Abstract
The platelet marginal band consists of a single peripheral microtubule (MT) that is wound in 8 to 12 coils and maintains discoid cell shape. About 90% of β-tubulin in the marginal band is of the divergent, megakaryocyte (MK)/platelet-restricted β1 isoform. β1-tubulin–null mice show reduced proplatelet formation, thrombocytopenia, and platelet spherocytosis. Here, we show that structural abnormalities in resting β1-tubulin—/— platelets include frequent kinks and breaks in the marginal band. Platelets derived from mice lacking the transcription factor GATA1 show similar defects, probably as a direct consequence of absent β1-tubulin. β1-tubulin+/— platelets have normal ratios of β-tubulin isotypes but the marginal band is half the normal thickness, which is sufficient to maintain elliptical cell shape. Thus, a threshold 50% or less of the normal amount of β1-tubulin is required to preserve marginal band integrity and cell shape. β1-tubulin—/— platelets have normal size and contents and show no defects in serotonin release or aggregation. Accordingly, the apparently isolated spherocytosis allows investigation of the role of discoid platelet shape in hemostasis. On agonist stimulation, the disorganized MTs in β1-tubulin—/— platelets fail to condense into central rings and instead are dispersed in short bundles and linear arrays. Nevertheless, intravital microscopy and flow chamber studies demonstrate full functionality of these spherocytic platelets under physiologic shear conditions. Together, these findings highlight the essential requirements of the MK/platelet-restricted β1-tubulin isoform in platelet structure and suggest that spherocytosis does not impair many aspects of platelet function.
Introduction
The marginal microtubule (MT) band of blood platelets maintains their characteristic discoid shape and appears to be an essential intermediate in the MT-driven process of proplatelet formation and platelet release.1-3 This marginal band consists of a single MT polymer that is wound in 8 to 12 coils in the cytoplasmic periphery and is located just beneath the plasma membrane.4-6 Its unique structure is generated during de novo platelet biogenesis at the ends of proplatelets, the megakaryocyte (MK) cytoplasmic extensions that branch and fragment into platelets.3,7,8
MTs are polymers assembled from heterodimers of globular α-tubulin and β-tubulin polypeptides that form linear protofilaments by parallel association.9,10 MTs generate intracellular force to drive diverse cellular functions, including chromosome segregation, vesicle and organelle transport, morphogenesis, and maintenance of cell shape.11 The several mammalian α- and β-tubulin genes12,13 share significant homology within each family, and most tubulin isotypes are expressed widely, albeit at different levels in various tissues. In contrast, β1-tubulin, the isoform that accounts for about 90% of β-tubulin in the platelet marginal band, has the most divergent sequence and is restricted in expression to the MK lineage.14-16 Most of the sequence divergence is concentrated in the C-terminal region, which is exposed on the outer surface of MTs and is believed to interact with a wide range of MT-associated proteins (MAPs).17,18
β1-tubulin levels increase with terminal MK differentiation, and the protein is absent from cells lacking the transcription factor NF-E2.16 β1-tubulin—/— mice in turn show markedly reduced proplatelet formation, thrombocytopenia, and platelets that are spherical rather than elliptical in shape.19 The total β-tubulin content in these platelets is significantly reduced, and the marginal band contains only 1 to 3 coils instead of the typical 8 to 12 coils. Thus, increased levels of β2- and β5-tubulin, the other isoforms normally present in MKs and platelets, fail to compensate adequately for the loss of β1-tubulin. Unexpectedly, β1-tubulin—/— platelets show an attenuated response to thrombin stimulation in vitro and hence implicate the marginal MT band in some aspects of platelet activation.19
The mechanisms and consequences of platelet spherocytosis are poorly understood, and β1-tubulin—/— mice provide the means to address these questions in a physiologic and genetic context. Here, we show that spherocytic platelets lacking the transcription factor GATA1 are also deficient in β1-tubulin, and we describe the physical and functional properties of resting and activated platelets with partial and complete β1-tubulin deficiency. Surprisingly, platelet spherocytosis per se has minimal consequences on hemostatic functions in vitro or in vivo. Our results imply that β1-tubulin is a critical determinant of platelet shape but not of cell size or content and that discoid shape in turn is not essential for the platelet functions we have examined in this study.
Materials and methods
Animals
β1-tubulin– and GATA1-deficient ΔneoΔgt animals were generated and maintained as described previously19,20 and handled in accordance with policies administered by Dana-Farber Cancer Institute and the Center for Blood Research Animal Care and Use Committees. Abnormalities in number and shape of β1-tubulin—/— platelets were similar in the inbred 129/Sv mouse strain and in mice maintained on a mixed 129/Sv-C57BL/6 genetic background (data not shown).
Platelet survival kinetics
Blood was obtained from the retro-orbital sinus of anesthetized mice, and platelet-rich plasma (PRP) was isolated by centrifugation at 200g for 8 minutes and repeat centrifugation of the supernatant and buffy coat at 200g for 6 minutes. Platelets were isolated by centrifugation of PRP at 850g for 5 minutes and washed and resuspended in 140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH 6. Platelets (109/mL) were labeled with 2.5 μM 5-chloromethyl fluorescein diacetate (CMFDA) as described,21 and 108 labeled platelets were injected via the lateral tail vein of wild-type recipient mice of the same genetic background. Blood was collected by retro-orbital venipuncture immediately (2 minutes) and 0.5, 2, 24, 48, and 72 hours after platelet transfusion, and PRP was analyzed by flow cytometry (FACScalibur, Becton Dickinson, San Jose, CA; 5 × 104 events/sample). Cells were gated by forward- and side-scatter characteristics, and the percentage of CMFDA-positive platelets at each time point was determined by regarding the fraction of labeled platelets at 2 minutes as 100%. Recovery of labeled platelets at 2 minutes21 was equivalent for wild-type (72.6% ± 8.2%) and β1-tubulin—/— (76.8% ± 11.3%) donors.
Light, electron, immunofluorescence, and confocal microscopy
Spleens and long bones harvested from adult mice were fixed in buffered 10% formalin phosphate, and bones were decalcified in HCl. Specimens were embedded in paraffin, and 7-μm sections cut onto glass slides were stained with hematoxylin and eosin. Transmission and rapid-freeze electron microscopy and confocal immunofluorescence were performed exactly as described previously.3,19,22
To determine the structure of the marginal MT band on activation, platelets were isolated from platelet-rich plasma by centrifugation at 800g for 5 minutes, resuspended in platelet buffer,19 incubated at 37°C for 30 minutes, and centrifuged onto poly-L-lysine–coated glass coverslips at 250g for 5 minutes at 37°C. Attached platelets were treated with 1 U/mL thrombin (Sigma, St Louis, MO), fixed for 20 minutes in 3.7% formaldehyde, permeabilized with 0.5% Triton X-100 in Hanks buffered saline solution (Gibco, Bethesda, MD) containing 0.1 mM EGTA (ethylene glycol tetraacetic acid), and blocked with 0.5% bovine serum albumin in phosphate-buffered saline (PBS).
Resting (Figures 1,2,3) or activated (Figure 7) platelets were incubated serially with either α-tubulin (Sigma; 1 μg/mL; Figures 1,2) or β-tubulin antibodies (5 μg/mL) for 2 hours and secondary antibody (5 μg/mL) for 1 hour, with extensive washes with PBS between and after the incubations. Controls were processed identically except for omission of the primary antibody. Images were obtained using a Zeiss (Thornwood, NY) Axiovert S100 microscope equipped with a × 100 differential interference contrast (DIC) oil immersion objective.
β1-tubulin—/— platelets have abnormal marginal MT band structure. Confocal immunofluorescence with anti–α-tubulin antibody (A) and rapid-freeze electron microscopy (C) of wild-type (+/+) platelets illustrate the normal appearance and thickness of the marginal band. In β1-tubulin—/— platelets (B,D-F), the same structure is thinner, usually deformed, frequently broken (arrows in E-F) or kinked (B, arrows in D), and generally disorganized (F); these features are never encountered in wild-type platelets. Scale bar (C-F) = 0.2 μm. The exposure time for panel B is longer than for panel A to account for the difference in MT band thickness and to allow delineation of the structural anomalies. Both +/+ (G) and β1-tubulin—/— (H) platelets show disassembled MTs at 4°C but regenerate a circumferential marginal band at 37°C. (I) The survival kinetics of transfused wild-type and β1-tubulin—/— platelets in recipient mice are indistinguishable. CMFDA-labeled platelets (108) were injected into +/+ recipients of the same genetic background, blood was collected at the indicated time points, and in vivo survival of the injected platelets (mean ± SD from 4 independent donors of each genotype) was assessed by flow cytometry. Original magnification for all immunofluorescence images, × 1000.
β1-tubulin—/— platelets have abnormal marginal MT band structure. Confocal immunofluorescence with anti–α-tubulin antibody (A) and rapid-freeze electron microscopy (C) of wild-type (+/+) platelets illustrate the normal appearance and thickness of the marginal band. In β1-tubulin—/— platelets (B,D-F), the same structure is thinner, usually deformed, frequently broken (arrows in E-F) or kinked (B, arrows in D), and generally disorganized (F); these features are never encountered in wild-type platelets. Scale bar (C-F) = 0.2 μm. The exposure time for panel B is longer than for panel A to account for the difference in MT band thickness and to allow delineation of the structural anomalies. Both +/+ (G) and β1-tubulin—/— (H) platelets show disassembled MTs at 4°C but regenerate a circumferential marginal band at 37°C. (I) The survival kinetics of transfused wild-type and β1-tubulin—/— platelets in recipient mice are indistinguishable. CMFDA-labeled platelets (108) were injected into +/+ recipients of the same genetic background, blood was collected at the indicated time points, and in vivo survival of the injected platelets (mean ± SD from 4 independent donors of each genotype) was assessed by flow cytometry. Original magnification for all immunofluorescence images, × 1000.
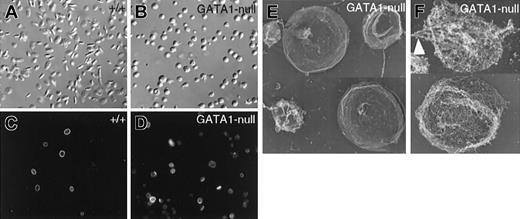
GATA-1–deficient platelets lack the characteristic discoid shape and harbor defective marginal bands. (A-B) Representative microscopic fields of resting platelet populations examined by differential interference contrast microscopy, showing the characteristic elliptical shape of normal (A) and spherocytosis of GATA-1–null (B) platelets. (C-D) Indirect immunofluorescence for α-tubulin. Most wild-type (+/+) platelets (C) contain prominent MT rings of relatively uniform diameter, whereas GATA-1 null platelets (D) contain marginal bands that stain faintly and are usually deformed or uncoiled. (E-F) Rapid-freeze electron microscopy of fixed (E) or permeabilized (F) GATA1-null platelets. Surface replicas (E) highlight the heterogeneity in size of GATA1-null platelets. MTs are frequently kinked (arrowhead in F), broken, or separated from the cell periphery.
GATA-1–deficient platelets lack the characteristic discoid shape and harbor defective marginal bands. (A-B) Representative microscopic fields of resting platelet populations examined by differential interference contrast microscopy, showing the characteristic elliptical shape of normal (A) and spherocytosis of GATA-1–null (B) platelets. (C-D) Indirect immunofluorescence for α-tubulin. Most wild-type (+/+) platelets (C) contain prominent MT rings of relatively uniform diameter, whereas GATA-1 null platelets (D) contain marginal bands that stain faintly and are usually deformed or uncoiled. (E-F) Rapid-freeze electron microscopy of fixed (E) or permeabilized (F) GATA1-null platelets. Surface replicas (E) highlight the heterogeneity in size of GATA1-null platelets. MTs are frequently kinked (arrowhead in F), broken, or separated from the cell periphery.
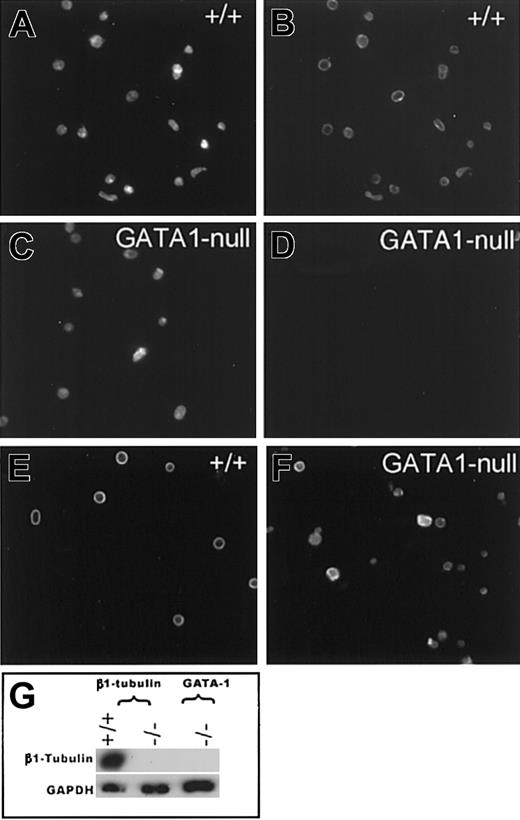
GATA1-deficient platelets lack β1-tubulin. Representative photomicrographs of platelet populations examined by Texas red–phalloidin (A,C) staining and indirect β-tubulin immunofluorescence (B,D-F). Wild-type (+/+) resting platelets show normal, bright staining for β1-tubulin (B), whereas GATA1-null platelets contain no detectable signal (D). Immunostaining with a mixture of β2 and β5 antibodies demonstrates that +/+ (E) and GATA1-null (F) resting platelets contain other β-tubulin isoforms. (G) Immunoblot analysis confirms absence of β1-tubulin in GATA1-deficient platelets. β1-tubulin knockout or wild-type cells and reaction with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody serve as controls.
GATA1-deficient platelets lack β1-tubulin. Representative photomicrographs of platelet populations examined by Texas red–phalloidin (A,C) staining and indirect β-tubulin immunofluorescence (B,D-F). Wild-type (+/+) resting platelets show normal, bright staining for β1-tubulin (B), whereas GATA1-null platelets contain no detectable signal (D). Immunostaining with a mixture of β2 and β5 antibodies demonstrates that +/+ (E) and GATA1-null (F) resting platelets contain other β-tubulin isoforms. (G) Immunoblot analysis confirms absence of β1-tubulin in GATA1-deficient platelets. β1-tubulin knockout or wild-type cells and reaction with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody serve as controls.
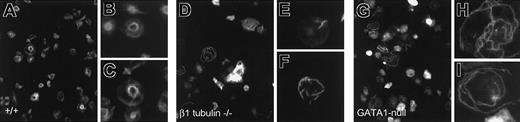
Defective reorganization of β1-tubulin—/—and GATA1-deficient marginal bands with thrombin-induced activation. Anti–α-tubulin immunostaining of wild-type (A-C), β1-tubulin—/— (D-F), and GATA1-null (G-I) platelets stimulated with thrombin and examined under low (A,D,G) or high (B-C,E-F,H-I) magnification. Wild-type platelets reorganize the MT coil into tight central rings with scant radiating fibers, whereas β1-tubulin– and GATA1-null platelets contain unraveled or completely disorganized MTs arranged randomly or in short bundles.
Defective reorganization of β1-tubulin—/—and GATA1-deficient marginal bands with thrombin-induced activation. Anti–α-tubulin immunostaining of wild-type (A-C), β1-tubulin—/— (D-F), and GATA1-null (G-I) platelets stimulated with thrombin and examined under low (A,D,G) or high (B-C,E-F,H-I) magnification. Wild-type platelets reorganize the MT coil into tight central rings with scant radiating fibers, whereas β1-tubulin– and GATA1-null platelets contain unraveled or completely disorganized MTs arranged randomly or in short bundles.
Immunoblot analysis
Platelet lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA), treated with 5% nonfat dry milk to block nonspecific binding, and then probed with a panel of antibodies directed against individual β-tubulin isoforms (generously provided by Drs. Sally Lewis and Nick Cowan, New York University, and Dr Richard Luduena, University of Texas), α-tubulin (Sigma), or glyceraldehyde 3-phosphate dehydrogenase (Biodesign International, Kennebunk, ME).
Platelet activation and aggregation studies
Blood was collected in 1-mL polypropylene tubes in 10% (final volume) acid-citrate-dextrose (38 mM citric acid, 75 mM trisodium citrate, 100 mM dextrose), and PRP was obtained by centrifugation at 220g for 7 minutes. For platelet aggregation in PRP, the plasma and buffy coat were gently transferred to a fresh polypropylene tube and centrifuged at 160g for 4 minutes. Simultaneously, platelet-poor plasma (PPP) was obtained by centrifuging the residual red blood cell concentrate at 2000g for 20 minutes, and the number of platelets in the PRP was adjusted to 2 × 108 platelets/mL using PPP.
For platelet aggregation using washed platelets, the plasma and buffy coat were transferred to a fresh polypropylene tube containing 0.7 mL washing buffer (129 mM NaCl, 13.6 mM trisodium citrate, 11.1 mM dextrose, 1.6 mM KH2PO4, pH 6.8) in the presence of 1 μM prostaglandin E1 (Sigma), and centrifuged at 160g for 4 minutes. The supernatant was transferred to 2 polypropylene tubes containing 3 mL washing buffer, centrifuged at 2000g for 10 minutes, and the pellet was resuspended in 137 mM NaCl, 4 mM KCl, 0.5 mM MgCl2, 0.5 mM sodium phosphate, 11.1 mM dextrose, 0.1% bovine serum albumin, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4. Aliquots (360 μL) of platelet suspension (2 × 108 platelets/mL for PRP or 3 × 108 washed platelets/mL) were stirred at 1100 rpm and activated by addition of agonists (10 μM adenosine diphosphate [ADP] final concentration or 1 U/mL or 0.05 U/mL thrombin) at 37°C using an aggregometer (Sienco, Morrison, CO). The extent of aggregation was measured and expressed in light transmission units.
To evaluate serotonin release, PRP was loaded with 0.5 μM [14C]5-hydroxytryptamine (5-HT; Amersham, Piscataway, NJ) for 30 minutes at ambient temperature. Platelets, isolated from PRP using a metrizamide gradient (Accurate, Westbury, NY), were stimulated in 150 μL volume with various concentrations of human thrombin (Sigma) for 2 minutes at 37°C, and the reaction was terminated by adding 50 μL of 6.8% formaldehyde and immediate centrifugation at 12 000g for 1 minute. The amount of [14C]5-HT released into the supernatant was determined by liquid scintillation counting, and results are expressed as the fraction of liberated 14C compared with the total present in unstimulated platelets.
In vivo thrombosis studies
Male mice (3.5-4.5 weeks old) were injected intravenously with calcein acetoxymethyl ester–labeled platelets (5 × 106/g body weight) of the same genotype, as described previously23 and anesthetized, and the mesentery was exposed through a midline abdominal incision. Vessels were monitored for 50 minutes after FeCl3 treatment or until cessation of blood flow lasted longer than 10 seconds. When indicated, mice were transfused with approximately 0.5 × 109 platelets (wild type or β1-tubulin–null) 18 hours before the start of the experiment.
Flow chamber studies
Mouse blood was collected into 0.3 vol PBS containing 30 U/mL heparin. PRP was obtained by centrifugation at 300g for 10 minutes at room temperature (RT). PRP was centrifuged at 1000g in the presence of prostacyclin (0.1 μg/mL) for 7 minutes at RT. After one washing step, pelleted platelets were resuspended in modified Tyrode-HEPES buffer (137 mM NaCl, 0.3 mM Na2 HPO4, 2 mM KCl, 12 mM NaHCO3, 5 mM HEPES, 5 mM glucose, 1 mmol/L CaCl2, pH 7.3) containing 0.35% bovine serum albumin (BSA). Platelets were then labeled with 2 μg/mL calcein acetoxymethyl ester for 20 minutes, washed once, and resuspended at a density of 1 × 106/μL in modified Tyrode-HEPES buffer. Platelet-poor whole blood was reconstituted with 3 × 105 labeled platelets/μL.
Perfusion of whole blood was performed in a parallel-plate flow chamber system. Briefly, a silicone gasket with a flow path height of 127 μm was placed between a flat perfusion chamber (Glycotech, Rockville, MD) and a 35-mm tissue culture dish (Corning, Corning, NY) coated with 50 μg/mL collagen (Horm) for 1 hour at ambient temperature. Perfusion was carried out at a wall shear rate of 1000 seconds—1 for 2 minutes. Platelet adhesion was visualized with an Axiovert 135 inverted microscope (Zeiss) equipped with a 100 W HBO fluorescent lamp source (OptiQuip, Highland Mills, NY) and a silicon-intensified tube camera (C 2400; Hamamatsu, Middlesex, NJ) connected to an S-VHS video recorder (AG-6730; Panasonic, Matsushita Electric, Osaka, Japan). Images were analyzed using NIH Image 1.61 software.
Results
Structural properties of platelets and of the marginal MT band in the absence of β1-tubulin
Germ line loss of β1-tubulin in mice results in spherical platelets with a marginal MT band that is substantially thinner than normal.19 Confocal immunofluorescence and rapid-freeze electron microscopy further reveal qualitative defects in the marginal MT band. In contrast to the uniform structure found in normal platelets (Figure 1A,C), the MT bundle in β1-tubulin–null cells is frequently kinked, bent, broken (Figure 1B,D-E), or completely disorganized (Figure 1F). Thus, either the normal thickness (8-12 coils) of the marginal band or a minimum amount of the β1-tubulin isoform is essential for the structural integrity of the platelet marginal band. Platelet counts in β1-tubulin—/— mice remain between 40% and 50% of normal throughout life19 (data not shown).
These findings raise the possibility that MT structural defects in the absence of β1-tubulin may lead to thrombocytopenia as a result of accelerated clearance from the circulation. However, we observe no difference in in vivo platelet survival kinetics between wild-type and β1-tubulin—/— mice (Figure 1I), consistent with published findings that murine and primate platelets whose shape is changed by activation still circulate normally.24-26 Additionally, mutant mice observed up to 1 year of age do not present evidence of ongoing platelet destruction in the form of splenomegaly or megakaryocytosis. Taken together, the data point to a primary defect in platelet synthesis, consistent with the observation of reduced proplatelet formation by cultured β1-tubulin—/— MKs.19
One useful way to understand the characteristics of platelet MTs is by assessing their ability to repolymerize after cold-induced depolymerization. In accordance with published results,1 normal platelets reassemble an apparently intact marginal ring after repeated cycles of cold and warm temperatures (Figure 1G). Platelets lacking β1-tubulin retain this capacity (Figure 1H), which indicates that reassembly of a marginal MT coil does not depend strictly on properties that are exclusive to the β1-tubulin isoform.
Absence of β1-tubulin is associated with platelet spherocytosis in an independent mouse model of thrombocytopenia and defective platelets
Blood platelets produced in the absence of the transcription factor GATA1 in mice20,27 or humans28,29 show a number of morphologic defects, including spherocytosis (Figure 2A-B) and size heterogeneity (Figure 2E). As a likely basis for the shape defect, the marginal MT band in GATA1-null platelets bears a striking resemblance to that seen in the isolated absence of β1-tubulin, with thin coils and frequent kinks or breaks (Figure 2C-D,F). GATA1 is an essential transcriptional regulator of MK differentiation and coordinates, directly or indirectly, expression of numerous MK-specific genes.30 To determine if β1-tubulin is one such gene, we assessed protein expression in platelets derived from GATA1-null mice. Immunofluorescence studies reveal the same features seen in β1-tubulin–null platelets, including reduced amounts of α-tubulin (Figure 2C-D) and β-tubulin (Figure 3E-F) proteins and absence of β1-tubulin (Figure 3C-D). Immunoblot analysis confirms the loss of β1-tubulin (Figure 3G). Thus, selective loss of the β1 isoform is associated with the phenotype of platelet spherocytosis and defective marginal bands common to 2 independent mutant mouse models. These findings highlight the importance of the marginal band and of β1-tubulin in maintaining platelet discoid shape.
Properties of platelets and of the marginal MT band in β1-tubulin+/— mice
Platelets from one patient with an unusual bleeding disorder lack a detectable marginal band and are spherocytic, but both the MT coil and discoid cell shape are restored on in vitro treatment with paclitaxel (Taxol), which promotes MT assembly.31 The salient structural features of platelets lacking β1-tubulin suggest the possibility that stabilization of MTs or stimulation of MT assembly might also restore the typical discoid cell shape. However, β1-tubulin—/— platelets fail to acquire the normal elliptical shape and remain spherical after paclitaxel treatment (Figure 4A). This finding suggests that the baseline spherocytosis in β1-tubulin—/— mice does not result simply from an unstable MT structure but from absence of β1-tubulin during platelet biogenesis.
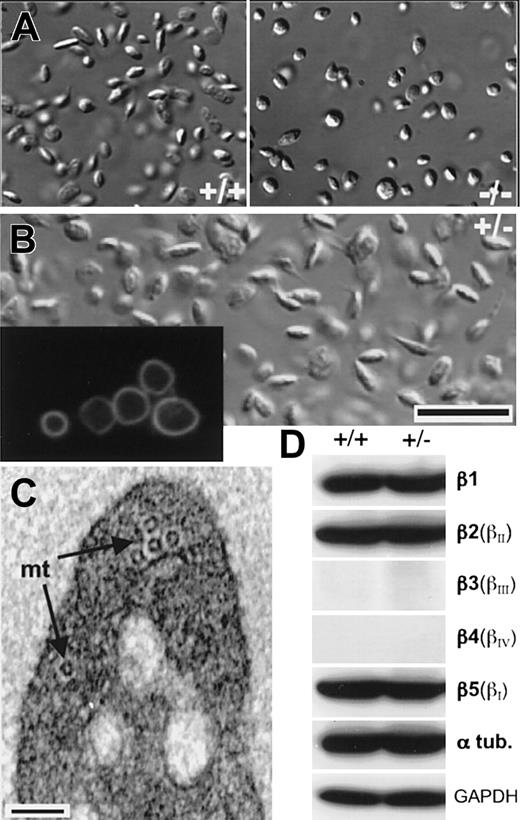
Response of β1-tubulin—/—platelets to treatment with paclitaxel and properties of β1-tubulin+/—platelets. (A) Treatment with the MT-stabilizing agent paclitaxel does not change the shape of resting wild-type platelets (+/+, left) or restore discoid morphology in β1-tubulin—/— platelets (right); original magnification, × 1000. (B) Differential interference contrast (DIC) micrograph of β1-tubulin+/— platelets in suspension, revealing the discoid resting shape. Inset (original magnification, × 1000) shows antitubulin immunofluorescence of cytocentrifuged β1-tubulin+/— platelets, which resemble wild-type platelets and lack the kinks and breaks seen in β1-tubulin—/— platelets. Scale bar = 6 μm. (C) Electron micrograph of resting β1-tubulin+/— platelet in transverse section, showing about half the number of microtubule (mt) coilings typically seen in the wild type. Scale bar = 0.1 μm. (D) Immunoblot analysis of platelet lysates to show relative proportions of all β-tubulin isotypes in wild-type (+/+) and β1-tubulin+/— platelets. αtub indicates α-tubulin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase (protein loading control).
Response of β1-tubulin—/—platelets to treatment with paclitaxel and properties of β1-tubulin+/—platelets. (A) Treatment with the MT-stabilizing agent paclitaxel does not change the shape of resting wild-type platelets (+/+, left) or restore discoid morphology in β1-tubulin—/— platelets (right); original magnification, × 1000. (B) Differential interference contrast (DIC) micrograph of β1-tubulin+/— platelets in suspension, revealing the discoid resting shape. Inset (original magnification, × 1000) shows antitubulin immunofluorescence of cytocentrifuged β1-tubulin+/— platelets, which resemble wild-type platelets and lack the kinks and breaks seen in β1-tubulin—/— platelets. Scale bar = 6 μm. (C) Electron micrograph of resting β1-tubulin+/— platelet in transverse section, showing about half the number of microtubule (mt) coilings typically seen in the wild type. Scale bar = 0.1 μm. (D) Immunoblot analysis of platelet lysates to show relative proportions of all β-tubulin isotypes in wild-type (+/+) and β1-tubulin+/— platelets. αtub indicates α-tubulin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase (protein loading control).
β1-tubulin levels are reduced approximately 50% in mature β1-tubulin+/— MKs, and, in some features, including proplatelet formation and platelet numbers, β1-tubulin+/— mice show a phenotype intermediate between the wild-type and homozygous null animals.19 To better understand the dosage requirement for β1-tubulin, we examined platelets from β1-tubulin+/— mice. Their marginal band contains, on average, about half the number of MT coils found in normal cells19 (Figure 4C). Despite this deficiency, β1-tubulin+/— platelets have the normal discoid appearance and lack kinks or breaks in the marginal band (Figure 4B); they also show the wild-type response to thrombin stimulation in vitro, as assessed by surface expression of P-selectin (data not shown). In the complete absence of β1-tubulin, MKs use unknown mechanisms to increase expression of the alternative isoforms β2 (βII) and β5 (βI), which then assemble into platelet MTs in higher than normal proportions and hence allow some degree of thrombopoiesis.19 In contrast, β1-tubulin+/— platelets reveal the same proportions of each β-tubulin isoform, and of α-tubulin, as seen in the wild type (Figure 4D). Thus, 50% of the normal amount of β1-tubulin in MKs is sufficient to impart discoid cell shape and to ensure adequate regulation of isotubulin ratios in blood platelets.
Structure of platelets lacking β1-tubulin
Loss of GATA1 is associated with multiple platelet defects, including considerable size heterogeneity, paucity of alpha-granules and dense granules, and disorganized internal membranes20,27 ; these defects reflect a broad failure in MK differentiation. In contrast, ultrastructural studies on β1-tubulin—/— platelets indicate that a defective marginal MT band and attendant spherocytosis may be the only physical abnormalities. The surface-connected canalicular and dense tubular systems are intact, internal membranes present typical profiles, and the complement of mitochondria and platelet-specific granules is normal (Figure 5). Average platelet size is also normal, as judged by electron microscopy (Figure 5) and flow cytometry (data not shown). Furthermore, ultrastructural analysis of β1-tubulin—/— MKs reveals normal development and organization of the internal membrane system and platelet-specific granules (Figure 5), although the peripheral, organelle-free zone frequently is abnormally wide. This cellular morphology is considered to represent a preterminal stage in MK maturation,6 and the findings in β1-tubulin—/— MKs may reflect the inherent inefficiencies in proplatelet formation.
Ultrastructure of β1-tubulin—/—platelets. (A) Wild-type resting platelet, shown to highlight the intact marginal MT band. (B-C) Resting β1-tubulin—/— platelets, showing their normal size and contents. DTS indicates dense tubular system; Gr, granules. (D) Electron micrograph showing the surface-connected canalicular system (SCCS) of β1-tubulin—/— platelets. Scale bars: A-D, 0.5 μm. (E-F) Light (E) and electron (F) micrographs of representative β1-tubulin—/— MKs, showing the common finding of dense cortical staining (arrows) in hematoxylin-and-eosin preparations and nearly normal cytoplasmic maturation, except for a slightly wider organelle-free peripheral zone (delineated by the H-shaped bar) in most MKs. Nuc indicates nucleus; panel E: original magnification, × 400; panel F: scale bar, 5 μm.
Ultrastructure of β1-tubulin—/—platelets. (A) Wild-type resting platelet, shown to highlight the intact marginal MT band. (B-C) Resting β1-tubulin—/— platelets, showing their normal size and contents. DTS indicates dense tubular system; Gr, granules. (D) Electron micrograph showing the surface-connected canalicular system (SCCS) of β1-tubulin—/— platelets. Scale bars: A-D, 0.5 μm. (E-F) Light (E) and electron (F) micrographs of representative β1-tubulin—/— MKs, showing the common finding of dense cortical staining (arrows) in hematoxylin-and-eosin preparations and nearly normal cytoplasmic maturation, except for a slightly wider organelle-free peripheral zone (delineated by the H-shaped bar) in most MKs. Nuc indicates nucleus; panel E: original magnification, × 400; panel F: scale bar, 5 μm.
Thus, the cellular requirement for β1-tubulin is not manifested until very late in MK maturation, and perhaps only at the proplatelet stage. Furthermore, the size and contents of blood platelets are not strictly dependent on the generation of a perfect marginal MT band; the rudimentary structure assembled in the absence of β1-tubulin is associated with qualitatively normal platelets. Although GATA1 and β1-tubulin–deficient platelets share some common features, only β1-tubulin—/— platelets show apparently isolated spherocytosis and hence provide an unprecedented opportunity to study the requirement for discoid cell shape in platelet functions.
Consequences of β1-tubulin loss and platelet spherocytosis on hemostatic functions
Surface expression of the activation marker P-selectin, which reflects fusion of platelet alpha-granules with plasma membranes, is mildly attenuated in β1-tubulin—/— but not in β1-tubulin+/— platelets19 (data not shown). These findings suggest that either a nearly intact marginal band or, more specifically, the β1-tubulin isoform, is essential for optimal platelet stimulation. To investigate this requirement further, we evaluated other parameters of platelet activation. β1-tubulin—/— platelets show normal thrombin-dependent serotonin release, even at the low doses at which P-selectin expression is reduced (Figure 6A). Moreover, both the velocity and amplitude of aggregation of platelet-rich plasma in response to adenosine diphosphate (ADP; Figure 6B) or of washed platelets in response to thrombin (Figure 6C) are the same for β1-tubulin—/— and control platelets. In standard aggregometry assays, onset of platelet aggregation is typically preceded by a transient decrease in light transmission that is believed to reflect rapid, agonist-induced change in platelet shape.32 In light of the resting spherical shape of β1-tubulin—/— platelets, this decline in light transmission is consistently blunted; this effect is highlighted under conditions that reveal activation-induced shape change without aggregation, ie, treating washed platelets with ADP (data not shown) or with a low dose of thrombin (Figure 6C, inset).
Parameters of β1-tubulin—/—platelet secretion and aggregation. (A) Normal [14C]5-HT secretion by stimulated β1-tubulin—/— platelets. Wild-type (□) and mutant (▪) platelets preloaded with [14C]5-HT were stimulated with the indicated concentrations of thrombin for 2 minutes, and [14C] release was determined as a fraction of total platelet content. Results are aggregated from 3 or 5 independent experiments and expressed as the mean ± SD for each dose of thrombin. (B-C) Results of aggregometry assays on fresh platelets from mice of the indicated β1-tubulin genotype stimulated in vitro with 10 μM ADP (B; platelet-rich plasma) or with 1 U/mL thrombin (C; washed platelets). To highlight the blunted shape change in the absence of β1-tubulin, washed platelets (inset in C) were separately stimulated with a low dose of thrombin (0.05 U/mL) that is insufficient to aggregate washed platelets.
Parameters of β1-tubulin—/—platelet secretion and aggregation. (A) Normal [14C]5-HT secretion by stimulated β1-tubulin—/— platelets. Wild-type (□) and mutant (▪) platelets preloaded with [14C]5-HT were stimulated with the indicated concentrations of thrombin for 2 minutes, and [14C] release was determined as a fraction of total platelet content. Results are aggregated from 3 or 5 independent experiments and expressed as the mean ± SD for each dose of thrombin. (B-C) Results of aggregometry assays on fresh platelets from mice of the indicated β1-tubulin genotype stimulated in vitro with 10 μM ADP (B; platelet-rich plasma) or with 1 U/mL thrombin (C; washed platelets). To highlight the blunted shape change in the absence of β1-tubulin, washed platelets (inset in C) were separately stimulated with a low dose of thrombin (0.05 U/mL) that is insufficient to aggregate washed platelets.
Activated platelets convert rapidly from discoid to spherical forms, extend surface filopodia and lamellipodia, condense previously dispersed organelles into the cell center, and rearrange the peripheral MT coil into a tight central ring.33,34 Radial arrays of individual MTs extending into the surface projections are also observed in some cells and may facilitate organelle transport to the platelet periphery. To investigate if spherocytic platelets exhibit normal activation-dependent MT reorganization, we used immunofluorescence microscopy to visualize the MT cytoskeletons after thrombin treatment (Figure 7). Whereas control (β1-tubulin+/+ and β1-tubulin+/—) platelets contain multiple MTs constricted into tight rings in the cell center (Figure 7A-C), the MTs in both β1-tubulin—/— (Figure 7D-F) and GATA1-deficient (Figure 7G-I) platelets lack this arrangement entirely. Instead, irregular MTs are unusually assembled in the periphery of spread platelets, occasionally rimming the cortex, and individual MTs are randomly dispersed or found in short bundles or fibers scattered within the cytoplasm. Sometimes the entire MT coil is unraveled (Figure 7D,G,I). These findings supply important correlation between absence of β1-tubulin and structural aspects of platelet activation. They further suggest that the abnormalities in resting and activated β1-tubulin—/— platelets may compromise hemostasis in vivo in ways that are not readily revealed in aggregation and other in vitro activation assays.
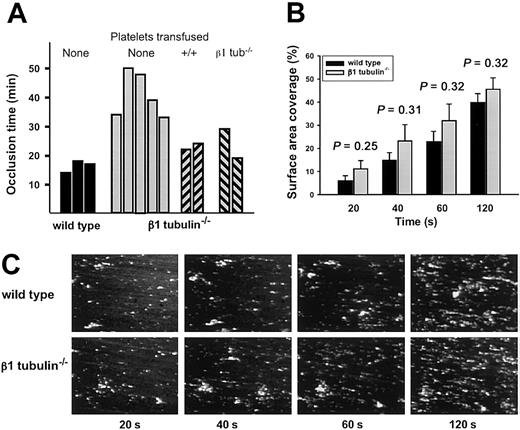
To study platelet function under physiologic shear conditions, we performed intravital microscopy in a model of ferric chloride–induced arterial thrombosis.23 Compared with wild-type controls, formation of occlusive thrombi is markedly delayed in injured arterioles of β1-tubulin—/— mice (Figure 8A). Although this finding superficially suggests a functional platelet defect, we considered the alternative possibility that the delay in vessel occlusion reflects the 50% or more reduced platelet count in β1-tubulin—/— mice. Indeed, transfusion of heterologous β1-tubulin—/— platelets (∼ 0.5 × 109 platelets per mouse) reduces the occlusion time (Figure 8A), indicating that when corrected for quantity the mutant platelets can function normally under the high shear rate conditions present in arterioles. To further investigate the capacity of β1-tubulin—/— platelets to form thrombi, we used whole blood to study platelet adhesion to a collagen surface under conditions of arterial shear rate (1000 second—1) in a flow chamber. Thrombus formation under these conditions is known to be a multistep process involving platelet receptors such as glycoproteins Ibα, VI, and IIb/IIIa, and their ligands.35,36 Because the number of adhering platelets in this assay depends on the platelet count, we ensured the use of equal numbers of platelets in wild-type and mutant samples (see “Materials and methods”). β1-tubulin—/— platelets show normal adhesion and thrombus formation during the 2 minutes of perfusion (Figure 8B-C), demonstrating that platelet activation and their adhesion to a prothrombotic surface or to each other are not measurably affected in this context.
Normal adhesion of β1-tubulin—/—platelets under physiologic shear conditions. (A) Arterial thrombosis model. Wild-type or β1-tubulin—/— mice were injected with fluorescently labeled platelets of matching genotype, and their mesenteries were exposed. When indicated, β1-tubulin–null mice were transfused with 0.5 × 109 wild-type or β1-tubulin–null platelets 18 hours prior to the start of the experiment. Arterioles (60-100 μm in diameter) were selected, vascular injury was provoked by superfusion with ferric chloride, and the time before blood flow ceased for more than 10 seconds was determined. Each bar represents an individual animal. (B-C) Parallel-plate flow chamber studies. Platelets from mice of the indicated genotype were prepared as described in “Materials and methods” and then perfused for 2 minutes over a collagen surface at a wall shear rate of 1000 seconds—1. (B) Surface-area coverage (%) of calcein fluorescence at different time points (20 seconds to 2 minutes) over the perfusion period; n = 5 independent animals of each genotype, and the statistical significance of observed differences between the 2 groups is indicated over each pair of bars. (C) Representative fluorescence images of a portion of the flow chamber for platelets derived from single mice.
Normal adhesion of β1-tubulin—/—platelets under physiologic shear conditions. (A) Arterial thrombosis model. Wild-type or β1-tubulin—/— mice were injected with fluorescently labeled platelets of matching genotype, and their mesenteries were exposed. When indicated, β1-tubulin–null mice were transfused with 0.5 × 109 wild-type or β1-tubulin–null platelets 18 hours prior to the start of the experiment. Arterioles (60-100 μm in diameter) were selected, vascular injury was provoked by superfusion with ferric chloride, and the time before blood flow ceased for more than 10 seconds was determined. Each bar represents an individual animal. (B-C) Parallel-plate flow chamber studies. Platelets from mice of the indicated genotype were prepared as described in “Materials and methods” and then perfused for 2 minutes over a collagen surface at a wall shear rate of 1000 seconds—1. (B) Surface-area coverage (%) of calcein fluorescence at different time points (20 seconds to 2 minutes) over the perfusion period; n = 5 independent animals of each genotype, and the statistical significance of observed differences between the 2 groups is indicated over each pair of bars. (C) Representative fluorescence images of a portion of the flow chamber for platelets derived from single mice.
Discussion
Platelet release from terminally differentiated MKs is driven in large part by MTs. In the final phase of cell maturation, MKs increase tubulin synthesis and reorganize cytoplasmic MTs, first in the cell periphery and subsequently in the proplatelet extensions where nascent blood platelets are assembled.3,8 The MK- and platelet-specific tubulin β1 is required for optimal thrombopoiesis.19 In its absence, chronic thrombocytopenia results from a primary defect in platelet synthesis and the platelet marginal band is thin and fragile. The present study of mouse platelets with complete and partial β1-tubulin deficiency provides new structural and functional insights into the platelet marginal band. Our analysis indicates that thrombopoiesis requires a threshold amount of cellular β1-tubulin to coordinate isotubulin stability and assembly and to generate a marginal band capable of supporting discoid platelet shape.
β1-tubulin is absent from MKs that lack the transcription factor NF-E2 and do not support normal platelet synthesis.16,37 In contrast, although the block in MK maturation seen in the absence of GATA1 is more profound in some ways, GATA1-deficient MKs are able to produce some circulating platelets and thus avoid lethal hemorrhage.20 These platelets are also uniformly spherical and have a thin, fragile marginal MT band. Among the many defects observed in GATA1-null platelets,27 at least these features appear to follow from absence of β1-tubulin and likely reflect a scenario wherein GATA1 regulates p45 NF-E2 expression and β1-tubulin in turn is an NF-E2–dependent gene. However, both GATA1 and NF-E2 control expression of many other essential genes.16,27 Although β1-tubulin—/— and GATA1-null platelets share the common feature of spherocytosis, platelet size and composition are severely affected in the absence of GATA127 but are normal in β1-tubulin—/— mice. This observation sheds useful light on mechanisms of platelet biogenesis. Teardrop-shaped precursors of the mature marginal band are among the earliest structures generated in the proplatelet,3 and cellular components must be partitioned precisely within nascent blood platelets. β1-tubulin–null mice reveal that an intact marginal MT band is not essential for these aspects of platelet assembly. Independently, it has been suggested that integrity of MK MTs is essential for proper cytoplasmic organization of the demarcation membrane system.38 Although this may yet be true, our observations indicate that MTs assembled in the absence of β1-tubulin can support the mature MK structure adequately.
The mechanisms by which the MT marginal band imparts an elliptical shape to resting platelets remain unclear but probably involve complex structural interactions with the actin cytoskeleton, the membrane skeleton, and the plasma membrane. The plasma membrane of resting platelets could be linked to the marginal band by virtue of a MAP, much as the MT-associated Tau protein is thought to connect MTs with the plasma membrane in neurons.39 Alternatively, tubulin subunits within the platelet marginal band may associate directly with the plasma membrane; the documented palmitoylation of α-tubulin in resting platelets40,41 provides a possible means to link the 2 structures directly. One might speculate on some other mechanisms based on events that unfold rapidly on platelet activation, when the transition from discoid to spherical shape is one of the earliest events.42 A necessary and transient rise in cytosolic calcium exposes actin-binding sites in gelsolin, with attendant severing of actin filaments and platelet shape change. Thus, the actin cytoskeleton likely also contributes toward resting platelet shape in a manner that depends on the tubulin composition of the marginal MT band.
The marked abnormality in activational reorganization of β1-tubulin—/— and GATA1-null MTs (Figure 7) may be especially interesting in this light. Normal platelets treated with MT depolymerizing agents (vinca alkaloids) during activation fail to undergo internal transformation, secondary aggregation, and secretion.43,44 This finding suggests that MT integrity is essential for physiologic platelet responses, perhaps much as it is needed for endothelial cells to secrete Weibel-Palade bodies, which are related to platelet α-granules.45 Nevertheless, treatment of chilled platelets with paclitaxel rearranges the MTs into irregular bundles and randomly dispersed individual tubules46 ; despite the failure of MTs to relocate into tight central rings, platelets still change shape and secrete granule contents. This finding suggests that reorganization of MTs into central coils per se is dispensable for platelet activation responses and that, as long as MTs are present, activation can occur almost normally. Although the full physiologic significance of MT reorganization in activated platelets remains unclear, our data demonstrate that β1-tubulin is required to reorganize platelet marginal bands correctly during platelet activation. The defective reorganization seen in β1-tubulin—/— and GATA1-null platelets may result from a limiting amount of MT polymers in these cells.
Both β1-tubulin– and GATA1-deficient platelets do contain an MT ring, however anomalous, so it is difficult to know how platelets might appear in the complete absence of this structure. We previously highlighted the likely integral role of the marginal band in thrombopoiesis3 and suggested that it may be impossible to produce platelets that lack a marginal band entirely. However, one patient with a lifelong history of easy bruising is reported to circulate spherocytic platelets virtually devoid of MT coils.31 Although these platelets are normal in size and content, their aggregation response toward most agonists is poor; MK morphology was not reported but interestingly, platelet MT coils and discoid shape are restored by addition of paclitaxel in vitro. This finding suggests that MTs are available for thrombopoiesis but are perhaps unstable in the circulating platelets. Further characterization of this unusual platelet disorder will add to the appreciation of thrombopoietic mechanisms.
Our studies lead to a surprising conclusion about the necessity of the MT marginal band in platelet structure and function. Spherocytic β1-tubulin—/— platelets have a normal life span in the circulation, secrete dense granule contents and aggregate normally in response to various stimuli, and facilitate hemostasis in injured arterioles. Thus, the discoid shape of blood platelets is apparently dispensable for many aspects of platelet function, as measured in vitro and in vivo, and this shape either serves roles we have not tested or may be a functionally irrelevant byproduct of the thrombopoietic mechanism. Although we previously regarded the prolonged bleeding time of β1-tubulin—/— mice as evidence against the latter possibility,19 the present data suggest that, like the delay in occlusion of injured arterioles (Figure 8), the prolonged bleeding time might also simply reflect thrombocytopenia. Alternatively, platelet spherocytosis may cause defects in platelet-endothelial interactions in ways we are unable to measure or impair platelet rolling over damaged blood vessels. There is also a discernible defect in surface P-selectin expression in response to low doses of thrombin.19
Finally, it is instructive to consider our findings in an evolutionary context. Anucleate blood platelets, found only in mammals, represent a relatively recent evolutionary step (approximately 400 million years)47 that presumably required deployment of original and sophisticated mechanisms to sense the conclusion of the intrinsic differentiation program and partition the MK cytoplasm. β1-tubulin, which is uniquely expressed in MKs and platelets, hence evolved in a context that demands both functional versatility and mechanisms to enable high expression levels late in MK ontogeny. High levels of lineage- and stage-specific β1-tubulin gene expression are achieved through the presumptively sequential action of GATA1 and NF-E2, whereas its functional specificity is implied by both the high sequence divergence48 and the clear requirement for β1-tubulin in ensuring proper ratios of tubulin isoforms.19 The correct isotubulin distribution in turn is necessary to generate proplatelets and platelets in the required numbers, to impart stability to the marginal band, and to maintain discoid platelet shape. Although our findings suggest that spherocytic platelets do not compromise hemostasis appreciably, it is likely that the evolutionary emergence of platelets and of β1-tubulin shares a common origin in the proplatelet-based mechanism of mammalian thrombopoiesis.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-11-3491.
Supported by research grants R01HL63143, R01HL68130, and P01 HL56949 from the National Institutes of Health, and Scholar Awards from the American Society of Hematology, the American Heart Association, and the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Heinz Schwer for his role in an early phase of this work; Kari Horney, Vanessa Stolzer, and Howard Mulhern for technical assistance; Sally Lewis, Nick Cowan, and Richard Luduena for reagents; and Kurt Barkalow and Harald Schulze for comments on the manuscript.



![Figure 6. Parameters of β1-tubulin—/— platelet secretion and aggregation. (A) Normal [14C]5-HT secretion by stimulated β1-tubulin—/— platelets. Wild-type (□) and mutant (▪) platelets preloaded with [14C]5-HT were stimulated with the indicated concentrations of thrombin for 2 minutes, and [14C] release was determined as a fraction of total platelet content. Results are aggregated from 3 or 5 independent experiments and expressed as the mean ± SD for each dose of thrombin. (B-C) Results of aggregometry assays on fresh platelets from mice of the indicated β1-tubulin genotype stimulated in vitro with 10 μM ADP (B; platelet-rich plasma) or with 1 U/mL thrombin (C; washed platelets). To highlight the blunted shape change in the absence of β1-tubulin, washed platelets (inset in C) were separately stimulated with a low dose of thrombin (0.05 U/mL) that is insufficient to aggregate washed platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/12/10.1182_blood-2002-11-3491/6/m_h81234498006.jpeg?Expires=1765927210&Signature=nIq5FjYEavxjiZiQcXIsAxmpH6M8tQ2KsR6zjZSLFg8ZF0jyNCwSj9k4p0MET5NOq~Mf0iiJB3~B21BwCryK8Kx61MDKCUbGxdd0B3d8rdEk5fBbJ98Qhf~UMwLC9HyaIb5aZa~x~iHlotGgk5K~apLDp0M0TXD013mBHkJ~mAxDPMstVcG5TRoDsdH0JtVEjxIVDHJfffpgR7r8fmQo0--9ODR0pEvtU5mKXOD07NTx2VMaN1SAvY17VVZq0Jdy3eLphl7F5arvGGatXt0StfrvfshqeS8Cqi1UCpUke4Xun-KWEVR510-yzWV6-cj5iNU-GJRJBTugj1jql3CV7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal