Idiopathic myelofibrosis (IM), also known as myelofibrosis with myeloid metaplasia, is a myeloproliferative disorder of clonal origin characterized by extramedullary hematopoiesis with a leukoerythroblastic blood picture, tear-drop erythrocytes, and progressive splenomegaly associated with bone marrow fibrosis.1 A number of data suggested that alterations of megakaryocytopoiesis, with accumulation of abnormal megakaryocytes in the hematopoietic tissues, could participate in the development of the disease.2 We read with great interest the recent article by Vannucchi et al3 as we have been involved in studies on the pathogenesis of IM for several years. In this paper, the authors reported that mice harboring the GATA-1low mutation develop a frank IM-like disease characterized by the presence of tear-drop poikilocytes and progenitors in the blood, collagen fibers in the marrow and in the spleen, and hematopoietic foci in the liver, and they suggested that the human IM might result from deregulated expression or mutations of GATA-1. Actually, transcription factor GATA-1, which interacts with the transcriptional cofactor, friend of GATA-1 (FOG),4 exerts a critical role in erythroid and megakaryocytic cell differentiation.5,6 Given the prominent place of the megakaryocyte (Mk) population in the pathogenesis of IM, we recently investigated alterations in the expression and/or functionality of these factors in hematopoietic cells from 20 patients. Using semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR), we observed an increased level of GATA-1 expression in patients' peripheral blood mononuclear cells (PBMC) compared with PBMC from normal blood; such an increase likely reflects the presence of a high number of circulating megakaryocytes and their precursors in patients' blood.7 Concerning CD34+ progenitor cells (Figure 1A), we did not observe any significant difference in GATA-1 expression level between CD34+ progenitor cells purified from patients' blood (as myelofibrosis precluded successful bone marrow aspiration) and CD34+ cells isolated from unmotilized peripheral blood and bone marrow of healthy individuals (yield of purity ≥ 97%).
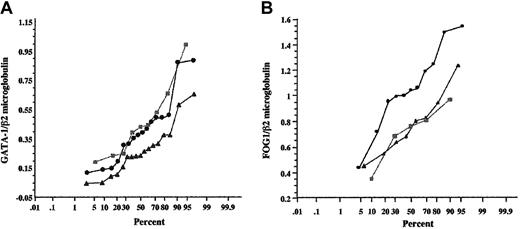
GATA-1 mRNA expression in patients' hematopoietic cells. Semiquantification of GATA-1 gene expression was performed after coamplification and normalization with an internal control β2-microglobulin. RT-PCR products were analyzed by 7% polyacrylamide gel electrophoresis and visualized by BET staining. The GATA-1–β2-microglobulin ratio (401 bp:274 bp) (A) and the FOG-1–β2-microglobulin ratio (275 bp:165 bp) (B) were calculated for patients' CD34+ cells (•), compared with CD34+ cells from healthy individual bone marrow (▴) and CD34+ cells from healthy subject peripheral blood (▪). The results were expressed as curves of cumulative frequencies, the y-axis corresponding to the GATA-1–β2-microglobulin ratio or FOG1–β2-microglobulin ratio, and the x-axis indicating the percentage of individuals.
GATA-1 mRNA expression in patients' hematopoietic cells. Semiquantification of GATA-1 gene expression was performed after coamplification and normalization with an internal control β2-microglobulin. RT-PCR products were analyzed by 7% polyacrylamide gel electrophoresis and visualized by BET staining. The GATA-1–β2-microglobulin ratio (401 bp:274 bp) (A) and the FOG-1–β2-microglobulin ratio (275 bp:165 bp) (B) were calculated for patients' CD34+ cells (•), compared with CD34+ cells from healthy individual bone marrow (▴) and CD34+ cells from healthy subject peripheral blood (▪). The results were expressed as curves of cumulative frequencies, the y-axis corresponding to the GATA-1–β2-microglobulin ratio or FOG1–β2-microglobulin ratio, and the x-axis indicating the percentage of individuals.
In contrast, the expression of cofactor FOG-18 was significantly increased (P = .02) in CD34+ cells from patients when compared with CD34+ cells from normal bone marrow and normal unmotilized peripheral blood. As GATA-1 also associates in a multiprotein complex with the stem cell leukemia gene (SCL),9 a crucial actor in early hematopoiesis and differentiation along the erythroid and megakaryocytic pathways,10 we sought for potential alteration in SCL expression. Our studies showed that SCL gene transcription is altered in patients' CD34+ progenitor cells and that the sublocalization of SCL protein is different in patients' megakaryocytic cells and megakaryocytes from healthy subjects, ie both nuclear and cytoplasmic versus solely nuclear, respectively.
Altogether, the interesting data by Vannucchi et al and our findings highlight the possible contribution of a deregulated expression of any of these interacting transcription factors and/or of altered interactions between these nuclear proteins to the pathogenesis of idiopathic myelofibrosis.
Little steps toward the identification of the myelofibrotic locus
Idiopathic myelofibrosis (IM) is a myeloproliferative disorder of clonal origin thought to be originated by either somatic or inherited mutations at a locus, the IM locus, that would confer proliferative advantage to a bipotent (erythroid [E], and megakaryocytic [Mk]) progenitor cell.1,2 This cell would eventually undergo transformation leading the disease toward its final leukemic phase. Almost all of the IM cases express at diagnosis one major cytogenetic defect, mostly del(13q), del(20q), or partial trisomy 1q.3 IM, however, is not characterized by any “unique” chromosomal abnormality. As such, the chromosomal abnormalities found to be associated with IM are considered secondary genetic events in the multistep progression of a disease whose primary genetic cause, the IM locus, is still to be identified.
The genes that are currently under investigation as candidates for the IM locus are represented by classic tumor suppressor genes, such as the retinoblastoma (RB1)4 and the p53 genes or the p16 gene and the RAS family of proto-oncogenes.5,6 Alternatively, genes that are involved in the control of proliferation of early hematopoietic cells, such as stem cell factor (SCF) and its receptor c-kit, and elements of the b-fibroblast growth factor (b-FGF) pathway7 are also being considered. Among those, SCF/c-kit are slightly favored because of the observation that the progenitor cells circulating in myeloproliferative disorders present both elevated expression8 and point mutation9,10 of c-kit. However, none of the many mouse mutants in the SCF and c-kit locus available has been described up to now to develop myelofibrosis. Furthermore it is not clear how any of these genes would specifically induce the abnormalities in the E and Mk pathway, which are the landmarks of the disease.
Hypothesis-driven research has suggested genes in the thrombopoietin (TPO) pathway, the growth factor which specifically regulates Mk production in vivo,11 as possible candidates for the IM locus. This hypothesis gained considerable favor when it was demonstrated that IM can be experimentally induced in mice by in vivo manipulation of the levels of TPO.12-14 However, a later study failed to find autocrine TPO production or mutations in the TPO receptor (Mpl) gene in 14 cases of human IM,15 leaving the relationship between possible presence of alterations in the TPO/Mpl pathway and the increased numbers of Mk observed in these patients still to be ascertained.
GATA-1 is a gene specifically involved in the regulation of the number of both E and Mk in vivo.16 Our paper,17 showing that GATA-1low (GATA-1tm2Sho) mutant mice develop a frank myelofibrotic syndrome with age, opens the possibility that genes involved in GATA-1 function might be directly responsible for the development of the disease. The letter by Martyré et al, showing that CD34+ cells circulating in the patients express lower levels of FOG-1 (one of the GATA-1 obligatory partners), further encourages research in this direction. Both reports are far away from having identified the IM locus. In fact, since the mouse develops IM very late in life, it is possible that in this case the GATA-1low mutation has a permissive role by inducing the hyper-proliferation of the progenitor cells that allows them to accumulate secondary mutations. On the other hand, since the letter by Dr Martyré et al does not provide any evidence for genetic abnormalities in the FOG-1 locus, it is possible that the alterations it describes are pleiotropic to an underlying genetic defect.
It must be said that many excellent experimental papers on the pathophysiology of IM have been recently published. Among those, especially worthy of mention is the report that forced expression of TPO transforms normal, but not transforming growth factor–βnull (TGF-βnull), stem cells into IM-inducing clones.18 All the data that are being accumulated, plus the existence of national and international registries of the disease, which have been carefully established in the mean time, raise great hope that by unifying the experimental efforts we might be finally very close to grasp the etiology of human IM.
Correspondence: Alessandro Maria Vannucchi, Department of Hematology, University of Florence, Florence, Italy; e-mail: a.vannucchi@dfc.unifi.it; and Anna Rita Migliaccio, Laboratory of Clinical Biochemistry, Istituto Superiore Sanità, Rome, Italy; e-mail: migliar@iss.it

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal