Under steady-state conditions, internalization of self-antigens embodied in apoptotic cells by dendritic cells (DCs) resident in peripheral tissue followed by DC migration and presentation of self-peptides to T cells in secondary lymphoid organs are key steps for induction and maintenance of peripheral T-cell tolerance. We show here that, besides this traffic of apoptotic cells mediated by peripheral tissue–resident DCs, splenic marginal zone DCs rapidly ingest circulating apoptotic leukocytes, process apoptotic cell–derived peptides into major histocompatibility complex class II (MHC-II) molecules, and acquire CD8α during their mobilization to T-cell areas of splenic follicles. Because apoptotic cells activate complement and some complement factors are opsonins for phagocytosis and play roles in the maintenance of peripheral tolerance, we investigated the role of complement receptors (CRs) in relation to phagocytosis of apoptotic cells by DCs. Apoptotic cell uptake by marginal zone DCs was mediated in part via CR3 (CD11b/CD18) and, to a lesser extent, CR4 (CD11c/CD18) and was reduced significantly in vivo in hypocomplementemic animals. Following phagocytosis of apoptotic cells, DCs exhibited decreased levels of mRNA and secretion of the proinflammatory cytokines interleukin 1α (IL-1α), IL-1β, IL-6, IL-12p70, and tumor necrosis factor α (TNF-α), without effect on the anti-inflammatory mediator transforming growth factor β1 (TGF-β1). This selective inhibitory effect was at least partially mediated through C3bi-CD11b/CD18 interaction. Characterization of apoptotic cell/DC interaction and its outcome provides insight into the mechanisms by which apoptotic cells affect DC function without disrupting peripheral tolerance.
Introduction
Continual transport of self-antigen (self-Ag) by migrating dendritic cells (DCs) to secondary lymphoid organs is a crucial step for induction/maintenance of peripheral tolerance.1 Apoptotic cells resulting from cellular turnover in peripheral tissues are an important source of self-Ag. Immature DCs endocytose apoptotic cells in vitro and DC-bearing apoptotic cells have been detected in vaginal epithelium and intestinal lamina propria in rodents.2-6 The observation by Huang et al6 that intestinal DC-bearing apoptotic epithelial cell fragments traffic to mesenteric lymph nodes under steady-state conditions suggests that endocytosis of apoptotic cells by DCs in peripheral tissues followed by transport and presentation of self-peptides to naive T lymphocytes in secondary lymphoid organs may be involved in T-cell peripheral tolerance. There are possible reasons to explain why, under steady-state conditions, endocytosis and processing of apoptotic cells by antigen-presenting cells (APCs) does not disrupt self-tolerance: (1) interaction with apoptotic cells does not induce DC maturation,7-9 and (2) ingestion of apoptotic cells by macrophages decreases secretion of proinflammatory factors and induces release of the anti-inflammatory interleukin 10 (IL-10) and transforming growth factor β1 (TGF-β1).10-14 We show here for the first time, that besides DCs migrating from peripheral tissues, DCs within the splenic marginal zone (MZ) internalize circulating apoptotic leukocytes efficiently in vivo. Following phagocytosis of these allogeneic cells, MZ DCs process allopeptides into major histocompatibility complex class II (MHC-II) molecules and acquire CD8α during their migration to the T-cell area of the follicle.
Although there is extensive information about the receptors used by macrophages during phagocytosis of apoptotic cells, little is known about the mechanisms used by DCs. The integrins, αvβ3 and αvβ5,and CD36 (the thrombospondin receptor) have been shown to play a role during internalization of apoptotic cells by DCs.2,4Myeloid DCs express high levels of the complement receptors (CRs) 3 (CD11b/CD18) and 4 (CD11c/CD18). Both (mainly CR3) are receptors for C3bi, an opsonin present on the surface of apoptotic bodies as a result of complement activation induced by the same apoptotic cells.15,16 Evidence indicates that C3bi is required for internalization of apoptotic cells by macrophages and for induction of immunosuppression and tolerance in certain murine models.15-17 These observations led us to investigate the role played by C3bi-CD11b/CD18 interaction during internalization of apoptotic cells by DCs and the effect on cytokine profile produced by DCs. Our results show that internalization of apoptotic cells by MZ DCs is mediated in part via CD11b/CD18 and, to a lesser extent CD11c/CD18, and is dependent on the presence of complement factors in vivo. After interaction with apoptotic cells, DCs secrete lower amounts of IL-1α, IL-1β, IL-6, IL-12p70, and tumor necrosis factor α (TNF-α) but maintain high levels of TGF-β1, IL-1 receptor antagonist (IL-1ra), and macrophage migration inhibitory factor (MIF) mRNAs. These cytokine changes occur at the level of mRNA transcription or stability. Interaction of C3bi deposited on the surface of apoptotic cells with the β2 integrin CD11b/CD18 on the DC surface is one of the mechanisms responsible for the cytokine switch.
Materials and methods
Mice
Ten- to 12-week-old C57BL/10 (B10; H2Kb, IAb, IEneg, H2Db) and BALB/c (H2Kd, IAd, IEd, H2Dd) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in the pathogen-free Central Animal Facility of the University of Pittsburgh Medical Center. Hypocomplementemia was induced by 3 intraperitoneal injections (8 hours apart) of 4 U/mouse/dose cobra venom factor (CVF) (Quidel, Santa Clara, CA), 18 hours before intravenous (tail vein) administration of apoptotic cells. Controls animals received 3 intraperitoneal injections of phosphate-buffered saline (PBS). Studies were approved by the Institutional Animal Care and Use Committee of the American Association for Accreditation of Laboratory Animal Care (AAALAC).
Reagents
Murine recombinant granulocyte-macrophage colony-stimulating factor (mrGM-CSF) was a gift from the Schering-Plough Research Institute (Kenilworth, NJ), and murine recombinant IL-4 (mrIL-4) was purchased from R & D Systems (Minneapolis, MN). Cytochalasin D, PKH67, and PKH26 were from Sigma (St Louis, MO). The annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit was from BD Pharmingen (San Diego, CA). Y-Ae mAb was kindly provided by Dr Charles A. Janeway (Yale University).
DCs
The method for isolation of spleen DCs was modified from that described by Inaba et al.18 19 Briefly, B10 spleens were flushed with 100U/mL collagenase (type IV, Sigma), teased apart with fine forceps, and digested with 400U/mL collagenase (30 minutes at 37°C). After digestion, cells were diluted in ice-cold Ca++-free 0.01 M EDTA (ethylenediaminetetraacetic acid) Hanks balanced salt solution (HBSS). Splenic DC-enriched suspensions (20%-30% DC purity) were obtained by gradient centrifugation (1800 rpm, 20 minutes at 4°C) over 16% (wt/vol) metrizamide (Sigma). Thereafter, DCs were washed with ice-cold Ca++-free 0.01 M EDTA HBSS, then labeled with bead-conjugated anti-CD11c monoclonal antibody (mAb; clone N418, Miltenyi Biotec, Auburn, CA), and immunobead sorted by positive selection using VS+ separation columns (Miltenyi Biotec). At the end of the procedure, the DC purity was 92% or greater assessed by CD11c and IAhi expression by 2-color flow cytometry.
Bone marrow (BM) DCs were generated as described.20Briefly, BM cells were removed from femurs and tibias of B10 mice and depleted of erythrocytes by hypotonic lysis. Erythroid precursors, T and B lymphocytes, natural killer (NK) cells, granulocytes, and IA+ cells were removed by complement depletion using a cocktail of mAbs (anti–TER-119, anti-CD3ε, anti-B220, anti–NK-1.1, anti-Gr1, and anti-IAb; BD Pharmingen) followed by incubation (45 minutes, 37°C) with low-toxicity rabbit complement (Cedarlane, Hornby, ON, Canada). Remaining BM cells were cultured in RPMI 1640 (Life Technologies, Grand Island, NY) in 75-cm2 flasks (5 × 106cells/flask) with 10% vol/vol heat-inactivated fetal calf serum (FCS; Life Technologies), glutamine, nonessential amino acids, sodium pyruvate, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2-mercaptoethanol (2-ME), and penicillin/streptomycin, supplemented with mrGM-CSF (1000 U/mL) and mrIL-4 (1000 U/mL). Culture medium and exogenous cytokines were renewed every other day.
Phagocytosis of apoptotic cells
BALB/c splenocytes were used as a source of apoptotic cells. Apoptosis was induced by UVB irradiation (ULTRA-LUM, Claremont, CA, lamp model UVB16). After culture in RPMI 1640 (or PBS) the percentage of splenocytes undergoing early apoptosis (annexin V+, propidium iodide–negative [PI−] cells) increased from 25% (1 hour after UV irradiation) to 85% (3 hours after UV irradiation) with less than 10% of cells in late apoptosis (annexin V+ PI+ cells). To avoid accumulation of cells in late apoptosis for in vitro studies, apoptotic splenocytes were mixed with DCs no longer than 1 hour after UV irradiation. For in vivo analysis, apoptotic cells were injected 3 hours following UV irradiation. For most of the in vitro studies, BALB/c splenocytes were labeled (red) with PKH26 before UV irradiation and then mixed with PKH67-GL–labeled (green), immunobead-sorted (B10) splenic or BM DCs. Apoptotic splenocytes and DCs were incubated in round-bottom polypropylene tubes at 5:1 or 2:1 ratios for 2 hours in the absence of serum or in the presence of different concentrations of normal or heat-inactivated (56°C, 45 minutes) B10 mouse serum. Thereafter, the cells were washed with ice-cold 0.1% sodium azide/PBS and fixed with 2% (vol/vol) paraformaldehyde. The percentage of green DCs with internalized/attached red apoptotic cells (double-positive cells) was analyzed by flow cytometry. For blocking experiments, DCs were preincubated (30 minutes at 4°C) with 10 or 25 μg/mL of each of the following azide-free/low-endotoxin mAbs (all from BD Pharmingen): anti-CD11b (clone M1/70), anti-CD11c (HL3), anti-CD18 (clone GAME-46), anti-CD14 (clone rmC5-3), anti-CD21/CD35 (clone 7G6), anti-CD35 (clone-8C12), anti-CD51 (clone H9.2B8), anti-CD61 (clone2G9.G2), or IgG control.
Immunofluorescence staining of tissue sections
Spleen blocks were embedded in Tissue-Tek OCT (Miles Laboratories, Elkhart, IN), snap frozen in isopentane/liquid nitrogen, and stored at −80°C. Cryostat sections (8 μm) and cytospins (Shandon cytocentrifuge; 230g) were fixed in 96% ethanol (10 minutes), blocked with 10% normal goat serum, and incubated overnight (4°C) with each of the following biotin-mAbs: anti-CD11c, anti-CD11b, anti-CD8α, anti-H2Dd (all from BD Pharmingen), anti–MOMA 1 (Bachem, King of Prussia, PA), anti-F4/80 (Bachem), or anti–ER-TR9 (Bachem). As a second step, slides were incubated with 1:3000 Cy3-streptavidin (Jackson Immunoresearch Lab, West Grove, PA), for 30 minutes at room temperature. Cell nuclei were stained with DAPI (4,6 diamidino-2-phenylindole; Molecular Probes, Eugene, OR). For some experiments, cytospins were stained with FITC-TUNEL (In Situ Cell Death Detection Kit, FITC, Roche, Indianapolis, IN) following by Cy3 anti-CD11c or Cy3 anti-H2Dd. Slides were fixed in 2% paraformaldehyde, mounted in glycerol/PBS, and examined with a Zeiss Axiovert 135 microscope equipped with appropriate filters and a cooled CCD camera (Photometrics CH250, Tucson, AZ). Signals from different fluorochromes were acquired independently, and montages edited using the Adobe Photoshop software program (Adobe Systems, Mountain View, CA).
Confocal and 2-photon confocal microscopy
CD11c+ immunobead-sorted splenic DCs from mice injected with PKH67-apoptotic cells that had been attached to poly-l-lysine–treated slides were fixed with 2% paraformaldehyde, labeled with Cy3-CD11c. They were imaged with a Leica TCS-NT confocal microscope (Leica Microsystems, Deerfield, IL) with filters for detection of Cy3 and FITC at 1024 × 1024 resolution with a section interval of 0.8 μm. Two-photon microscopy was used to analyze the intrasplenic localization of PKH67-BALB/c apoptotic cells in B10 recipients. Then, 3-mm3 spleen fragments were fixed with 2% paraformaldehyde for 2 hours. Single x-y–axis images were acquired using a multiphoton laser scanning confocal microscope comprising a titanium-sapphire ultrafast tunable laser (Coherent Mira Model 900-F), Olympus Fluoview confocal scanning electronics, an Olympus IX70 inverted system microscope, and custom built input-power attenuation and external photomultiplier detection systems. Single-plane image acquisition used 2-photon excitation at 870 nm with an Olympus 20 ×, UApo 0.7NA water immersion objective.
RPA
The analysis of cytokine mRNAs transcribed by DCs was performed by RNAse protection assay (RPA) as described.20 RNA was isolated using a total RNA Isolation Kit (BD Pharmingen) from 5 × 106 BM DCs (per experimental variable) purified by negative selection by immunomagnetic sorting. The RPA was performed using the RiboQuant Multi-Probe RPA System (BD Pharmingen). Two multiprobe customized template kits containing cDNAs encoding mouse IL-1α, IL-1β, IL-1ra, IL-4, IL-6, IL-10, IL-12p35, IL-12p40, interferon α (IFN-α), IFN-β, IFN-γ, TNF-α, TGFβ1, GM-CSF, MIF, and the housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as templates for the T7 polymerase-directed synthesis of γ-32P]-UTP–labeled antisense RNA probes. Hybridization (16 hours at 56°C) of 5 μg of each target mRNA with the antisense RNA probe sets was followed by RNAse and proteinase K treatment, phenol-chloroform extraction, and ammonium acetate precipitation of protected RNA duplexes. In each RPA, the corresponding antisense RNA probe set (3 × 103 cpm) was included as molecular weight standard, and to examine probe set integrity. Mouse RNA (positive control) and RNA degradation controls were included. Yeast tRNA served as negative control. Samples were electrophoresed on acrylamide-urea sequencing gels. Dried gels were exposed on Fujifilm x-ray film, at −80°C. Quantification of bands was performed by densitometry (Personal densitometers 1; Molecular Dynamics, Sunnyvale, CA). The signals from specific mRNAs were normalized to signals from housekeeping genes (L32 and GADPH) run on each lane to adjust for loading differences.
Rosetting and polystyrene bead assays
Erythocytes bearing IgM and mouse C3bi were prepared as described.21,22 Briefly, sheep erythrocytes (Colorado Serum, Denver, CO) were washed 3 times in veronal-buffered saline (VBS, Sigma), and a 5% vol/vol erythrocyte suspension incubated with a 1:100 subagglutinating dilution of antisheep red blood cell IgM mAb (Cedarlane). The sensitized erythrocytes (EAs) were washed in VBS and a 5% EA suspension incubated with one tenth mouse (B10) serum at 37°C for 30 minutes. Under these conditions, most of the bound C3 generated is primarily in the C3bi form.23 In preliminary experiments (not shown), 90% to 95% of immature BM DCs rosetted C3biEAs (and did not bind EAs), a phenomenon blocked by anti-CD11b (M1-70) mAb. Based on the fact that spleen and BM DCs do not express CR for C1q, C3b, C3d, C3dg, or C4b (ie, C1qR,24 CR1, and CR2) and that the I domain of CD11b (blocked by M1/70 mAb) is the main C3bi receptor expressed by DCs, these results confirmed the presence of murine C3bi on C3biEAs (no commercial mAb against mouse C3bi is available). DCs were incubated with C3biEAs or EAs (erythrocyte/DC ratio = 25:1) for 16 hours at 37°C in culture medium with 2% wt/vol bovine serum albumin (BSA fraction V; protease free, Roche) and 0.5 mg/mL soybean trypsin inhibitor (Sigma). Polystyrene beads with bound anti–rat IgG (Dynabeads M-450; Dynal, Lake Success, NY) were coated with 10 μg/mL anti-CD11b (M1/70), anti-CD18 (GAME-46), anti-CD11a (leukocyte function associated antigen 1 [LFA-1]), or irrelevant IgG in PBS. DCs were incubated with mAb-coated beads (bead/DC ratio = 8:1) first for 3 hours at 4°C and then for 13 hours at 37°C.
Statistical analysis
Results are expressed as mean ± SD. Comparisons between different means were performed by ANOVA, followed by the Student Newman Keuls test. Comparison between 2 means was performed by Student ttest. A P < .05 was considered significant.
Results
Preferential uptake of apoptotic cells by spleen MZ DCs
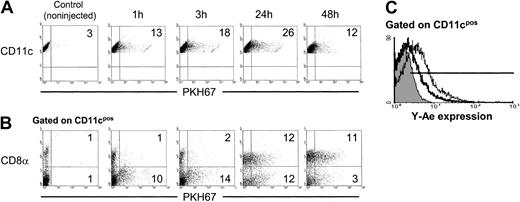
The kinetics of phagocytosis of apoptotic cells by splenic DCs was analyzed in vivo. As early as 1 hour after intravenous injection of 2 × 107 PKH67 apoptotic (BALB/c) splenocytes in allogeneic (B10) recipients, 10% to 15% of splenic CD11c+DCs internalized apoptotic cells as determined by flow cytometry, with maximum levels of endocytosis 24 hours after apoptotic cell injection (Figure 1A). After 1 hour or 3 hours of injection, CD8α− DCs were the main DC subset that had internalized apoptotic cells. Whereas the percentage of CD8α+ DCs with apoptotic cell inclusions increased after 24 hours, a proportional decrease in the number of CD8α−DCs with apoptotic cells was observed (Figure 1B). This effect may be ascribed to either up-regulation of CD8α by CD8α− DCs, or to transfer of PKH67-apoptotic cells from CD8α− to CD8α+ DCs. The capacity of spleen DCs to process and present allopeptides contained in the apoptotic splenocytes was examined with Y-Ae mAb. This recognizes the IAb(B10)–IEα52-68 peptide (IE from BALB/c) complex.25 Y-Ae positivity was detected in splenic DCs from animals intravenously injected with BALB/c apoptotic splenocytes at 24 hours (35% ± 10% of splenic DCs) and at 48 hours (62% ± 15% of splenic DCs) after injection (4 independent experiments) and not in DCs from B10 mice injected with IEα− (B10) apoptotic leukocytes (Figure 1C).
Kinetics of apoptotic cell uptake by splenic DCs in vivo.
(A) A quantity of 2 × 107 PKH67-labeled BALB/c apoptotic splenocytes was injected intravenously into B10 mice. Internalization of apoptotic cells by DCs was analyzed by flow cytometry at different time points. (B) Splenic DC-enriched suspensions were labeled with phycoerythrin anti-CD11c and Cychrome anti-CD8α mAbs and analyzed by flow cytometry. (C) Expression of the IAb-IEα52-68 peptide detected with Y-Ae mAb on CD11c+ spleen (B10) DCs 24 hours (thick line) or 48 hours (thin line) after intravenous injection of (BALB/c) apoptotic splenocytes. As control, splenic DCs from animals injected with IEα− (B10) apoptotic leukocytes were included (gray histogram). Numbers on dot plots represent percentages of DCs that have internalized PKH67+ apoptotic cells. Data are representative of 4 independent experiments.
Kinetics of apoptotic cell uptake by splenic DCs in vivo.
(A) A quantity of 2 × 107 PKH67-labeled BALB/c apoptotic splenocytes was injected intravenously into B10 mice. Internalization of apoptotic cells by DCs was analyzed by flow cytometry at different time points. (B) Splenic DC-enriched suspensions were labeled with phycoerythrin anti-CD11c and Cychrome anti-CD8α mAbs and analyzed by flow cytometry. (C) Expression of the IAb-IEα52-68 peptide detected with Y-Ae mAb on CD11c+ spleen (B10) DCs 24 hours (thick line) or 48 hours (thin line) after intravenous injection of (BALB/c) apoptotic splenocytes. As control, splenic DCs from animals injected with IEα− (B10) apoptotic leukocytes were included (gray histogram). Numbers on dot plots represent percentages of DCs that have internalized PKH67+ apoptotic cells. Data are representative of 4 independent experiments.
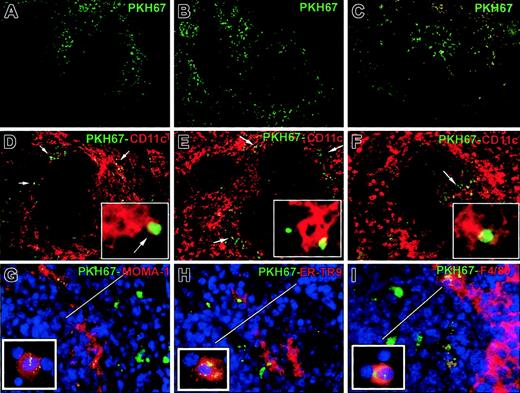
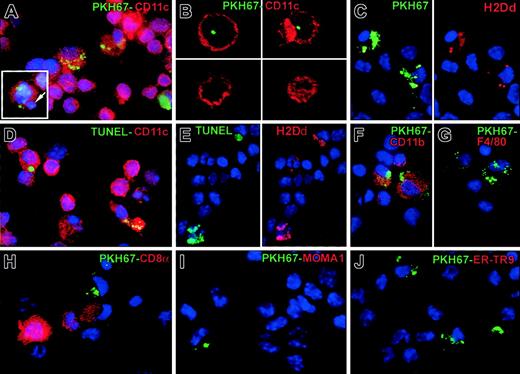
Three-dimensional analysis by 2-photon confocal microscopy, followed by 2-color immunofluorescence, showed that circulating apoptotic cells accumulated initially (first 24 hours) at the periphery of splenic follicles, mostly within CD11c+ DCs of the MZ (Figure 2A-B,D-E). By 48 to 72 hours, DCs with PKH67 inclusions mobilized to the T-cell area of the follicle center (Figure 2C,F). The preferential entrapment of circulating apoptotic cells into the MZ was confirmed by labeling MOMA-1+ metallophillic macrophages (inner limit of MZ) and red pulp F4/80hi macrophages (outer limit). MOMA-1+ metallophillic macrophages, ER-TR9+ MZ macrophages, and F4/80hi red pulp macrophages also internalized apoptotic cells (Figure 2G-I). Analysis of immunobead-sorted splenic DCs from B10 mice injected intravenously with BALB/c PKH67-apoptotic cells by confocal microscopy demonstrated PKH67 fragments within the cytoplasm of CD11c+ DCs (Figure 3A-B). Coexpression of donor (BALB/c) H2 molecules (H2Dd) and PKH67 or TUNEL in CD11c+splenic DCs confirmed that donor apoptotic cells (H2Dd+, TUNEL+) were indeed taken up by MZ DCs (Figure 3C-E). This observation excluded the possibility that PKH67 could have been transferred passively between PKH67-apoptotic cells and DCs. Cytospin preparations of DCs isolated 1 hour after injection of apoptotic cells confirmed that the apoptotic cells were initially internalized by CD8α− CD11b+ F4/80dimER-TR9− MOMA-1− DCs (Figure 3F,J).
Entrapment of circulating apoptotic cells by MZ DCs.
Uptake of apoptotic cells by DCs was studied by 2-photon confocal microscopy on spleen blocks (A-C) and by immunofluorescence on spleen sections (D-F), 6 hours (A,D), 24 hours (B,E), and 48 hours (C,F) after intravenous injection of PKH67-labeled (green) BALB/c apoptotic splenocytes in B10 mice. Early entrapment of apoptotic cells (green) by MZ DCs (CD11c+ in red) at the periphery of splenic follicles 6 hours (A,D) and 24 hours (B,E) after intravenous injection is evident. Staining with Cy3–anti-CD11c shows DCs with internalized apoptotic cell fragments (DCs in red, apoptotic cell fragments in green, panels D-F and insets). After 48 hours, DCs with green apoptotic cells mobilized to the center of the follicle (C,F) close to the central arteriole of the T-cell area (arrow in panel F). MOMA-1+ metallophillic macrophages (G), ER-TR9+MZ macrophages (H), and F4/80+ macrophages of the red pulp also internalized apoptotic cells (in green; detail of cytospins in insets in panels G-I). Arrows in panels D to F show apoptotic cells in green. Bars in panels G to I indicate the approximate width of the MZ. Four animals were analyzed per time point. Yellow image denotes overlapping red and green images. Nuclei were counterstained with DAPI. Original magnification, × 200 for panels A to F; × 400 for panels G to I; all insets, × 1000.
Entrapment of circulating apoptotic cells by MZ DCs.
Uptake of apoptotic cells by DCs was studied by 2-photon confocal microscopy on spleen blocks (A-C) and by immunofluorescence on spleen sections (D-F), 6 hours (A,D), 24 hours (B,E), and 48 hours (C,F) after intravenous injection of PKH67-labeled (green) BALB/c apoptotic splenocytes in B10 mice. Early entrapment of apoptotic cells (green) by MZ DCs (CD11c+ in red) at the periphery of splenic follicles 6 hours (A,D) and 24 hours (B,E) after intravenous injection is evident. Staining with Cy3–anti-CD11c shows DCs with internalized apoptotic cell fragments (DCs in red, apoptotic cell fragments in green, panels D-F and insets). After 48 hours, DCs with green apoptotic cells mobilized to the center of the follicle (C,F) close to the central arteriole of the T-cell area (arrow in panel F). MOMA-1+ metallophillic macrophages (G), ER-TR9+MZ macrophages (H), and F4/80+ macrophages of the red pulp also internalized apoptotic cells (in green; detail of cytospins in insets in panels G-I). Arrows in panels D to F show apoptotic cells in green. Bars in panels G to I indicate the approximate width of the MZ. Four animals were analyzed per time point. Yellow image denotes overlapping red and green images. Nuclei were counterstained with DAPI. Original magnification, × 200 for panels A to F; × 400 for panels G to I; all insets, × 1000.
Uptake of apoptotic cells by splenic DCs in vivo.
Internalization of apoptotic cells by splenic DCs was analyzed in cytospins of immunobead-sorted DCs 1 hour after injection of PKH67-(green) apoptotic (BALB/c) splenocytes in (B10) mice. (A) CD11c+ DCs with apoptotic cell fragments (green) and with DAPI+ intracytoplasmic inclusions, likely DNA from ingested apoptotic cells (in blue indicated by arrow in inset). (B) Serial sections analyzed by confocal microscopy confirmed the intracellular localization of PKH67+ fragments in splenic CD11c+ DCs. (C) The donor origin (BALB/c) of the intracytoplasmic inclusions in (B10) DCs was confirmed by H2Dd expression (in red) in PKH67+ (green) fragments. (D-E) FITC-TUNEL staining in combination with Cy3–anti-CD11c or Cy3–anti-H2Dd confirmed the presence of donor (BALB/c)-derived apoptotic cells within (B10) DCs. (F-J) One hour after intravenous injection of apoptotic cells, DCs that internalized apoptotic cells were CD11bhi, F4/80lo/−, CD8α−, MOMA-1−, or ER-TR9−. Images are representative of 5 animals analyzed. Nuclei were counterstained with DAPI. Original magnification for all panels, × 1000; inset A, × 1000.
Uptake of apoptotic cells by splenic DCs in vivo.
Internalization of apoptotic cells by splenic DCs was analyzed in cytospins of immunobead-sorted DCs 1 hour after injection of PKH67-(green) apoptotic (BALB/c) splenocytes in (B10) mice. (A) CD11c+ DCs with apoptotic cell fragments (green) and with DAPI+ intracytoplasmic inclusions, likely DNA from ingested apoptotic cells (in blue indicated by arrow in inset). (B) Serial sections analyzed by confocal microscopy confirmed the intracellular localization of PKH67+ fragments in splenic CD11c+ DCs. (C) The donor origin (BALB/c) of the intracytoplasmic inclusions in (B10) DCs was confirmed by H2Dd expression (in red) in PKH67+ (green) fragments. (D-E) FITC-TUNEL staining in combination with Cy3–anti-CD11c or Cy3–anti-H2Dd confirmed the presence of donor (BALB/c)-derived apoptotic cells within (B10) DCs. (F-J) One hour after intravenous injection of apoptotic cells, DCs that internalized apoptotic cells were CD11bhi, F4/80lo/−, CD8α−, MOMA-1−, or ER-TR9−. Images are representative of 5 animals analyzed. Nuclei were counterstained with DAPI. Original magnification for all panels, × 1000; inset A, × 1000.
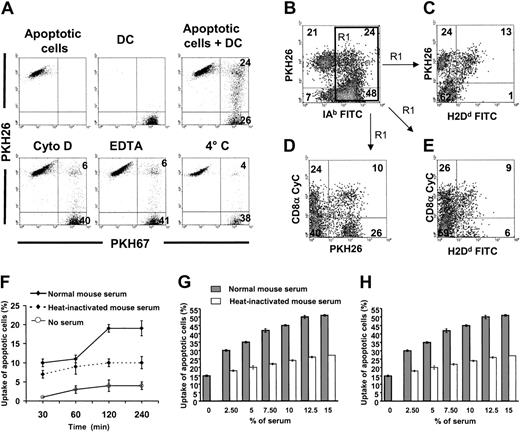
Uptake of apoptotic cells by CD8α− and CD8α+ splenic DCs in vitro
The fact that in vivo, 1 to 3 hours after intravenous injection, CD8α− DCs exhibited a higher level of phagocytosis of apoptotic cells than CD8α+ DCs may have been due to more efficient endocytosis or to a more appropriate anatomic localization of the former cells. The proximity of CD8α− DCs to the marginal sinus, through which circulating cells traffic, may favor the interaction of circulating apoptotic cells with CD8α−DCs rather than with CD8α+ DCs, which are more numerous in T-cell areas.26-28 In vitro experiments showed that 18% to 50% (33% ± 5%) of freshly isolated PKH67-splenic (B10) DCs phagocytosed PKH26-apoptotic (BALB/c) splenocytes within 2 hours, as determined by the presence of double-positive DCs by flow cytometry (Figure 4A). Phagocytosis assays were performed in the presence of 10% normal B10 mouse serum. The fact that uptake of apoptotic cells by splenic DCs decreased in the presence of 2 mM EDTA, 10 μm cytochalasin D, or at 4°C, confirmed that the assay detected internalization of apoptotic cells rather than binding of apoptotic cells to DCs (Figure 4A). To test the phagocytic capacity of CD8α+ DCs, unlabeled splenic (B10) DCs were mixed with (BALB/c) PKH26 apoptotic cells (2 hours, 37°C), fixed in paraformaldehyde, and labeled with Cychrome–anti-CD8α and FITC–anti-IAb or FITC–anti-H2Dd(anti-H2Dd mAb cannot penetrate the DC membrane, and thus labels free or partially internalized BALB/c apoptotic cells). With this approach, it was possible to distinguish: (1) free apoptotic cells (PKH26+, H-2Dd+, FSClow, SSClow); (2) apoptotic cells attached to or partially endocytosed by B10 DC (PKH26+, H-2Dd+, IAb+, FSChi, SSCint), and (3) apoptotic cells completely internalized by B10 DC (PKH26+, H-2Dd−, IAb+, FSChi, SSCint; Figure 4B-E). Although most of the apoptotic cells associated with B10 DCs were completely internalized (PKH26+, IAb+, and H2Dd−), the majority of the apoptotic cells associated with CD8α+ DCs remained outside the DC (PKH26+, H2Dd+; Figure4B,C and 4D,E, respectively). This result demonstrates that the entrapment of circulating apoptotic splenocytes in vivo is initially performed mainly by CD8α− DCs that acquire CD8α expression during their migration to the follicular T-cell area (Figures 1 and 2A-F).
Internalization of apoptotic cells by spleen DCs in vitro: role of serum factors.
(A) PKH67 immunobead-sorted splenic CD11c+ DCs (purity = 96%) were cocultured with PKH26 BALB/c apoptotic splenocytes under different conditions and results were analyzed by flow cytometry. After 2 hours of coculture 33% ± 14% (4 experiments) splenic DCs internalized/bound PKH26 apoptotic cells, a phenomenon blocked by cytochalasin D (Cyto D), EDTA, or at 4°C. (B-C) In 4 experiments, 31% ± 4% IAb+ splenic DCs associated with BALB/c (H2Dd+, PKH26+) apoptotic splenocytes (top right quadrant in panel B). Interestingly, a fraction of these were labeled by anti-H2Dd FITC mAb (top right quadrant in panel C). Because H2Dd is only expressed by the BALB/c apoptotic splenocytes and anti-H2Dd FITC cannot penetrate the DC membrane, this result demonstrates that some apoptotic cells are attached to the DC surface without being internalized. (D-E) The fact that PKH26+ apoptotic cells associated with CD8α− DCs were not reached by anti-H2Dd FITC suggests that they were internalized by DCs. By contrast, apoptotic cells associated with CD8α+ DCs were labeled by anti-H2Dd FITC mAb (E, top right quadrant), a fact that indicates that apoptotic cells were still attached to the DC surface instead of being internalized. Results in panels C to E were gated on IAb+ DCs of panel B (region 1 = R1 in panel B). Numbers inside quadrants indicate percentages of cells. Results are representative of 4 independent experiments. (F-H) Role of heat-labile serum factors in uptake of apoptotic cells by splenic DCs. (F-G) PKH26 splenic (B10) DCs were cocultured with PKH67 apoptotic (BALB/c) splenocytes under different conditions and uptake was assessed by 2-color flow cytometry. (H) Similar experiments were performed with in vitro–generated immunomagnetic bead-sorted immature (B10) BM DCs cocultured with apoptotic (BALB/c) cells. Uptake of apoptotic cells (percents) in the vertical axis of panels F to H represent the percentages of DCs that have captured apoptotic cells.
Internalization of apoptotic cells by spleen DCs in vitro: role of serum factors.
(A) PKH67 immunobead-sorted splenic CD11c+ DCs (purity = 96%) were cocultured with PKH26 BALB/c apoptotic splenocytes under different conditions and results were analyzed by flow cytometry. After 2 hours of coculture 33% ± 14% (4 experiments) splenic DCs internalized/bound PKH26 apoptotic cells, a phenomenon blocked by cytochalasin D (Cyto D), EDTA, or at 4°C. (B-C) In 4 experiments, 31% ± 4% IAb+ splenic DCs associated with BALB/c (H2Dd+, PKH26+) apoptotic splenocytes (top right quadrant in panel B). Interestingly, a fraction of these were labeled by anti-H2Dd FITC mAb (top right quadrant in panel C). Because H2Dd is only expressed by the BALB/c apoptotic splenocytes and anti-H2Dd FITC cannot penetrate the DC membrane, this result demonstrates that some apoptotic cells are attached to the DC surface without being internalized. (D-E) The fact that PKH26+ apoptotic cells associated with CD8α− DCs were not reached by anti-H2Dd FITC suggests that they were internalized by DCs. By contrast, apoptotic cells associated with CD8α+ DCs were labeled by anti-H2Dd FITC mAb (E, top right quadrant), a fact that indicates that apoptotic cells were still attached to the DC surface instead of being internalized. Results in panels C to E were gated on IAb+ DCs of panel B (region 1 = R1 in panel B). Numbers inside quadrants indicate percentages of cells. Results are representative of 4 independent experiments. (F-H) Role of heat-labile serum factors in uptake of apoptotic cells by splenic DCs. (F-G) PKH26 splenic (B10) DCs were cocultured with PKH67 apoptotic (BALB/c) splenocytes under different conditions and uptake was assessed by 2-color flow cytometry. (H) Similar experiments were performed with in vitro–generated immunomagnetic bead-sorted immature (B10) BM DCs cocultured with apoptotic (BALB/c) cells. Uptake of apoptotic cells (percents) in the vertical axis of panels F to H represent the percentages of DCs that have captured apoptotic cells.
Increased uptake of apoptotic cells by splenic DCs in the presence of normal serum
The role of heat-labile serum factors in the internalization of apoptotic cells by splenic DCs was analyzed in vitro in the absence of serum, or in the presence of normal (noncomplement inactivated) or heat-inactivated (56°C, 45 minutes) mouse serum. In the presence of 10% normal (B10) mouse serum, PKH67-splenic (B10) DCs exhibited an increase in their ability to phagocytose PKH26-apoptotic (BALB/c) splenocytes of at least 3-fold compared with their activity in the absence of serum. This effect reached a plateau at 12.5% serum concentration and after 2 hours of incubation (Figure 4F-G). Depletion of heat-labile serum protein activity decreased the number of DCs that phagocytosed apoptotic cells 2-fold (Figure 4G), an effect that was dependent on time and serum concentration. Similar results were obtained when syngeneic (B10) splenocytes or thymocytes were used as sources of apoptotic cells (data not shown). Reduced uptake of apoptotic cells in the presence of heat-inactivated serum suggests that complement factors (ie, C1q, C3bi that are known to enhance the process) may be involved in the adhesion/internalization of apoptotic cells by splenic DCs.15,16,29 The fact that similar results were obtained with BM-derived immature CD11c+ CD86− (B10) DCs, which did not require purification by positive selection with anti-CD11c mAb (clone N418), demonstrated that purification with N418 mAb did not interfere with C3bi recognition by DCs (Figure 4H), as shown previously.30
Role of CRs in internalization of apoptotic cells by splenic DCs
Because heat-labile serum factors optimized the uptake of apoptotic cells by DCs and because C3bi on the surface of apoptotic cells facilitates their phagocytosis by macrophages,15 16we assayed the role of the C3bi receptors CD11b/CD18 and CD11c/CD18 on uptake of apoptotic cells by splenic DCs. PHK67-splenic (B10) DCs were mixed with PHK26-apoptotic (BALB/c) splenocytes in the presence of mAbs (10-25 μg/mL) specific for CRs. Apoptotic cells were preincubated (60-90 minutes) with normal (B10) mouse serum, washed, and then used for phagocytosis assays in the presence of 10% complement-inactivated mouse (B10) serum. This approach prevented DC death from antibody-dependent complement activation. Inhibition of up to 40% of apoptotic cell uptake by splenic DCs was observed following blocking of the α and β chains of CD11b/CD18 (CR3) with mAbs M1/70 and GAME-46, respectively (Figure 5A). A less pronounced inhibition of apoptotic cell uptake was detected after blocking CD11c with mAb HL3. Inhibition was not detected with either irrelevant IgG, mAbs directed against CRs not expressed by murine DCs (anti-CD21/35 or anti-CD21), or anti-CD14 mAb (involved in uptake of apoptotic cells by macrophages but not expressed by DCs; Figure 5A). As positive controls, blocking of the αv (CD51) and β3 (CD61) integrins with specific mAb also inhibited the uptake of apoptotic cells by DCs.
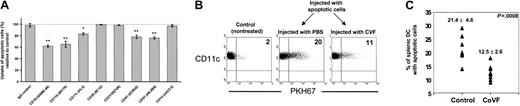
Role of CRs and complement in uptake of apoptotic cells by splenic DCs.
(A) Internalization of apoptotic BALB/c splenocytes by purified splenic B10 DCs in the presence of mAb specific for CR or controls. Apoptotic cells were preincubated with normal mouse B10 serum before analysis, and phagocytosis assays were conducted in the presence of vol/vol 10% heat-inactivated mouse B10 serum. Uptake of apoptotic cells (percent) relative to control represents the percentage of DCs with apoptotic cells in each experimental condition compared with the control group performed in the absence of mAbs and considered as 100% of phagocytosis (dotted line). Data represent the means ± SDs of 3 experiments; *P ≤ .01; **P ≤ .001. (B-C) B10 mice made hypocomplementemic with CVF were injected intravenously with PKH67-BALB/c apoptotic splenocytes. After 12 hours, the animals were killed and splenic DC-enriched suspensions were stained with phycoerythrin–anti-CD11c mAb. As controls, non-CVF–treated animals injected with the same dose of apoptotic cells were included. Data in panel B are from one animal representative of each group (n = 8). The percentages of splenic DCs with apoptotic cells in individual animals of each group and their means ± SDs are displayed in panel C.
Role of CRs and complement in uptake of apoptotic cells by splenic DCs.
(A) Internalization of apoptotic BALB/c splenocytes by purified splenic B10 DCs in the presence of mAb specific for CR or controls. Apoptotic cells were preincubated with normal mouse B10 serum before analysis, and phagocytosis assays were conducted in the presence of vol/vol 10% heat-inactivated mouse B10 serum. Uptake of apoptotic cells (percent) relative to control represents the percentage of DCs with apoptotic cells in each experimental condition compared with the control group performed in the absence of mAbs and considered as 100% of phagocytosis (dotted line). Data represent the means ± SDs of 3 experiments; *P ≤ .01; **P ≤ .001. (B-C) B10 mice made hypocomplementemic with CVF were injected intravenously with PKH67-BALB/c apoptotic splenocytes. After 12 hours, the animals were killed and splenic DC-enriched suspensions were stained with phycoerythrin–anti-CD11c mAb. As controls, non-CVF–treated animals injected with the same dose of apoptotic cells were included. Data in panel B are from one animal representative of each group (n = 8). The percentages of splenic DCs with apoptotic cells in individual animals of each group and their means ± SDs are displayed in panel C.
The role of complement factors in the uptake of apoptotic cells by splenic DCs was analyzed in vivo in (B10) mice made hypocomplementemic with CVF before intravenous injection of 2 × 107PKH67-apoptotic (BALB/c) splenocytes. CVF is a C3-like polypeptide that functions as a C3 convertase resistant to inactivation by factors H and I, a fact that, in mice, results in a severe inactivation of C3 (and secondary C5) without effect on factors like C1q that precede C3 in the complement cascade.31 After 12 hours, the percentage of splenic DCs that internalized circulating apoptotic cells was reduced significantly (P < .001) in hypocomplementemic mice compared with non-CVF–treated controls (Figure 5B-C). Thus complement factors derived from C3 or later in the complement cascade are involved in the uptake of circulating apoptotic cells by splenic MZ DCs in vivo.
Interaction with apoptotic cells regulates the pattern of DC cytokines
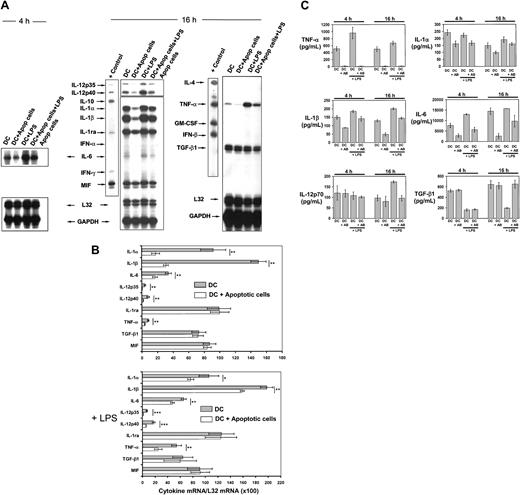
The effect that apoptotic cells exert on cytokine gene transcription and secretion by DCs was analyzed by RPA and enzyme-linked immunosorbent assay (ELISA), respectively. Due to the high numbers of DCs required to perform semiquantitative mRNA analysis by RPA, we used immature BM-derived (B10) DCs negatively selected by immunomagnetic sorting (purity of CD11c+ CD86−DC < 95%). These BM DCs exhibit similar characteristics to MZ DCs (CD8α− CD11b+ CD11c+CD86−/lo F4/80dim ER-TR9−MOMA-1− and take up apoptotic cells with similar efficiency; Figure 4G-H). BM DCs were cocultured with BALB/c apoptotic splenocytes (1:4 ratio) for 4 or 16 hours and the level of cytokine mRNAs assessed by RPA. Interaction/ingestion of apoptotic cells reduced the levels of IL-1α, IL-1β, IL-6, IL-12p35, IL-12p40, and TNF-α mRNAs (Figure 6A-B). A proportional decrease in cytokine mRNA copies was also detected in DCs cocultured with apoptotic splenocytes followed by stimulation with 200 ng/mL lipopolysaccharide (LPS; Figure 6A-B). Ingestion of apoptotic cells exerted minimal or no effect on the levels of IL-1ra, MIF, and TGF-β1 mRNAs. The absence of detectable mRNA in extracts from equivalent numbers of apoptotic cells used in the phagocytosis assays demonstrated that the changes in cytokine mRNA levels were not caused by mRNA from apoptotic cells (Figure 6A). Similar results were obtained by ELISA for the amounts of cytokines secreted by DCs in the culture supernatant. Levels of TNF-α, IL-1α, IL-1β, and IL-6 decreased significantly following incubation of DCs with apoptotic cells, even on stimulation with LPS (200 ng/mL; Figure 6C). Incubation with apoptotic cells blocked the stimulatory effect of LPS on IL-12p70 secretion and the inhibition that LPS exerted on TGF-β1 secretion (Figure 6C).
Effect of apoptotic cells on DC cytokine gene transcription and secretion.
(A) Comparative RPA analysis of cytokine mRNA transcribed by DCs following phagocytosis of apoptotic splenocytes. mRNA was isolated from immunomagnetic bead-sorted BM DCs after coincubation with apoptotic cells for 4 or 16 hours, in the absence or presence of 200 μg/mL LPS. For most cytokines, the changes in mRNA levels were evident after 16 hours with the exception of IL-6. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometry analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). Densitometric values were pooled from 3 separate experiments; *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Quantitation by ELISA of cytokines secreted by DCs after phagocytosis of apoptotic splenocytes in the presence or absence of 200 μg/mL LPS. Basal levels of TGF-β1 in the culture medium were deducted. Data are displayed as the means ± SDs of 3 different experiments.
Effect of apoptotic cells on DC cytokine gene transcription and secretion.
(A) Comparative RPA analysis of cytokine mRNA transcribed by DCs following phagocytosis of apoptotic splenocytes. mRNA was isolated from immunomagnetic bead-sorted BM DCs after coincubation with apoptotic cells for 4 or 16 hours, in the absence or presence of 200 μg/mL LPS. For most cytokines, the changes in mRNA levels were evident after 16 hours with the exception of IL-6. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometry analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). Densitometric values were pooled from 3 separate experiments; *P ≤ .05; **P ≤ .01; ***P ≤ .001. (C) Quantitation by ELISA of cytokines secreted by DCs after phagocytosis of apoptotic splenocytes in the presence or absence of 200 μg/mL LPS. Basal levels of TGF-β1 in the culture medium were deducted. Data are displayed as the means ± SDs of 3 different experiments.
Interaction of C3bi with CD11b/CD18 mimics the effects of apoptotic cells on DC cytokines
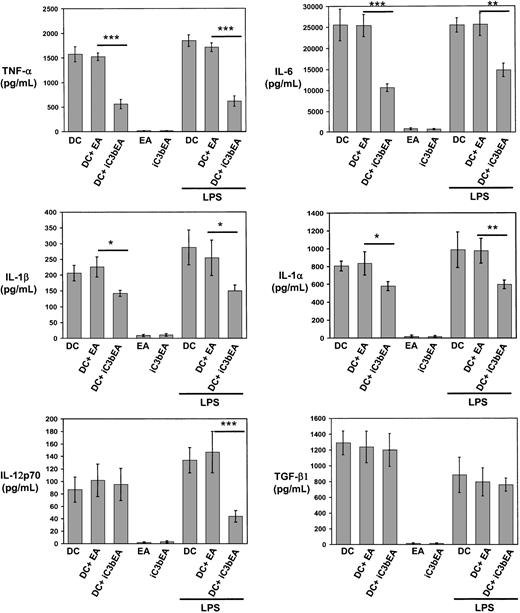
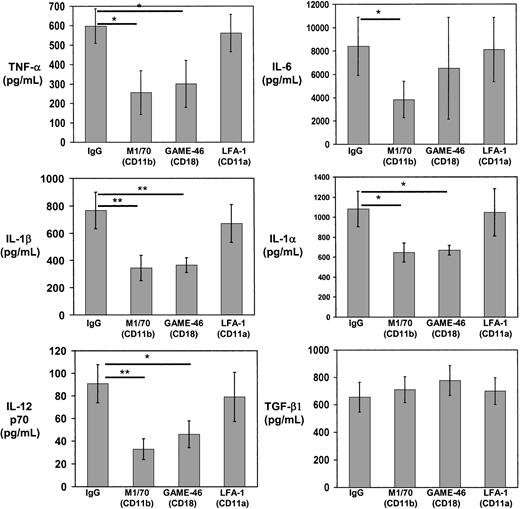
Binding of C3bi on the apoptotic cell surface to CD11b/CD18 on DCs may be involved in recognition/internalization of apoptotic cells and may also instruct DCs to regulate cytokine production, as occurs in macrophages.32 33 To analyze the effect of C3bi on DC cytokines, immunobead-sorted immature BM DCs were mixed with C3biEA (prepared with mouse serum) or EAs in rosetting assays, or with medium alone. The levels of TNF-α, IL-6, IL-1α, and IL-1β decreased significantly when DCs were incubated with C3biEA, even in the presence of LPS (200 ng/mL; Figure7). Similarly, incubation with C3biEA decreased the levels of IL-12p70 secreted by BM DCs in response to LPS stimulation. C3biEA interaction did not modify the levels of TGF-β1 secreted by DCs. To establish whether the effect of C3bi on DC cytokines was mediated via CD11b/CD18, immature BM DCs were incubated with anti-CD11b mAb (M1/70)–coated 4.5-μm polystyrene beads in the presence of 200 ng/mL LPS. BM DCs incubated with anti-CD11b (M1/70) beads decreased significantly their secretion of TNF-α, IL-6, IL-1α, IL1-β, and IL-12p70 compared with DCs incubated with irrelevant IgG-coated beads (Figure 8). A similar reduction (with the exception of IL-6) was induced by anti-CD18 mAb (GAME-46)–coated beads. As control, incubation with anti-CD11a mAb (LFA-1)–coated beads did not affect cytokine levels.
C3bi interaction decreases secretion of proinflammatory cytokines by DCs.
Quantitation of cytokines secreted by immunomagnetic bead-sorted BM DCs following interaction with C3biEAs or EAs in the absence or presence of 200 μg/mL LPS. As controls, C3biEAs or EAs (without DCs) were included. Basal levels of TGF-β1 in the culture medium were deducted from the results. Results are illustrated as the means ± SDs of 3 different experiments, *P ≤ .05; **P ≤ .01, and ***P ≤ .001.
C3bi interaction decreases secretion of proinflammatory cytokines by DCs.
Quantitation of cytokines secreted by immunomagnetic bead-sorted BM DCs following interaction with C3biEAs or EAs in the absence or presence of 200 μg/mL LPS. As controls, C3biEAs or EAs (without DCs) were included. Basal levels of TGF-β1 in the culture medium were deducted from the results. Results are illustrated as the means ± SDs of 3 different experiments, *P ≤ .05; **P ≤ .01, and ***P ≤ .001.
CD11b/CD18 mimics the effect of C3bi on DC cytokines.
Quantitation of cytokines released by immunomagnetic bead-sorted BM DCs after interaction with polysterene beads coated with anti-CD11b (M1/70), anti-CD18 (GAME-46), anti-CD11a (LFA-1), or irrelevant IgG. Basal levels of TGF-β1 in the culture medium were deducted. Results are illustrated as the means ± SDs of 3 different experiments; *P ≤ .05 and **P ≤ .01.
CD11b/CD18 mimics the effect of C3bi on DC cytokines.
Quantitation of cytokines released by immunomagnetic bead-sorted BM DCs after interaction with polysterene beads coated with anti-CD11b (M1/70), anti-CD18 (GAME-46), anti-CD11a (LFA-1), or irrelevant IgG. Basal levels of TGF-β1 in the culture medium were deducted. Results are illustrated as the means ± SDs of 3 different experiments; *P ≤ .05 and **P ≤ .01.
Discussion
Models of peripheral tolerance have demonstrated that migratory BM-derived APCs, which constitutively transport and process peripheral tissue–specific self-Ag to lymph nodes, are able to silence Ag-specific T-cell–receptor transgenic CD4+ or CD8+ T cells.34,35 Interestingly, migratory DCs internalize and transport apoptotic intestinal epithelial cells to T-cell areas of mesenteric lymph nodes.6 These and other observations suggest that, in the absence of inflammation, tissue-resident DCs take up apoptotic cells, process the self-Ag contained within them and, without receiving a maturation or danger signal, migrate constitutively via lymphatic vessels to lymph nodes.18,19 Once in the T-cell area, DCs maintain peripheral tolerance by induction of self-Ag–specific T-cell anergy/deletion or generation of T-regulatory cells.1 36-40
In the present study, we demonstrate that besides the constitutive traffic of apoptotic cell fragments within DCs from the periphery to lymph nodes via lymphatic vessels, circulating apoptotic cells may also be internalized by spleen-resident MZ DCs. This alternative and efficient mechanism may account for the billions of apoptotic leukocytes that are removed from the circulation every day without breaching self-tolerance. MZ DCs are in a strategic localization to capture blood-borne particulate antigen, including circulating apoptotic leukocytes.41 We show here that MZ DCs rapidly internalize circulating allogeneic PKH-67+ apoptotic leukocytes. Several facts confirmed that the fragments found within MZ DCs were apoptotic cells. Most of the PKH-67+ inclusions stained specifically for the donor allogeneic antigen H2Dd and TUNEL, and some of the TUNEL+inclusions coexpressed H2Dd. Our results also reveal that the uptake of circulating apoptotic cells is initially performed mainly by CD8α− DCs that, during the ensuing 24 to 72 hours, up-regulate surface CD8α expression, process allogeneic antigen–derived peptides into IA molecules, and mobilize to the T-cell area in the center of the splenic follicle. These findings agree with the fact that MZ DCs, unlike interdigitating DCs of the T-cell areas, are highly phagocytic in vivo and therefore they are depleted following phagocytosis of macrophage-depleting liposomes containing clodronate.42
Our results support the recent demonstration that splenic CD8α− DCs give rise to CD8α+ DCs, the fact that DC-bearing apoptotic cells are occasionally detected in T-cell areas of lymph nodes, and the ability of CD8α+ DCs to cross-prime apoptotic cell–derived antigen 24 hours after intravenous injection of haptenated apoptotic cells.6,28,43 By contrast, at the time of writing, Iyoda et al24 reported that only CD8α+ DCs within lymphoid and nonlymphoid tissues engulf various particles including apoptotic cells in situ. This latter observation conflicts with the reported lower phagocytic capacity of CD8α+ DCs compared with CD8α−DCs, and contradicts accounts of the internalization of apoptotic cells by murine CD8α− BM- or human monocyte-derived DCs.2-5 The fact that Iyoda et al did not formally demonstrate that the cell fragments associated with CD8α+DCs were indeed apoptotic (by TUNEL or ultrastructure) or that the apoptotic cells were inside the DCs (and not clustered on the surface of CD8α+ DCs), together with different kinetics of analysis, may in part explain the discrepancies between the latter findings and the present observations.24
It is likely that the consequences of apoptotic cell clearance for tolerance versus immunity depend on the interaction of apoptotic cells with DC surface receptors, on the fact that apoptotic cells are rapidly internalized preventing the release of intracellular proteases that activate APCs, and on the DC microenvironment where the endocytosis takes place.44-47 The cell membrane of apoptotic cells undergoes molecular changes that allow its recognition by receptors expressed by phagocytes.44-46 Among them, CD36 (thrombospondin receptor) and the integrins αvβ3 (vitronectin receptor) and αvβ5 are required during internalization of apoptotic cells by DCs.2,4 We demonstrate here that CR3 and to a lesser extent CR4, also play a role in interaction/phagocytosis of apoptotic cells by MZ DCs. Interestingly, unlike CD8α+ DCs, splenic CD8α+ DCs express high levels of CR3, a fact that correlates with their higher phagocytic capacity for apoptotic cells in the presence of complement factors. It is known that apoptotic cells activate both the classical and alternative complement pathways.15,16 Externalization of phosphatidylserine and binding of C1q are in part responsible for complement activation and deposition of C3bi on apoptotic cells.15,16,48 As occurs with macrophages, we have shown here that heat-labile serum factors optimize the uptake of apoptotic cells by spleen and BM DCs.15,16 The use of heat-inactivated serum in previous studies may have overlooked the role of complement in the uptake of apoptotic cells by DCs. We used DCs, apoptotic leukocytes, and serum all from the mouse to obviate lack of cross-reactivity between human and mouse complement factors.21 We also avoided the use of polystyrene plates that may interfere with CD11b/CD18-C3bi binding.49 These differences and the fact that diverse receptors may be used by different phagocytes to target distinct apoptotic cells may explain recent discrepancies concerning the role of CD11b/CD18 in phagocytosis of apoptotic cells by macrophages.15,16 50
We show here that after DCs internalized apoptotic cells they produced significantly lower levels of IL-1α, IL-1β, IL-6, and TNF-α and the Th1-driving factor IL-12p70, with little or no effect on immunoregulatory mediators like TGF-β1, IL-1ra, and MIF. This inhibitory effect on DC cytokines was even present following stimulation with LPS. At least part of the inhibition occurred at the level of mRNA transcription or stability. Because BM DCs did not produce IL-10 and because TGF-β1 levels secreted by DCs did not increase further after interaction with apoptotic cells, it is unlikely that in our model IL-10 or TGF-β1 caused the changes in cytokine production, as occurs in macrophages.13,20 In macrophages, the interaction of apoptotic cells with CD36, CD47, and the recently described phosphatidylserine-specific receptor on the phagocyte surface has been shown to decrease secretion of proinflammatory cytokines.11,51,52 Of related interest, engagement of CD47 or CD36 decreases secretion of IL-6, IL-12p70, and TNF-α by human monocyte-derived DCs.53,54 We investigated whether the signaling through C3bi receptors expressed by DCs with its natural ligand acts in concert with other apoptotic cell receptors in decreasing the levels of proinflammatory cytokines. This was of particular interest based on the fact that binding of C3bi to CD11b/CD18 inhibits IL-12p70 secretion by macrophages and that cutaneous deposition of C3bi plays a crucial role in UVB-irradiated skin immunosuppression through its interaction with a subpopulation of dermal macrophages.17,32,33 55 Our results show that C3bi binding to CD11b/CD18 on DCs suppresses markedly the secretion of proinflammatory cytokines without affecting TGF-β1 levels, an effect that was even present following activation of DCs with LPS.
In conclusion, our results demonstrate that, under steady-state conditions, DCs of the splenic MZ rapidly take up apoptotic leukocytes from the circulation. Together with other ligands, C3bi deposition optimizes apoptotic cell uptake and instructs DCs both to reduce the synthesis of proinflammatory and Th1-driving cytokines, and to maintain high levels of the immunoregulatory factor TGF-β1. After interaction with apoptotic cells, MZ DCs differentiate into CD8α+ DCs, process antigenic peptides derived from apoptotic cells, and mobilize to T-cell areas. This cytokine switch may help to explain the diminished antigen-driven T-cell stimulation of DCs that have ingested apoptotic cells and may be one of the mechanisms by which processing of apoptotic cells by DCs does not breach self-tolerance.7,9 It also may explain the recent observations that intravenous injection of donor apoptotic splenocytes enhances BM engraftment across major histocompatibility barriers and that intravenous administration of antigen-coupled apoptotic splenocytes induces active cutaneous immune unresponsiveness.43 56
We thank Bridget L. Colvin, Susan Specht, and Jan Urso for technical assistance. We thank the Schering-Plough Research Institute for the gifts of cytokines and Dr Charles A Janeway (Yale University) for the Y-Ae mAb.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1769.
Supported by grants from National Institutes of Health: R21 HL69725 (A.E.M.); R01 AI43916 and P01 CA73743 (L.D.F.); R01 DK49745 and R01 AI41011 (A.W.T.); and the Dermatology Foundation (A.T.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adrian E. Morelli or Angus W. Thomson, E1504 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15213; e-mail:morelli@imap.pitt.edu or thomsonaw@msx.upmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal