Abstract
Activation of complement cascade via the antibody-mediated classical pathway can initiate red blood cell (RBC) destruction, causing transfusion reactions and hemolytic anemia. In the present study, we have assessed the ability of a human recombinant soluble form of complement receptor 1 (sCR1) to inhibit complement-mediated RBC destruction in vitro and in vivo. Using an in vitro alloimmune incompatibility model, sCR1 inhibited complement activation and prevented hemolysis. Following transfusion of human group O RBCs into mice lacking detectable pre-existing antibodies against the transfused RBCs, systemic coadministration of 10 mg/kg sCR1, a dose well tolerated in human subjects for prevention of tissue injury, completely inhibited the in vivo clearance of the transfused RBCs and surface C3 deposition in the first hour after transfusion, correlating with the half-life of sCR1 in the circulation. Treatment with sCR1 increased the survival of transfused human group A RBCs in the circulation of mice with pre-existing anti-A for 2 hours after transfusion by 50%, reduced intravascular hemolysis, and lowered the levels of complement deposition (C3 and C4), but not immunoglobulin G (IgG) or IgM, on the transfused cells by 100-fold. We further identified potential functional domains in CR1 that can act to limit complement-mediated RBC destruction in vitro and in vivo. Collectively, our data highlight a potential use of CR1-based inhibitors for prevention of complement-dependent immune hemolysis.
Introduction
The complement system is an important mediator of the host immune response to infection and tissue damage, but may cause substantial injury when activated inappropriately.1 The pathobiologic effects of complement are mediated directly by the formation of the cytolytic membrane attack complex (C5b-9), and indirectly by the generation of anaphylatoxins C3a and C5a. Furthermore, complement activation can result in formation of opsonins (C3b), promoting the engulfment of target cells by phagocytes. In immune destruction of red blood cells (RBCs), complement plays a critical role, being involved in both intravascular and extravascular hemolysis.2 Generally, in the presence of a potent, complement-binding antibody and large numbers of closely situated red cell antigens, complement activation can proceed to completion, resulting in intravascular hemolysis. This type of RBC destruction is usually seen following transfusion of ABO-incompatible RBCs, and although there are numerous highly sensitive systems to detect very low levels of antibody, ABO incompatibility remains the major cause of death as a result of transfusion.3 The majority of blood group antibodies (including both alloantibodies and autoantibodies) that can fix complement, however, activate complement up to the C3 stage but do not go on to act as hemolysins. Nevertheless, because C3 is activated, RBCs can become coated with C3b, sequestered in the spleen and liver and phagocytosed by tissue macrophages, thereby causing extravascular hemolysis. Although antibody-coated red cells can also be destroyed extravascularly without complement activation, red cell removal is enhanced considerably when C3 is present on red cells in addition to immunoglobulin G (IgG). Indeed, as many as 50% of patients with immune hemolytic diseases have both IgG and complement on their red cells.4 Because of the contribution of complement in RBC destruction, we have taken the approach of activation using recombinant complement inhibitory molecules to block complement-mediated immune hemolysis, and thereby prevent some of its associated life-threatening effects.
Human complement receptor 1 (CR1, CD35) is a single chain transmembrane glycoprotein of molecular weight 160 kd to 250 kd (depending on allotype) and is a member of the regulators of complement activators (RCA) family of proteins that prevent excessive complement activation by inhibiting key enzymes, C3 and C5 convertases of the 3 complement pathways, namely the classical, alternative, and lectin.5 Specifically, CR1 binds to C4b and C3b and, as a consequence, plays a key role in immune complex clearance.6,7 Through its C4b/C3b binding, it causes the displacement of the catalytic subunits of the C3 and C5 convertases (decay accelerating activity), thereby inhibiting complement activation at the cell surface.8,9 CR1 also possesses cofactor activity for the serum protease factor I that cleaves C3b and C4b such that they no longer can participate in the complement cascade.10 Weisman et al demonstrated that a soluble recombinant form of human CR1 (sCR1), lacking the transmemebrane and cytoplasmic tail, retained the complement regulatory functions of full-length CR1, and acted as an effective fluid-phase complement inhibitor in vivo.11 Human sCR1 has prevented complement-mediated tissue injury in mice and other animal models and is currently in human clinical trials for treatment of complement-mediated acute lung injury and acute respiratory distress syndrome, being administered intraveneously.12,13 Despite the role of sCR1 as a global inhibitor of all complement activation pathways, the antibacterial defenses of the patients undergoing treatment have not thus far been compromised.14 This underscores the usefulness of sCR1 as a therapeutic agent. The potential of sCR1 for inhibiting complement-mediated RBC destruction such as following transfusion immunization has important clinical implications but has remained unexplored.
The recombinant sCR1 used in animal and human studies is composed of 30 short consensus repeat (SCR) domains of 60 to 70 amino acids and the first 28 SCRs can be further organized in groups of 7 into 4 long homologous repeats (LHRs) A through D, each of about 450 amino acids.15 Although several in vitro CR1 structure-function studies have been performed,16 there are currently no reports addressing the in vivo role of the individual functional domains of CR1, such as the LHRs, for complement inhibition in animal models and, therefore, their potential as complement inhibitors. Such in vivo structure-function studies are crucial for future development of structure-based inhibitors.
In this study, we have used an in vitro model of blood group alloimmune incompatibility to test the ability of CR1 derivatives to inhibit complement-dependent red cell destruction. By developing a mouse model of transfusion reaction, we have demonstrated the effectiveness of sCR1 to inhibit complement activation and prolong RBC survival in vivo. We have also identified LHR-A as a functional domain in CR1 that can limit complement-mediated RBC destruction in vivo.
Materials and methods
Recombinant protein preparation
Stable 293T cells expressing His-tagged human CR1 truncations were prepared and incubated with Opti-MEM (InVitrogen, Carlsbad, CA) as described previously.17 Supernatants were harvested, incubated with Ni-NTA agarose beads (Qiagen, Valencia, CA), and eluted with phosphate-buffered saline (PBS) containing 500 mM imidazol. Eluted fractions were analyzed by immunoblotting as described previously using known concentrations of myc-tagged control, Positope (InVitrogen), to estimate protein concentrations.17
Ligand binding studies
Approximately 5 μg of CR1 derivatives were first immobilized on nickel-agarose beads (Qiagen) and incubated separately with 100 ng purified C3b and C4b (Advanced Research Technologies, San Diego, CA) in low ionic binding buffer (25 mM NaCl). After several washes, the proteins were eluted using 500 mM imidazole and analyzed by Western blotting with anti-C3 or anti-C4 (Advanced Research Technologies).
Cofactor I assays
Purified C3b or C4b (80 ng; Advanced Research Technologies) were incubated in low ionic buffer (containing 25 mM NaCl) with 60 ng factor I (Advanced Research Technologies) in the absence (mock) or presence of 10 ng or 100 ng LHR-A, LHR-B, LHR-C, and LHR-D. After incubation at 37°C for 1 hour, the samples were run under reducing conditions and immunoblotted with anti-C3 or anti-C4 (Advanced Research Technologies).
Hemolysis and C3 deposition assays in vitro
Fresh human sera from O volunteers, as a source of complement, were preincubated with mock or different concentrations of CR1 derivatives for 15 minutes at room temperature. As a control, we preincubated the sera with 10 mM EDTA (ethylenediaminetetraacetic acid) to inhibit all complement activation pathways or 10 mM EGTA (ethyleneglycotetraacetic acid) and 2 mM MgCl2 to inhibit the antibody-dependent classical pathway. RBCs (108/mL), sensitized with human plasma containing complement-fixing antibodies anti-Lea, anti-Leb, and anti-I (all identified by the Reference Laboratory, New York Blood Center) and 3 examples of anti-A (from volunteer group O donors) were added to a final serum concentration of 30% and incubated for 30 minutes at 37°C. Although most anti-I and anti-Le antibodies react optimally at lower temperatures, these antibodies were able to react at 37°C in our studies, probably because the experiments were set up at lower temperatures. Thus, the antibodies appeared to exhibit bithermic reactivity.18 Sera from a total of 5 different group O individuals were used throughout these studies, although not all sera were tested for each antibody-sensitized RBC experiment. However, each experiment was repeated at least twice and each antibody-sensitized RBC was tested against at least one serum. Hemolysis was assessed by spectrophotometric measurement of hemoglobin in the supernatants and the mock-treated samples were used as control: the amount of hemolysis in the experimental samples was expressed as a percentage of the control (taken as 100%). The remaining unlysed RBCs were stained with fluorescein isothiocyanate (FITC)–conjugated goat anti–human C3 (ICN, Aurora, OH) and FITC-conjugated goat anti–human C4 (ICN) and analyzed by fluorescence activated cell sorting (FACS) analysis. The fluorescence intensity values for complement deposition (C3 and C4) on mock-treated samples were used as control and expressed as 100%. Complement deposition on the remaining samples were then calculated as a percentage of the control. Similar results were obtained with a panel of anti-C3 and anti-C4 from other commercial companies.
Transfusion of human RBCs into mice
Human RBCs were labeled with the fluorescent dye PKH-26 (Sigma, St Louis, MO) according to manufacturer's instructions and resuspended in known amounts of recombinant CR1 derivatives or mock to give a final hematocrit of 50%. C57Bl/6Ly5.1 male or female mice (25 g; 7 to 9 weeks old) were first injected via the tail vein with 300 μL of different concentrations of recombinant CR1 derivatives or mock. After 2 minutes, 200 μL of the stained RBCs at 50% hematocrit was administered intravenously. Blood samples (25 μL) were obtained by retro-orbital sinus bleeding at different time points after the transfusion and the clearance of fluorescent RBCs was measured by flow cytometry. The fraction of fluorescent human RBCs in the total number of recipient RBCs studied (300 000 per sample) was determined. We also measured the fraction of transfused cells by staining the blood samples with FITC-conjugated antihuman glycophorin A (GPA) (Dako, Glostrup, Denmark) that specifically labels human RBCs. Given that mice weighing 25 g have a total blood volume of approximately 2 mL, these experimental mice were transfused with a volume equivalent to 10% of their total blood volume. Control experiments with unlabeled human RBCs detected by staining with anti-GPA and flow cytometry gave similar results, while transfusion of PKH-labeled mouse RBCs resulted in survival of transfused cells for up to 2 weeks after transfusion, indicating that PKH labeling per se did not affect the survival of transfused RBCs.
Measurements of intravascular hemolysis
We measured the hemolysis (hemoglobin release) in the plasma spectrophotometrically at optical density (OD) 405 and subtracted the values for the pretransfusion levels for each mouse. The levels of released hemoglobin in CR1-treated mice were then compared with those of mock-treated animals at each time point and were expressed as a percentage of hemolysis of the samples from mock-treated mice.
Detection of antibody and complement components on transfused RBCs
We analyzed the amount of complement deposition and antibody sensitization on the transfused human RBCs by 2-color flow cytometry. Collected RBCs from each time point were stained with R-PE–conjugated anti–human GPA (Dako) and one of the following antibodies: an FITC-conjugated goat anti–mouse C3 (ICN), FITC-conjugated rabbit anti–human C3c (Dako), FITC-conjugated goat anti–human C4 (ICN), FITC-conjugated rabbit anti–human C4c (Dako), FITC-conjugated anti–mouse IgG (Vector Laboratories, Burlingame, CA), and FITC-conjugated anti–mouse IgM (Vector Laboratories). We found that antibodies to human C4 and C4c were cross-reactive with murine C4. The level of reactivity with each given antibody was calculated as the median of FL-1 fluorescence intensity of human GPA-positive RBCs. To detect antibody coating on the transfused human group O RBCs in circulation, we also made elutes from the collected blood by an acid elution method using the Elu-Kit II (Gamma Biologicals, Houston, TX) according to manufacturer's instructions, but no antibody activity was demonstrated.
Statistical analysis
The significance of differences between groups of mice were calculated using analysis of variance (Anova) test and only P values < .05 were considered significant.
Results
Biochemical analysis of complement inhibitory functions of CR1
In order to assess the ability of CR1 derivatives to prevent complement activation by human blood group antibodies, we expressed several histidine-tagged truncated human CR1 soluble proteins in 293T cells (Figure 1A) and tested their complement inhibitory activities biochemically (Figure 5B-D) and by in vitro assays (Figure 2). The rationale to use smaller truncated CR1 proteins was based on previous in vitro structure-function studies that have identified potential complement inhibitory activities within the LHRs.15,16 Since the complement-inhibiting activities of CR1 are dependent on interaction with its ligands, namely C3b and C4b,6,7,9 we first tested the ligand-binding activities of our recombinant CR1 derivatives. Because the CR1 derivatives are His-tagged, they were first immobilized onto nickel agarose beads and after incubation with C4b or C3b in low ionic binding conditions, the proteins were eluted and analyzed by immunoblotting with anti-C3 or anti-C4. Under reducing conditions, C4b migrates as 3 major bands (Figure 1B, upper blot) of 94 kd to 96 kd (α chain), 72 kd to 77 kd (β chain), and 32 kd to 35 kd (γ chain, not shown), whereas C3b migrates as 2 bands of 105 kd (α′ chain) and 75 kd (β chain) (Figure 1B, lower blot). Using the pull-down assay, we found that LHR-A, LHR-B, and LHR-C and sCR1 bound C4b (Figure 1B, upper blot) and that only LHR-B, LHR-C, and sCR1 bound C3b (Figure 1B, lower blot). Our C3b/C4b ligand binding data are consistent with previous data,20,21 demonstrating that our CR1 recombinant proteins have functional activity.
Biochemical analysis of CR1 derivatives. (A) Schematic representation of CR1 truncations. The top diagram depicts the full-length CR1 protein consisting of a signal peptide followed by tandemly arranged 30 SCRs (shown as joined boxes).15,19 sCR1 lacks the transmembrane and cytoplasmic tails; LHR-A through LHR-D are truncations of 7 SCRs each and LHR-D+ consists of LHR-D and the last 2 SCRs. (B) Pull-down assays with C4b and C3b and CR1 derivatives: LHR-A, LHR-B, LHR-C, LHR-D, LHR-D+, sCR1, and mock as a control. The input (first lane) C4b (upper blot) and C3b (lower blot) are indicated by arrows. Both C3b and C4b were eluted from sCR1, LHR-B, and LHR-C. C4b was eluted, but C3b was not eluted from LHR-A. Although similar amounts of LHR-D and LHR-D+ were used in the pull-down assays, neither one bound to C3b or C4b. (C-D) Cofactor activity of LHRs for cleavage of C4b (C) and C3b (D). Purified C4b or C3b was incubated with factor I alone (first lane, In C4b or In C3b, respectively), or in the presence of 2 different amounts (100 ng or 10 ng, indicated as a gradient) of CR1 derivatives: LHR-A, LHR-B, LHR-C, LHR-D, and mock as a control. The position of degradation products of C3b and C4b are indicated by arrows.
Biochemical analysis of CR1 derivatives. (A) Schematic representation of CR1 truncations. The top diagram depicts the full-length CR1 protein consisting of a signal peptide followed by tandemly arranged 30 SCRs (shown as joined boxes).15,19 sCR1 lacks the transmembrane and cytoplasmic tails; LHR-A through LHR-D are truncations of 7 SCRs each and LHR-D+ consists of LHR-D and the last 2 SCRs. (B) Pull-down assays with C4b and C3b and CR1 derivatives: LHR-A, LHR-B, LHR-C, LHR-D, LHR-D+, sCR1, and mock as a control. The input (first lane) C4b (upper blot) and C3b (lower blot) are indicated by arrows. Both C3b and C4b were eluted from sCR1, LHR-B, and LHR-C. C4b was eluted, but C3b was not eluted from LHR-A. Although similar amounts of LHR-D and LHR-D+ were used in the pull-down assays, neither one bound to C3b or C4b. (C-D) Cofactor activity of LHRs for cleavage of C4b (C) and C3b (D). Purified C4b or C3b was incubated with factor I alone (first lane, In C4b or In C3b, respectively), or in the presence of 2 different amounts (100 ng or 10 ng, indicated as a gradient) of CR1 derivatives: LHR-A, LHR-B, LHR-C, LHR-D, and mock as a control. The position of degradation products of C3b and C4b are indicated by arrows.
Inhibition of complement activation by CR1 derivatives. (A) The amount of hemoglobin released in the plasma of human group A–transfused mice treated without (mock, n = 15) or with different CR1 derivatives (sCR1, n = 9; LHR-A, n = 9; LHR-B, n = 5) was measured at OD 405 at the indicated times after transfusion and was subtracted from the pretransfusion levels. Data are presented as the percentage of hemolysis in the plasma of mock-treated mice at indicated time points after transfusion with the error bars depicting SEM. (B-D) The level of reactivity of anti-C4 (B), anti-C3, using FITC-conjugated anti–mouse C3 (C), and anti-IgG (D), with transfused human group A RBCs at indicated times after transfusion was measured by flow cytometry and is presented in fluorescence intensity units on the y-axis. Mock, n = 15; sCR1, n = 9; LHR-A, n = 9; and LHR-B, n = 5. (E) The level of reactivity of anti-C3, with transfused group O RBCs in fluorescence units. Mock, n = 8; sCR1, n = 8; LHR-A, n = 4; and LHR-B, n = 6.
Inhibition of complement activation by CR1 derivatives. (A) The amount of hemoglobin released in the plasma of human group A–transfused mice treated without (mock, n = 15) or with different CR1 derivatives (sCR1, n = 9; LHR-A, n = 9; LHR-B, n = 5) was measured at OD 405 at the indicated times after transfusion and was subtracted from the pretransfusion levels. Data are presented as the percentage of hemolysis in the plasma of mock-treated mice at indicated time points after transfusion with the error bars depicting SEM. (B-D) The level of reactivity of anti-C4 (B), anti-C3, using FITC-conjugated anti–mouse C3 (C), and anti-IgG (D), with transfused human group A RBCs at indicated times after transfusion was measured by flow cytometry and is presented in fluorescence intensity units on the y-axis. Mock, n = 15; sCR1, n = 9; LHR-A, n = 9; and LHR-B, n = 5. (E) The level of reactivity of anti-C3, with transfused group O RBCs in fluorescence units. Mock, n = 8; sCR1, n = 8; LHR-A, n = 4; and LHR-B, n = 6.
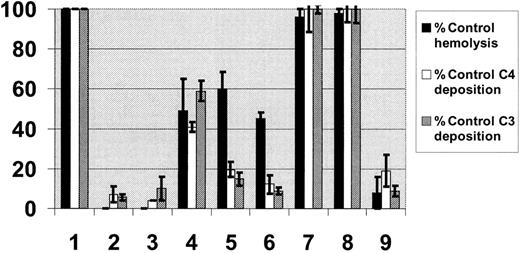
Functional analysis of CR1 derivatives in vitro. RBCs, sensitized with 6 different complement-fixing antibodies, were incubated with fresh sera as source of complement, which had been pretreated with mock (lane 1) or known complement inhibitors: either 10 mM EDTA (lane 2) to block all complement activation pathways or 10 mM EGTA in the presence of 2 mM MgCl2 (lane 3) to block the classical pathway; or CR1 derivatives all at a final concentration of 1 μM: LHR-A (lane 4), LHR-B (lane 5), LHR-C (lane 6), LHR-D (lane 7), LHR-D+ (lane 8), and sCR1 (lane 9). Hemolysis (hemoglobin release) in the supernatants was measured spectrophotometrically at 405 nm. The reactivity of remaining RBCs with anti-C3 using FITC-conjugated human anti-C3, or anti-C4 using FITC-conjugated human anti-C4, was measured by flow cytometry. Data are presented as percentage of control (Mock) hemolysis (▪), C4 (□), or C3 (▦) reactivities. Each series of experiments with a given antibody-sensitized antibody was repeated at least 2 times so that each bar represents the mean ± standard error of the mean (SEM) of least 12 experiments.
Functional analysis of CR1 derivatives in vitro. RBCs, sensitized with 6 different complement-fixing antibodies, were incubated with fresh sera as source of complement, which had been pretreated with mock (lane 1) or known complement inhibitors: either 10 mM EDTA (lane 2) to block all complement activation pathways or 10 mM EGTA in the presence of 2 mM MgCl2 (lane 3) to block the classical pathway; or CR1 derivatives all at a final concentration of 1 μM: LHR-A (lane 4), LHR-B (lane 5), LHR-C (lane 6), LHR-D (lane 7), LHR-D+ (lane 8), and sCR1 (lane 9). Hemolysis (hemoglobin release) in the supernatants was measured spectrophotometrically at 405 nm. The reactivity of remaining RBCs with anti-C3 using FITC-conjugated human anti-C3, or anti-C4 using FITC-conjugated human anti-C4, was measured by flow cytometry. Data are presented as percentage of control (Mock) hemolysis (▪), C4 (□), or C3 (▦) reactivities. Each series of experiments with a given antibody-sensitized antibody was repeated at least 2 times so that each bar represents the mean ± standard error of the mean (SEM) of least 12 experiments.
A characteristic anticomplement activity of CR1 is its ability to act as a cofactor for factor I–mediated degradation of C4b and C3b.8,10,11 To assay the cofactor activities of individual LHRs, the recombinant proteins were incubated with C3b and C4b and the fragmentation of the α chains of C4b and C3b were analyzed (Figure 1C-D, respectively). Addition of LHR-B and LHR-C consistently cleaved C4b to C4d (46 kd) and C4c (30 kd) (Figure 1C). Similarly, addition of LHR-B and LHR-C cleaved C3b to iC3b, characterized by the 68-kd and 43kd fragments and to C3dg of 40 kd and C3c of 30 kd (Figure 1D), consistent with previous data.22-24 LHR-A had only weak cofactor activity for C3b cleavage since only iC3b was generated using high protein concentrations (Figure 1D).
Complement inhibition by CR1 derivatives in an in vitro model of alloimmune incompatibility
Having shown that the recombinant CR1 derivatives possess anticomplement activity, we next tested the ability of these molecules to prevent complement activation by human blood group antibodies (Figure 2). We specifically activated the classical complement pathway using RBCs sensitized with complement-fixing blood group antibodies (anti-Lea, anti-Leb, anti-I, and 3 examples of anti-A) and human sera in the absence or presence of CR1 derivatives. We found that sCR1 inhibited hemolysis of antigen-positive RBCs as well as surface complement (C4 and C3) deposition (Figure 2, lane 9). LHR-A (lane 4), LHR-B (lane 5), and LHR-C (lane 6) also inhibited hemolysis, albeit less effectively than sCR1. LHR-B (lane 5), LHR-C (lane 6) and, to a lesser extent, LHR-A (lane 4), inhibited surface complement deposition. These results demonstrate that sCR1 is indeed capable of inhibiting RBC destruction by complement-fixing blood group antibodies in an in vitro model of alloimmune incompatibility and that the potential inhibitory domains reside in LHR-A, LHR-B, and LHR-C.
Protective effects of sCR1 in a mouse model of complement-mediated RBC destruction
Our objective was to develop a mouse model of antibody-mediated transfusion reactions that would enable us to investigate whether CR1 derivatives could prevent RBCs from complement-dependent immune destruction, and thereby prolong the survival of transfused RBCs in vivo. Because the mouse blood group systems are not well characterized, we decided to transfuse human RBCs into mice with pre-existing antibodies directed against specific human blood group antigens. Certain strains of mice have naturally occurring cross-reactive antibodies against ABO antigens.25,26 We found that plasma samples from C57Bl/6Ly5.1 mice were specifically reactive with human group A RBCs in vivo with hemagglutination titers of 1 in 16. Interestingly, the plasma from these mice was nonreactive with human group O RBCs as evaluated serologically or by flow cytometry. Nevertheless, RBCs from one species can trigger heterologous complement activation in other species,27,28 and we therefore expected that the complement cascade would be activated following transfusion of human group O RBCs in this xeno-transfusion model. We also hypothesized that transfusion of human group A RBCs to C57Bl/6Ly5.1 mice would result in more severe complement activation than following transfusion of the control human group O RBCs due to presence of pre-existing anti-A–like antibodies.
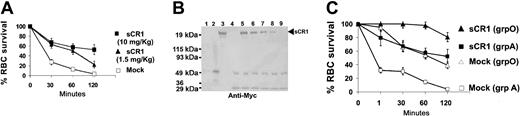
Injection of human group A RBCs at an amount equivalent to 10% of the total mouse blood volume into C57Bl/6Ly5.1 mice resulted in rapid elimination of the transfused RBCs from the circulation, with kinetics similar to the clearance of group A–incompatible RBCs in humans with pre-existing anti-A antibodies (Figure 3A, αgmockαh). When we intravenously coinjected 10 mg/kg of sCR1 at the time of transfusion, the survival of transfused RBCs was considerably prolonged up to 2 hours after transfusion (P < .05 as compared with mock-treated mice, Figure 3A) and correlated with the circulating half-life of sCR1 (Figure 3B). Furthermore, we found that administration of lower doses of sCR1 (1.5 mg/kg) was less effective in prolonging transfused RBC survival, particularly at the later time point of 2 hours after transfusion (P > .05 as compared with mock-treated mice at 120 minutes after transfusion, Figure 3A). All subsequent experiments were performed by administering sCR1 at a dose of 10 mg/kg. It is of note that sCR1 tested in human subjects is well tolerated at this dose.14 Transfusion of mice with control human group O RBCs with or without coinjection of sCR1 is shown in Figure 3C. Transfused group O RBCs were eliminated from the circulation of mock-treated recipients at a much slower rate compared with the rate of clearance of transfused group A RBCs (P < .05, Figure 3C). Nevertheless, all the transfused group O cells were removed from the peripheral blood by 5 hours after transfusion (data not shown). sCR1 treatment completely prevented the clearance of the transfused group O RBCs in the first hour after transfusion (Figure 3C), at which time sCR1 levels had reached half the original concentration in the mouse blood circulation (Figure 3B). Altogether, these data demonstrate the effectiveness of sCR1 in prolonging survival of incompatible RBCs in vivo and support its potential use as a therapeutic agent for the prevention of complement-mediated RBC destruction following transfusions.
sCR1 prolongs the survival of transfused human RBCs in mice. (A) Fluorescently labeled human group A RBCs equivalent to 10% of total mouse blood volume were injected intravenously into C57Bl/6Ly5.1 mice without (Mock, □, n = 15) or with sCR1 given at a dose of 1.5 mg/kg (▴, n = 5) or 10 mg/kg (▪, n = 9), where n is the number of animals per group. At times indicated, venous blood was sampled and analyzed by flow cytometry for the fraction of fluorescent RBCs. To show the clearance kinetics, injected RBCs at time 0 were taken as 100% and the remaining RBCs were calculated at different time points as the average for each group of mice (error bars depict the SEM). sCR1 at a dose of 10 mg/kg increased the survival of transfused RBCs at all time points (P < .05 as compared with Mock), but at a dose of 1.5 mg/kg, the increased RBC survival was only significant at earlier points (P < .05), and not at 120 minutes after transfusion. (B) Representative immunoblot of plasma from transfused mice coinjected with sCR1 at a dose of 10 mg/kg at the following time points: 1 minute (lane 5), 15 minutes (lane 6), 30 minutes (lane 7), 60 minutes (lane 8), and 120 minutes (lane 9) after injection. To estimate plasma concentrations of sCR1, known concentrations of myc-positope (100 ng in lane1 and 500 ng in lane 2) were used. As a negative control, plasma from noninjected mice (lane 4) was used and as a positive control, injected recombinant sCR1 (lane 3) was used. (C) Comparison of the survival of human group O (triangles) versus group A (squares; identical data as in A) RBCs in mice treated without or with sCR1 administration at a dose of 10 mg/kg. Prolonged RBC survival in mice treated with sCR1 (P < .05 at all time points).
sCR1 prolongs the survival of transfused human RBCs in mice. (A) Fluorescently labeled human group A RBCs equivalent to 10% of total mouse blood volume were injected intravenously into C57Bl/6Ly5.1 mice without (Mock, □, n = 15) or with sCR1 given at a dose of 1.5 mg/kg (▴, n = 5) or 10 mg/kg (▪, n = 9), where n is the number of animals per group. At times indicated, venous blood was sampled and analyzed by flow cytometry for the fraction of fluorescent RBCs. To show the clearance kinetics, injected RBCs at time 0 were taken as 100% and the remaining RBCs were calculated at different time points as the average for each group of mice (error bars depict the SEM). sCR1 at a dose of 10 mg/kg increased the survival of transfused RBCs at all time points (P < .05 as compared with Mock), but at a dose of 1.5 mg/kg, the increased RBC survival was only significant at earlier points (P < .05), and not at 120 minutes after transfusion. (B) Representative immunoblot of plasma from transfused mice coinjected with sCR1 at a dose of 10 mg/kg at the following time points: 1 minute (lane 5), 15 minutes (lane 6), 30 minutes (lane 7), 60 minutes (lane 8), and 120 minutes (lane 9) after injection. To estimate plasma concentrations of sCR1, known concentrations of myc-positope (100 ng in lane1 and 500 ng in lane 2) were used. As a negative control, plasma from noninjected mice (lane 4) was used and as a positive control, injected recombinant sCR1 (lane 3) was used. (C) Comparison of the survival of human group O (triangles) versus group A (squares; identical data as in A) RBCs in mice treated without or with sCR1 administration at a dose of 10 mg/kg. Prolonged RBC survival in mice treated with sCR1 (P < .05 at all time points).
Role of CR1 functional domains in vivo
We next tested the effectiveness of the different functional regions of CR1 in inhibiting transfusion reactions in vivo. Specifically, we compared the complement inhibitory activities of LHR-A, which possesses weak cofactor activity, versus LHR-B, which possesses strong cofactor activity in our mouse transfusion model. Because LHRs are one-fourth the size of sCR1, we injected LHRs at a dose of 2.5 mg/kg to achieve comparable molar concentrations of LHRs to that of sCR1 in the circulation. We found that both LHR-A and LHR-B had similar half-lives to that of sCR1 in mouse circulation (data not shown). However, treatment with LHR-B resulted in rapid elimination of the transfused group A (Figure 4A) and group O (Figure 4B) RBCs in mice, similar to that seen in the mock-treated mice. In contrast, the transfused RBCs were cleared much slower in the presence of LHR-A in both groups of mice (P < .05 as compared with LHR-B and mock-treated mice, Figure 4A-B), although not as effectively as that seen in sCR1-treated animals (Figure 4A-B). These data indicate that LHR-A, but not LHR-B, possesses a functional domain that by itself can prolong the survival of incompatible RBCs in mice.
LHR-A, but not LHR-B, prolongs the survival of transfused RBCs in vivo. (A) Survival of human group A RBCs in mice treated with LHR-A (administered at 2.5 mg/kg, n = 9, ⋄) is significantly greater than in mice treated with LHR-B (administered at 2.5 mg/kg, n = 5, ○)(P < .05 at all time points). For comparison, the survival of transfused RBCs from mice treated with mock (n = 15, □) and sCR1 (10 mg/kg, n = 9, ▪) from Figure 3A are shown. (B) Survival of human group O RBCs in mice treated with LHR-A (2.5 mg/kg, n = 4, ⋄) is significantly greater than in mice treated with LHR-B (2.5 mg/kg, n = 6, ○)(P < .05 at all time points). For comparison, the survival curves of mock (n = 8, ▵) and sCR1 (n = 8, ▴) from Figure 3C are included.
LHR-A, but not LHR-B, prolongs the survival of transfused RBCs in vivo. (A) Survival of human group A RBCs in mice treated with LHR-A (administered at 2.5 mg/kg, n = 9, ⋄) is significantly greater than in mice treated with LHR-B (administered at 2.5 mg/kg, n = 5, ○)(P < .05 at all time points). For comparison, the survival of transfused RBCs from mice treated with mock (n = 15, □) and sCR1 (10 mg/kg, n = 9, ▪) from Figure 3A are shown. (B) Survival of human group O RBCs in mice treated with LHR-A (2.5 mg/kg, n = 4, ⋄) is significantly greater than in mice treated with LHR-B (2.5 mg/kg, n = 6, ○)(P < .05 at all time points). For comparison, the survival curves of mock (n = 8, ▵) and sCR1 (n = 8, ▴) from Figure 3C are included.
Analysis of in vivo complement regulation by CR1 derivatives
Immune-mediated RBC destruction can occur intravascularly or extravascularly.2 Intravascular RBC destruction is associated with severe forms of transfusion reactions such as hemolytic transfusion reactions due to ABO incompatibility in humans, whereas transfusion reactions as a result of extravascular hemolysis are generally less severe.29 To investigate the mechanism by which CR1 derivatives limit RBC destruction in the transfusion models in vivo, we measured the amount of intravascular hemolysis in plasma as well as the levels of antibody and complement sensitization of the transfused RBCs for up to 2 hours after transfusion. We found high levels of hemolysis in the plasma of mice transfused with group A RBCs. The extent of intravascular hemolysis in mice treated with sCR1 and LHR-A, but not LHR-B, was reduced by 50% (Figure 5A). In addition, lower levels of C4 and C3 were deposited on the circulating transfused group A RBCs of mice treated with sCR1 and LHR-A, but not LHR-B, as compared with mice injected with mock alone (Figure 5B-C, respectively). We found that the transfused group A cells were coated with IgG, and as expected, CR1 derivatives (sCR1, LHR-A, or LHR-B) did not affect the levels of antibody sensitization of these cells (Figure 5D). Similarly, we found sensitization of transfused RBCs with IgM, but the levels were not affected by CR1 derivatives (data not shown).
We did not find significant intravascular hemolysis in mice transfused with group O RBCs in the absence of CR1 derivatives (data not shown), indicating a less severe type of transfusion reaction. Consistent with this, we found that the levels of C3 deposited on the circulating human group O RBCs of mock-treated mice were overall lower by a factor of 100 compared with the amounts of C3 deposited on group A RBCs of mock-treated mice (Figure 5E). Treatment with sCR1 and LHR-A, but not LHR-B, resulted in the reduction of C3 deposition on the circulating transfused group O RBCs (Figure 5E). We did not detect antibody (IgM or IgG) coating or C4 deposition on the transfused group O RBCs in the absence or presence of CR1 derivatives (data not shown). In addition, the plasma of these mice taken 5 hours after transfusion had no detectable antibodies against human group O RBCs (data not shown).
Discussion
Our present study has demonstrated that CR1 derivatives can reduce complement-mediated RBC destruction both in vitro and in vivo. Moreover, we have found that LHR-A, but not LHR-B, possesses a functional complement inhibitory domain that can limit RBC destruction in an in vivo mouse model of transfusion reactions.
To assess the effectiveness of CR1 derivatives in inhibiting complement-mediated RBC destruction in vivo, we have developed a xeno-transfusion model in the C57Bl/6Ly5.1 mouse strain. By transfusing human group O RBCs into mice, the mouse complement system was activated, resulting in RBC destruction within minutes. Although we could not detect any antibodies on the transfused group O RBCs, it is still possible that xeno-antibodies, at levels that are below our detection systems, were responsible for activating complement and RBC destruction in this setting. Analogous to this situation is the finding that in rare cases no detectable antibodies can be found in patients who have experienced hemolytic transfusion reactions, despite the use of sensitive antibody detection systems.30 It is also possible that the alternative complement pathway was activated following transfusion of human group O cells, even though we could not detect any factor B, which is specific to the alternative activation pathway, on the transfused cells (data not shown). Following transfusion of group A RBCs with pre-existing anti-A–like antibodies into mice, the complement cascade was activated more severely, probably due to a combination of the anti-A–like antibodies and complement activation via the alternative and/or classical pathways as a result of xeno-transfusion. It is important to note that there are similarities between anti-A antibodies and xeno-antibodies; both are preexisting in the recipient, their specificity is directed against carbohydrate antigens, and they can fix complement.30,31 Moreover, it is likely that once the classical pathway is activated severely as seen in acute hemolytic transfusion reactions, it can lead to the recruitment and activation of the alternative pathway. We therefore believe that, despite being a xeno-transfusion model, our mouse transfusion reaction studies can be used as a system for the study of acute and severe complement activation and RBC destruction due to pre-existing complement-fixing antibodies.
Following transfusion of human group O RBCs, we found that treatment with sCR1, and specifically LHR-A, inhibited C3 sensitization of the transfused RBCs, thereby prolonging their survival in the circulation. Indeed, RBC destruction was completely prevented in the first hour following addition of sCR1.
Treatment with sCR1 and LHR-A in mice transfused with human group A RBCs reduced the levels of complement deposition on the transfused RBCs by inhibiting the C4 and C3 activation steps, resulting in lower amounts of intravascular hemolysis and prolonged RBC survival. Elegant studies performed by Davenport and his colleagues have demonstrated the generation of a number of inflammatory cytokines in an in vitro model of ABO incompatibility.32-34 We have preliminary evidence indicating that the circulating levels of a number of these cytokines are indeed reduced to background levels following treatment with sCR1 and LHR-A (K.Y. et al, unpublished data, October 2002).
Unlike LHR-B, LHR-A has minimal cofactor activity (this study and Krych et al24 ) but possesses decay-accelerating activity for C3 convertases of the alternative and classical pathways.35 In our studies, individually expressed LHR-A as well as LHR-B reduced complement-dependent hemolysis in vitro. However, we found that LHR-A, but not LHR-B, inhibited complement activation and limited RBC destruction in vivo. It is possible that the differences in the results obtained by the in vitro and in vivo studies may be due to the species differences between murine and human complement systems. Alternatively, the differences may be due to discrepancies between in vitro and in vivo systems. Indeed, it has been shown that in vitro mouse sera have low hemolytic activity against human RBCs,28 whereas following transfusion of human RBCs in vivo, the mouse complement activation can lead to hemolysis of the transfused RBCs intravascularly. In this context, our studies clearly show that, despite the effectiveness of cofactor activity of CR1 (present in LHR-B) to inhibit human complement-mediated hemolysis in vitro, it plays no role in prevention of complement-mediated RBC destruction in an in vivo mouse model of transfusion reaction. It therefore follows that the decay-accelerating activity of CR1 for C3 convertases, associated with LHR-A,35 is important in inhibiting RBC destruction in this in vivo murine system.
The sCR1 preparation used in human clinical trials had a circulating half-life of about 30 hours12,13 ; in contrast, our recombinant preparation had a half-life of about 1 hour. This is likely due to the expression systems used to prepare the recombinant proteins, because it has been shown that altering the culture conditions to grow the recombinant sCR1 can affect its circulating half-life.12,13 It will be interesting to directly compare the clearance rates of the sCR1 preparations used in the human clinical trials with our preparation. Nevertheless, we found that the survival of transfused RBCs in vivo correlated with the circulating half-life of CR1 derivatives. We therefore believe that we can achieve prolonged RBC survival by administering recombinant proteins with longer half-lives. For example, by making CR1 chimeras with IgG, we can expect to create CR1 fusion partners that have longer survival times in the circulation due to the prolonged plasma half-life of the Fc moiety.36 Moreover, through structure-function studies, we can produce more potent CR1-based inhibitors as has been shown by the generation of LHR-A mutants with improved active sites.16
Collectively, our data highlight a potential therapeutic role for CR1 derivatives to control complement-dependent immune hemolysis. It is important to note that in most common cases of immune-mediated RBC destruction, antibodies alone are responsible for hemolysis. However, the patient groups that would benefit from intervention by complement inhibitors are mainly those undergoing chronic transfusions such as patients with sickle cell disease or thalassemias, who have a high incidence of alloimmunization against minor RBC antigens,37,38 and may have developed multiple alloantibodies including complement-fixing antibodies. By treating these patients prophylactically with complement inhibitors, it may no longer be necessary to match for the antigens whose corresponding antibodies are known to fix complement (eg, Jk antigens), thereby increasing the chances of finding suitable units of blood for the patients. Similarly, prophylactic treatment with sCR1 may permit mismatched RBC transfusions to trauma patients for whom there is no time to type and match blood.
A major concern in the clinical application of complement inhibitors is that they may compromise the host defense system, although the patients undergoing clinical trials with sCR1 have not shown a higher incidence of bacterial infections.14 By identifying the functional regions of CR1 in vivo, our goal is to develop effective CR1 structure-based small synthetic molecules, lacking immunogenecity, that would permit the administration of lower drug doses and offer better efficacy in down-regulating complement activation.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3068.
Supported in part by grants to K.Y. from the National Institutes of Health (R01 HL69102), the American Heart Association Grant-in-Aid Heritage Affiliate, and the National Blood Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Cheng-Han Huang (New York Blood Center), Mohandas Narla (New York Blood Center), Richard Davey (New York Blood Center), and Nikos Yannoutsos (Rockefeller University) for helpful discussions.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal