Abstract
The transcription factor LMO2 is believed to exert its effect through the formation of protein-protein interactions with other DNA-binding factors such as GATA-1 and TAL1. Although LMO2 has been shown to be critical for the formation of the erythroid cell lineage, the gene is also expressed in a number of nonerythroid tissues. In this report, we demonstrate that the more distal of the 2 promoters for the LMO2 gene is highly restricted in its pattern of expression, directing the hematopoietic-specific expression of this gene. Deletion and mutation analyses have identified a critical cis element in the first untranslated exon of the gene. This element is a consensus-binding site for a small family of basic leucine zipper proteins containing a proline and acidic amino acid–rich (PAR) domain. Although all 3 members of this family are produced in erythroid cells, only 2 of these proteins, thyrotroph embryonic factor and hepatic leukemia factor, can activate transcription from this LMO2 promoter element. These findings represent a novel mechanism in erythroid gene regulation because PAR proteins have not previously been implicated in this process.
Introduction
In the differentiation of hematopoietic stem cells, cells must proceed from multipotent progenitors through several restricted lineage `decisions' to give rise to mature blood cells. This process involves specification of the transcriptional profile.1,2 Recent studies have demonstrated that this specification uses not only the activation of lineage-specific transcripts, but also the repression of gene expression relevant for other lineages. Previous models of transcriptional activation have involved protein factors binding to their cognate DNA recognition sites and individually impinging on the basal transcription machinery to modulate the activity of a given promoter. Recent studies, however, have demonstrated more intricate mechanisms of gene regulation. These current models incorporate multiprotein complexes of transcription factors that may bind to a series of sites or act to sequester components, thereby modulating gene expression.
Although many of the factors in such complexes are capable of specifically binding DNA, other components serve as bridging molecules. LMO2 is an example of the latter type of protein and is a member of the LIM-only family of genes. The LMO2 protein consists of 2 LIM domains, cysteine- and histidine-rich protein motifs that are structurally similar to zinc finger DNA-binding domains.3,4 To date, however, no evidence of direct DNA binding by LMO proteins has been found. Rather, the LIM domains have been associated with protein-protein interactions.5-7
Such protein interactions have been implicated in the assembly of an erythroid-specific complex of transcription factors. For example, LMO2 and TAL1 can be found as a complex in erythroid cells8 and LMO2 will also assemble with GATA-1. However, efforts to demonstrate a stable association between GATA-1 and TAL1 have been unsuccessful.9 Evidence for a model in which LMO2 interacts with both GATA-1 and TAL1, serving as a protein link between these 2 DNA-binding proteins, has now been provided.10 These studies were based on binding assays using consensus DNA-binding sites; actual target genes for this large multiprotein complex were not identified. Our laboratory has provided support for this model of transcriptional activation by a GATA-1/TAL1/LMO2 complex with the report of a cis element in an endogenous promoter that potentially serves as an in vivo target sequence for such a complex.11 The site is present in the promoter region of the erythroid Krüppel-like factor (EKLF) gene and consists of a TAL1-binding site flanked by 2 GATA-1 sites. The EKLF gene encodes a transcription factor that is required for expression of the adult hemoglobin gene.
In additional experiments designed to study the physiologic role of Lmo2, a homozygous null mutation in the coding region of the Lmo2 gene was created in mice.12 This mutation resulted in a lack of yolk sac erythropoiesis and embryonic lethality at embryonic day 8.5. Moreover, the analysis of chimeric animals produced from homozygous deficient (Lmo2–/Lmo2–) embryonic stem (ES) cells demonstrated a requirement for Lmo2 in adult hematopoiesis13 and angiogenic remodeling of the vasculature.14 Thus, expression of the LMO2 gene is a key factor in hematopoiesis.
Our current work with the LMO2 promoter has led to the investigation of another family of transcription factors not previously associated with hematopoietic gene regulation. This group of basic leucine zipper (bZIP) proteins is defined by the presence of a proline and acidic amino acid–rich protein (PAR) domain and consists of 3 family members—hepatic leukemia factor (HLF), D-site binding protein (DBP) from the albumin gene, and thyrotroph embryonic factor (TEF). In this report we describe a mechanism that leads to hematopoietic-specific expression of the human LMO2 gene and demonstrate the novel observation that a member of the PAR family plays a critical role in this expression.
Materials and methods
Northern blot analysis of LMO2
A rat tissue poly(A) Northern blot was prepared as previously described15 using 2 μg RNA per lane. We also prepared a blot with 20 μg total RNA from K562 and RAG (renal adenocarcinoma) cells. The Human Immune System MTN Blot II was purchased from Clontech (Palo Alto, CA). This blot contains 2 μg poly(A) RNA per lane. All blots were probed with polymerase chain reaction (PCR) fragments corresponding to the distal promoter region, stripped, and reprobed with a portion of the coding region. The primers used to generate the 260–base pair (bp) distal-specific probe from human gDNA were 5′-TCCTCAAAGAACAGCAACGGG-3′ (+28 to +48, exon 116) and 5′-GCAGATGATGTAATCCTGGTGGC-3′ (+265 to +288, exon 116). The coding region probe that recognizes transcripts from both the distal and the more proximal promoter was a 230-bp fragment generated by PCR with the primers 5′-GAGGAATTCATGTCCTCGGCCATCGAAAG-3′ and 5′-GAGGTCGACCTAAAGCTTTATCATCCCATTGATC-3′. These primers amplify the entire coding region of the LMO2 gene and contain restriction enzyme sites for subsequent cloning of the fragment. An Lmo2-expressed sequence tag (EST; IMAGE clone no. 708405) was used as the template in the PCR process. The probes were labeled to approximately equal specific activities and the manufacturer's protocol (Clontech) was followed concerning hybridization solutions and stripping and reuse of the blots. An actin probe was also used as a control for the loading and integrity of RNA on the blots.
Isolation of the LMO2 gene promoter
A human BAC library was obtained from Research Genetics (Carlsbad, CA) and screened by PCR with a sequence tagged sites (STS) marker for LMO2. The primer set was left = 5′-GGGGAGGTGTTCACTGAAGA-3′, right = 5′CTATGGATGGGCTGTGGC-3′ (Genbank ID Z56500, UniSTS SGC31579). Subclones were derived from the positive BAC clone and subjected to nucleotide sequence analysis.
Construction of reporter plasmids
The –3190LMOCAT used for the generation of transgenic mice was constructed as follows: a HindIII-BamHI fragment containing the chloramphenicol acetyl transferase (CAT) gene was cloned into the corresponding sites in pGEM7 (Promega, Madison, WI). A 3190-bp AccI (blunted)–HindIII LMO2 fragment was then ligated into the SmaI and HindIII sites of this vector.
A luciferase reporter was used in the analysis of the LMO2 promoter in tissue culture cells. –3190LMOLUC is a 3190-bp XhoI and HindIII fragment from –3190LMOCAT cloned into the XhoI and HindIII sites of the pGL3 basic vector (Promega). –2468LMOLUC was produced with a ClaI-HindIII fragment, –512LMOLUC is a KpnI-HindIII fragment and –125LMOLUC is a PstI-HindIII fragment, all cloned in the pGL3 vector. The –125ΔBglLMOLUC and –3190ΔBglLMOLUC are the corresponding constructs with an internal deletion of the BglII-HindIII fragment spanning the sequence from +249 to +428.
To create 3′ deletions in the +249/+428 region, specific primers were used to amplify the area. The GL1 primer from the pGL3 vector was used in combination with either the primer 5′-GGAAGCTTCCCTTGCAATCGTCCATTC-3′ or 5′-GGAAGCTTAATCACCACTTCTTCTG-3′ for –125/+338LMOLUC or –125/+378LMOLUC, respectively. Finally, for the –125/288LMOLUC construct, a double-stranded oligomer was synthesized consisting of the sequence from +249 to +288 with BglII-HindIII overhangs. This was ligated to the BglII-HindIII sites of –125LMOLUC replacing the +249 to +428 sequences.
Construction of the –125/EboxMLMOLUC, –125/EboxCALMOLUC, –125/EboxTGLMOLUC, and –125/PARMLMOLUC was accomplished using PCR mutagenesis with the oligos listed in Figure 6. The –125/S8MLMOLUC was produced with the primer 5′-CTGCTAGCTTCTCTGATGAATGG-3′. The TATA-PARLUC and TATA-PARMLUC constructs were made using concatamers of the 2 oligomers listed in Figure 9A ligated 3′ of the E1b minimal TATA box sequence (5′-AGGTATATAATG-3′) in the pGL3 basic vector. All constructs were subjected to nucleotide sequence analysis to verify the appropriate insertions.
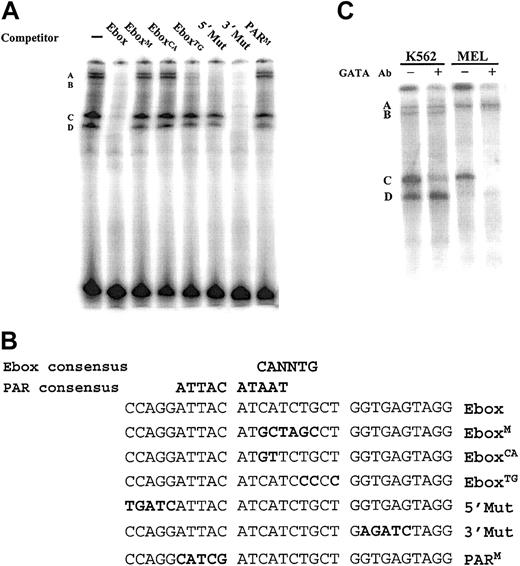
An erythroid cell nuclear factor specifically binds a PAR motif overlapping the Ebox site in the LMO2 promoter. (A) EMSA was performed with a labeled oligonucleotide spanning the Ebox and PAR sites and nuclear extracts from K562 cells. Specific protein-DNA complexes are labeled A-D. (B) The double-stranded oligos listed were used as competitors. Mutations in the sequence are shown in bold. Critical nucleotides are indicated by the inability of a mutant oligomer to compete for the radiolabeled binding to the wild-type 30-mer. (C) A similar EMSA using the Ebox wild-type probe and nuclear extracts from K562 and MEL cells and carried out in the presence and absence of an antibody directed against GATA-1. The disappearance of the band labeled C in the presence of the antibody indicates a GATA-1 binding complex.
An erythroid cell nuclear factor specifically binds a PAR motif overlapping the Ebox site in the LMO2 promoter. (A) EMSA was performed with a labeled oligonucleotide spanning the Ebox and PAR sites and nuclear extracts from K562 cells. Specific protein-DNA complexes are labeled A-D. (B) The double-stranded oligos listed were used as competitors. Mutations in the sequence are shown in bold. Critical nucleotides are indicated by the inability of a mutant oligomer to compete for the radiolabeled binding to the wild-type 30-mer. (C) A similar EMSA using the Ebox wild-type probe and nuclear extracts from K562 and MEL cells and carried out in the presence and absence of an antibody directed against GATA-1. The disappearance of the band labeled C in the presence of the antibody indicates a GATA-1 binding complex.
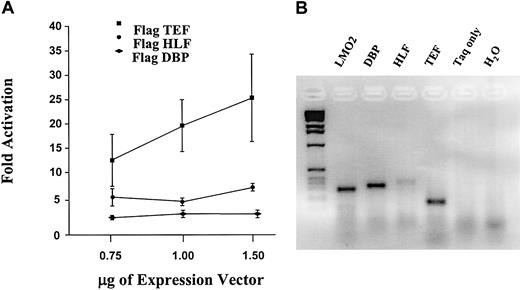
TEF produces the highest transcriptional activation through the LMO2 PAR-binding site. (A) Increasing amounts of FLAG-tagged PAR expression constructs were cotransfected into K562 cells with the TATA-PAR reporter construct. The level of TATA-PAR luciferase expression in the absence of PAR proteins was set as 1. A Western blot analysis was also performed to ensure approximately equal expression of the factors from each construct. (B) Endogenous coexpression of LMO2 and the PAR genes in primary erythroid cells was demonstrated by RT-PCR analysis of RNA from human reticulocytes. The 2 control lanes were from reactions incubated with the DBP primers; similar negative results were obtained with the other primer sets.
TEF produces the highest transcriptional activation through the LMO2 PAR-binding site. (A) Increasing amounts of FLAG-tagged PAR expression constructs were cotransfected into K562 cells with the TATA-PAR reporter construct. The level of TATA-PAR luciferase expression in the absence of PAR proteins was set as 1. A Western blot analysis was also performed to ensure approximately equal expression of the factors from each construct. (B) Endogenous coexpression of LMO2 and the PAR genes in primary erythroid cells was demonstrated by RT-PCR analysis of RNA from human reticulocytes. The 2 control lanes were from reactions incubated with the DBP primers; similar negative results were obtained with the other primer sets.
Primers used to Flag tag the PAR cDNAs were as follows: for DBP the 5′ Flag oligo CAGGATCCATGGACTACAAGGACGACGATGACAAAGCGCGGCCTCTG and the 3′ oligo CAGAGTTGCCTTGCGCTCCTTTTCC; for the HLF the 5′ Flag oligo CAGGATCCATGGACTACAAGGACGACGATGACAAAGAGAAAATGTCCCGAC and the 3′ oligo TGCATACAGTTCGGAGATGGGATG were used. TEF was cloned into the pFLAG Mac (Sigma, St Louis, MO) vector using the 5′ oligo designated hTEFXho39 AACTCGAGTCCGACGCGGGCGGCGGAAAGAAG and the 3′ oligo hTEF296rv GATGGTCTTGTCCCAGATG. This construct was digested with EcoRV and BamHI and the FlagTEF sequence was cloned to pcDNA3.1 (Invitrogen, Carlsbad, CA). The DBP and HLF constructs were also cloned into the pcDNA3.1 vector.
Transgenic mice
The –3190LMOCAT construct was purified (Qiagen, Valencia, CA) before injection and transgenic animals were produced in the FVB/N mouse strain. Putative transgenic animals were screened by PCR. DNA from established lines was analyzed by Southern blotting to determine copy number and to ensure the transgene was intact and free of rearrangements. Blood and bone marrow samples were collected and placed in a phosphate-buffered saline/heparin solution. The marrow samples were obtained from the femurs of F1 mice. The samples were centrifuged, and the cell pellet was lysed by freeze-thaw in a 0.1M Tris (tris(hydroxymethyl)aminomethane), pH 7.5, solution. F1 transgenic animals were also mated to produce timed pregnancies and the embryos collected at 10.5 days or 14.5 days after coitus. The yolk sacs were collected from 10.5-day embryos and fetal liver samples were obtained from the 14.5-day time points. These samples represent the sites of hematopoiesis at the 2 developmental time points. Transgenic embryos and fetuses were selected based on PCR assay of a portion of the embryo body. Tissue extracts were prepared from adult F1 animals or the F1 fetal samples by homogenization in the Tris solution, followed by freeze-thaw lysis. CAT assays were performed both by CAT enzyme-linked immunosorbent assay (ELISA; Roche, Indianapolis, IN) and thin-layer chromatography using the FAST CAT Yellow kit from Molecular Probes (Eugene, OR). Results were normalized for protein levels using a bicinchoninic acid assay (Pierce Chemicals, Rockford, IL).
Transfections and reporter assays
K562 cells were plated at 2 × 106 cells/well in a 6-well plate and a total of 2 μg DNA and 7 μL of the TransIT LT-1 lipofection reagent in serum-free media (Mirus, Madison, WI) were mixed and added to the cells. HEK293 and COS cells were plated at 5 × 105 cells/well and 2 × 105 cells/well, respectively, in 12-well plates. For these cells, approximately 24 hours later a mix of 0.5 μg DNA and 3 μL FuGene (Roche) reagent in serum-free media were added to the cells. Cells were harvested 18 to 24 hours later and lysed in 65 μL of lysis buffer (Tropix, Bedford, MA). The β-galactosidase activity was measured by mixing 2 μL of this cell extract with Galacto-Star (Tropix) reagent and luciferase activity was measured using 20 to 25 μLof cell extract and the Luciferase assay system from Promega. The samples were assayed in a 96-well plate using a Luminoskan Ascent luminometer (Thermo Labsystems, Franklin, MA).
The DBP, TEF, and HLF expression vectors used in the cotransfection experiments were the generous gift of Dr Hitoshi Okamura (Kobe University Graduate School of Medicine, Japan) who has previously used these constructs successfully in promoter transactivation experiments.17 Our FLAG-tagged versions of these clones were used in cotransfection experiments to generate dose-response curves. In these assays, 0.75 to 1.5 μg of each FLAG-PAR expression vector was used in the presence of 0.5 μg of the reporter construct and 1.5 μg of the β-galactosidase transfection control plasmid. The BSKSII plasmid (Clontech) was added as needed to equalize the amount of DNA added to each well.
Coexpression of LMO2 and the genes of the PAR family in human erythroid cells was demonstrated by reverse transcription–PCR (RT-PCR). The primers sets used were: LMO2, FW 5′-TCCTCAAAGAACAGCAACGGG-3′ and RV 5′-CGAATTCTCAGAGCAGTTGTTACAG-3′; DBP, FW 5′-GGAGGAGCAGAAGGATGAGAAATAC-3′ and RV 5′-ACAGGGCAGGAAGGGAACAG-3′; HLF, FW 5′-TAGCACCACACGCAAACCAACC-3′ and RV 5′-AAGCAGAGAGTCCTGGAAGAGTAGG-3′; and TEF, FW 5′-AAACCGTGTCCAGCACAGAATC-3′ and RV 5′-CAGGTCCTCCTCAGCAAACTTG-3′. Human reticulocyte RNA was used as the template and RT-PCRs were carried out using a SuperScript One-Step RT-PCR kit from Invitrogen.
Electrophoretic mobility shift assays
Nuclear extracts were purchased from Geneka (Montreal, QE, Canada). All binding assays were done in a total volume of 30 μL in the presence of 1 × 105 cpm 32P-labeled probe containing 8 to 10 μg nuclear extract, 250 mM KCl, 20 mM Tris (pH 7.9), 60 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesuflonic acid; pH 7.8), 10% glycerol, 0.5 mM dithiothreitol (DTT), 6 mM EDTA (ethylenediaminetetraacetic acid), and 3 μg dI/dC, with or without 100 ng specific competitor. All reactions were incubated on ice for 15 minutes without probe and for 15 minutes after the addition of probe. In the supershift assay, 100 ng GATA-1 rat monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added at the time of probe addition. The DNA-protein complexes were separated on 5% polyacrylamide gels at 150 V for 4 hours at 4°C.
Results
The distal LMO2 promoter directs hematopoietic-specific gene expression
The genomic structure of LMO2 includes 3 untranslated 5′ exons (1-3) with the initiator methionine appearing in exon 4 (Figure 1A). Two promoters have been identified for the LMO2 gene.16 The more proximal promoter is located within exon 3 of the larger transcript, therefore both transcripts result in identical, translated LMO2 proteins. Analyses using RT-PCR of T-cell acute lymphoblastic leukemia (T-ALL) patient cell lines, fetal liver, and adult kidney indicated that the proximal promoter was broadly used, whereas the distal promoter was only active in fetal liver and some of the T-ALL cell lines,16 suggesting the possibility of hematopoietic-specific expression via the distal promoter.
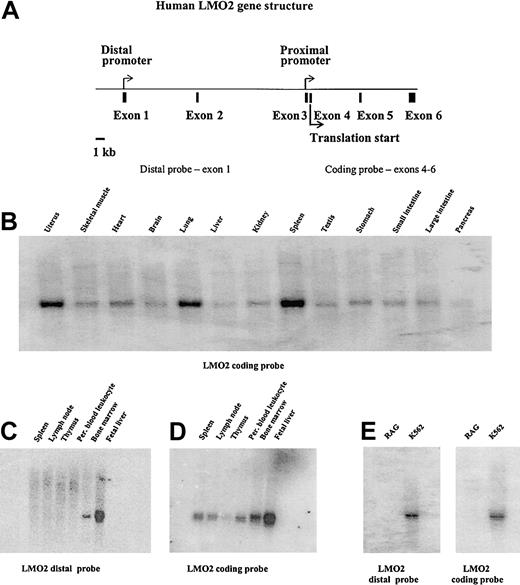
Northern blot analyses demonstrating the tissue distribution of LMO2 gene expression and specificity of the distal promoter. (A) Diagram of the human LMO2 gene structure. The 2 promoters are marked by arrows; the translation start site in exon 4 is also indicated. Panels B and D were hybridized with a probe from the coding region of the gene encompassing exons 4 to 6. This probe recognizes transcripts from either the distal or proximal promoter. Panel C is the same blot as shown in panel D, but is hybridized with a probe specific for the distal promoter transcript. A similar analysis of the blot shown in panel B gave no hybridization signal. Panel E was hybridized with both the coding probe and the distal promoter probe as shown.
Northern blot analyses demonstrating the tissue distribution of LMO2 gene expression and specificity of the distal promoter. (A) Diagram of the human LMO2 gene structure. The 2 promoters are marked by arrows; the translation start site in exon 4 is also indicated. Panels B and D were hybridized with a probe from the coding region of the gene encompassing exons 4 to 6. This probe recognizes transcripts from either the distal or proximal promoter. Panel C is the same blot as shown in panel D, but is hybridized with a probe specific for the distal promoter transcript. A similar analysis of the blot shown in panel B gave no hybridization signal. Panel E was hybridized with both the coding probe and the distal promoter probe as shown.
To more carefully analyze the role of LMO2 in hematopoietic cells, we first investigated the potential tissue-specific difference in promoter usage by Northern blot analysis. Probes that either recognized the LMO2 coding region, and therefore both transcripts, or the unique sequence contained in the transcript from the distal promoter were used. Figure 1B illustrates typical results with the coding region probe. We observe low levels of expression in numerous tissues, whereas significant expression is present in spleen, lung, and uterus. These results are consistent with previously published results.18,19 When a probe specific for the distal promoter transcript was used, no expression was detected in any of these tissues (data not shown). However, Northern blot analysis using this distal probe did demonstrate expression in bone marrow and fetal liver (Figure 1C). No hybridizing bands were detected in RNA derived from spleen, lymph nodes, thymus, or peripheral blood leukocytes. Figure 1D illustrates this same blot probed with the coding region portion of the LMO2 gene and demonstrates expression in nearly all hematopoietic tissues. Furthermore, an analysis of a number of tissue culture cell lines confirms the erythroid expression using the distal probe. Figure 1E, for example, demonstrates LMO2 expression from the distal promoter in the erythroid K562 cell line with no expression in the RAG renal cell carcinoma line. From this analysis we conclude that the proximal LMO2 promoter directs expression of the LMO2 protein in a large number of tissues, whereas the distal promoter is active only in hematopoietic cells. Because we are most interested in the determinants and transcription factor hierarchies involved in erythroid-specific gene expression, we next focused our analysis on the distal LMO2 promoter.
To clone the distal promoter, an STS marker mapping to LMO2 was used in a PCR-based assay to screen a BAC library. From the BAC clones positive in this screen, we subcloned and sequenced fragments spanning both the distal and proximal LMO2 promoters. To determine the critical regulatory elements leading to the hematopoietic-specific expression from the distal LMO2 promoter, we tested the largest promoter construct in transgenic mice to establish whether this starting construct was sufficient to recapitulate the endogenous expression pattern. A reporter construct was prepared containing the distal promoter with 3 kilobase (kb) of flanking sequence driving expression of the CAT gene. This construct was used to produce 3 lines of transgenic mice. In each case, expression of the transgene was only observed in the bone marrow and blood of adult animals. Table 1 contains expression data from line 4.2 for all the tissues analyzed; only the positive values from line 7.2 and 7.6 are shown for comparison purposes. In addition, we examined the developmental expression of the LMO2 reporter transgene by performing CAT assays on extracts of 14.5-days-after-coitus (dpc) fetal liver and 10.5-dpc yolk sac samples. The LMO2 distal promoter directed expression in these tissues, with the more robust reporter activity detected in the 14.5-dpc liver samples.
Normalized expression data from transgenic mice carrying the –3190LMOCAT construct
Tissue sample . | Relative CAT activity . |
|---|---|
| Line 7.2* | |
| Adult blood | 9 |
| Adult bone marrow | 100 |
| Embryonic yolk sac | 3 |
| Fetal liver | 34 |
| Line 7.6† | |
| Adult blood | 40 |
| Adult bone marrow | 43 |
| Embryonic yolk sac | 3 |
| Fetal liver | 29 |
| Line 4.2‡ | |
| Adult blood | 1 |
| Adult bone marrow | 93 |
| Adult brain | < 0.1 |
| Adult heart | < 0.1 |
| Adult liver | < 0.1 |
| Adult lung | < 0.1 |
| Adult kidney | < 0.1 |
| Adult thymus | < 0.1 |
| Embryonic yolk sac | Not done |
| Fetal liver | Not done |
Tissue sample . | Relative CAT activity . |
|---|---|
| Line 7.2* | |
| Adult blood | 9 |
| Adult bone marrow | 100 |
| Embryonic yolk sac | 3 |
| Fetal liver | 34 |
| Line 7.6† | |
| Adult blood | 40 |
| Adult bone marrow | 43 |
| Embryonic yolk sac | 3 |
| Fetal liver | 29 |
| Line 4.2‡ | |
| Adult blood | 1 |
| Adult bone marrow | 93 |
| Adult brain | < 0.1 |
| Adult heart | < 0.1 |
| Adult liver | < 0.1 |
| Adult lung | < 0.1 |
| Adult kidney | < 0.1 |
| Adult thymus | < 0.1 |
| Embryonic yolk sac | Not done |
| Fetal liver | Not done |
Copy number 20.
Copy number 40.
Copy number 20.
Expression from the LMO2 distal promoter is dependent on an element within the transcribed portion of the gene
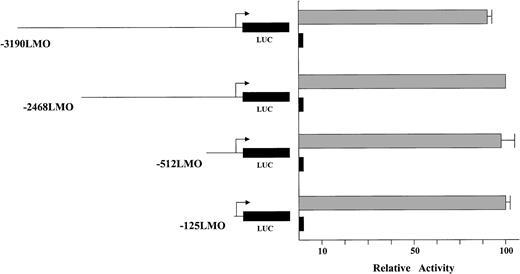
A series of deletion constructs was generated starting with the 3-kb promoter assayed in the transgenic mice and removing bases to within 125 bp of the transcription start site. These reporter constructs were assayed in transient transfection experiments in erythroid (K562) and control (COS) cell lines. First, the LMO2 distal promoter is virtually silent in the control cell line regardless of the construct, whereas expression in K562 cells is present (Figure 2). Second, the level of reporter gene activity is maintained in the erythroid cells throughout the deletion series. The data suggest that sequences regulating expression in K562 cells are not contained in the 5′ flanking region.
Deletion constructs of the LMO2 distal promoter assayed in K562 and COS cells. The gray boxes indicate the K562 data, the black boxes are the expressed level in COS cells. The values were normalized for transfection efficiencies using a cotransfected β-galactosidase construct. The longest construct recapitulates expression of the endogenous gene. Moreover, the level of the reporter gene activity is maintained in the erythroid cells throughout the deletion series.
Deletion constructs of the LMO2 distal promoter assayed in K562 and COS cells. The gray boxes indicate the K562 data, the black boxes are the expressed level in COS cells. The values were normalized for transfection efficiencies using a cotransfected β-galactosidase construct. The longest construct recapitulates expression of the endogenous gene. Moreover, the level of the reporter gene activity is maintained in the erythroid cells throughout the deletion series.
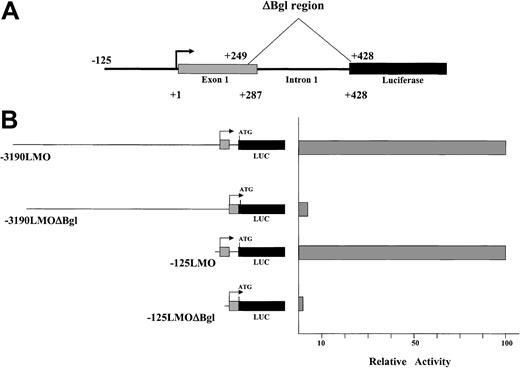
In each of the tested constructs, the 3′ end of the promoter region extends past the transcription start site, through the first untranslated exon for LMO2 and into the first intervening sequence (IVS). This region is magnified for the –125 construct in Figure 3A. Because exon 1 is noncoding it does not contain an ATG and thus does not interfere with translation of the luciferase reporter. Because a number of genes contain regulatory elements within an intron, this region was included to test for its ability to contribute to the LMO2 promoter activity. We produced a deletion to eliminate 180 bp encompassing the IVS1 region and a portion of exon 1 (shown in Figure 3 as ΔBgl). This deletion was produced in both the –125 and –3190 constructs and assayed in K562 cells. In each case, as illustrated in Figure 3, the result was the reduction of reporter gene expression to less than 5% of the level supported by the full-length construct. Thus, a critical element for activity of the LMO2 distal promoter resides within the transcribed portion of this gene.
A critical element resides in a fragment spanning a part of untranslated exon 1 and IVS1. (A) Deletion of the 180-bp ΔBgl fragment is diagrammed. (B) The elimination of this region significantly reduces expression in K562 cells.
A critical element resides in a fragment spanning a part of untranslated exon 1 and IVS1. (A) Deletion of the 180-bp ΔBgl fragment is diagrammed. (B) The elimination of this region significantly reduces expression in K562 cells.
An overlapping PAR/Ebox site is functionally important and specifically binds erythroid nuclear factors
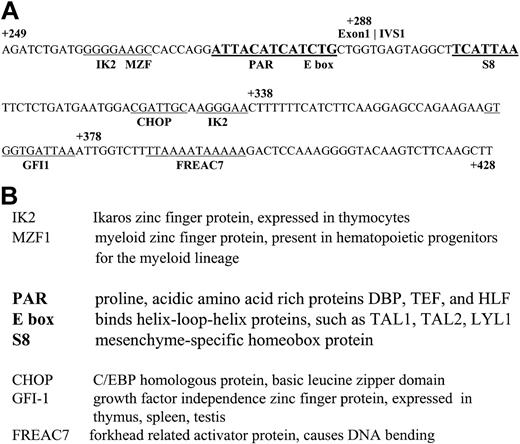
The nucleotide sequence of this 180-bp region was surveyed for the presence of known transcription factor–binding sites using the TransFac database and the MatInspector program (Genomatix, Munich, Germany).20 Although there are many sites present in this region and noted on the sequence shown in Figure 4, we refined our analysis through a comparison between the human and mouse genomic LMO2 sequences. Although regulatory features need not be identical between mouse and human genes, these sites were investigated as a reasonable starting point to screen for functionally relevant cis elements. The evolutionarily conserved sites thus identified include the bolded regions denoted as PAR, Ebox, and S8 in Figure 4A. Figure 4B briefly defines these binding proteins as well as the nonconserved sites in this region. The presence of a conserved Ebox site was of particular interest because this represents a potential binding site for TAL1, another important factor in erythroid development. The Ebox motif is a CANNTG palindrome that is recognized by factors containing the helix-loop-helix structural feature (HLH proteins) such as MYC and USF, as well as TAL1.
Nucleotide sequence of the ΔBgl region from the LMO2 distal promoter. (A) Transcription factor consensus sites are underlined. Sites in bold are highly conserved between the mouse and human sequences. (B) A description of the factors that bind to these consensus sites.
Nucleotide sequence of the ΔBgl region from the LMO2 distal promoter. (A) Transcription factor consensus sites are underlined. Sites in bold are highly conserved between the mouse and human sequences. (B) A description of the factors that bind to these consensus sites.
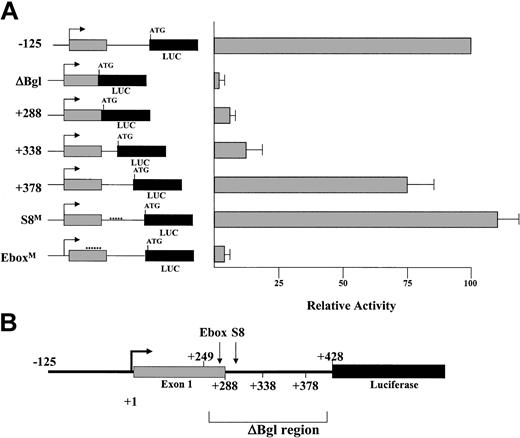
To fully investigate elements throughout the region, a series of deletion constructs was prepared, eliminating 40- to 50-bp sections starting from the 3′ end of the construct (Figure 5A). In addition, constructs were prepared in which site-directed mutagenesis was used to replace 5 of the 6 nucleotides in the Ebox motif (EboxM), and separately, to produce a similar mutation in the conserved S8 binding site (S8M). This latter sequence is a consensus site for homeobox proteins possessing a paired-type DNA-binding domain. All of these mutational and deletion constructs were derived from the –125LMO CAT reporter and are diagrammed in Figure 5A-B.
Mutations in the conserved Ebox motif disrupt LMO2 promoter activity in K562 cells. (A) Diagram of the position of a deletion series within the ΔBgl region of the LMO2 gene promoter. The position of the Ebox and S8 motifs subjected to site-specific mutation are also noted. (B) Illustration of the activity of the constructs derived from this region, assayed in K562 cells.
Mutations in the conserved Ebox motif disrupt LMO2 promoter activity in K562 cells. (A) Diagram of the position of a deletion series within the ΔBgl region of the LMO2 gene promoter. The position of the Ebox and S8 motifs subjected to site-specific mutation are also noted. (B) Illustration of the activity of the constructs derived from this region, assayed in K562 cells.
Each construct was assayed by transient transfection in K562 cells and compared to the level of reporter activity obtained with the intact –125LMO LUC construct. Analysis of the 3′ deletion constructs confirms the importance of the Ebox region, but also indicates that additional elements must contribute to restore the full activity of this promoter (Figure 5B). That is, in the erythroid K562 cells, restoration of the Ebox alone is ineffective (note +288 construct), and substantial activity does not return until the sequences from +249 to +378 are reinstated in the +378 construct. Within the context of the entire –125 promoter construct, point mutations in the Ebox-binding motif led to significant attenuation of reporter gene expression. In contrast, mutations of the S8-binding motif had no effect on reporter expression.
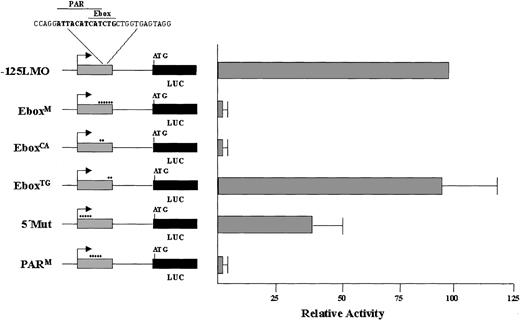
To determine the factors binding to the Ebox region, electrophoretic mobility shift assays (EMSAs) were performed with a labeled 30-mer centered on the Ebox, using nuclear extracts from K562 cells, and a series of mutant double-stranded oligomers as competitors. The nucleotide sequences of these oligomers and a representative binding assay are shown (Figure 6A-B). Four DNA-protein complexes are evident in the binding assay, consisting of a slow mobility doublet labeled A/B and 2 faster mobility bands labeled C and D. The complexes are specifically competed by the addition of unlabeled probe (Ebox), but not by the inclusion of oligomers containing either a 6-bp replacement of the Ebox (EboxM) or a smaller replacement of only the critical CA nucleotides in the Ebox consensus sequence (EboxCA). Of note, however, a similar mutation replacing the TG nucleotides still effectively competed in the formation of the complexes labeled A/B. Because mutations in either the CA or the TG sites would disrupt binding of an Ebox-type transcription factor, the EboxTG result indicated such a protein was most likely not involved at this site.
These data suggest the possibility that the critical factors could be binding to the PAR-binding motif that overlaps the Ebox site. We therefore focused on the 9 of 10-bp match to the consensus site for this subfamily of bZIP proteins containing a PAR domain. As shown in Figure 6, a 5-bp replacement mutation produced within the PAR consensus site (PARM), and upstream of the Ebox site, renders the oligomer ineffective as a competitor. Thus, mutations in this binding site disrupt DNA-protein complex formation. In addition, radiolabeling of the PARM mutant and use of this 30-mer as the binding template resulted in no complex formation in a parallel EMSA (data not shown). Additional mutations in regions flanking the PAR motif (5′Mut, 3′Mut) still successfully compete for binding to the upper A/B bands, although the 5′Mut does appear to contribute to C/D complex formation.
Because genes expressed in erythroid cells generally use GATA-1 in their regulatory repertoire, the involvement of GATA-1 as a participant in the formation of the DNA-protein complexes was investigated. Supershift analyses were performed with an antibody to GATA-1 using both K562 and MEL nuclear extracts (Figure 6C). In both assays formation of the complex labeled “C” is inhibited, indicating the presence of GATA-1. No change was observed in the complexes in the region of the A/B doublet.
These binding assays define the PAR site as a critical element for the binding of erythroid nuclear factors. However, it is known that the demonstration of specific binding in an EMSA does not always correlate with functional activity. Point mutations corresponding to the oligomers used in the EMSA analysis were introduced into the –125LMO luciferase construct, and the promoter activity of these mutants was assayed in K562 cells (Figure 7). Mutation of the PAR site (PARM) led to significant reduction in reporter gene expression and the decrease in expression of this mutant is comparable to mutation of the entire Ebox (EboxM). Furthermore, the transcriptional activity of these constructs exactly correlates with the ability to form the A/B doublet in the EMSAs. Those mutations that were ineffective in the EMSA competition (EboxM, EboxCA, and PARM) also ablated promoter function (compare Figures 6 and 7). Mutations that affect the presence of the lower 2 bands (C and D) have only a small effect on promoter activity. Thus, although GATA-1 may be involved in the regulation of the LMO2 gene, because its binding generates the `C' complex, it is producing only a modest effect in our assays.
Site-specific mutations indicate a PAR site overlapping the Ebox motif is critical for LMO2 promoter activity in K562 cells. The nucleotide substitutions used in the EMSA shown in Figure 6 were introduced into –125LMO-Luc constructs and analyzed in K562 cells.
Site-specific mutations indicate a PAR site overlapping the Ebox motif is critical for LMO2 promoter activity in K562 cells. The nucleotide substitutions used in the EMSA shown in Figure 6 were introduced into –125LMO-Luc constructs and analyzed in K562 cells.
The PAR-domain protein TEF specifically activates transcription via a binding motif in the LMO2 distal promoter
Because our binding and functional data indicated the PAR-binding domain was a critical element for the distal LMO2 promoter, the potential role for the known factors within this group was investigated. Because DBP, HLF, and TEF have not been studied in erythroid cells, an RT-PCR analysis was performed in K562 cells to determine whether they are endogenously expressed in these cells. Transcripts were detected for all 3 gene products (data not shown), suggesting they are all potential candidates in the regulation of the LMO2 distal promoter. Moreover, a similar analysis using RNA derived from human reticulocytes also revealed the expression of all 3 factors, DBP, HLF, and TEF, as well as the distal LMO2 transcript, in uncultured primary erythroid cells (Figure 9B).
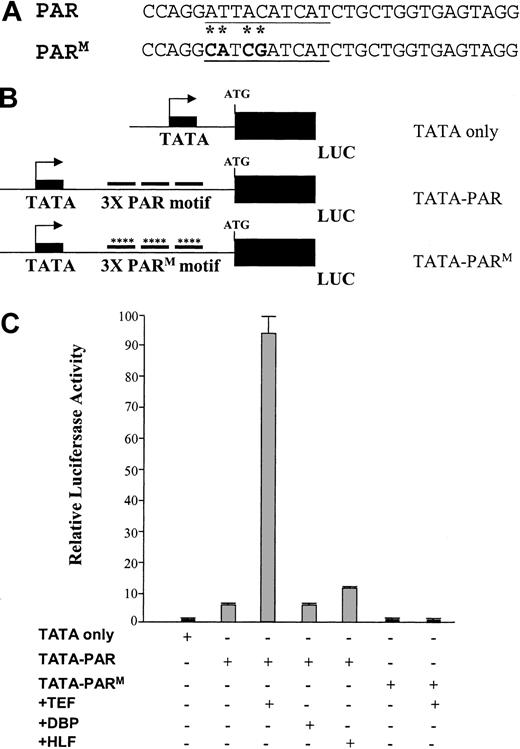
Cotransfection experiments were therefore carried out to investigate the ability of these proteins to activate transcription through the LMO2 PAR-binding domain. The constructs for this study consist of the E1b minimal promoter driving the luciferase reporter gene, and 3 copies of either the wild-type or mutant PAR 30-mer sequence positioned downstream of the promoter to mimic the spatial arrangement of the LMO2 distal promoter (Figure 8A-B). These constructs were cotransfected into HEK293 cells with expression constructs for each of the PAR proteins.17 Introduction of the plasmid encoding the TEF protein results in a 10-fold increase in reporter gene activity in the presence of the wild-type PAR domain (TATA-PAR; Figure 8C). In contrast, the other factors, HLF and DBP, have little or no effect in this assay even though all 3 proteins have been shown to bind the same consensus nucleotide sequence.21-23 Furthermore, mutations in the PAR sequence that eliminate DNA protein complex formation are similarly inactive in this assay. That is, the TATA-PARM construct is unresponsive to the addition of TEF (Figure 8C).
A PAR domain protein enhances promoter activity through the PAR motif from the LMO2 gene. (A) The 30-mer oligonucleotides representing the wild-type and mutated PAR domains. The consensus PAR-binding site is underlined; the mutated nucleotides are shown in bold with an asterisk. (B) Schematic diagram of the minimal promoter constructs containing 3 copies of the oligomers. (C) Analysis of the reporter gene activity in transient cotransfection experiments in HEK 293 cells.
A PAR domain protein enhances promoter activity through the PAR motif from the LMO2 gene. (A) The 30-mer oligonucleotides representing the wild-type and mutated PAR domains. The consensus PAR-binding site is underlined; the mutated nucleotides are shown in bold with an asterisk. (B) Schematic diagram of the minimal promoter constructs containing 3 copies of the oligomers. (C) Analysis of the reporter gene activity in transient cotransfection experiments in HEK 293 cells.
An increase in promoter activity was also noted between the TATA-PAR construct and the TATA-only plasmid. This is most easily explained as an effect by endogenous TEF. As with the K562 cells, RT-PCR assays demonstrate the presence of all 3 PAR transcripts in the 293 cells used in this experiment (data not shown). Conversely, analysis of the TATA-PARM construct shows no such induction compared to TATA alone nor is there an effect when TEF is cotransfected in the presence of this mutant-binding site.
This analysis with the PAR factors suggested that TEF was a more potent activator at the distal LMO2-binding site than either HLF or DBP. To further assess the role of these factors, we introduced N-terminal epitope tags into the PAR expression constructs and repeated the cotransfection experiments with increasing amounts of these vectors. Because commercial antibodies are not available for the PAR factors, these FLAG tags provided a means of verifying that each protein was expressed; Western blot analysis using the FLAG antibody demonstrated that all 3 PAR proteins were highly expressed as expected and at comparable levels to each other (data not shown). A dose-response curve for the activation of the TATA-PAR construct by these FLAG-PAR factors is shown in Figure 9A. The human erythroid K562 cell line was used for this assay. As with the previous analysis in the human 293 cells, the largest activation is observed with TEF; DBP has virtually no activity, and HLF produces an intermediate effect.
These results demonstrate that a PAR domain protein is capable of specifically activating transcription through a critical binding motif from the human LMO2 distal promoter. Although all 3 PAR family members are coexpressed with LMO2 in hematopoietic cells (Figure 9B), only TEF and, to a lesser extent, HLF have an effect on LMO2 promoter activity. This is a novel mechanism of regulation because the PAR proteins have previously not been implicated in hematopoietic-specific gene expression.
Discussion
In this report, we have focused on the hematopoietic-specific expression of the human LMO2 gene. We have determined that of the 2 known promoters for this gene, the activity of the distal promoter is restricted to yolk sac, fetal liver, and adult bone marrow. Furthermore, because our Northern blots have been performed with approximately equal probe specific activities, the level of the hybridization signals suggest that transcription from this distal promoter is significant in the tissues where it is active. Interestingly, this genomic arrangement with 2 promoters, one broadly used and a second promoter directing a more limited pattern of expression, occurs in a number of genes involved in hematopoiesis. For example, GATA-1, -2, -3, -4, and -6, all have 2 promoters, one of which directs tissue-restricted expression.24-28
Gene-targeting studies demonstrate that LMO2 plays a pivotal role in hematopoiesis. Our studies are directed at understanding at the molecular level, the hierarchy and interplay of transcription factors involved in this system. Our initial work with the LMO2 gene promoter focused on the conserved Ebox motif as a likely candidate element in the regulation of LMO2 because this sequence was a consensus-binding site for TAL1. TAL1, GATA-1, and LMO2 are all important transcription factors in erythropoiesis, and a model with TAL1 regulating LMO2 was an attractive hypothesis. As a result of the data presented here, however, the PAR family of genes, and TEF and HLF in particular, must now be considered in this analysis. TEF was originally described in the developing anterior pituitary gland29 and was demonstrated to bind to an element in the thyroid-stimulating hormone-β promoter and activate transcription of this gene.29 Subsequent studies have shown the TEF gene to be widely expressed in adult tissues including liver, brain, kidney, spleen, testes, and lung.30,31 HLF is predominantly expressed in liver and kidney with lower levels in the lung and brain.32 This transcription factor has similarities to LMO2 in that it is not normally expressed in lymphoid cells and is involved in a chromosomal translocation that is associated with acute lymphoblastic leukemia. In the case of HLF, however, the leukemia is of B-cell origin and the translocation results in the expression of a fusion protein with the transactivation domain of E2A fused to the DNA-binding domain of HLF.33 In our studies, TEF consistently demonstrates the highest transactivation of the reporter gene. However, HLF also produces an approximately 5-fold activation and thus could also be involved in the regulation of LMO2.
It is important to note that because the PAR proteins are bZIP factors, they function as dimers and can heterodimerize as well as form homodimers.29,34,35 Because in many cases, such as in K562 and 293 cells as shown here, all 3 family members are present, the possibility exists for a number of different functional complexes. Begbie et al36 have presented data demonstrating a synergistic effect on the transactivation of the factor VIII and factor IX genes by DBP and HLF. Although our data suggest both HLF and TEF may be functionally active, we have no evidence at this time for a similar synergistic or even additive effect by PAR proteins in the regulation of the LMO2 gene. Cotransfection experiments carried out with combinations of PAR factors have not resulted in an activation above that of TEF alone (K.P.A., unpublished results, 2002).
Finally, although the PAR-binding motif is essential for promoter activity, the data presented in Figure 5 demonstrate that this site alone is not sufficient. That is, the constructs +288 and +338 contain an intact PAR site, but express minimal transcriptional activity. The addition of 40 more base pairs in the +378 construct, however, restores this activity. Interestingly, there is a growth factor–independent 1 (GFI-1) consensus site in this region (Figure 4). GFI-1 is a zinc finger transcription factor that was originally identified in a T-cell lymphoma line. More recently, Saleque et al37 have demonstrated that GFI-1 is essential for development of the erythroid and megakaryocytic lineages of the hematopoietic system through their analysis of a Gfi-1 null mouse. No in vivo gene targets are currently known for GFI-1, but the involvement of both LMO2 and GFI-1 in the hematopoietic system suggests the LMO2 gene as a possible candidate.
Hematopoiesis involves the progressive commitment of pluripotent stem cells to specific cell lineages. A number of transcription factors participate in and establish the various lineage-specific transcription profiles as this differentiation process proceeds. Reports demonstrating LMO2 interactions with 9 different factors9,10,38-43 suggest LMO2 plays numerous and varied roles in this cascade of events. Our data implicating a PAR protein in the regulation of this important gene identifies another family of factors to be considered as we examine the interplay of nuclear factors during hematopoiesis.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-09-2702.
Supported in part by the National Leukemia Research Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Hitoshi Okamura for generously providing the expression vectors for DBP, TEF, and HLF. Our thanks to Suzan Hammond for technical expertise in the transfection studies and Matthew Anderson for his assistance with the CAT assays.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal