupled to horseradish peroxidase. The negative control consisted of human myeloma IgG protein. Values shown are the differences between the absorbance of specific binding and the background for each concentration and are represented as means of triplicate wells ± SDs. (B) IVIg binds to dendritic cells. Five-day-old DCs were incubated with 0.15 mM intact IVIg (left) or F(ab′)2 fragments (middle) of IVIg for 48 hours. The negative control consisted of human myeloma IgG protein (right). Binding of IVIg or F(ab′)2 fragments was then revealed using DTAF-conjugated goat antihuman IgG.
Discussion
In this report, we show that normal immunoglobulin G for therapeutic use (intravenous immunoglobulin, IVIg) inhibits the maturation of DCs in vitro and abrogates the capacity of mature DCs to secrete IL-12 on activation while enhancing IL-10 production. IVIg-induced down-regulation of costimulatory molecules associated with the modulation of cytokine secretion resulted in the inhibition of autoreactive and alloreactive T-cell activation and proliferation. Modulation of DC maturation and function by IVIg is of relevance to its immunomodulatory effects in controlling specific immune responses.
Several immunomodulatory agents, including glucocorticoids, prostaglandin E2 (PGE2), N-acetyl-l-cysteine, IL-10, and vitamin D3, have been shown to exert suppressive effects on DC.19-26 Here we demonstrate that IVIg interferes with the differentiation of DCs from monocytes in vitro, resulting in the down-regulation of CD1a, CD83, and HLA-DR, along with that of the costimulatory molecules CD80, CD86, and CD40. The expression of CD14 on cells is, however, unaffected by IVIg or is slightly higher than that of untreated control cells, and differentiated cells are negative for CD16. Whereas glucocorticoids, IL-10, and vitamin D3enhance membrane expression of CD14 and CD16 in monocyte-derived DCs, our results indicate that IVIg does inhibit the differentiation of DCs from monocytes but, unlike these agents, does not promote DC differentiation toward macrophages.
DCs exhibit the unique property of priming T cells through up-regulation of the costimulatory molecules B7 and CD40 and secretion of IL-12.1 The interaction of B7 with CD28 on T cells increases the efficacy of signaling through the T-cell receptor (TCR), which correlates with T-cell activation.27,28 Disruption of the costimulatory pathways has been shown to be effective in inhibiting the pathogenic process in several models of autoimmune diseases and in allograft rejection.27,29-31 Here, we show that IVIg induced the down-regulation of costimulatory molecules on DCs and modulated cytokine secretion that resulted in the inhibition of autoreactive and alloreactive T-cell activation and proliferation. The inhibition of expression of costimulatory molecules on DCs by IVIg is thus intriguing given the critical role of costimulatory signals delivered by CD40, CD80, and CD86 for optimal T-cell activation. It is tempting to hypothesize that the beneficial effect of IVIg in autoimmune diseases and in graft-versus-host disease in allogeneic transplantation8 32-36 may be associated with the ability of IVIg to render DCs inactive and to block immune responses.
Monocytes cultured in the presence of GM-CSF and IL-4 give rise to immature DCs that undergo further phenotypic changes after stimulation with maturation signals, including TNF-α, LPS, CD40L, or engagement of Fas.37 38 Here we report on a blocking effect of IVIg on the constitutive and the LPS-mediated maturation of DCs, including reduction in the expression of surface costimulatory molecules crucial for T-cell activation. Stimulation of IVIg-pretreated DCs with LPS, however, generated a subpopulation of DCs with levels of HLA-DR, CD86, CD80, and CD40 similar to those observed on LPS-stimulated non-IVIg–treated cells. We further observed that such high levels of costimulatory molecules were restricted to individual molecules on each cell and did not extend to all costimulatory molecules required for T-cell activation. Thus, IVIg treatment of DCs resulted in heterogeneous populations of cells with respect to the nature of the expressed costimulatory molecules. The lack of simultaneous expression by DCs of all costimulatory molecules may contribute to the inhibitory effect on MLR of the pretreatment of DCs with IVIg.
The present study further demonstrates that IVIg modulates the secretion of cytokines critical for the maturation of DCs and for T-cell responses. Thus, IVIg abrogated the secretion of IL-12, whereas IL-10 secretion was up-regulated. IL-10 secretion by unstimulated DCs was enhanced 4-fold by IVIg, which was further up-regulated by 8-fold on stimulation with LPS. This latter effect of IVIg is of potential relevance considering its beneficial effect in autoimmune conditions and graft-versus host disease.32,39 Insufficient IL-12 production and decreased expression of CD80 by APCs, with concomitant increased secretion of IL-10 that in turn blocks DC maturation and inhibits IL-12 production during antigen presentation, have been implicated in the induction of anergy and tolerance of T cells.40-42 The fact that IVIg blocks phenotypic and functional maturation was further substantiated by its ability to block autologous and allogeneic MLR. Interestingly, the inhibitory effect of IVIg on autologous CD4+ T-cell proliferation (ie, up to 33-fold inhibition) was more striking than that on allogeneic MLR (ie, up to 2-fold). A direct effect of soluble IVIg on T cells is excluded because IVIg-treated DCs were washed extensively before coculture.
During the maturation process of DCs, together with costimulatory molecules the expression of CD83 is up-regulated.1,2Recent reports suggest that CD83 is not merely a marker of maturation—it also plays an essential functional role in DC-mediated immune response.43 As reported recently, soluble extracellular CD83 domain inhibits DC maturation and DC-mediated T-cell proliferation.43,44 It is unclear at this stage whether IVIg treatment of DC would down-regulate the synthesis of mRNA for CD83 or would inhibit the transport of the protein or enhance the release of the soluble form of CD83 from activated DCs. Although treatment of immature DCs with HSA, an irrelevant protein, resulted in a down-regulated expression of CD83, the cells responded well to maturation signals by LPS treatment, including the up-regulation of CD83, similar to what was observed in the presence of medium alone (control). Further, there were no differences between the 2 groups (HSA and medium alone) in cytokine secretion and MLR assays, suggesting the specificity of the effects of IVIg. Lechmann et al43observed that T-cell proliferation was inhibited when soluble extracellular CD83 was present in the culture. Given that in our setup IVIg-treated DCs were washed extensively before use for MLR assays yet we still observed MLR inhibition, we believe that the down-regulated expression of CD83, along with other costimulatory molecules, rather than a direct effect of soluble CD83 in the cultures was responsible for the inhibitory function of IVIg-treated DCs in MLR.
Taken together, the results from this and from previous studies suggest that normal circulating immunoglobulins ensure the maintenance of lymphocyte homeostasis by involving multiple mechanisms. We have previously shown that IVIg induces apoptosis of B and T lymphocytes in a Fas-dependent manner.45,46 Here, we show that IVIg down-regulates signaling molecules on DCs that are essential for rescuing T cells from Fas-mediated apoptosis.47
IVIg preparations contain variable amounts of monomers and dimers.48 The IVIg preparation (Sandoglobulin) used in the present study contained largely monomeric forms of IgG. However, as reported earlier, the therapeutic efficiency of IVIg preparations may depend on the dimers of IgG.48 49 We observed an inhibitory effect on DC function by monomer-enriched fractions of IVIg, but it is possible that dimer-enriched IVIg preparations may exert similar or even stronger effects on DCs.
We observe that the inhibitory effect of IVIg on DC maturation involves the Fc and F(ab′)2 fragments. Some of the beneficial effects of IVIg in autoimmune and other immune-mediated conditions have been attributed to Fc fragments of IVIg.50,51 Recent studies in an animal model of idiopathic thrombocytopenic purpura suggest that IVIg increases the expression of the Fcγ receptor IIB, an inhibitory receptor.51 Thus, interaction between Fc fragments of IVIg and Fc receptor may induce inhibitory effects on DC through immunoreceptor tyrosine-based inhibitory motifs. The relative expression of activation and inhibitory isoforms of FcγRII (CD32) have not yet been analyzed on human DCs. Thus, the characterization of patterns of expression and function of FcγR on human DCs should help in better understanding Fc-mediated effects of IVIg on DC.52-55
One of the F(ab′)2 dependent inhibitory effects of IVIg on DCs may involve the anticytokine (IL-4 and GM-CSF) and anti-LPS nature of IVIg. As reported earlier, pools of normal human IgG contain variable amounts of specific and high-avidity antibody to certain cytokines.18 Other lines of evidence point out that the neutralization of cytokines and LPS by antibodies in IVIg may not be the exclusive mechanism by which IVIg exerts its role. Thus, we observed no difference in the inhibitory capacity of IVIg on DCs, either when IVIg was added together with cytokines or with LPS or when it was added after preincubating the cells with cytokines (data not shown) or with LPS for 3 hours. Further, IVIg has been shown to interact with several immunologically relevant molecules, such as HLA, Fas, CD4, RGD motif of integrin molecules, and T-cell receptor,45 56-61 some of which are also expressed on DCs. By binding to some of these molecules, IVIg may participate in the modulation of function of DCs. Indeed, we observed that intact IgG and F(ab′)2 fragments of IVIg bind to DCs, indicating receptor-mediated interactions of IVIg on DCs. The distinction between Fc-dependent and variable region–dependent mechanisms remains artificial in that several functions of IVIg are amplified, or indeed made possible, by cooperative Fc binding to FcR on cells targeted by the relevant variable regions. Identification of molecules on DC surfaces that participate in the modulation of DCs by IVIg and of the ensuing signal-transduction events will further contribute to elucidate the mechanisms underlying the complex immunoregulatory effects of IVIg and will rationalize its therapeutic use.
We thank W. R. Heath of The Walter and Eliza Hall Institute of Medical Research (Parkville, Australia), Muriel Andrieu (INSERM U 445, Paris, France) for critically reading the manuscript, and S. Delignat and M. F. Belair for technical support. IVIg was a kind gift from ZLB Bioplasma AG, Bern, Switzerland.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-05-1447.
Supported by Institut National de la Santé et de la Recherche Médicale (INSERM) and Centre National de la Recherche Scientifique (CNRS) France and by a grant from ZLB Bioplasma AG, Bern, Switzerland. J.B. and C.C. are recipients of fellowships from the Ministère de l'Education Nationale. N.M. is a recipient of fellowship from Agence Nationale de Recherches sur le Sida.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Srini V. Kaveri, INSERM U 430, Institut des Cordeliers, 15, rue de l'Ecole de Médecine, 75006 Paris, France; e-mail:srini.kaveri@u430.bhdc.jussieu.fr.

![Fig. 6. Effects of IVIg treatment on DC-mediated T-cell proliferation. / IVIg treatment abrogates the capacity of DCs to stimulate autologous (A-B) and allogeneic (C-D) T cells. Immature DCs obtained by culturing monocytes for 5 days were cultured in the presence of IVIg (0.15 mM) or HSA (0.15 mM) or in the absence of IVIg (Ctl) for an additional 48 hours. Graded doses of DCs were used to stimulate autologous (A) and allogeneic (C) CD4+ T cells (1 × 105cells/well) in MLR. In a second type of experiment, immature DCs were treated for 12 hours with IVIg or HSA followed by stimulation with LPS (1 μg/mL) or treated with LPS alone for 48 hours to obtain mature DCs followed by stimulation of autologous (B) and allogeneic (D) T cells. Thymidine incorporation was measured on day 5 by a 16-hour pulse with 1 μCi (0.037 MBq) [3H]thymidine. Results are shown as means ± SDs of triplicate values. Statistical significance as determined by unpaired Student t test (*P < .05; **P < .001) is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-05-1447/4/m_h80233700006.jpeg?Expires=1769101512&Signature=kZozWKKPEk-o94XtbUcLuSbCaJWmR6wKlidfvjE82fhmGPC3rrcNiruqrenHFYI009rr-~3gogmHFqnSWZ86QMtIV-W~BtUgkixgvCUeYhtrrch1B7F4BtBkP7CXvpTSXIo~lqoeq6-8k1kGJxirp3kzCdKDN2iAte7hNkDwSo9OC1whpPy16mkW4N4D3IB0R1hilsk8LA3Ui8VA8qAPS05IAqrTiNisMbzUjjyZcN8vBMTaSIvkrD~u7Kw1orgZ5d4M2~Xq6T9uN8tE--qlSE0GicxdFBKnwHi9kq5isRNnXEqG-ul070yeVUwtewLczYLgggEfqax4zTM5vmVMZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal