The potential immunosuppressive effect of an anti-CD154 monoclonal antibody (mAb) on the pathogenic autoreactive T-cell response was evaluated using an in vitro culture system with glycoprotein IIb/IIIa (GPIIb/IIIa)–reactive T cells from patients with immune thrombocytopenic purpura (ITP). The anti-CD154 mAb did not inhibit T-cell proliferation, but suppressed anti-GPIIb/IIIa antibody production, in bulk peripheral blood mononuclear cell cultures stimulated with GPIIb/IIIa. Repeated antigenic stimulation of GPIIb/IIIa-reactive CD4+ T-cell lines in the presence of anti-CD154 mAb resulted in the loss of proliferative capacity and helper function for promoting anti-GPIIb/IIIa antibody production. These anergic T-cell lines showed a cytokine profile of low interferon γ and high interleukin 10 and suppressed anti-GPIIb/IIIa antibody production. Our results indicate that blockade of the CD40/CD154 interaction induces generation of autoantigen-specific anergic CD4+ T cells with regulatory function and could be a therapeutic option for suppressing pathogenic autoimmune responses in patients with ITP.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by increased platelet destruction caused by antiplatelet autoantibodies, which mainly target glycoprotein IIb/IIIa (GPIIb/IIIa).1,2 We have recently found that GPIIb/IIIa-reactive CD4+ T cells from patients with ITP have helper activity that promotes production of anti-GPIIb/IIIa antibodies capable of binding to normal platelets, indicating that these autoreactive T cells are involved in the pathogenic process of ITP.3-5 Therefore, the GPIIb/IIIa-reactive T cell is a reasonable target for a therapeutic strategy that selectively suppresses the pathogenic autoimmune response in patients with ITP. One candidate strategy is the disruption of a costimulatory signal by blocking the interaction between CD40 on antigen-presenting cells (APCs) and CD154 (also known as CD40 ligand) on activated CD4+ T cells; this interaction is essential for the T cell–dependent humoral immune response.6,7 The efficacy of blocking this interaction therapeutically with anti-CD154 monoclonal antibody (mAb) has been shown in animal models for various autoimmune diseases.8-10 Thus, blockade of the CD40/CD154 interaction has been proposed as a strategy for treating autoimmune diseases11 and is currently being used in preclinical and clinical studies.12,13 In animal models, the CD40/CD154 blockade inhibits the expansion and effector functions of pathogenic autoreactive T cells9,10 and even induces long-term antigen-specific tolerance,14 although the details of the immunoregulatory action remain to be elucidated. In this study, to examine the potential of applying CD40/CD154-targeted intervention to the treatment of ITP, we investigated the ability of anti-CD154 mAb to suppress the T-cell response to GPIIb/IIIa in patients with ITP using in vitro culture systems.
Study design
Patients
We studied cells from 5 patients with ITP (ITP1, 10, 11, 14, and 19), all of whom had been enrolled in our previous study.4All samples were obtained after the patients gave their written informed consent, approved by the Institutional Review Board of Keio University School of Medicine, Tokyo.
Effects of anti-CD154 mAb on GPIIb/IIIa-induced T-cell responses in bulk T-cell cultures
Peripheral blood mononuclear cells (PBMCs) were used to analyze the direct effects of anti-CD154 mAb on T-cell proliferation and anti-GPIIb/IIIa antibody production induced by trypsin-digested human GPIIb/IIIa.3 IgG anti-GPIIb/IIIa antibodies in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using purified human GPIIb/IIIa as an antigen.3 Serial dilutions of anti-CD154 mAb (0.25-2 μg/mL; Ancell, Bayport, MN), or anti–HLA-DR, anti–HLA-DQ, or isotype-matched control mAb (1 μg/mL; Leinco Technologies, Ballwin, MO) were added at the initiation of the cultures.15
Repeated treatment of GPIIb/IIIa-reactive T-cell lines with anti-CD154 mAb
We used 2 GPIIb/IIIa-reactive CD4+T-cell lines, SiM4 and WY9, generated from ITP14 and ITP10, respectively. These lines had a Th0 cytokine profile with the capacity to induce production of anti-GPIIb/IIIa antibodies that bound to intact platelets.4 SiM4 and WY9 recognized recombinant glutathione S-transferase fusion proteins encompassing amino acids 18-259 of GPIIbα (IIbα18-259) and 22-262 of GPIIIa (IIIa22-262), respectively, in an HLA-DR–restricted manner.4 Two tetanus toxoid (TT)–reactive CD4+ T-cell lines generated from a healthy donor were used as controls.16 After being rested for 10 days, T-cell lines (2 × 105) were stimulated with IIbα11-259, IIIa22-262, or TT (5 μg/mL), interleukin 2 (IL-2; 50 U/mL), and irradiated autologous lymphoblastoid B-cell line (106) as APCs for 7 days in the presence of anti-CD154 or isotype-control mAb (2 μg/mL). This treatment was repeated for up to 5 rounds. The viable T cells were recovered and subsequently examined for their ability to proliferate and promote anti-GPIIb/IIIa antibody production from autologous B cells in response to antigenic stimulation.4 In some experiments, anti-CD154 mAb-treated GPIIb/IIIa-reactive T-cell line WY9 was washed twice, serially diluted, and added to cultures of untreated WY9 and autologous B cells to evaluate its effect on anti-GPIIb/IIIa antibody production in the presence or absence of anti–IL-10 mAb (10 μg/mL; R & D Systems, Minneapolis, MN). To determine cytokine profiles of GPIIb/IIIa-reactive T-cell lines treated with anti-CD154 or isotype-control mAb, T cells were stimulated with phorbol myristate acetate and ionomycin for 48 hours, and the amounts of interferon γ (IFN-γ), IL-2, IL-4, IL-6, and IL-10 in culture supernatants were measured using commercial ELISA kits (Biosource International, Camarillo, CA).16
Statistical analysis
All comparisons were tested for statistical significance using the Mann-Whitney U test.
Results and discussion
The effects of anti-CD154 mAb on T-cell responses induced by trypsin-digested GPIIb/IIIa were examined in PBMC cultures from 5 patients with ITP, and similar results were obtained from all samples. As shown in Figure 1A-B, anti-CD154 mAb had no effect on the proliferative response of T cells with GPIIb/IIIa, but inhibited anti-GPIIb/IIIa antibody production in a dose-dependent manner. A similar inhibitory effect of anti-CD154 mAb on in vitro T cell–dependent antibody production was reported for an anti-TT antibody response in human splenocyte cultures17 as well as autoantibody responses in patients with autoimmune diseases.15,18 19
Effects of anti-CD154 mAb on GPIIb/IIIa-induced T-cell responses in bulk PBMC cultures and GPIIb/IIIa-reactive CD4+ T-cell lines.
(A) PBMCs from patient ITP14 were stimulated with trypsin-digested GPIIb/IIIa (5 μg/mL) for 7 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.5, 1, and 2 μg/mL), or isotype-matched control mAb (2 μg/mL), and [3H]thymidine incorporation was measured by liquid scintillation counting. The results shown are the means ± SDs of quadruplicate values. Asterisk indicates significant inhibition of T-cell proliferation by the mAb treatment in comparison with the culture with isotype-control mAb. (B) PBMCs from ITP14 were cultured with trypsin-digested GPIIb/IIIa (5 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.25, 0.5, and 1 μg/mL), or isotype-matched control mAb (1 μg/mL), and IgG anti-GPIIb/IIIa antibody levels were measured by ELISA. The results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of IgG anti-GPIIb/IIIa antibody production by the mAb treatment compared with the culture with isotype-matched control mAb. (C) GPIIb/IIIa-reactive CD4+ T-cell lines SiM4 and WY9 (2 × 105) were repeatedly treated with irradiated autologous lymphoblastoid B-cell line (106), recombinant fragment (IIbα18-252 for SiM4 or IIIa22-262 for WY9; 5 μg/mL), and IL-2 (50 U/mL) in the presence of anti-CD154 or isotype-control mAb (2 μg/mL), and examined for their capacity to proliferate on stimulation with the antigenic recombinant fragment or glutathione S-transferase in quadruplicate cultures. Results are expressed as a stimulation index, which was calculated as the mean counts per minute incorporated into cultures with the antigenic recombinant fragment divided by the mean counts per minute incorporated into the cultures with glutathione S-transferase. A result representative of 3 independent experiments is shown. (D) Lines SiM4 and WY9, which were repeatedly treated with anti-CD154 or isotype-matched control mAb, were examined for their capacity to stimulate autologous B cells to produce IgG anti-GPIIb/IIIa antibodies on stimulation with IIbα18-252 (SiM4) or IIIa22-262 (WY9). Results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of anti-GPIIb/IIIa antibody production by treatment with anti-CD154 mAb compared with the treatment with isotype-control mAb. The IgG anti-GPIIb/IIIa antibody levels in cultures of B cells alone were 0.020 ± 0.005 (SiM4) and 0.085 ± 0.011 (WY9). The results were similar in 3 independent experiments.
Effects of anti-CD154 mAb on GPIIb/IIIa-induced T-cell responses in bulk PBMC cultures and GPIIb/IIIa-reactive CD4+ T-cell lines.
(A) PBMCs from patient ITP14 were stimulated with trypsin-digested GPIIb/IIIa (5 μg/mL) for 7 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.5, 1, and 2 μg/mL), or isotype-matched control mAb (2 μg/mL), and [3H]thymidine incorporation was measured by liquid scintillation counting. The results shown are the means ± SDs of quadruplicate values. Asterisk indicates significant inhibition of T-cell proliferation by the mAb treatment in comparison with the culture with isotype-control mAb. (B) PBMCs from ITP14 were cultured with trypsin-digested GPIIb/IIIa (5 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.25, 0.5, and 1 μg/mL), or isotype-matched control mAb (1 μg/mL), and IgG anti-GPIIb/IIIa antibody levels were measured by ELISA. The results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of IgG anti-GPIIb/IIIa antibody production by the mAb treatment compared with the culture with isotype-matched control mAb. (C) GPIIb/IIIa-reactive CD4+ T-cell lines SiM4 and WY9 (2 × 105) were repeatedly treated with irradiated autologous lymphoblastoid B-cell line (106), recombinant fragment (IIbα18-252 for SiM4 or IIIa22-262 for WY9; 5 μg/mL), and IL-2 (50 U/mL) in the presence of anti-CD154 or isotype-control mAb (2 μg/mL), and examined for their capacity to proliferate on stimulation with the antigenic recombinant fragment or glutathione S-transferase in quadruplicate cultures. Results are expressed as a stimulation index, which was calculated as the mean counts per minute incorporated into cultures with the antigenic recombinant fragment divided by the mean counts per minute incorporated into the cultures with glutathione S-transferase. A result representative of 3 independent experiments is shown. (D) Lines SiM4 and WY9, which were repeatedly treated with anti-CD154 or isotype-matched control mAb, were examined for their capacity to stimulate autologous B cells to produce IgG anti-GPIIb/IIIa antibodies on stimulation with IIbα18-252 (SiM4) or IIIa22-262 (WY9). Results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of anti-GPIIb/IIIa antibody production by treatment with anti-CD154 mAb compared with the treatment with isotype-control mAb. The IgG anti-GPIIb/IIIa antibody levels in cultures of B cells alone were 0.020 ± 0.005 (SiM4) and 0.085 ± 0.011 (WY9). The results were similar in 3 independent experiments.
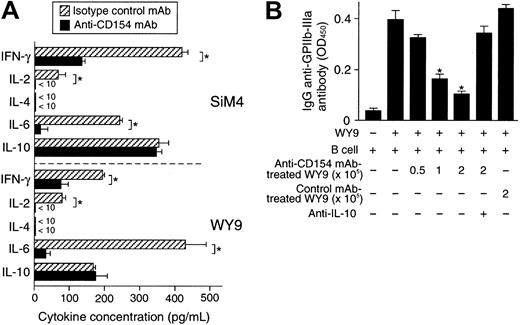
It has been proposed that failure to deliver the essential costimulatory signal during the T cell-APC interaction would result in a state of T-cell anergy, a cellular state in which T cells fail to proliferate when optimally restimulated by an antigen.20To test whether blockade of the CD40/CD154 interaction induces an anergic state in autoreactive T cells, GPIIb/IIIa-reactive T-cell lines were repeatedly stimulated by a GPIIb/IIIa fragment, APCs, and anti-CD154 mAb. The number of viable cells recovered apparently decreased after 2 rounds of the anti-CD154 mAb treatment, and too few T cells to analyze were recovered after 4 rounds of treatment. As shown in Figure 1C, antigen-specific proliferation of GPIIb/IIIa-reactive T cells gradually decreased after repeated anti-CD154 mAb treatment, but not after treatment with isotype-matched control mAb. The anti-CD154 mAb-treated T cells lost their helper activity for promoting anti-GPIIb/IIIa antibody production (Figure 1D). The treatment of GPIIb/IIIa-reactive T cells with TT or without antigen and the treatment of TT-reactive T-cell lines with IIbα18-259 failed to induce an anergic state, indicating that anti-CD154 mAb-induced T-cell anergy is antigen dependent and antigen specific. In GPIIb/IIIa-reactive T-cell lines treated twice with anti-CD154 mAb, production of IFN-γ, IL-2, and IL-6 was greatly reduced, but IL-10 production was preserved (Figure 2A), resulting in a cytokine profile with low IFN-γ and high IL-10, similar to the profile of CD4+ regulatory T cells.21 The anergic GPIIb/IIIa-reactive T-cell line WY9 inhibited anti-GPIIb/IIIa antibody production mediated by untreated WY9 (Figure 2B). Neutralization of IL-10 by anti–IL-10 mAb abolished the suppressive effect of the anti-CD154 mAb-treated anergic T cells, indicating that the effector function of GPIIb/IIIa-reactive CD4+ T cells could be suppressed by bystander GPIIb/IIIa-reactive anergic T cells, partly through the relatively augmented IL-10 production.
Cytokine profiles and regulatory function of GPIIb/IIIa-reactive CD4+ T-cell lines treated twice with anti-CD154 mAb.
GPIIb/IIIa-reactive CD4+ T-cell lines SiM4 and WY9 were cultured twice with autologous lymphoblastoid B-cell line, antigenic recombinant fragment, and IL-2 in the presence of anti-CD154 or isotype-matched control mAb. (A) The T-cell lines treated with anti-CD154 or isotype-matched control mAb (2 × 105) were stimulated with phorbol myristate acetate (1 μg/mL) and ionomycin (25 ng/mL) for 48 hours, and the levels of IFN-γ, IL-2, IL-4, IL-6, and IL-10 in the culture supernatants were measured by ELISA. The results shown are the means ± SDs of triplicate values. Asterisks indicate significant differences in cytokine levels between the anti-CD154 mAb–treated and isotype-matched control mAb–treated T-cell lines. The results of 1 of 2 experiments with similar results are shown. (B) Line WY9 (3 × 105) was cultured with autologous B cells (3 × 105), IIIa22-262 (5 μg/mL), and pokeweed mitogen (1 μg/mL) for 10 days in the presence or absence of serially diluted WY9-treated twice with anti-CD154 mAb (0.5 to 2 × 105) or treated with isotype-matched control mAb (2 × 105). Anti–IL-10 mAb (10 μg/mL) was added at the start of some cultures. IgG anti-GPIIb/IIIa antibodies in culture supernatants were measured by ELISA. The results shown are the means ± SDs of duplicate values. Asterisks indicate significant difference between anti-GPIIb/IIIa antibody levels in cultures with and without the anti-CD154 mAb–treated WY9 cells. The results of 1 of 2 experiments with similar results are shown.
Cytokine profiles and regulatory function of GPIIb/IIIa-reactive CD4+ T-cell lines treated twice with anti-CD154 mAb.
GPIIb/IIIa-reactive CD4+ T-cell lines SiM4 and WY9 were cultured twice with autologous lymphoblastoid B-cell line, antigenic recombinant fragment, and IL-2 in the presence of anti-CD154 or isotype-matched control mAb. (A) The T-cell lines treated with anti-CD154 or isotype-matched control mAb (2 × 105) were stimulated with phorbol myristate acetate (1 μg/mL) and ionomycin (25 ng/mL) for 48 hours, and the levels of IFN-γ, IL-2, IL-4, IL-6, and IL-10 in the culture supernatants were measured by ELISA. The results shown are the means ± SDs of triplicate values. Asterisks indicate significant differences in cytokine levels between the anti-CD154 mAb–treated and isotype-matched control mAb–treated T-cell lines. The results of 1 of 2 experiments with similar results are shown. (B) Line WY9 (3 × 105) was cultured with autologous B cells (3 × 105), IIIa22-262 (5 μg/mL), and pokeweed mitogen (1 μg/mL) for 10 days in the presence or absence of serially diluted WY9-treated twice with anti-CD154 mAb (0.5 to 2 × 105) or treated with isotype-matched control mAb (2 × 105). Anti–IL-10 mAb (10 μg/mL) was added at the start of some cultures. IgG anti-GPIIb/IIIa antibodies in culture supernatants were measured by ELISA. The results shown are the means ± SDs of duplicate values. Asterisks indicate significant difference between anti-GPIIb/IIIa antibody levels in cultures with and without the anti-CD154 mAb–treated WY9 cells. The results of 1 of 2 experiments with similar results are shown.
Several mechanisms for the in vivo suppressive effects of anti-CD154 mAb on the specific T-cell response have been proposed, including suppression of Th1-type immune response,9,10up-regulation of cytotoxic T lymphocyte–associated antigen 4 on T cells,10 and induction of regulatory cells sharing properties of natural killer and dendritic cells.22 Here, using a human in vitro culture system, we found a novel mechanism, that is, suppression of T-cell effector function through the induction of antigen-specific anergic T cells with potential regulatory function. The in vitro suppressive effect of anti-CD154 mAb on the pathogenic GPIIb/IIIa-reactive T-cell responses strongly suggests potential utility of CD40/CD154-targeted immune interventions in therapy for ITP and other autoimmune diseases. However, anti-CD154 mAb has been reported to induce thromboembolic events in primates and humans.23 Therefore, when anti-CD154 mAb is used therapeutically, we have to pay special attention to its potential effects on formation and stabilization of thrombi, which are mediated by CD154 expressed on activated platelets.24 25
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-07-2157.
Supported by the Keio University Medical Science Fund, by a grant from the Japanese Ministry of Health and Welfare, and by the Terumo Life Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masataka Kuwana, Institute for Advanced Medical Research, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail:kuwanam@sc.itc.keio.ac.jp.

![Fig. 1. Effects of anti-CD154 mAb on GPIIb/IIIa-induced T-cell responses in bulk PBMC cultures and GPIIb/IIIa-reactive CD4+ T-cell lines. / (A) PBMCs from patient ITP14 were stimulated with trypsin-digested GPIIb/IIIa (5 μg/mL) for 7 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.5, 1, and 2 μg/mL), or isotype-matched control mAb (2 μg/mL), and [3H]thymidine incorporation was measured by liquid scintillation counting. The results shown are the means ± SDs of quadruplicate values. Asterisk indicates significant inhibition of T-cell proliferation by the mAb treatment in comparison with the culture with isotype-control mAb. (B) PBMCs from ITP14 were cultured with trypsin-digested GPIIb/IIIa (5 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days in the presence or absence of anti–HLA-DR (1 μg/mL), anti–HLA-DQ (1 μg/mL), anti-CD154 (0.25, 0.5, and 1 μg/mL), or isotype-matched control mAb (1 μg/mL), and IgG anti-GPIIb/IIIa antibody levels were measured by ELISA. The results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of IgG anti-GPIIb/IIIa antibody production by the mAb treatment compared with the culture with isotype-matched control mAb. (C) GPIIb/IIIa-reactive CD4+ T-cell lines SiM4 and WY9 (2 × 105) were repeatedly treated with irradiated autologous lymphoblastoid B-cell line (106), recombinant fragment (IIbα18-252 for SiM4 or IIIa22-262 for WY9; 5 μg/mL), and IL-2 (50 U/mL) in the presence of anti-CD154 or isotype-control mAb (2 μg/mL), and examined for their capacity to proliferate on stimulation with the antigenic recombinant fragment or glutathione S-transferase in quadruplicate cultures. Results are expressed as a stimulation index, which was calculated as the mean counts per minute incorporated into cultures with the antigenic recombinant fragment divided by the mean counts per minute incorporated into the cultures with glutathione S-transferase. A result representative of 3 independent experiments is shown. (D) Lines SiM4 and WY9, which were repeatedly treated with anti-CD154 or isotype-matched control mAb, were examined for their capacity to stimulate autologous B cells to produce IgG anti-GPIIb/IIIa antibodies on stimulation with IIbα18-252 (SiM4) or IIIa22-262 (WY9). Results shown are the means ± SDs of duplicate values. Asterisks indicate significant inhibition of anti-GPIIb/IIIa antibody production by treatment with anti-CD154 mAb compared with the treatment with isotype-control mAb. The IgG anti-GPIIb/IIIa antibody levels in cultures of B cells alone were 0.020 ± 0.005 (SiM4) and 0.085 ± 0.011 (WY9). The results were similar in 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-07-2157/4/m_h80233683001.jpeg?Expires=1769258594&Signature=Dgy3yPMI5v6CI4vXfH38Wolsxx37~Kf9NLNJSVf~jofAUIp-aKMRsl4zDNT43czbZ2~vLtezjIKrKm8N3mdJOG7xOmrmd2Z-1fjmca7MIoNB-yshk1xMrV6E49vPrjkTZ~3m4D6EfgOrUOpjdyjgTmg7dBQmk3ZXoKDtm3lM9h2fMDtrtAMikPCkm0-n3L~7hbbJhPgZd8oHB5nCca7mwlzIxA-k7V5hXxc4ZDM38pmI58oz~6zn7i9K6LRqUSk8tkLG8b-OUWSzpeHgTAxUIpzu8VREr-UDfimQrSBagLtD6GU23r~G~IWjUNU95XVwQheKz~P4xsI5CbWb3qjJlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal