Transfusion-related acute lung injury (TRALI) is a life-threatening complication of hemotherapy. We report a series of 90 TRALI reactions in 81 patients secondary to transfusion with whole blood platelets (72 reactions), apheresis platelets (2), packed red cells (15), and plasma (1). The overall prevalence was 1 in 1120 cellular components. To examine the epidemiology of TRALI, we completed a nested case-control study of the first 46 patients with TRALI compared with 225 controls who had received transfusions. We then completed a prospective analysis of possible biologic response modifiers responsible for 51 of the TRALI cases, including human leukocyte antigen (HLA) class I, class II, and granulocyte antibodies in donors and neutrophil (PMN) priming activity in the plasma of the implicated units and recipients. Two groups were at risk: patients with hematologic malignancies (P < .0004) and patients with cardiac disease (P < .0006). TRALI was associated with older platelets (P = .014). In the prospective study, antileukocyte antibodies were found in only 3.6% of cases. The implicated blood components had greater PMN priming activity than controls (P < .05), and compared with pretransfusion samples, TRALI patients' plasma demonstrated increases in both interleukin 6 (IL-6) and lipid (neutral lipids and lysophosphatidylcholines) priming activity (P < .05). We conclude that TRALI may be more frequent than previously recognized and that patient susceptibility, product age, and increased levels of bioactive lipids in components may predispose patients to TRALI. TRALI, like the acute respiratory distress syndrome, may be a 2-event phenomenon with both recipient predisposition and factors in the stored units playing major roles.
Introduction
Transfusion-related acute lung injury (TRALI) consists of the insidious onset of pulmonary insufficiency, manifested by severe dyspnea, hypoxia, and radiographic evidence of pulmonary edema with normal cardiac function.1 It has been reported to be a relatively rare complication of transfusion, occurring with 1 of 5000 transfused units, and occurs within 6 hours of the transfusion.1 The initial reports of TRALI implicated whole blood, packed red blood cells, platelet concentrates, and plasma in the genesis of TRALI.1,2 The pathogenesis of TRALI has been related to the infusion of antileukocyte antibodies, resulting in activation of the complement cascade, pulmonary leukostasis, and neutrophil (PMN)–mediated lung injury.1-6Although the majority of these major histocompatibility complex (MHC) class I, MHC class II, or antigranulocyte antibodies are thought to be of donor origin, the presence of these immunoglobulins in the transfusion recipient has also been postulated to cause TRALI through activation of transfused PMNs.1-7 An animal model of TRALI showed that infusion of specific anti-PMN antibodies with PMNs that expressed this antigen resulted in acute lung injury; however, infusion of antineutrophil antibodies with PMNs that did not contain this antigen did not cause injury.8 Although this model provides experimental evidence for cases of TRALI in which specific antibodies are infused into a recipient whose granulocytes express antigen(s) recognized by these antibodies, a number of TRALI reactions do not have an immunologic etiology.8,9Moreover, in reports of TRALI many of the antileukocyte antibodies detected were not specific for human leukocyte antigen (HLA) or granulocyte antigens, nor was it clear that the recipients' leukocytes expressed the specific antigens to which these immunoglobulins were directed.5 8-13
A study of 10 patients with TRALI compared with 10 patients with uncomplicated febrile and urticarial transfusion reactions linked TRALI to the transfusion of lipids with significant PMN priming activity.9 These compounds were identified as a mixture of lysophosphatidylcholines (lyso-PCs) that accumulate during routine storage of all cellular blood components, reaching a relative maximum by component outdate, and cause acute lung injury in an animal model.9,14-16 Clinical studies have documented that older platelet concentrates are associated with both increased prevalence and severity of transfusion reactions, and cytokines that accumulate during storage were proposed as the responsible mediators.17-19Two of these cytokines, interleukin 6 (IL-6) and interleukin 8 (IL-8), stimulate PMNs and may play a role in TRALI.17-24
TRALI is clinically identical to the adult respiratory distress syndrome (ARDS), which has been defined as a 2-event, PMN-mediated syndrome.1,9,25-28 Therefore, when investigation of the donors in the first 17 TRALI cases failed to identify antileukocyte antibodies, and laboratory examination of 8 recipients (including 2 patients with recurrent TRALI reactions to products from different donors) also was negative for such antibodies, we sought another etiology. We hypothesize that significant numbers of TRALI cases, similar to ARDS, are the result of 2 insults, with the first insult related to the clinical condition of the patient and the second to the infusion of biologic response modifiers within the blood component.26,28 29 We studied a total of 90 TRALI cases, which occurred in 81 patients. The first part of the study was a retrospective, nested case-control study comprising 46 TRALI cases and 225 concomitantly transfused controls. It had as its focus the identification of patient and product factors that might be associated with TRALI. The second part of the study was a prospective evaluation of possible biologic response modifiers related to TRALI and comprised 51 consecutive TRALI reactions. The biologic response modifiers included donor antibodies to HLA and granulocyte antigens and bioactive lipids and cytokines in both the transfused products and the TRALI patients.
Patients, materials, and methods
Patient study population
Materials
Unless otherwise specified, all reagents were purchased from Sigma Chemical (St Louis, MO). Sterile couplers; sterile saline for injection, United States Pharmacopeia (USP); and sterile water for injection, USP, were purchased from Baxter Healthcare Corporation (Deerfield, IL). All buffers were made from injection-grade, USP solutions in sterile water obtained from the following manufacturers: 10% CaCl2, Fujisawa USA, Deerfield, IL; 23.4% NaCl, 20 Meq/mL KCl, and 50% MgSO4, American Regent Laboratories, Shirley, NY; and sodium phosphates and 50% dextrose, Abbott Laboratories, North Chicago, IL. A high-pressure liquid chromatograph with a model 200 quaternary isocratic pump and a model 235 diode array detector were purchased from Perkin Elmer, Norwalk, CT. Test tubes, Nunc microplates, formaldehyde, and solvents for extraction and separation of lipids were obtained from Fisher Scientific (Pittsburgh, PA). Undiluted rabbit complement was obtained from Cedarlane (Hornby, ON, Canada). Eosin dye was purchased from Mallinckrodt (Paris, KY). Rabbit complement and Dynabeads HLA Cell Prep II were purchased from GTI (Brookfield, WI) and Dynal (Biotech, Lake Success, NY), respectively. Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and IL-8 were procured from R&D Systems, Minneapolis, MN. WEB 2170 was the kind gift of Boerhinger Ingelheim Pharmaceuticals (Ridgefield, CT).
Diagnostic criteria
The medical or surgical staff, both nurses and physicians, reported all suspected transfusion reactions to the Transfusion Medicine Service at UAH. In all cases, the Transfusion Medicine staff made the diagnosis following a detailed review of the patient's chart and interviews with the involved medical personnel and, where possible, family members who witnessed the reaction. All patients in this study met the criteria for TRALI.1,5 9 TRALI was diagnosed in cases in which (1) respiratory insufficiency (tachypnea, shortness of breath, increased work of breathing, and cyanosis) accompanied by significant O2 desaturation, quantified by pulse oximetry or arterial blood gas measurement, was the predominant presenting symptom; (2) the degree of respiratory compromise required immediate medical intervention; (3) onset of symptoms was temporally related to transfusion (within 4 hours; most occurred within 10 to 30 minutes); and (4) no other clinical cause (volume overload, allergic manifestation, or sepsis) was evident for the pulmonary compromise. None of the patients demonstrated wheezing, stridor, or urticaria. Volume overload was ruled out by clinical examination, a review of the fluids administered, and, where applicable, chest x-ray findings of pulmonary edema in the presence of normal heart size. A number of patients were excluded from the study because volume overload could not be ruled out. Most TRALI reactions had accompanying fever and systemic hypertension; hypotension was documented in a few unusually severe cases. As major respiratory compromise is usually not the presenting manifestation of transfusion-associated bacterial sepsis, cultures of the implicated products and of the patient were done only if the patient's temperature rose by more than 2°C and persisted or if fever was accompanied by rigors and/or vasomotor instability. All bacterial cultures were negative. Care was taken to exclude other causes of respiratory compromise, including cardiac compromise. All cases of TRALI were reviewed by one of us (L.K.B.).
Epidemiology and a prospective study of TRALI
In compliance with UAH Institutional Review Board requirements, demographic and clinical laboratory data were collected retrospectively on 46 sequential TRALI patients who had received platelets separated from whole blood by centrifugation (WB-PLTs) and a group of 225 randomly selected, hospitalized control subjects who received transfusions of WB-PLTs during the same time period who did not experience a transfusion reaction. The object of the nested case-control study was to identify possible patient factors (age, sex, underlying diagnoses, previous transfusion reaction, type of transfusion reaction) or product factors (age, ABO antigen mismatch with recipient) associated with TRALI. Logistic regression analysis was performed with SAS software (SPSS, Plover, WI), using a level of statistical significance ofP < .05.
The prospective portion of the study (51 patients) included 17 cases that were also part of the nested case-control study plus 34 additional, sequential cases of TRALI. It is important to note that the prospective portion of the study evaluated the role of biologic response modifiers possibly associated with TRALI and the retrospective study analyzed only the demographic and clinical laboratory data. All patients who received transfusions over the study period had a pretransfusion plasma sample drawn, and a posttransfusion plasma sample was obtained at the time TRALI was recognized. Ten cases of TRALI were not included owing to an inadequate pretransfusion plasma sample or unwillingness to participate in the study.
Sample collection
Whole blood (5-10 mL) anticoagulated with EDTA (ethylenediaminetetraacetic acid) was prospectively collected from all patients receiving transfusions at UAH as part of routine typing. At the time TRALI was recognized, an additional plasma sample (anticoagulated with EDTA) was obtained from the patient, and a 5- to 10-mL sample was collected from the implicated blood product. Samples were obtained from all implicated platelet concentrates, but because 13 of 15 of the implicated packed red blood cell (PRBC) units were fully transfused, the studies on PRBC-induced TRALI were limited to pre- and posttransfusion patient plasma samples and donor testing for the measurement of antileukocyte antibodies. Because these PRBC donor samples were not from the implicated product, they were not included in the prospective analysis. Plasma was isolated and stored at −70°C.
HLA antibody testing
Prospectively, anti–HLA-A and -BB (class I) and antigranulocyte antibody testing was performed on samples from 104 implicated blood components (one pool) and 24 control samples from the nested case-control study: 86 samples were tested in Portland, OR, at the Red Cross Regional Tissue Typing Laboratory, and 42 were tested in Edmonton at the Regional Tissue Typing Laboratory, UAH. Unless otherwise specified, all reactions took place at room temperature. The methodology employed at both institutions for detection of lymphocytotoxic antibodies to HLA-AB antigens was identical and was completed as previously described with an additional wash of lymphocyte targets to remove residual dimethyl sulfoxide (DMSO).30 These assays employed a reference panel of lymphocytes from 25 to 50 donors, plated on plastic microplates. The reactions were completed as previously described, and the presence of anti-HLA/A antibodies was evaluated by light microscopy.30
In Portland, 75 of the 104 samples from implicated blood components were tested for the presence of anti–HLA class II antibodies by means of a dual-color fluorescent bead assay. Briefly, the HLA class II+ cells were isolated from peripheral blood with Dynabeads HLA Cell Prep II. Cells were added to the plates containing the unit samples. The mixture was incubated for 30 minutes, undiluted class II rabbit complement and 1% ethidium bromide were added, and the reaction proceeded for 30 minutes. Plates were washed with Hanks buffered saline solution and evaluated by fluorescent microscopy. There were not sufficient platelet plasma samples from the control patients to permit anti–HLA class II antibody testing.
Granulocyte antibody testing
The detection of antigranulocyte antibodies from the implicated blood components was performed by 2 related methodologies. In Portland, a panel of 6 to 8 granulocytes and an agglutination-based technique were used. Donor granulocytes were isolated from whole blood by Ficoll-Hypaque gradient centrifugation and hypotonic lysis of contaminating red blood cells (RBCs). Plasma was incubated at 56°C for 20 minutes to inactivate complement, and particulates were removed. Isolated PMNs (6.5-8.5 × 106) were incubated with differing serum concentrations for 60 minutes at 31°C, and antibodies were detected by light microscopy evaluation of PMN aggregation compared with the negative control. Positive controls comprised sera from patients with known immunoglobulins directed against NA1, NA2, NB1, and 5b (gift of David Stroncek, NIH). In Edmonton, antigranulocyte antibodies were detected with a panel of 5 or 6 granulocytes. Donor granulocytes were isolated from whole blood by discontinuous Percoll gradient centrifugation, washed, fixed with 2% formalin for 5 minutes, and washed again. Granulocytes (1.0 × 106) were incubated for 60 minutes with 100 μL of test plasma, washed, and then incubated with filtered fluorescein isothiocyanate (FITC)–conjugated F(ab)2 rabbit antihuman IgG (heavy and light chain–specific) for 60 minutes at 4°C in the dark. The granulocytes were then washed, a phosphate-buffered saline (PBS)–glycerol (3:1) solution was added, and the PMNs were mounted on slides. The presence of bound immunoglobulins was determined by fluorescent microscopy in comparison with control plasma.
PMN isolation and priming
After obtaining informed consent under a protocol approved by the Combined Multiple Institution Review Board, University of Colorado Health Sciences Center, whole blood was drawn from healthy donors, and PMNs were isolated by standard techniques.15 The PMN priming reactions have been described in detail, and priming was defined as the augmentation of the formylmethionylleucylphenylalanine (fMLP)–activated respiratory burst in buffer- or vehicle-primed PMNs.9 Each plasma sample was tested for the capacity to prime PMNs. Additionally, chloroform extracts and HPLC fractions of the plasma lipids, resuspended in 1.25% fatty acid–free, globulin-free human albumin, were also tested for their ability to prime the PMN oxidase.
Extraction and separation of lipids
Lipids were extracted from plasma samples as described.15,31 The chloroform-soluble phase was removed, dried, and the lipids were solubilized either in 1.25% essentially fatty acid–free, globulin-free human albumin, for PMN priming experiments, or in hexane/isopropanol/water, for HPLC separation.16
HPLC was performed using a normal phase system developed for phospholipid class separation at determined retention times using purified lipid standards.16,32 The individual fractions were dried, resuspended in 1.25% fatty acid–free human albumin, and assayed for their ability to prime the PMN oxidase.16 PMNs primed with 1.25% albumin served as controls.
Measurement of IL-6 and IL-8
IL-6 and IL-8 were measured in duplicate, using ELISA kit assays that were unaffected by lysed hemoglobin.
Data analysis
Statistical differences (P < .05) among groups were measured by a paired analysis of variance (ANOVA) for repeated measurements or an independent ANOVA for comparison of independent groups, followed by a Newman-Keuls post hoc analysis for multiple comparisons.33 34 All data are expressed as the mean ± the standard error of the mean.
Results
Clinical features of TRALI
From July 1, 1991, to June 30, 1995, 90 confirmed TRALI cases were diagnosed in 81 patients at UAH, Edmonton, Alberta, Canada (Table1). All identified cases fit previous clinical criteria for diagnosis of TRALI.1,2,7,14 28 All patients with TRALI presented with respiratory compromise and severe hypoxemia, often accompanied by visible cyanosis. The reactions were temporally associated with the infusion of a blood product (usually occurring within 30 minutes of transfusion), and there were no other evident causes for the respiratory insufficiency. Volume overload, allergic reaction, and transfusion-related sepsis were ruled out clinically or by review of the medical record as described. Fever, sometimes accompanied by rigors, was an almost constant concomitant symptom, and hypotension was rare. All reactions were treated with supplemental O2 in conjunction with antipyretics, corticosteroids, and diphenhydramine in most cases. Mechanical ventilation was required in 3 cases. The acute respiratory distress was ameliorated rapidly and resolved in hours. In a single case, TRALI precipitated the patient's death by leading to recurrent myocardial infarction and cardiogenic shock. Seven patients had recurrent TRALI reactions, which in all cases were to products from different donors. Two patients had 3 TRALI reactions each: the first patient to 3 separate WB-PLT transfusions and the second to 2 separate WB-PLT transfusions and to 1 A-PLT transfusion. Three other patients had 2 TRALI reactions each, all to WB-PLT transfusions. The last 2 patients had 2 TRALI reactions each: the first to WB-PLTs and PRBCs, and the second to 2 separate PRBC transfusions. These recurrent reactions usually occurred within hours to days of the initial reaction. When recurrent TRALI reactions were recognized, subsequent transfusions for at-risk patients consisted of washed PRBCs or the freshest available A-PLTs, without recurrence.
Implicated blood products in the 48-month study period (July 1991-June 1995) and the relative prevalence of TRALI per unit transfused of each
| Implicated component . | No. of TRALI reactions . | No. of units transfused . | Prevalence, % (ratio) . |
|---|---|---|---|
| WB-PLTs | 72 | 31 074 | 0.23 (1 of 432) |
| A-PLTs | 2 | 2 447 | 0.082 (1 of 1 224) |
| PRBCs | 15 | 66 161 | 0.023 (1 of 4 410) |
| FFP | 1 | 19 411 | 0.0052 (1 of 19 411) |
| All cellular components | 89 | 99 682 | 0.089 (1 of 1 120) |
| All components | 90 | 119 093 | 0.076 (1/ of 1 323) |
| Implicated component . | No. of TRALI reactions . | No. of units transfused . | Prevalence, % (ratio) . |
|---|---|---|---|
| WB-PLTs | 72 | 31 074 | 0.23 (1 of 432) |
| A-PLTs | 2 | 2 447 | 0.082 (1 of 1 224) |
| PRBCs | 15 | 66 161 | 0.023 (1 of 4 410) |
| FFP | 1 | 19 411 | 0.0052 (1 of 19 411) |
| All cellular components | 89 | 99 682 | 0.089 (1 of 1 120) |
| All components | 90 | 119 093 | 0.076 (1/ of 1 323) |
WB-PLTs indicates platelets separated from whole blood by centrifugation; A-PLTs, platelets collected by apheresis on a Cobe Spectra apheresis apparatus (Gambro BCT, Lakewood, CO); PRBCs, packed red blood cells; and FFP, fresh frozen plasma.
Blood components associated with TRALI
The implicated blood components in the 90 TRALI reactions consisted of 72 units of WB-PLTs, 2 units of A-PLTs, 15 units of PRBC s, and 1 unit of FFP. None of these products except for the A-PLTs were leukoreduced prior to storage, and poststorage leukoreduction was employed in 3 transfusions (2 of WB-PLTs, 1 of PRBCs) for patients with documented recurrent febrile reactions. Prior to June 1992, PRBCs were supplied in citrate phosphate dextrose adenine solution–1 (CPDA-1) anticoagulant, and after that time they were supplied in AS-3 (Nutricel) additive solution. There was no difference in the prevalence of TRALI reactions to PRBCs between these products (data not shown). WB-PLTs were normally administered rapidly and sequentially at the bedside, at a dosage of 1 unit per 10 kg body weight, with a single pooled transfusion. The relative prevalence of TRALI reactions was highest for WB-PLTs (2.4 of 1000) and lowest for plasma (1 of 19 000) (Table 1).
Nested case-control study
From July 1991 to May 1993, 46 consecutive patients had recognized TRALI reactions to whole blood–derived platelet concentrates (WB-PLTs; Table 2). In the case of 3 patients who had multiple TRALI reactions to WB-PLTs, only the first reaction was used in the study. Patient and blood product variables from these TRALI patients were compared by logistic regression analysis with those of a control group of 225 patients who had received concurrent transfusions of WB-PLTs who did not experience transfusion reactions. The variables assessed in this study included recipient demographics; recipient diagnosis; ABO compatibility of recipient and donor blood groups; total number of units transfused during the hospitalization; history of previous transfusions, including the number and type of other transfusion reactions; the incremental rise in platelet count; and the age of the WB-PLT unit transfused. The results demonstrate that TRALI was not associated with recipients' age or sex, nor was it associated with incompatibility of recipient and donor blood groups, the number of previous transfusions, or the number or type of previous transfusion reactions (data not shown). Furthermore, 35 of 46 TRALI patients had increases of more than 10 × 109/L in their posttransfusion platelet count (next count done within 24 hours of the implicated transfusion), with the average increase being 34 × 109/L, similar to those of controls. There was no clinical evidence of bleeding or platelet refractoriness in any of the TRALI or control patients. Two patient groups were at particular risk for developing TRALI reactions: patients with hematologic malignancies (P = .0004), the majority being in the induction phase of chemotherapy, and patients with cardiac disease (P = .0006) who required cardiopulmonary bypass ooperations. TRALI was also correlated with longer WB-PLT storage time: implicated units were stored for 4.5 ± 0.2 days, versus a storage time of 4.2 ± 0.1 days for control units (P = .014).
Clinical characteristics of the 46 TRALI patients from the nested case-control study
| Principal diagnosis . | No. of patients younger than 17 y (n = 15) . | No. of patients older than 16 y (n = 31) . |
|---|---|---|
| Hematologic malignancy* | ||
| ALL, induction chemotherapy | 6 | 0 |
| ALL, consolidation chemotherapy | 1 | 0 |
| AML, induction chemotherapy | 2 | 5 |
| AML, consolidation chemotherapy | 1 (with febrile reaction) | 0 |
| NHL, induction chemotherapy | 1 | 1 |
| NHL, consolidation chemotherapy | 1 | 1 (with GI bleed) |
| Myelodsyplastic syndrome | 0 | 3 (1 with GI bleed) |
| Nonhematological malignancy | ||
| Optic glioma, receiving chemotherapy | 1 | 0 |
| Osteogenic sarcoma, receiving chemotherapy | 1 (with lung abscess) | 0 |
| Prostate cancer with metastasis | 0 | 1 (with DIC) |
| Lung cancer with metastasis | 0 | 1 |
| Cardiac disease* | ||
| Cardiac surgery with cardiopulmonary bypass | 1 | 11 (1 with wound infection) |
| Acute myocardial infarction, bleeding due to platelet dysfunction | 0 | 1 |
| Other | ||
| GI bleed | 0 | 2 (both with cirrhosis) |
| Multiple trauma | 0 | 1 (with HITT) |
| ITP/SLE | 0 | 2 (1 with infected graft) |
| Infection | 0 | 2† |
| Previous transfusion reactions | ||
| None | 9 | 29 |
| Allergic | 4 | 0 |
| Febrile | 2 | 2 |
| Platelet age (oldest product in implicated transfusion), d* | ||
| 2 | 1 | 0 |
| 3 | 1 | 3 |
| 4 | 3 | 9 |
| 5 | 9 | 19 |
| Principal diagnosis . | No. of patients younger than 17 y (n = 15) . | No. of patients older than 16 y (n = 31) . |
|---|---|---|
| Hematologic malignancy* | ||
| ALL, induction chemotherapy | 6 | 0 |
| ALL, consolidation chemotherapy | 1 | 0 |
| AML, induction chemotherapy | 2 | 5 |
| AML, consolidation chemotherapy | 1 (with febrile reaction) | 0 |
| NHL, induction chemotherapy | 1 | 1 |
| NHL, consolidation chemotherapy | 1 | 1 (with GI bleed) |
| Myelodsyplastic syndrome | 0 | 3 (1 with GI bleed) |
| Nonhematological malignancy | ||
| Optic glioma, receiving chemotherapy | 1 | 0 |
| Osteogenic sarcoma, receiving chemotherapy | 1 (with lung abscess) | 0 |
| Prostate cancer with metastasis | 0 | 1 (with DIC) |
| Lung cancer with metastasis | 0 | 1 |
| Cardiac disease* | ||
| Cardiac surgery with cardiopulmonary bypass | 1 | 11 (1 with wound infection) |
| Acute myocardial infarction, bleeding due to platelet dysfunction | 0 | 1 |
| Other | ||
| GI bleed | 0 | 2 (both with cirrhosis) |
| Multiple trauma | 0 | 1 (with HITT) |
| ITP/SLE | 0 | 2 (1 with infected graft) |
| Infection | 0 | 2† |
| Previous transfusion reactions | ||
| None | 9 | 29 |
| Allergic | 4 | 0 |
| Febrile | 2 | 2 |
| Platelet age (oldest product in implicated transfusion), d* | ||
| 2 | 1 | 0 |
| 3 | 1 | 3 |
| 4 | 3 | 9 |
| 5 | 9 | 19 |
Significant concomitant comorbidities are shown in parentheses. All but one patient had absolute neutrophil counts greater than 500/μL. ALL indicates acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; NHL, non-Hodgkin lymphoma; GI, gastrointestinal; ITP/SLE, idiopathic thrombocytopenic purpura/systemic lupus erythematosus; DIC, disseminated intravascular coagulation; and HITT, heparin-induced thrombocytopenia with thrombosis.
Statistically significantly different from the 225 control patients (P < .05). See “Nested case-control study.”
One patient had cholangitis, one had cellulitis.
Evaluation of immunoglobulins in the implicated blood products
To evaluate the role of antileukocyte antibodies in TRALI, we tested a total of 104 samples from donors implicated in 28 prospectively identified TRALI reactions as well as products from 24 control donors from the nested case-control study, representing 5 WB-PLT transfusions. Samples were evaluated for the presence of antibodies to HLA class I, HLA class II, and granulocyte antigens (Table 3). One donor had a moderately positive, significant anti–HLA class I antibody that was typed to the A26 locus. No other TRALI or control donors tested positive for anti–HLA class I antibodies. Even assuming that all weak, nonspecific reactions were significant, antileukocyte antibodies in donor plasma could be demonstrated in only 7 (25%) of 28 TRALI cases. If reactivity similar to that of positive controls is considered significant, this was demonstrable in only 3 (10.7%) of 28 cases. If only reactivity with definable specificity is considered significant, this antigen specificity was demonstrable in only 1 (3.6%) of 28 cases. It is also notable that frequency of donor antigranulocyte and anti–HLA class I antibodies did not differ significantly between the 28 TRALI cases and the 5 control cases. In addition, plasma from 13 of 15 PRBC units was not tested for the presence of antileukocyte antibodies owing to insufficient sample size. Samples were drawn from the donors of these implicated units, and none of the samples tested demonstrated anti-HLA or antigranulocyte antibodies (results not shown).
Antileukocyte antibodies detected in plasma from TRALI and control donors
| . | No. of cases . | Test results for total n . | No. of cases with positive results . |
|---|---|---|---|
| Antigranulocyte antibodies | |||
| TRALI donors (n = 104) | 28 | 16 weakly positive3-150 | 4 |
| Control donors (n = 24) | 5 (no reaction) | 3 weakly positive | 2 |
| Anti–HLA class I antibodies | |||
| TRALI donors (n = 104) | 28 | 1 moderately positive3-151 | 1 |
| Control donors (n = 24) | 5 (no reaction) | 0 positive | 2 |
| Anti–HLA class II antibodies (n = 75 TRALI donors) | 20 | 5 moderately positive3-152 | 5 |
| . | No. of cases . | Test results for total n . | No. of cases with positive results . |
|---|---|---|---|
| Antigranulocyte antibodies | |||
| TRALI donors (n = 104) | 28 | 16 weakly positive3-150 | 4 |
| Control donors (n = 24) | 5 (no reaction) | 3 weakly positive | 2 |
| Anti–HLA class I antibodies | |||
| TRALI donors (n = 104) | 28 | 1 moderately positive3-151 | 1 |
| Control donors (n = 24) | 5 (no reaction) | 0 positive | 2 |
| Anti–HLA class II antibodies (n = 75 TRALI donors) | 20 | 5 moderately positive3-152 | 5 |
Antibody testing was performed as described in “Patients, materials, and methods.”
All reactions were weak without definable specificity. Panel cells included cells positive for NA1, NA2, NB1, and NB2 antigens. Positive control antibodies included anti-NA1, anti-NA2, and anti-5b and gave strong reactions with appropriate control granulocytes.
Moderate reactions with anti-A26 specificity defined. Positive controls gave moderate to strong reactions.
Target cells included all serologically defined class II, DR types, except DR 12 and DR 14. Positive controls gave moderate to strong reactions. Of the 5 TRALI donors with positive reactions, 2 had strongly positive reactions with no definable specificity (both implicated plasmas were negative for antigranulocyte and anti-HLA class I antibodies) and 3 had weak or questionable reactions (all 3 implicated plasmas also tested nonspecifically weakly/questionably positive for antigranulocyte antibodies and were negative for anti-HLA class I antibodies).
Analysis of the lipid priming activity from the implicated WB-PLT concentrates
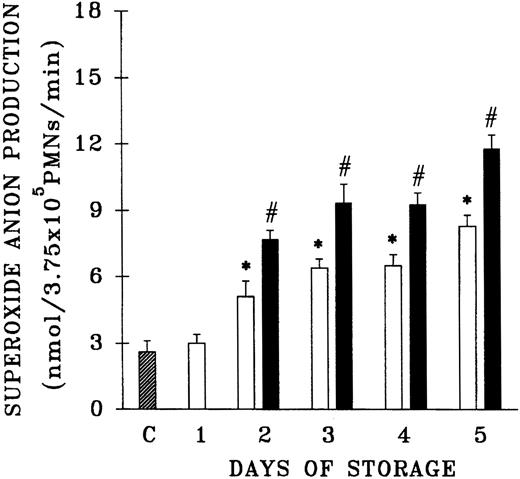
Because previous work that demonstrated the accumulation of PMN priming activity employed blood components from a single center, the plasma samples from 61 platelet units that differed in storage duration and had been given to hospitalized patients without untoward events were evaluated for the presence of PMN priming activity.15,16 Fresh plasma and day-1 WB-PLT plasma did not contain PMN priming activity different from that of buffer-treated controls (Figure 1). However, day-2 through day-5 plasma samples contained significant amounts of PMN priming activity compared with buffer-treated controls or day-1 samples (P < .05). Moreover, 64% ± 8% of this priming activity could be inhibited by pretreatment of the PMNs with WEB 2170, a lipid receptor antagonist,35-37 and 67% ± 14% of the plasma priming activity was chloroform extractable, defining it as a lipid, a result identical to previous reports.15 16
Plasma priming activity of control platelet concentrates vs platelet concentrates implicated in TRALI reactions as a function of storage time.
The buffer priming of fMLP (1 μM)–activated PMNs (C) is compared with that of PMNs pretreated with 10% plasma from units transfused to patients who did not have transfusion reactions (control platelets; ■), activated with fMLP, and with the plasma fraction of platelets implicated in TRALI reactions (implicated platelets; ▪), activated with fMLP, both as a function of storage time. * indicates statistically significant differences (P < .05) between the control platelet priming activity and priming activity from both the buffer-treated controls and day-1 platelet plasma. # indicates a statistically significant difference (P < .05) between the plasma priming activity of implicated platelet units and the plasma priming activity of control platelets, both as a function of storage time. Each bar represents a sample size of at least 8.
Plasma priming activity of control platelet concentrates vs platelet concentrates implicated in TRALI reactions as a function of storage time.
The buffer priming of fMLP (1 μM)–activated PMNs (C) is compared with that of PMNs pretreated with 10% plasma from units transfused to patients who did not have transfusion reactions (control platelets; ■), activated with fMLP, and with the plasma fraction of platelets implicated in TRALI reactions (implicated platelets; ▪), activated with fMLP, both as a function of storage time. * indicates statistically significant differences (P < .05) between the control platelet priming activity and priming activity from both the buffer-treated controls and day-1 platelet plasma. # indicates a statistically significant difference (P < .05) between the plasma priming activity of implicated platelet units and the plasma priming activity of control platelets, both as a function of storage time. Each bar represents a sample size of at least 8.
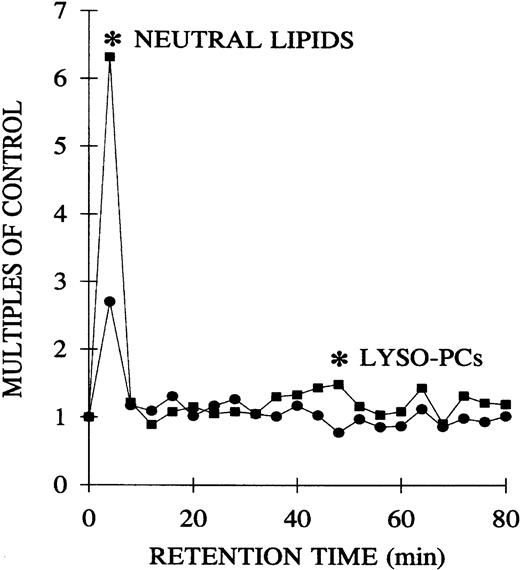
Since previous data documented that PMN priming activity accumulates in the plasma during routine storage of platelets, we quantified the amount of PMN priming activity in the implicated WB-PLT units and compared this activity with activity in the control group of platelets that were not implicated in transfusion reactions, as described above (Figure 1).16 The amount of lipid priming activity was higher in the implicated units than in control platelet units stored for the same duration, buffer-treated controls, and day-1 WB-PLT plasma (P < .05 for all groups days 2-5). Moreover, 66% ± 10% of the priming activity in the implicated group was chloroform extractable. HPLC separation of the lipids by phospholipid class demonstrated that the lipid activity from both the implicated and control platelets was a single peak of priming activity at the retention time of lyso-PCs, a result identical to previous reports (Figure 2).16
Separation of the lipid priming activity by phospholipid class from the plasma of day-5 control platelets and day-5 implicated platelets.
Lipids were extracted and separated by means of a normal-phase HPLC system. The resulting 4-minute fractions were resolubilized in 1.25% albumin and tested for their ability to prime the fMLP-activated respiratory burst in PMNs from healthy donors compared with albumin-treated controls. One peak of activity was found at the retention time of lyso-PCs in both the control and implicated WB-PLT units. The figure is representative of 6 control and 6 implicated WB-PLT units, which underwent identical analysis.
Separation of the lipid priming activity by phospholipid class from the plasma of day-5 control platelets and day-5 implicated platelets.
Lipids were extracted and separated by means of a normal-phase HPLC system. The resulting 4-minute fractions were resolubilized in 1.25% albumin and tested for their ability to prime the fMLP-activated respiratory burst in PMNs from healthy donors compared with albumin-treated controls. One peak of activity was found at the retention time of lyso-PCs in both the control and implicated WB-PLT units. The figure is representative of 6 control and 6 implicated WB-PLT units, which underwent identical analysis.
The PMN priming activity of patient plasma samples
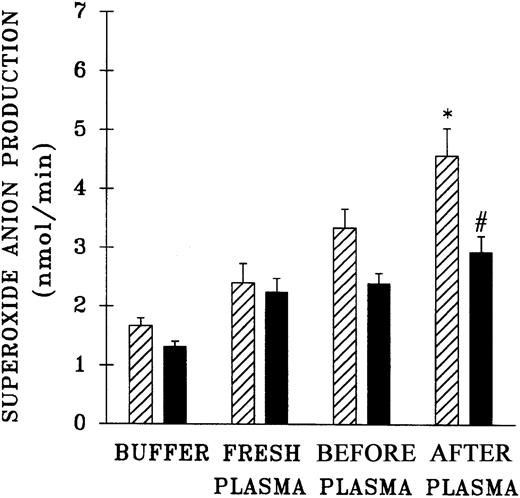
The priming activity of both pretransfusion plasma and postreaction plasma drawn at the time TRALI was clinically recognized was quantified and compared with the activity of fresh plasma and buffer-treated controls (Figure 3). Incubation of PMNs with the postreaction plasma significantly augmented the fMLP response compared with controls and pretransfusion plasma samples (P < .05). It is noteworthy that the pretransfusion plasma also enhanced the fMLP-activated respiratory burst, but this enhancement approached significance and was not different from the priming activity of the buffer-treated controls (P = .06). Preincubation of PMNs with WEB 2170 partially abrogated the priming activity of the postreaction plasma, by 54% ± 12%, compared with fMLP-activated controls (P < .05).
Priming activity of TRALI patient plasma at the time the reaction was recognized compared with activity of fresh plasma from healthy donors, their pretransfusion plasma samples, and buffer-primed controls.
▨ indicates neutrophils preincubated with buffer and ▪ indicates PMNs that were preincubated with WEB 2170 to block the PAF receptor. The plasma-treated groups (fresh, before, and after) were primed with 10% plasma for 5 minutes and then activated with 1 μM fMLP, in contrast to buffer-primed controls. The pretransfusion plasma (before) and posttransfusion plasma samples (after) were drawn from the same patient, the pretransfusion sample prior to the transfusion at the time of blood typing and the posttransfusion sample at the time TRALI was recognized. * indicates a statistically significant (P < .05) difference compared with the fresh plasma, the paired pretransfusion plasma sample, and the buffer-treated control. # indicates a statistically significant (P < .05) difference between PMNs pretreated with WEB 2170 and buffer-treated PMNs. The sample size was 10 for fresh plasma and 34 for the TRALI patients' pre- and posttransfusion samples.
Priming activity of TRALI patient plasma at the time the reaction was recognized compared with activity of fresh plasma from healthy donors, their pretransfusion plasma samples, and buffer-primed controls.
▨ indicates neutrophils preincubated with buffer and ▪ indicates PMNs that were preincubated with WEB 2170 to block the PAF receptor. The plasma-treated groups (fresh, before, and after) were primed with 10% plasma for 5 minutes and then activated with 1 μM fMLP, in contrast to buffer-primed controls. The pretransfusion plasma (before) and posttransfusion plasma samples (after) were drawn from the same patient, the pretransfusion sample prior to the transfusion at the time of blood typing and the posttransfusion sample at the time TRALI was recognized. * indicates a statistically significant (P < .05) difference compared with the fresh plasma, the paired pretransfusion plasma sample, and the buffer-treated control. # indicates a statistically significant (P < .05) difference between PMNs pretreated with WEB 2170 and buffer-treated PMNs. The sample size was 10 for fresh plasma and 34 for the TRALI patients' pre- and posttransfusion samples.
Normal-phase HPLC separation of the lipid priming activity from TRALI patients
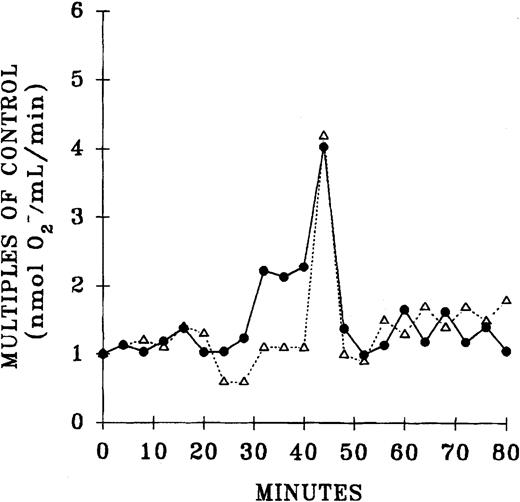
The plasma lipids of both the pretransfusion and postreaction samples from TRALI patients were extracted and separated by phospholipid class by normal-phase HPLC.15 The lipids were dried, solubilized in albumin, and their priming activity assayed with the use of PMNs from healthy donors. Compared with the pretransfusion samples, lipids, at the retention times of neutral lipids and lyso-PCs, significantly primed the fMLP-activated respiratory burst: pretransfusion neutral lipids, 2.7 ± 0.9, versus posttransfusion neutral lipids, 6.3 ± 2.2 (2.3-fold ± 0.5-fold;P < .05), and pretransfusion lyso-PCs, 0.8 ± 0.1, versus posttransfusion lyso-PCs, 1.5 ± 0.3 (1.8-fold ± 0.1-fold; P < .05) (Figure4).
Separation of the lipid PMN priming activity from the plasma of TRALI patients before transfusion, at the time of blood typing, and after transfusion, at the time TRALI was recognized, as a function of the retention time of lipids separated by normal phase HPLC.
Lipids were extracted from paired pretransfusion (●) and posttransfusion (▪) plasma samples, separated by normal-phase HPLC, resolubilized in 1.25% albumin, and tested for their ability to prime the fMLP activation of the oxidase of PMNs from healthy donors compared with albumin-treated controls. Two peaks of PMN priming activity are present at the retention times of neutral lipids and lyso-PCs that are not present before transfusion. * indicates statistical significance (P < .05) between the pretransfusion plasma and posttransfusion plasma groups. The sample size is 6 for both groups.
Separation of the lipid PMN priming activity from the plasma of TRALI patients before transfusion, at the time of blood typing, and after transfusion, at the time TRALI was recognized, as a function of the retention time of lipids separated by normal phase HPLC.
Lipids were extracted from paired pretransfusion (●) and posttransfusion (▪) plasma samples, separated by normal-phase HPLC, resolubilized in 1.25% albumin, and tested for their ability to prime the fMLP activation of the oxidase of PMNs from healthy donors compared with albumin-treated controls. Two peaks of PMN priming activity are present at the retention times of neutral lipids and lyso-PCs that are not present before transfusion. * indicates statistical significance (P < .05) between the pretransfusion plasma and posttransfusion plasma groups. The sample size is 6 for both groups.
Measurement of IL-6 and IL-8 in implicated WB-PLTs and patient samples
Since cytokines have been implicated in reactions precipitated by the transfusion of WB-PLTs,17-19 22 both IL-6 and IL-8 levels were measured in the implicated WB-PLT units and in both the pre- and posttransfusion plasma samples from TRALI patients. IL-6 levels were increased as a function of the storage time of the implicated platelet concentrates, but did not reach levels statistically different from the plasma levels of healthy control patients until day 5 of storage (day 5, 251.5 ± 103 pg/mL; controls, 1.6 ± 0.9 pg/mL; P < .05) (Figure5A). Similarly, IL-8 levels in the implicated platelets demonstrated a general increase as a function of storage time that was not statistically different from the plasma values of healthy control subjects until day 5 (day 5, 4816 ± 1815 pg/mL; controls, 50 ± 29 pg/mL; P < .05) (Figure5B).
Plasma levels of interleukin 6 (IL-6) and IL-8 from platelet units implicated in TRALI reactions.
Levels of IL-6 and IL-8 (pg/mL) are depicted as a function of storage time and are compared with plasma levels drawn from 10 healthy control donors (C, at left of each panel). * indicates a statistically significant (P < .05) difference compared with levels from the healthy controls. Each measurement represents a sample size of 11 to 34.
Plasma levels of interleukin 6 (IL-6) and IL-8 from platelet units implicated in TRALI reactions.
Levels of IL-6 and IL-8 (pg/mL) are depicted as a function of storage time and are compared with plasma levels drawn from 10 healthy control donors (C, at left of each panel). * indicates a statistically significant (P < .05) difference compared with levels from the healthy controls. Each measurement represents a sample size of 11 to 34.
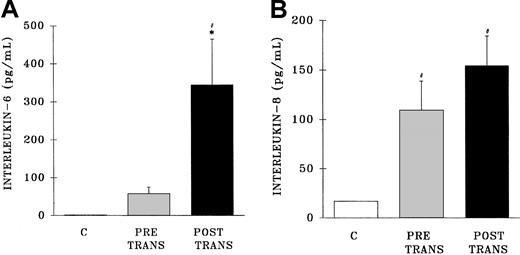
Measurement of IL-6 in the postreaction plasma samples demonstrated an increase in IL-6 levels compared with both pretransfusion samples and samples from healthy control subjects (postreaction, 345 ± 115 pg/mL; pretransfusion, 58 ± 17 pg/mL; healthy controls, 1.0 ± 2.3 pg/mL; P < .05) (Figure6A). Conversely, postreaction IL-8 levels were not increased compared with the levels found in the pretransfusion samples; however, IL-8 concentrations were higher in both the pre-and posttransfusion patient samples than in healthy control samples (postreaction, 155 ± 20 pg/mL; pretransfusion, 109 ± 29 pg/mL; healthy controls, 17 ± 1 pg/mL) (Figure 6B).
Plasma levels of interleukin 6 and interleukin 8 from healthy control donors and TRALI patients before transfusion and after transfusion at the time TRALI was recognized.
* indicates statistical significance (P < .05) compared with the pretransfusion (pretrans) sample and # indicates statistical significance (P < .05) compared with the healthy control sample (C, at left of each panel). The sample size was 32 for both the pretransfusion plasma and posttransfusion plasma groups.
Plasma levels of interleukin 6 and interleukin 8 from healthy control donors and TRALI patients before transfusion and after transfusion at the time TRALI was recognized.
* indicates statistical significance (P < .05) compared with the pretransfusion (pretrans) sample and # indicates statistical significance (P < .05) compared with the healthy control sample (C, at left of each panel). The sample size was 32 for both the pretransfusion plasma and posttransfusion plasma groups.
Discussion
Although TRALI has been thought to be relatively rare, a previous report indicated that TRALI occurred in 1 in 2000 transfusions of cellular components.9 In the present study the prevalence of TRALI was 1 in 1120 for all cellular components transfused and 1 in 1323 for all components transfused, a 4-fold increase over the initial reported prevalence of 1 in 5000 and supportive of the higher prevalence.1,9 These findings suggest that TRALI may be an underrecognized complication of hemotherapy, as previously postulated.2 The nested case-control study found that patients with underlying hematologic malignancy and those with cardiac disease requiring coronary bypass surgery appear to be at risk for the development of TRALI. There was also a slight but statistically significant increase in platelet age in TRALI cases compared with controls. This modest increase in storage time suggests that older stored platelets may be associated with TRALI and is consistent with a possible role for a biologic response modifier (or modifiers) generated during storage that increases in concentration proportionally to component age. Taken together with the increased amount of lipid priming activity in these units, these results suggest that older, stored platelet concentrates may have the potential to cause TRALI in susceptible hosts.
A possible antibody-mediated etiology of TRALI was conservatively found (as determined by the presence of any demonstrable antigranulocyte or anti–HLA class I or class II reactivity in implicated donor plasma) in only 25% of prospectively studied TRALI cases. Moreover, if only reactivity with definable specificity is considered significant, such reactivity was demonstrable in only 3.6% of cases. All available units were tested, one as a pool of 10 units, such that no reactions escaped testing for antileukocyte antibodies. Furthermore, the frequency of donor antigranulocyte and anti–HLA class I antibodies did not differ significantly between the 28 TRALI cases and the 5 control cases, for control donors were as likely to test positive for antileukocyte antibodies as TRALI donors. This finding is consistent with previous reports that documented the frequent presence of antileukocyte antibodies in healthy blood donors.38 In addition, there was a marked disparity in the prevalence of TRALI reactions among different blood components, with all cellular components (including PRBCs that contain little plasma) being 17 times more likely to be associated with TRALI than plasma alone. There was also discordance in this series between the one donor who exhibited a strongly positive, specific anti-A26 antibody and the more frequent weakly positive, nonspecific antigranulocyte reactivity found in both TRALI and control donors. This nonspecific reactivity in both control and implicated units suggests a possible artifact. Such discordance was striking given other studies, which showed a high concordance in TRALI donors of anti–HLA class I antibodies (72%) and antigranulocyte antibodies (89%), with anti–HLA class I antibodies being found in the same donors as the antigranulocyte antibodies in all but 2 cases.1 A similar concordance with lymphocytotoxic and granulocyte agglutinating antibodies of TRALI donors was also described.39
The presence of PMN priming activity was evaluated in the implicated units and compared with activity in control units of similar storage age. Both control and implicated platelet concentrates had PMN priming agents in the plasma fraction; however, the priming activity in the implicated units was significantly greater. The majority of this PMN priming activity was lipid, as found in previous reports.15,16 Moreover, the posttransfusion plasma from patients with TRALI contained significant PMN priming activity compared with the pretransfusion plasma, almost identical to the lipid priming activity found in posttransfusion samples in a retrospective series of TRALI cases.9
Analysis of the priming activity in the posttransfusion plasma demonstrated that there are 2 lipid classes that comprise this activity: the first at the retention time of neutral lipids and the second at the retention time of lyso-PCs. Compared with the pretransfusion plasma samples, the priming activity of the neutral lipids in the posttransfusion plasma was augmented 2.3-fold ± 0.5-fold, for there was some basal neutral lipid priming activity in the pretransfusion samples. The second peak of priming activity found in the posttransfusion plasma was not present in the pretransfusion samples and may possibly represent the infusion of high levels of lyso-PCs present in the platelet concentrates. These experiments were performed on paired pre- and posttransfusion plasma from the same patients to avoid investigator bias.
The possible role of cytokines infused with WB-PLT units was also investigated. Higher levels of both IL-6 and IL-8 were infused with the implicated day-5 platelets that resulted in TRALI reactions. IL-6 concentrations at day 5 of storage (251 ± 103 pg/mL) were similar to IL-6 levels (214-1088 pg/mL), which have been linked to the genesis of febrile transfusion reactions but not TRALI.17,22Concentrations of IL-6 that affected PMNs were several orders of magnitude higher in vitro.21,23 IL-8 levels were significantly elevated in the implicated WB-PLTs on day 5 only, similar to the levels reported by Stack and Snyder,40 and at these concentrations IL-8 has been reported to affect PMN function in vitro.20,21 Moreover, levels of IL-6, but not IL-8, were increased in TRALI patients. However, 32% of the TRALI reactions were to WB-PLTs stored for less than 5 days that did not have significantly elevated levels of either IL-6 or IL-8. Thus, IL-6 and IL-8 did not accumulate in high enough levels in the implicated WB-PLTs to affect PMNs, and their association with TRALI remains undefined. Furthermore, the increased levels of IL-6 in TRALI patients may have been reflective of lung injury, for increased levels of circulating IL-6 are a sensitive, but nonspecific, indicator of tissue damage.41 42
TRALI is clinically identical to ARDS.1,14,25-28,43,44Animal models of both TRALI and ARDS have shown that these PMN-mediated conditions may be the result of at least 2 independent events.14,25-28 The first event begins with activation of the pulmonary vascular endothelium, resulting in the release of chemokines and an increase in adhesion molecules on the endothelial surface.28 These chemokines prime and attract PMNs to the endothelial surface, where they firmly adhere and undergo cytoskeletal changes that result in rigid nondistensible PMNs unable to traverse the pulmonary microcirculation.28,45-47 This first event is then followed by a second event that causes activation of the microbicidal arsenal of these primed PMNs, which results in endothelial damage, capillary leak, and pulmonary damage.25,28,46 In TRALI, the first “event” is likely the clinical status of the patient, which may include active infection or sepsis, recent surgery (especially cardiopulmonary bypass surgery, which systemically primes PMNs and activates a variety of endothelial tissue beds), or traumatic injury.48,49 The second event is the transfusion of a lipid, cytokine, or antibody that activates these primed, adherent PMNs.14,26-29 Although priming and activation of PMNs are operationally defined as separate processes in vitro, it is important to realize that both are part of normal PMN physiology in the recruitment of PMNs from the circulation to sites of infection or inflammation in the tissues, where PMNs are essential for eradication of microbial invaders.14,25-29 However, in both TRALI and ARDS, similar signals come from the intravascular space, rather than from the tissues, resulting in adherence or trapping of primed PMNs in the microvasculature without the ability to transmigrate into the extravascular space.28 This priming of PMNs alters their reactivity, such that agents that do not cause assembly of the oxidase and release of cytotoxic agents in quiescent PMNs can cause activation of the microbicidal arsenal in primed PMNs.28,50,51Therefore, priming changes the PMN phenotype to “hyperreactive,” and a second, normally innocuous PMN priming agent, including lyso-PCs, could then activate these primed, adherent PMNs, which have maximal cytotoxic potential, culminating in acute lung injury (ALI).28,50 In vitro experiments have demonstrated that lyso-PCs have the capacity to activate the microbicidal arsenal of primed, but not quiescent, PMNs, causing destruction of human pulmonary endothelium.50-52 These events are the basis of the 2-insult animal models of ARDS, and lyso-PCs are etiologic in such models.14,25 27
These studies were performed some years ago, and transfusion practices have changed to include the routine use of A-PLTS and leukoreduced PRBCs. Despite these changes, the present study is still relevant, because (1) lyso-PCs are generated during storage of A-PLTs; (2) many centers worldwide still employ WB-PLTs; and (3) our results suggest that the magnitude of the role played by antileukocyte antibodies, especially anti-HLA antibodies, in the pathogenesis of TRALI reactions may be minor. Confirmation of these results would carry significant implications for the prevention of TRALI from the perspectives of public health and blood product manufacturing and would suggest that the major focus in preventing TRALI should not be on identification and exclusion of donors with anti-HLA antibodies, but rather on changes in manufacturing processes to inhibit the accumulation of lipids during storage.
In conclusion, TRALI, like ARDS, appears to be the result of 2 insults.25-27 Lipids in stored components may represent the second of these 2 required insults, and this prospective study has confirmed the presence of such lipids in the implicated component and in the patient at the time TRALI was recognized. Blood transfusions have been linked to the generation of ALI and are also a robust predictor of postinjury multiple organ failure (MOF), which includes ALI, in traumatically injured patients.53-55 Moreover, older, stored PRBC units are associated with the development of MOF in injured patients.52 Careful selection of components for transfusion in patients at risk for TRALI should be considered, including the use of washed or fresh components. Following recognition of the morbidity attendant on TRALI, the Food and Drug Administration has considered measures to preclude these reactions, including exclusion of multiparous women as donors. Given our failure to implicate antileukocyte antibodies as a major pathogenetic mechanism in this prospective series, this recommendation would seem unwarranted, and additional prospective and in vitro studies are required to definitively establish the role such antibodies play in TRALI.
Thanks to Shamim Shivji (HLA Laboratory, University of Alberta) for able technical assistance in performing the granulocyte immunofluorescence assays.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-03-0958.
Supported by Bonfils Blood Center; The Stacy Marie True Memorial Trust; a grant from the National Blood Foundation; a Professional Development Award from The Children's Hospital, Denver, CO; a Clinical Associate Physician Award (M01-RR00069) from the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health (NIH); a Transfusion Medicine Academic Award (K07-HL02036) from the National Heart, Lungs, and Blood Institute (NHLBI), NIH; and a grant (HL59355) from NHLBI, NIH.
Portions of this work were presented at the annual meetings of the American Society of Hematology, Seattle, WA, December 1-5, 1995; and Orlando, FL, December 7-11, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lynn K. Boshkov, Department of Pathology, Oregon Health Sciences University, L471, 3181 SW Sam Jackson Park Rd, Portland, OR 97201-3098; e-mail: boshkovl@ohsu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal