Abstract
Human cytomegalovirus (HCMV) has developed multiple strategies to escape immune recognition. Here, we demonstrate that HCMV down-regulates HLA-DR expression in infected interferon γ (IFN-γ)–stimulated fibroblasts at 1 day after infection. Decreased HLA-DR expression was not observed on cells infected with an HCMV strain lacking the pp65 gene (RVAD65), but was observed on cells transfected with the pp65 gene. HLA-DR expression accumulated in vacuoles near the nucleus in HCMV-infected, but not in uninfected or RVAD65-infected cells. In addition, the HLA-DR α-chain, but not the β-chain or HLA-DM, was degraded in HCMV-infected but not in RVAD65-infected cells. Thus, the HCMV protein pp65 mediates decreased expression of HLA-DR, by mediating an accumulation of HLA class II molecules in lysosomes that results in degradation of the HLA-DR α-chain.
Introduction
Human cytomegalovirus (HCMV) belongs to the β herpes family, and a primary infection is followed by latency and/or a persistent infection.1 Although HCMV infection is usually asymptomatic in healthy individuals, the virus can cause severe morbidity and mortality in immunocompromised patients, eg, patients who have received a transplant and patients with AIDS. T cells play an important role in combating intracellular pathogens. Activation of T cells depends on their ability to recognize foreign peptides associated with either HLA class I (cytotoxic T cells, CTL) or HLA class II molecules (CD4+ T cell). Although HLA class I molecules are expressed by all nucleated cells, HLA class II molecules are present only on antigen-presenting cells, such as dendritic cells, macrophages, and B cells. However, HLA class II expression can be induced on other cells by, for example, interferon γ (IFN-γ) stimulation. Both the inducible and the constitutive HLA class II expression is tightly regulated, and multiple proteins are involved in this process.2 Increased or induced HLA class II expression by IFN-γ3 is mediated through activation of the Jak/Stat signaling pathway and transcription of the class II transactivator (CIITA),4 which initiates HLA class II transcription. Furthermore, several proteins are responsible for controlling the transportation and peptide loading of HLA class II molecules. Three important proteins in this process are HLA-DM, the invariant chain (Ii), and the class II–associated invariant chain peptide (CLIP). Although the invariant chain is involved in the correct folding of the HLA class II complex in the endoplasmic reticulum (ER) and transportation of the complex to the class II compartment (MIIC), prevention of peptide binding in the ER is mediated by CLIP. HLA-DM catalyses the release of CLIP, and the binding of antigenic peptides to the cleft in the HLA class II molecules present in the MIIC.5
CD4+ T cells play a key role in the early activation of CTL as well as in B-cell activation. HCMV immediately early (IE)–specific CD4+ T cells have been shown to produce cytokines, which inhibit HCMV replication in U373 MG cells,6 and CD4+ T cells can also control and clear murine CMV (MCMV) infection.7,8 Thus, immune evasion strategies affecting HLA class II expression and antigen presentation to CD4+ T cells would be of utmost importance for the virus to avoid early immune recognition. Although several different HCMV proteins9-13 that interfere with HLA class I expression have been identified, the mechanisms and the viral proteins involved in down-regulation of HLA class II molecules on HCMV-infected cells are less characterized. Several studies have shown that HCMV and MCMV inhibit both the inducible14-16 and the constitutive HLA class II expression17-21 on infected cells. HCMV also blocks the IFN-γ–induced HLA class II molecule expression in infected endothelial cells, possibly by interfering with the Jak/Stat signaling pathway,14 which prevents transcription of CIITA and translocation of HLA class II molecules. However, the HCMV proteins mediating this effect are presently unknown. Although Tomazin et al17 have suggested that down-regulation of HLA-DR on stable HLA-DR–transfected U373 cells is mediated by the HCMV gene US2, which resulted in degradation of the HLA-DR α- and HLA-DM α-chains, Gewurz et al22 did not observe an association between US2 and HLA-DM or HLA-DR. The HCMV protein US2 also induces a degradation of HLA class I molecules, hence the US2 protein decreases the expression of HLA class I and class II molecules in infected cells by completely different actions. We recently demonstrated that HCMV-infected macrophages exhibited a reduced expression of HLA class II molecules, which was mediated by at least 2 different mechanisms, at an early (1 day after infection) and at a late (4 days after infection) time point after infection.19 The late effect was caused by one or several genes in the US region, possibly by the action of the US2 protein.17 However, the early effect on HLA class II expression appeared to be mediated by a structural component carried by the virus, because UV-inactivated HCMV-infected macrophages also demonstrated a decreased expression of HLA class II molecules at 1 day after infection.
Because the level of HCMV infection of macrophages is low, and hence macrophages are not suited to use in studies of molecular mechanisms that could explain the down-regulation of HLA-DR, we, therefore, further examined the early mechanism responsible for the HCMV-induced reduced HLA-DR expression in IFN-γ–stimulated human fibroblasts. We demonstrate that HCMV reduces HLA-DR expression on infected fibroblasts at 1 day after infection, which was not observed on cells infected with an HCMV pp65 knockout strain (RVAD65). Furthermore, HLA-DR transcription was not blocked in infected cells. Instead, HLA class II molecules were accumulated in lysosomes near the nucleus, and the HLA-DR α-chain was degraded in HCMV, but not in RVAD65-infected cells.
Materials and methods
Cells
Human lung fibroblasts (kindly provided by V. Sundquist, Karolinska Institute, Sweden) were maintained in Dulbecco modified Eagle medium (DMEM; GIBCO BRL, Grand Island, NY) with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin at 37°C and 5% CO2 and used until passage 27. For induction of HLA class II expression, fibroblasts were stimulated with 400 to 600 U IFN-γ/mL for at least 48 hours.
Establishment of mature dendritic cells
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated as previously described,23 and cells were washed and cultured into cell flasks at a concentration of 5 × 106 cells/mL in RPMI medium supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco BRL), 10% FCS and incubated at 37°C for 2 hours. The nonadherent cells were removed, the cultures were extensively washed, and the monocyte-enriched cells were further cultured in RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 10% FCS, stimulated with interleukin 4 (IL-4; 1000 U/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL) (both from R&D Systems, Minneapolis, MN), and cultured for 6 days. Maturation of dendritic cells (DCs) was induced after 6 days by addition of tumor necrosis factor α (TNF-α; 50 ng/mL; R&D Systems) for 24 to 48 hours.
HCMV infection
Five different HCMV strains were used for infection of fibroblasts; AD169 and Towne (laboratory strains), HA (a clinical isolate), and 2 mutant HCMV strains; UL18ΔHCMV (AD169 deletion mutant lacking the UL18 gene, kindly provided by Helena Browne, University of Cambridge, United Kingdom), and RVAD65 (AD169 deletion mutant lacking the pp65 gene24 ). The cells were infected with a multiplicity of infection (MOI) of 5 and cultured in DMEM with 5% FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin at 37°C and 5% CO2. Mature DCs were infected with AD169 or RVAD65 at an MOI of 25 to 40 for 24 hours and then analyzed for the expression of HLA-DR at the cell surface or the intracellular expression of an early HCMV antigen (pp52, clone CCH2; Dakopatts, Glostrup, Denmark). Cell-free viral stocks of the different HCMV strains were prepared from supernatants of fibroblast cultures, frozen, and stored until use at –70°C. Virus titers were determined by plaque assays as previously described.25
Flow cytometric analysis
A fluorescence-activated cell sorter (FACSort; Becton Dickinson, San Jose, CA) was used to analyze uninfected and HCMV-infected IFN-γ–stimulated fibroblasts for the expression of HLA-DR, HLA-DM, the invariant chain (CD74, Ii), Jak-1, Jak-2, and Stat 1. The following antibodies were used: phycoerythrin (PE)–conjugated anti–HLA-DR, PE-conjugated anti–HLA-DM, fluorescein isothiocyanate (FITC)–conjugated anti-CD74 (invariant chain), anti-Stat 1, anti-Jak 1 (all purchased from Pharmingen, Becton Dickinson Immunosystem, CA), anti–Jak-2 (Santa Cruz Biotechnology, Santa Cruz, CA), and isotype controls (Pharmingen, Becton Dickinson Immunosystem). As secondary antibodies, we used FITC-conjugated F(ab)2 fragments of rabbit-antimouse or swine-antirabbit antibodies (both from Dakopatts). Fixation and permeabilization for intracellular staining of cells for expression of HLA-DM, the invariant chain, Jak 1/2, and Stat 1 was performed using the Cytofix/Cytoperm Kit (Pharmingen, Becton Dickinson Immunosystem) according to manufacturer's instructions. Data were analyzed using CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA), and the expression of the different proteins in uninfected and HCMV-infected cells was measured as the mean channel value in linear value of the respective antibody compared with the isotype control.
Transfection
Fibroblasts were transfected using the polyethylenimine (PEI) method, which is briefly performed as follows: cells were plated and transfected at a cell confluency of 50% to 70%. A mixture of 2 μg DNA 1.2 μL PEI (PEI stock 22.5 mg 25 kD PEI [Aldrich]/10 mL dH20, pH 7), and then dH20 was added to a final volume of 20 μL. The mix was left for 10 minutes at room temperature before being added to the cell cultures. The vectors used were pCDNA3 alone or with insert of the HCMV genes, pp28 or pp65, and resulted in a transfection efficacy of 15% to 50% in individual experiments. Cells were analyzed for the expression of HLA-DR at 24 hours after the transfection.
RT-PCR
RNA from uninfected and HCMV-infected fibroblasts was prepared 1 day after infection by lysing cells with Trizol (Gibco BRL), and, thereafter, RNA was prepared according to manufacturer's instructions. cDNA was synthesized using a first strand cDNA kit (Pharmacia LKB Biotechnology, Uppsala, Sweden) according to manufacturer's instructions. Specific primer pairs for HLA-DR (5′-AAA GCG CTC CAA CTA TAC TCC GA-3′, 5′-ACC CTG CAG TCG TAA ACG TCC-3′) were used in a reverse transcriptase–polymerase chain reaction (RT-PCR). As a positive control for the detection of DNA or RNA, primers specific for glucose-6-phosphatese dehydrogenase (G6PD) gene were used as a positive control for each sample. DNA and cDNA samples, from uninfected and HCMV-infected HL cells, were included as positive and negative controls. Serial dilutions (1:1 to 1:10 000) of the templates were used in semiquantitative PCR analyses using the same primer pairs. The PCR products were visualized on 2% agarose gels.
Immunohistochemistry
IFN-γ–stimulated fibroblasts were cultured on chamber-slides (LAB-TEK, Labora Cemicon, Sweden) and fixed either with 3% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 10 minutes at room temperature. Fixed cells were permeabilized with 0.3% Triton X-100 and then washed with PBS. To decrease nonspecific binding, cells were treated with a blocking solution (Protein Block Serum-Free; Dakopatts) according to manufacturer's instructions. Thereafter, cells were stained with antibodies specific for HLA-DR, HLA-DM, Ii, (all from Becton Dickinson Immunosystem; directly conjugated), rab 3, rab 4, rab 6, rab 7, LAMP-1 (all from Santa Cruz Biotechnology), and HCMV pp65 (mouse–anti-pp65; Argene Biosoft, Varilhes, France) and examined using either a fluorescence or a confocal microscope. The antibodies were diluted in antibody diluent solution (Dakopatts) to decrease unspecific binding. As secondary antibodies, we used FITC- or PE-conjugated F(ab)2 fragments of rabbit-antimouse, swine-antirabbit, or rabbit-antigoat antibodies (Dakopatts).
Immunoprecipitation and Western blot
Fibroblasts were incubated in culture medium in the presence of IFN-γ (400 U/mL) for 2 days, before being infected with HCMV. At 1 or 2 days after infection, cells were scraped in an immunoprecipitation (IP) buffer (50 mM Tris (tris(hydroxymethyl)aminomethane)–HCL, 150 mM NaCl, 1% NP40, 5 mM EDTA (ethylenediaminetetraacetic acid), 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM PMSF (phenylmethanesulfonyl fluoride), and 1 mm DTT (dithiothreitol)). The cell lysates was transferred to Eppendorf tubes, incubated on ice for 10 minutes, before being centrifuged at maximum speed, for 10 minutes at 4°C. The supernatant was transferred to a new tube and pre-absorbed to protein G-Sepharose CL-4B. Thereafter, HLA-DR was immunoprecipitated by incubating 1 μg HLA-DR–specific antibody (Becton Dickinson Immunosystem) with 100 μg precleared cell lysate, 100 μL IP buffer, and 50 μL protein G-Sepharose CL-4B (Pharmacia) at 4°C overnight with continuous mixing. The pellet was washed 4 times, the precipitates were boiled for 5 minutes in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and centrifuged before being applied on SDS-PAGE gels. Proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). The membranes were incubated with anti–HLA-DR α-chain (clone TAL.1B5), anti–HLA-DR β-chain (clone DK22) (both from Dakopatts) or anti–HLA-DM (clone MAP.DM1; Pharmingen) antibodies at 1:200, respectively, at 4°C overnight. Blots were washed 4 times with 0.03% Tween-20 in PBS and incubated with sheep antimouse immunoglobulin G horseradish peroxidase (IgG-HRP; 1:1000; Amersham Pharmacia Biotech) for 1 to 2 hours at room temperature. Blots were washed, and the bands were visualized by using electrogenerated chemiluminescence (ECL) reagents (Amersham Pharmacia Biotech) and Hyperfilm ECL (Amersham Pharmacia Biotech).
Radiolabeling
Fibroblasts were treated with IFN-γ for 72 hours, and then infected with AD169 or RVAD65 (MOI 10) or mock infected. After 4 hours, cells were labeled with 35S-methionine/cysteine (5.55 MBq/mL [150 μCi/mL]) for 30 minutes, and then the label was chased for 120 minutes. Cells were lysed as described in “Immunoprecipitation and Western blot,” and the HLA-DR α-chain was immunoprecipitated by incubating 1 μg HLA-DR α-chain–specific antibody (clone TAL.1B5; Dakopatts) with 100 μg precleared cell lysate, 100 μL IP buffer, and 50 μL protein G-Sepharose CL-4B (Pharmacia) at 4°C overnight with continuous mixing. The pellet was washed 4 times, the precipitates were boiled for 5 minutes in SDS-PAGE sample buffer and centrifuged before being applied on SDS-PAGE gels. Proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech).
Results
HCMV protein pp65 mediates reduced HLA-DR expression in HCMV-infected IFN-γ–stimulated fibroblasts
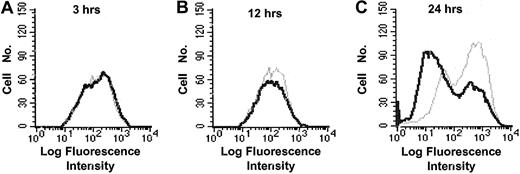
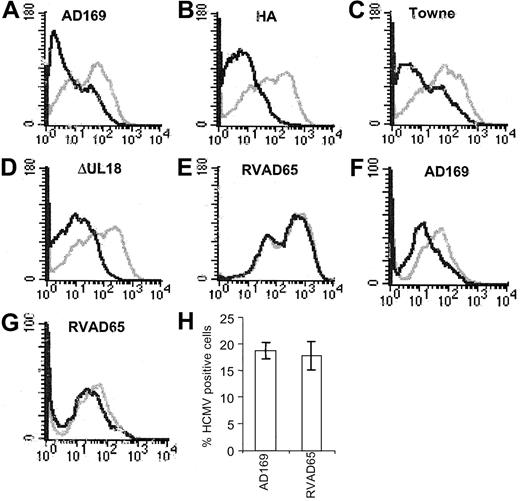
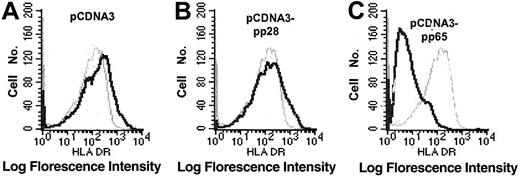
Previous studies have demonstrated decreased expression of the inducible14,15 and the constitutive17,19 HLA class II expression on HCMV-infected cells. We recently showed that HCMV decreased the expression of HLA class II molecules on HCMV-infected macrophages by an early and a late mechanism.19 Here, we further examined the early mechanism responsible for the effect on HLA class II expression. The experiments were performed using fibroblasts instead of macrophages, because a high and “uniform” infection level is required to be able to examine the molecular mechanism(s) and to identify the viral protein(s) that is responsible for the reduced HLA class II molecule expression. Fibroblasts were, therefore, stimulated with IFN-γ, infected with HCMV AD169, and analyzed for the expression of HLA-DR at 3, 12, and 24 hours after infection. Although HLA-DR expression was unaffected at 3 and 12 hours after infection (Figure 1A-B), decreased HLA-DR surface expression was observed at 24 hours after infection (Figure 1C). We also examined the ability of the different HCMV strains, Towne (a laboratory strain), HA (a clinical isolate), and the 2 deletion mutant strains of AD169, RVAD65 (which lacks the pp65 gene) and UL18ΔHCMV (which lacks the UL18 gene), to affect HLA-DR expression 1 day after infection. A reduced surface expression of HLA-DR was demonstrated on cells infected with either AD169, Towne, HA, or UL18ΔHCMV 1 day after infection (Figure 2A-D). In contrast, an effect on HLA-DR expression was not observed on RVAD65-infected cells at this time point (Figure 2E). Examination of fibroblasts infected with the different HCMV strains at 1 day after infection did not reveal a difference in the infection level at 1 day after infection (> 95% IE expression) or in viral growth at 7 days after infection (data not shown). To further evaluate the in vivo relevance of pp65's ability to affect HLA-DR expression in professional antigen-presenting cells, mature dendritic cells were infected with the HCMV strains AD169 or RVAD65. Interestingly, a reduced HLA-DR expression was demonstrated on AD169-infected dendritic cells, but not on RVAD65-infected cells as compared with mock-infected cells (Figure 2F-G, respectively). However, the ability of dendritic cells to become infected with the strain AD169 appeared to be donor specific. In general, we observed 1% to 5% HCMV-positive cells in dendritic cell culture infected with AD169 or RVAD65. However, in approximately 20% of the donors, we observed an infection level of 17% to 20% (pp52-positive cells), and in these cells a reduced HLA-DR expression was observed on AD169-infected cells, but not on RVAD65-infected dendritic cells at 1 day after infection (Figure 2H). To further confirm the role of pp65 in the down-regulation of HLA-DR at 1 day after infection, fibroblasts were transfected with vectors containing either the pp65 (pCDNA3-pp65) or the pp28 (pCDNA3-pp28) genes, or with the vector alone that served as a negative control. Although HLA-DR expression was not affected in IFN-γ–stimulated fibroblasts transfected with pCDNA-3 or pCDNA-3-pp28 (Figure 3A-B, respectively), pCDNA3-pp65–transfected cells demonstrated a significant decreased expression of HLA-DR (Figure 3C). Thus, these results suggest that the HCMV protein pp65 is involved in reduction of HLA-DR expression at an early time point after infection.
Down-regulation of HLA-DR at 1 day after infection on HCMV-infected cells. The expression of HLA-DR was analyzed at 3 to 24 hours after infection using flow cytometry. The expression of HLA-DR on mock-infected cells (gray line) and HCMV-infected cells (black line) at 3, 12, and 24 hours after infection is shown in panels A, B, and C, respectively.
Down-regulation of HLA-DR at 1 day after infection on HCMV-infected cells. The expression of HLA-DR was analyzed at 3 to 24 hours after infection using flow cytometry. The expression of HLA-DR on mock-infected cells (gray line) and HCMV-infected cells (black line) at 3, 12, and 24 hours after infection is shown in panels A, B, and C, respectively.
The RVAD65 HCMV strain does not affect the expression of HLA-DR. Fibroblasts infected with AD169, HA, Towne, UL18ΔHCMV, or RVAD765 were examined for the expression of HLA-DR at 1 day after infection. The level of expression of HLA-DR in AD169 (A), HA (B), Towne (C), UL18ΔHCMV (D), RVAD65 (E), or infected cells (black lines) was compared with the HLA-DR expression on uninfected cells (gray lines). Dendritic cells were mock infected or infected with AD169 or RVAD65 and analyzed for the expression of HLA-DR at 1 day after infection (F-G, respectively). Panel H represents the percentage of dendritic cells (±SD) stained positive for HCMV early antigens, where a significantly reduced HLA-DR expression was observed on the infected dendritic cell cultures (MOI 25).
The RVAD65 HCMV strain does not affect the expression of HLA-DR. Fibroblasts infected with AD169, HA, Towne, UL18ΔHCMV, or RVAD765 were examined for the expression of HLA-DR at 1 day after infection. The level of expression of HLA-DR in AD169 (A), HA (B), Towne (C), UL18ΔHCMV (D), RVAD65 (E), or infected cells (black lines) was compared with the HLA-DR expression on uninfected cells (gray lines). Dendritic cells were mock infected or infected with AD169 or RVAD65 and analyzed for the expression of HLA-DR at 1 day after infection (F-G, respectively). Panel H represents the percentage of dendritic cells (±SD) stained positive for HCMV early antigens, where a significantly reduced HLA-DR expression was observed on the infected dendritic cell cultures (MOI 25).
The HCMV protein pp65 mediates down-regulation of HLA-DR. To examine whether pp65 directly inhibited HLA-DR expression, cells were transfected with a pCDNA3 vector, containing the pp28 or pp65 genes, respectively. The expression level of HLA-DR in cells transfected with either pCDNA3 alone (A; black line) or pCDNA3-pp28 (B; black line), or pCDNA3-pp65 (C; black line) was compared with untreated cells (gray lines). The figure shows 1 representative example of 4 experiments.
The HCMV protein pp65 mediates down-regulation of HLA-DR. To examine whether pp65 directly inhibited HLA-DR expression, cells were transfected with a pCDNA3 vector, containing the pp28 or pp65 genes, respectively. The expression level of HLA-DR in cells transfected with either pCDNA3 alone (A; black line) or pCDNA3-pp28 (B; black line), or pCDNA3-pp65 (C; black line) was compared with untreated cells (gray lines). The figure shows 1 representative example of 4 experiments.
Reduced expression of HLA-DR does not involve an effect on HLA-DM, the invariant chain (Ii), or proteins involved in the Jak/Stat signaling pathway
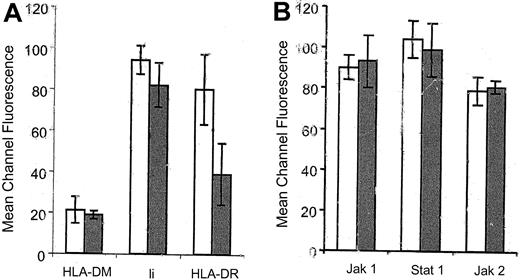
Reduced HLA-DR expression has been found to be mediated by at least 2 different mechanisms in HCMV-infected cells. It was first shown that HCMV inhibited the inducible HLA-DR expression by interfering with the Jak/Stat signaling pathway.14,15 However, the HCMV protein(s) that mediates this effect has not yet been identified. Thereafter, the HCMV protein US2 was shown to mediate down-regulation of HLA-DR expression by degradation of the HLA-DR and the HLA-DM α-chains.17 Therefore, we analyzed HCMV-infected IFN-γ–stimulated fibroblasts for the intracellular expression of HLA-DM, Ii, Jak-1, Jak-2, and Stat-1 at 1 day after infection, by flow cytometry. At 1 day after infection, a difference in the expression of these proteins was not observed in HCMV-infected fibroblasts as compared with uninfected cells (Figure 4). In addition, Western blot experiments did not reveal a difference in the protein levels of Jak-1, Jak-2, and Stat-1α in infected cells as compared with uninfected cells (data not shown). These results suggest that pp65 mediated reduced HLA-DR expression on infected cells and did not involve an effect on the levels of expression of HLA-DM, the Ii, or the proteins involved in the Jak/Stat signaling pathway.
The expression of HLA-DM, the invariant chain, Jak-1, Jak-2, and Stat-1 is not affected in HCMV-infected cells at 1 day after infection. The expression of HLA-DM, Ii, Jak1, Jak-2, and Stat-1 was analyzed by flow cytometry at 1 day after infection in HCMV-infected cells (gray bars) as compared with uninfected cells (white bars).
The expression of HLA-DM, the invariant chain, Jak-1, Jak-2, and Stat-1 is not affected in HCMV-infected cells at 1 day after infection. The expression of HLA-DM, Ii, Jak1, Jak-2, and Stat-1 was analyzed by flow cytometry at 1 day after infection in HCMV-infected cells (gray bars) as compared with uninfected cells (white bars).
HCMV blocks transportation of HLA-DR to the cell surface and pp65 appears to mediate a degradation of the HLA-DR α-chain
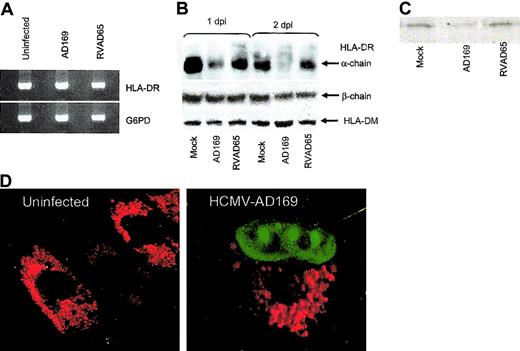
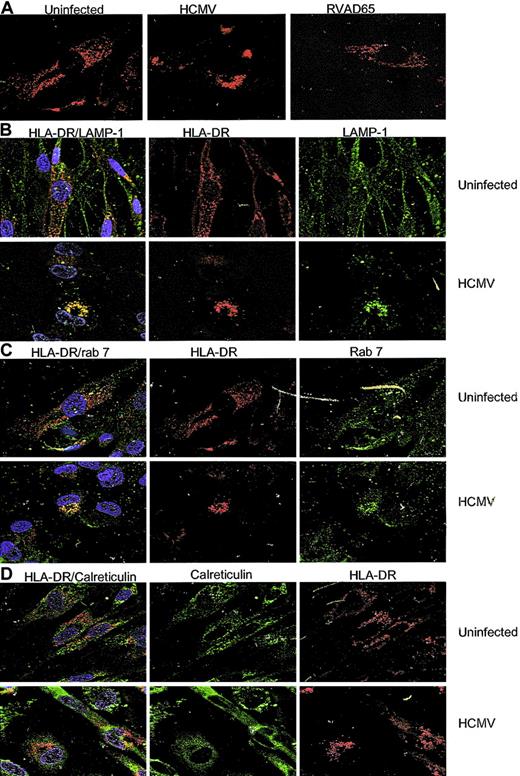
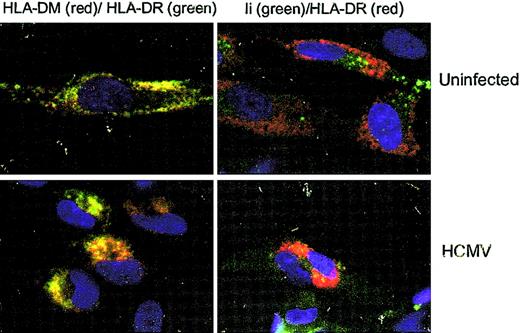
To examine whether decreased expression of HLA-DR at the cell surface on infected cells was caused by an inhibition of HLA-DR transcription or blocking of the transportation of HLA-DR molecules to the cell surface, an RT-PCR assay using serial dilutions of the templates was performed on uninfected and HCMV-infected IFN-γ–stimulated fibroblasts at 1 day after infection. Using this RT-PCR technique, we found that HLA-DR transcription was not affected in either HCMV- or RVAD65-infected cells as compared with uninfected cells (Figure 5A). Instead, at 1 day after infection, immunoprecipitation experiments demonstrated that the protein level of the HLA-DR α-chain was decreased in HCMV-infected, but not in RVAD65-infected cells, as compared with uninfected cells (Figure 5B). This experiment was performed using an antibody reacting against the monomorphic α-chain of HLA-DR. At 2 days after infection, the HLA-DR α-chain could not be detected in HCMV-infected cells (Figure 5B), but the protein was present both in RVAD65-infected cells and -uninfected cells. However, the expression of the HLA-DR β-chain or HLA-DM molecules was not affected either at 1 or 2 days after infection in HCMV- or RVAD65-infected cells (Figure 5B). However, because it is unknown whether the antibody specific for HLA-DM binds to the α-orthe β-chain, or both, we cannot exclude the possibility that one of the chains may be affected in HCMV-infected cells. Further, in pulse chase experiments, the HLA-DR α-chain could not be detected by immunoprecipitation of 35S-labled–infected cells, after 4 hours of infection, but was detected in uninfected and RVAD65-infected cells (Figure 5C). We, therefore, performed confocal microscopy analysis to analyze the distribution of HLA-DR expression in uninfected, HCMV-(AD169) and RVAD65-infected cells. Interestingly, a significant difference in HLA-DR expression was observed in HCMV-infected cells as compared with uninfected cells at 1 day after infection (Figure 5D). The pp65 protein was localized to the nucleus at 3, 6, 12 (data not shown), and 24 hours (Figure 5D) in HCMV-infected cells, as has previously been reported by several investigators.26,27 Although the intracellular staining pattern of HLA-DR was similar in uninfected (Figure 6A) and RVAD65-infected cells (Figure 6A), the expression of HLA-DR was condensed in vacuoles near the nucleus in HCMV-infected cells (Figure 6A). Further immunofluorescence analyses revealed that HLA-DR expression was colocalized with lysosomal associated membrane glycoprotein-1 (LAMP-1; Figure 6B) and rab 7 (Figure 6C) in HCMV-infected cells, but not with the ER resident protein calreticulin (Figure 6D), rab 1, rab 3, rab 4, rab 5, or rab 6 (data not shown). Interestingly, a colocalization with rab 7 and LAMP-1 was not found in uninfected cells (Figure 6B-C) or in RVAD65-infected cells (data not shown). In addition, the expression of HLA-DM but not the Ii, was also accumulated in vacuoles near the nucleus in HCMV-infected cells (Figure 7).
HCMV specifically affects the HLA-DR α-chain in infected cells. Fibroblasts were examined for the presence of HLA-DR mRNA in uninfected and HCMV-infected cells at 1 day after infection, using an RT-PCR assay. The transcription of HLA-DR in HCMV-AD169, RVAD65-infected fibroblasts, or uninfected cells is shown in panel A (n = 5). To analyze whether HCMV induces degradation of HLA-DR molecules, immunoprecipitation experiments were performed. The presence of HLA-DM or the HLA-DR α-or β-chain is shown in panel B at 1 and 2 days after infection (n = 3). The effect on newly synthesized HLA-DR α molecules was examined in cells after 4 hours of infection and 2 hours chase in mock-infected, AD169-, or RVAD65-infected cells (C). The distribution of HLA-DR and the expression of pp65 were examined by confocal microscopy. The HLA-DR expression in mock-infected cells and HCMV-infected cells at 1 day after infection is shown in panel D. The HCMV protein pp65 was present in the nucleus in HCMV-infected cells (D) at 1 day after infection. Original magnification, × 40.
HCMV specifically affects the HLA-DR α-chain in infected cells. Fibroblasts were examined for the presence of HLA-DR mRNA in uninfected and HCMV-infected cells at 1 day after infection, using an RT-PCR assay. The transcription of HLA-DR in HCMV-AD169, RVAD65-infected fibroblasts, or uninfected cells is shown in panel A (n = 5). To analyze whether HCMV induces degradation of HLA-DR molecules, immunoprecipitation experiments were performed. The presence of HLA-DM or the HLA-DR α-or β-chain is shown in panel B at 1 and 2 days after infection (n = 3). The effect on newly synthesized HLA-DR α molecules was examined in cells after 4 hours of infection and 2 hours chase in mock-infected, AD169-, or RVAD65-infected cells (C). The distribution of HLA-DR and the expression of pp65 were examined by confocal microscopy. The HLA-DR expression in mock-infected cells and HCMV-infected cells at 1 day after infection is shown in panel D. The HCMV protein pp65 was present in the nucleus in HCMV-infected cells (D) at 1 day after infection. Original magnification, × 40.
The transportation of HLA-DR is blocked in HCMV-infected cells. Confocal microscopy analysis was performed to examine the distribution of HLA-DR in HCMV, RVAD65-infected, and mock-infected cells at 1 day after infection. The staining pattern of HLA-DR is shown in panel A. Colocalization studies of HLA-DR (red) with LAMP-1 (green), rab 7 (green), or calreticulin (green) in uninfected and HCMV-infected cells is shown in panels B, C, and D, respectively. Original magnification, × 40.
The transportation of HLA-DR is blocked in HCMV-infected cells. Confocal microscopy analysis was performed to examine the distribution of HLA-DR in HCMV, RVAD65-infected, and mock-infected cells at 1 day after infection. The staining pattern of HLA-DR is shown in panel A. Colocalization studies of HLA-DR (red) with LAMP-1 (green), rab 7 (green), or calreticulin (green) in uninfected and HCMV-infected cells is shown in panels B, C, and D, respectively. Original magnification, × 40.
HLA-DM accumulates in vacuoles near the nucleus in HCMV-infected cells. The distribution of HLA-DM (red) and Ii (green), and its colocalization with HLA-DR was examined in mock-infected and HCMV-infected cells at 1 day after infection by confocal microscopy. Original magnification, × 40.
HLA-DM accumulates in vacuoles near the nucleus in HCMV-infected cells. The distribution of HLA-DM (red) and Ii (green), and its colocalization with HLA-DR was examined in mock-infected and HCMV-infected cells at 1 day after infection by confocal microscopy. Original magnification, × 40.
Discussion
HCMV has developed multiple immune evasion strategies, which enable the virus to coexist with its host. CD4+ T cells are important in initiating an immune response to virus infections, because they produce cytokines necessary for activation of CTL, and/or provide costimulatory signals to B cells and macrophages. Therefore, an ability of the virus to escape CD4+ T cells enhances the possibility for the virus to persist in its host. Here, we demonstrate that HCMV infection causes a reduction in the expression of HLA-DR 1 day after infection, which was mediated by the viral protein pp65. Decreased HLA-DR expression at the cell surface was neither caused by an interference with the HLA-DR transcription or degradation of proteins involved in peptide loading or transportation, such as HLA-DM, Ii, or the Jak/Stat proteins. Instead, HLA-DR molecules were trapped in vacuoles positive for rab 7 and LAMP-1 in infected cells, but not in uninfected cells or RVAD65-infected cells. These markers are both known to be present on lysosomes,28,29 which suggest that HLA-DR and HLA-DM were transported to lysosomes in infected cells. Furthermore, the HLA-DR α-, but not the β-chain or HLA-DM molecules, appeared to be degraded in HCMV-infected cells, and newly synthesized HLA-DR α-chains could not be detected very early after infection. Because accumulation and degradation of HLA-DR molecules did not occur in RVAD65-infected cells, these results suggest that pp65 mediated a redirection of the transportation of HLA-DR molecules to lysosomes, which results in degradation of the molecule's α-chain in fibroblasts. Fibroblasts are most likely not cells that are important in initiating an immune response against HCMV infection. However, these cells are useful as an experimental model to identify viral proteins and mechanisms responsible for down-regulation of HLA-DR in HCMV-infected cells. Hence, the relevance of the pp65 protein in initiating an inhibitory effect on the immune response could be disputed and is further highlighted by the conflicting evidence on the role of US2 protein to affect HLA-DR molecules in different cell types.17,22,30 We have previously reported that HCMV does indeed affect the constitutive expression of HLA-DR molecules at 1 day after infection, on HCMV-infected macrophages that resulted in a decreased ability of the infected cells to trigger an immune response.19 Here, we provide further evidence that HCMV-infected dendritic cells, but not dendritic cells infected with the pp65 knockout strain, reduced HLA-DR expression at 1 day after infection, which further implies an important role of pp65 to affect HLA class II molecule expression in vivo at an early time point after infection. Previous studies that have examined different aspects of HCMV infection of dendritic cells have used endothelial cell–adapted HCMV isolates, because the infection level appears to be negligible using the HCMV strain AD169. Here, we found that at an MOI of 25 to 40 of AD169 generally infects 1% to 5% of the donors. However, in 20% of the donors, we observed an infection rate of 17% to 20%. In such experiments, we did observe a significant down-regulation of HLA class II molecule expression. The reason for the discrepancy of the susceptibility of dendritic cells to AD169 is unknown but appears to be dependent on undefined donor-related factors.
The transcription, peptide loading, folding, and transportation of HLA-DR molecules to the cell surface are tightly controlled, and several different proteins take part in this process. The invariant chain directs the HLA class II complex into the endocytic pathway, where degradation of the invariant chain results in the transient formation of a class II αβ dimer with CLIP (a residual fragment of the invariant chain) in the peptide-binding groove of the HLA class II molecule.31 In the class II compartment, HLA-DM catalyzes the dissociation of CLIP5 and allows peptide loading before the HLA class II molecule is transported to the cell surface. HCMV-infected cells are known to exhibit reduced HLA class II molecule expression,14,17-19 and HCMV's ability to affect HLA class II expression may include mechanisms that affect several different parts in this process. Although at least 3 different mechanisms that reduce HLA class II expression have been observed14,15,17-19 in HCMV-infected cells, only the HCMV protein US2 has been linked to mediate reduced HLA class II expression.17 However, Gewurz et al22 did not observe a binding between US2 and HLA-DR or HLA-DM. This discrepancy between these 2 different studies may be explained by the fact that distinct regions of US2 mediate the interaction class II molecules, or that another factor is required to stabilize the interaction between US2 and HLA-DR, or HLA-DM. It is also plausible that US2 can be induced to be associated with HLA-DR and HLA-DM when overexpressed in the ER. Furthermore, this may indicate that viral proteins interact indirectly with HLA-DR molecules, causing down-regulation of HLA-DR at the cell surface. In addition, HLA class II complexes in dendritic cells were recently shown to be unaffected by an US2-mediated attack.30 Thus, HCMV may use different mechanisms for down-regulation of HLA class II molecules in different cell types. In this study, we provide evidence that the HCMV protein pp65 mediates decreased HLA class II expression at early time points after infection by inhibiting the transportation of HLA-DR molecules to the cell surface by redirecting HLA-DR molecules into lysosomes in HCMV-infected fibroblasts. Interestingly, both pp65 and US217 induce degradation of the HLA-DR α-chains. However, the distribution of HLA-DR in US2-expressing cells is presently unknown, and whether these 2 proteins cooperate, or act independently, requires further investigation.
The exact mechanisms by which pp65 mediates decreased expression of HLA-DR in infected cells are not yet clear. The pp65 protein has a nuclear localization signal26,27 and is present mainly in the nucleus in HCMV-infected cells. We did not observe a colocalization of pp65 and HLA-DR in HCMV-infected cells. Thus, the ability of the pp65 protein to interact directly with HLA-DR to inhibit transportation is unlikely. However, pp65 may disturb the transportation of HLA-DR molecules by affecting intracellular signaling motifs on the HLA-DR molecules, by inducing the expression, or by altering the function of another protein or factor that mediates the inhibitory effect on HLA-DR transportation. The pp65 protein has been associated with kinase activity32,33 and is regarded as a serine-threonine protein kinase.34 Because the phosphorylation state of a protein may determine the intracellular trafficking, one could speculate whether pp65, with its kinase activity, may alter the phosphorylation state of the HLA-DR molecules and thereby redirect their transportation to lysosomes. The ability of the virus to prevent transportation of proteins has also been reported for HLA class I, where US3 by an unknown mechanism, retains the HLA class I complex in the ER, and prevents transportation of the molecule to the cell surface.9 Furthermore, US2 and US11 transport the HLA class I heavy chain out from the ER to the cytosol where it becomes degraded.13 These observations reflect that HCMV uses different viral proteins to affect transportation of various molecules in infected cells.
Most likely, several other HCMV proteins and host-encoded proteins will be identified to participate in down-regulation of HLA class II molecules at different time points after HCMV infection, because at least 4 different HCMV proteins (US2, US3, US6, and US11) have been shown to mediate decreased expression of HLA class I molecules.9-12 Although pp65 is known to both induce a strong humoral35 and cellular immune response during natural infection,36-39 we here show that the pp65 protein does also enable virus-infected cells to escape immune recognition. The function of this process for viral replication is presently unknown. In mice, dense bodies of HCMV induced both humoral and cellular immune responses in the absence of viral gene expression.40 However, whether pp65 can act to reduce murine MHC class II molecule expression is presently unknown.
Interestingly, both pp65 and US2 have been shown to affect activation of both CD4+ and CD8+ T cells, by a direct or indirect interference with HLA class I and class II molecule antigen-presenting pathways. Although pp65 has been shown to reduce presentation of IE peptides to CTLs,41 US2 mediates degradation of both the heavy chain of HLA class I molecules,10 and the α-chain of HLA-DR molecules.17 The possibility of one viral protein to interfere with different parts of the immune response enables the virus to efficiently impair immune recognition. Because our findings demonstrate that the HCMV protein pp65 mediates down-regulation of HLA class II molecules and degradation of the HLA-DR α-chain, these results bring up the interesting hypothesis that pp65 transduced into the cells by virus infection/viral particles may participate in the early virus immune evasion strategies. The pp65 protein may, however, also act in the late phase of infection, when high levels of the protein will be produced. Engagement of this mechanism will most likely enhance the ability of the virus to replicate and persist in its host.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-05-1504.
Supported by grants from the Swedish Medical Research Council (12615-01A, 00793-36B, and 12615-04A), the Swedish Society of Medicine (98020633), the Tobias Foundation (1313/98, 20/01, and 33/02), the Swedish Children Cancer Research Foundation (1998/065 and 2001-046), Emil and Wera Cornells Foundation, the Heart and Lung Foundation (1999-41-305 and 2001-41-486), and the Deutsche Forschungsgemeinschaft (SFB 490) (B.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Olle Olerup for helpful discussions in the designing of HLA-DR primers, and Dr Erna Möller for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal