Abstract
Erythropoiesis is characterized by 2 waves of production during mouse embryogenesis: a primitive one originating from the yolk sac (YS) and a definitive one produced from both the YS and the embryo proper. How the latter wave is generated remains unclear. To investigate our hypothesis that endothelial cells (ECs) could generate erythroid cells, we designed a method to label ECs at 10 days after coitus. This labeling method associates 2 techniques: an intracardiac inoculation that allows molecules to be delivered into the bloodstream followed by a whole-embryo culture period. DiI-conjugated acetylated low-density lipoproteins (Ac-LDL-DiI) were used to specifically tag ECs from the inside. One hour after inoculation, DiI staining was found along the entire endothelial tree. Fluorescence-activated cell sorter (FACS) analysis revealed that DiI+ cells were CD31+, CD34+, and CD45–, an antigen makeup characteristic of the endothelial lineage. Twelve hours after inoculation, 43% of DiI+ circulating cells belonged to the erythroid lineage. These cells expressed Ter119 and displayed an adult globin chain arrangement; thus they belonged to the definitive lineage as confirmed in erythroid colony formation. The remaining cells likely represent committed white blood cells or multipotent progenitors, as revealed by a mixed-colony formation. Beyond the 29-somite stage, the proportion of DiI+ erythroid cells gradually decreased. These results demonstrate the generation of hematopoietic cells from an endothelial intermediate, using in vivo tracing. We provide evidence for a release of these cells into the circulation and hypothesize that these cells are able to colonize the fetal liver and generate definitive erythrocytes in vivo.
Introduction
During mouse embryogenesis, hematopoiesis begins in the extraembryonic yolk sac (YS) at 7.5 days after coitus (dpc), shifting to fetal liver at midgestation, then to spleen, and finally to bone marrow shortly before birth. Classical embryological experiments, clonal culture assays, and genetic studies in the mouse have demonstrated the existence of 2 distinct waves of hematopoietic emergence: a transient one, mainly restricted to erythropoiesis occurring in the YS blood islands prior to circulation between YS and embryo, and a definitive one originating from both the YS and the embryo proper. Consistent with pioneer studies on the avian model, the embryonic site has been identified in the aortic region—the so-called para-aortic splanchnopleura (p-Sp)/aortagonad-mesonephros (AGM) region—and was shown to be capable of producing hematopoietic cells (HCs) including hematopoietic stem cells (HSCs).1,2 In addition, in both avian and mouse species, the YS was shown to be able to produce definitive HSCs, including cells of erythroid lineage, at the same time as or even slighly before the AGM formation.3-8
Primitive and definitive erythropoiesis yields mature erythrocytes distinguishable by their morphology and the hemoglobin types they express. Mature primitive erythrocytes are nucleated cells containing embryonic as well as adult hemoglobins, whereas mature definitive erythrocytes are smaller enucleated red blood cells committed to adult hemoglobin synthesis.9-11
In the YS as well as in the aorta, HCs and ECs develop in close association. This proximity has prompted earlier embryologists to assume the existence of a putative ancestral mesodermal progenitor for ECs and HCs, the so-called hemangioblast.12 In addition to the shared expression of several markers, gene targeting experiments have yielded insights into the relationships between the 2 cell types. One major example is Flk-1, which was shown to be required for both HC and EC commitment.13,14 Moreover, embryonic stem (ES) cell technology made it possible to demonstrate the derivation of both ECs and HCs from a single cell.15 Such a notion of a common precursor has been challenged by the identification of the aorta as an intraembryonic site for HC production. Indeed, at the time of hematopoietic emergence, the vessel is already formed and aortic ECs express specific markers. Similar to the hemangioblast hypothesis, it was proposed that vessel ECs would be at the origin of the intraembryonic hematopoietic production by a mechanism involving a switch from ECs to HCs.16 This hypothesis is supported by several facts. Single TEK+ cells in the AGM region were able to generate both cell types.17 Cells positive for podocalyxin-like protein-1 (PCLP-1), isolated from the AGM region at 11.5 dpc, can generate both ECs and HCs when cocultured on OP9 stromal cells.18 VE-cadherin+/CD45-/Ter119- ECs isolated from mouse embryos at 9.5 dpc can generate blood cells including lymphocytes.19 In human embryos, CD34+/CD45- cells isolated from YS or AGM region can give rise to HCs.20 Although strongly suggesting that vascular ECs can give rise to HCs, all these studies have used in vitro systems, so that the in vivo situation remained unclear. Recently, Dzierzak and colleagues provided first indirect evidence for the generation of HCs by ECs. Ly-6Ais one of the earliest genes expressed in HSCs. Using a transgenic mouse harboring the green fluorescent protein (GFP) reporter gene under the control of the Ly-6Apromoter/enhancer, these authors showed that the first Ly-6A expression was restricted to cells inserted into the endothelium layer, thus strongly suggesting a derivation of HCs by ECs.21
The only direct evidence arguing for this affiliation came from the avian embryo. Using specific markers for one or the other cell type, Jaffredo et al showed that HC- and EC-specific markers were mutually exclusive at the time of cluster emergence.22 Using lineage tracing experiments, these authors were able to demonstrate that aortic HCs derive from ECs. The endothelial tree is tagged from the inside by intracardiac inoculation so that the compound is delivered in direct contact with the endothelium. Two different tracers were used: Ac-LDL, which is specifically and rapidly endocytosed by ECs, and nonreplicative retroviral vectors carrying a lacZ reporter gene.22,23
We have applied the Ac-LDL–mediated tracing method to tag the mouse vascular tree in vivo when definitive hematopoiesis occurred. We have designed a system comprising a step of Ac-LDL intracardiac delivery followed by a whole-embryo culture period.24 We have followed the EC derivatives with special attention to erythropoiesis. As early as one hour following inoculation, the whole vascular tree was labeled. The cells incorporating Ac-LDL-DiI were shown to be CD34+/CD31+/CD45-. Twelve hours after inoculation, Ac-LDL-DiI–labeled erythroid cells and progenitors were detected in the circulation. The erythroid cells displayed adult-type globins, whereas the progenitor cells were capable of generating mixed colonies when cultured in appropriated conditions. We demonstrate here, for the first time, the in vivo generation of HCs from ECs in mouse embryo. Moreover, the time course of our experiments shows that a large number of these EC-derived HCs can readily undergo erythropoiesis without giving rise to multipotent progenitors. We put forward the hypothesis that, once released into the circulation, these cells are able to colonize the fetal liver and give rise to definitive erythrocytes in vivo.
Materials and methods
Inoculation method
ICR mice were purchased from Nihon SLC (Hamamatsu, Japan). Noon was taken to be 0.5 dpc for plugged mice. Embryos at 10.0 dpc were isolated under a stereomicroscope in phosphate-buffered saline (PBS). Embryos were divided into 3 groups: 22 to 24, 29 to 31, and 34 to 36 somite stage. Embryos at the 22 to 24 somite stage were used in all experiments, and embryos at both the 29 to 31 and 34 to 36 somite stage were used for cytospin preparation of sorted DiI+ cells. Embryos were isolated from the uterus. The deciduo capsularis and the Reichert membrane were removed. The YS was cut along YS arteries. The amnion was opened for intracardiac inoculation. Ac-LDL-DiI solution (0.1 to 0.3 μL, Biomedical Technologies, Stoughton, MA) was inoculated into the circulation using a fine glass needle pulled out on a micropipette puller. Inoculated embryos were submitted to a whole-embryo culture system.
Whole mouse embryo culture
Embryos selected for the absence of damage on the YS and the embryo proper were cultured using the whole mouse embryo culture system from Ikemoto Scientific Technology (Tokyo, Japan), described previously.24 Inoculated embryos were transferred immediately into culture bottles containing 100% rat serum supplemented with glucose (2 mg/mL). The culture bottles were attached to a rotator drum and turned at 20 rpm at 37°C with a continuous supply of gas mixture (60% O2 and 5% CO2 balanced with N2) for 12 hours in the dark. After culture, inoculated embryos were collected, transferred into PBS, and observed under a stereomicroscope with a filter of 550 nm. Several morphological criteria were checked for correct development: number of somites, crown-rump length and head length, blood flow, and beating heart.25,26 Embryos exhibiting abnormal development or hemorrhage after cutting the YS or cardiac inoculation were not used.
The 10.0-dpc embryos isolated from the right uterine horn of a mother anesthetized with pentobarbitol (Dainihonseiyaku, Osaka, Japan) were inoculated and cultured for 12 hours as described in the previous paragraph. After embryo isolation, the mother's abdomen was closed so that it could recover. As controls, embryos carried by the left uterine horn were maintained for an additional 12 hours in the same mother until they reached 10.5 dpc. At that point they were collected and compared to the inoculated/cultured siblings. The number of somites and size as well as blood compositions were compared. Blood samples were cytocentrifuged on Cytospin 2 (Shandon, Pittsburgh, PA) and stained with May-Grünwald-Giemsa (Muto Chemica, Tokyo, Japan).
Histology
Embryos cultured for 1 hour were fixed in 4% paraformaldehyde in PBS (Sigma-Aldrich, St Louis, MO) for 8 to 12 hours at 4°C. Fixed embryos were hydrated with sucrose (Wako, Japan), embedded in OCT compound (catalog no. 4584, Tissue-Tek, Torrance, CA), frozen with dry ice, and sectioned at 7 μm. Sections were observed under a microscope equipped with epifluorescence (Eclipse E600, Nikon, Tokyo, Japan).
Flow cytometry and cell sorting
Inoculated embryos were rinsed 3 times before blood samples were collected by cutting blood vessels. Embryos were separated into YS, AGM region, and remnants. Individual tissues were incubated in a Dispase solution (catalog no. 295825, Roche Diagnostics, Basel, Switzerland) for 30 minutes, washed, and subsequently incubated with cell dissociation buffer containing EDTA/EGTA (ethylenediaminetetraacetic acid/ethylene glycol tetraacetic acid; catalog no. 13159, Invitrogen, Carlsbad, CA), as described previously.19 For surface antigen detection, cell suspensions were incubated on ice in the presence of fluorescein isothiocyanate (FITC)–conjugated anti–Mac-1 monoclonal antibodies (mAbs) and biotin-conjugated anti-Ter119, CD31, CD34, and CD45 mAbs purchased from Pharmingen (San Diego, CA). When biotin-conjugated mAbs were used, cells were washed and incubated with FITC-(Pharmingen) or R-phycoerythrin covalently linked to cyanin 5.1 (Immunotech, Marseille, France)–conjugated streptavidin. When nonhematopoietic samples were analyzed, both dead cells, revealed with TO-PRO-1 staining (Molecular Probes, Eugene, OR), and macrophages, revealed using an anti–Mac-1 antibody, were excluded. Flow cytometry was performed using a FACScan (Becton Dickinson, San Jose, CA). DiI+ cells were sorted from blood samples by FACSVantage (Becton Dickinson, Mountain View, CA), cytocentrifuged, and stained as described in the previous paragraph.
Methylcellulose clonal culture
Culture medium (1 mL) containing 1 × 104 sorted DiI+ cells, α minimal essential medium (Flow Laboratories, Rockville, MD), 1.2% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal bovine serum (Hyclone Laboratories, Logan, UT), 1% deionized fraction V bovine serum albumin (Sigma Chemical, St Louis, MO), 100 μmol/L 2-mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), and cytokines (100 ng/mL mouse stem cell factor [SCF], 10 ng/mL mouse interleukin 3 [IL-3] and 2 U/mL human erythropoietin [Epo, kindly provided by Kirin Brewery, Tokyo, Japan]) were plated in 35-mm suspension culture dishes (no. 1008, Falcon, Lincoln Park, NJ). The dishes were incubated at 37°C in a humidified atmosphere supplemented with 5% CO2. Erythroid colonies and bursts were counted at days 3 and 7, respectively. To detect multipotent progenitors, cytokines (100 ng/mL human IL-6 [Tosoh, Kanagawa, Japan], 20 ng/mL human thrombopoietin [TPO; Kirin] and 10 ng/mL human granulocyte colony-stimulating factor (G-CSF; Kirin]) were added to the conditions described in the previous paragraph. Mixed colonies were counted at day 7.
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
Total cellular RNA was isolated from individual erythroid bursts or 1 × 104 sorted DiI+ cells, using the RNeasy Mini Kit (Qiagen, Tokyo, Japan). First-strand cDNA was prepared according to the manufacturer's instructions (Perkin Elmer Cetus, Norwalk, CT). Briefly, oligo (dT)–primed cDNA was prepared using AMV reverse transcriptase (GIBCO-BRL, Gaithersburg, MD) in a reaction volume of 20 μL under conditions recommended by the manufacturer. PCR conditions were optimized for each primer set to maintain amplification in the linear range. Primer pairs were as follows: βH1, 5′ forward sequence (5′)5′-AGTCCCCATGGAGTCAAAGA-3′ and 3′ reverse sequence (3′) 5′-CTCAAGGAGACCTTTGCTCA-3′ β-major, (5′ forward) 5′-CTGACAGATGCTCTCTTTGGG-3′ and (3′ reverse) 5′-CA CAACCCCAGAAACAGACA-3′.27
Results
Development of cultured mouse embryos inoculated with Ac-LDL-DiI
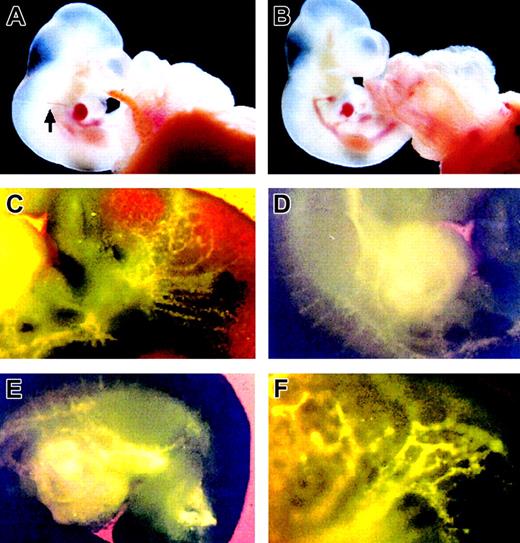
Embryos at 10 dpc were inoculated with Ac-LDL-DiI as shown in Figure 1A and submitted to whole-embryo culture. Approximately 50% of the inoculated embryos developed normally according to the criteria described in the previous paragraph and were selected for further experimental analysis. Figure 1B shows the inoculated embryo after 12 hours of whole-embryo culture. As shown in Figures 1C-E, the whole endothelial tree was labeled by DiI as soon as 1 hour after inoculation. To make sure that the culture system did not impair development of the embryos, the control experiment was examined by using littermates isolated from one mother having a bicornate uterus. A first batch of embryos was isolated from the right uterine horn and submitted to the experimental procedure. After the operation, the mother was resuscitated so that the second batch of embryos, remaining in the left uterine horn, could undergo further development. The inoculated/cultured and noninoculated/noncultured embryos from the same mother were compared following the criteria described in the previous paragraph. The number of somites in the inoculated/cultured embryos (n = 4) was 31.5 ± 0.6, and the number of somites in the noninoculated/noncultured embryos (n = 4) was 31.8 ± 1.5. Their size and blood composition were identical (data not shown). No differences were visible in the 2 samples, indicating that the experimental scheme had no influence on embryonic development.
Appearance of a mouse embryo inoculated with Ac-LDL-DiI at 10 dpc after 12 hours' whole-embryo culture. (A) An embryo before culture was inoculated with Ac-LDL-DiI into the heart. Arrow points out the injection needle. (B) An embryo after 12 hours' culture. (C-E) Vessel labeling after Ac-LDL-DiI staining 1 hour after inoculation observed under ultraviolet transillumination with the appropriate filter. (C) Region of the branchial arches. (D) Region of the heart. Note the delicate staining of the segmental arteries. (E) Truncal region. (F) magnified view of the upper part of the embryo.
Appearance of a mouse embryo inoculated with Ac-LDL-DiI at 10 dpc after 12 hours' whole-embryo culture. (A) An embryo before culture was inoculated with Ac-LDL-DiI into the heart. Arrow points out the injection needle. (B) An embryo after 12 hours' culture. (C-E) Vessel labeling after Ac-LDL-DiI staining 1 hour after inoculation observed under ultraviolet transillumination with the appropriate filter. (C) Region of the branchial arches. (D) Region of the heart. Note the delicate staining of the segmental arteries. (E) Truncal region. (F) magnified view of the upper part of the embryo.
Characterization of cell types incorporating Ac-LDL
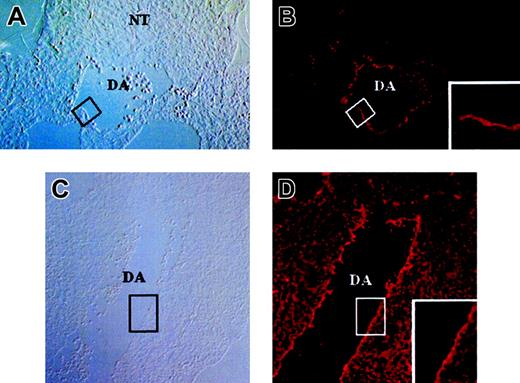
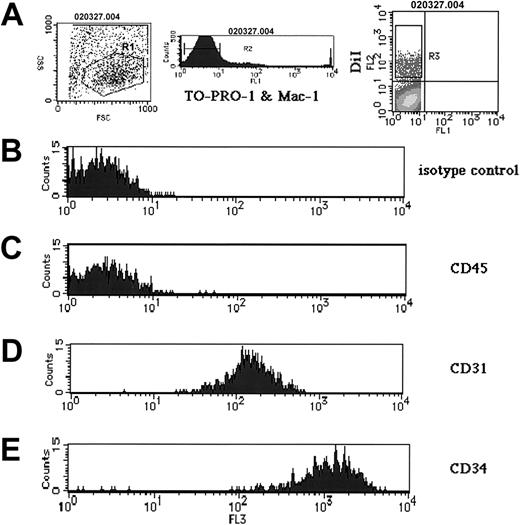
DiI staining displayed a dotted pattern, as shown in Figure 1F, in accord with the reported scavenger receptor–mediated endocytosis of Ac-LDL-DiI and the sequestration of Ac-LDL into vesicles in ECs.28,29 Inoculated embryos were sectioned and DiI staining was examined. DiI staining was restricted to the endothelial layer of arteries and veins (Figure 2). Since macrophages are also known to endocytose Ac-LDL, their presence among DiI+ cells was examined by flow cytometry, using the anti–Mac-1 monoclonal antibody. One hour after inoculation, YS, AGM region, and the rest of the body were dissociated and DiI+ cells were analyzed. As shown in Figure 3A (right panel), a large number of DiI+ cells were observed. Among those, only a few expressed Mac-1 (data not shown). After removal of both dead cells incorporating TO-PRO-1 and Mac-1+ cells, DiI+ cells were analyzed for expression of CD45 (the panleukocyte antigen that recognizes white blood cells and multipotent progenitor cells) and CD31 (PECAM) and CD34, which recognize both ECs and immature HCs. As shown in Figure 3B-E, most of the DiI+ cells were CD31+ and CD34+ but did not express CD45, an antigen makeup that is a signature of an endothelial lineage, as previously reported.20
Sections through Ac-LDL-DiI–inoculated embryos 1 hour after inoculation. Sections are 7 μm thick. (A-B) Cross-section at the level of the dorsal aorta. (A) Phase contrast. (B) Fluorescence revealing the DiI signal. Boxes in panels A and B indicate the region magnified in panel B. The signal is restricted to the internal-most cells, thus the endothelial layer. (C-D) Cross-section at the midtrunk level. (C) Phase contrast. (D) DiI. Boxes in panels C and D indicate the region magnified in panel D. DA indicates dorsal aorta; NT, neural tube. For all images, original magnification, × 40.
Sections through Ac-LDL-DiI–inoculated embryos 1 hour after inoculation. Sections are 7 μm thick. (A-B) Cross-section at the level of the dorsal aorta. (A) Phase contrast. (B) Fluorescence revealing the DiI signal. Boxes in panels A and B indicate the region magnified in panel B. The signal is restricted to the internal-most cells, thus the endothelial layer. (C-D) Cross-section at the midtrunk level. (C) Phase contrast. (D) DiI. Boxes in panels C and D indicate the region magnified in panel D. DA indicates dorsal aorta; NT, neural tube. For all images, original magnification, × 40.
Flow cytometry analysis on DiI+ isolated cells of YS after 1 hour of culture. The tissues of the inoculated embryos after 1 hour of culture were incubated in a dispased solution and examined by flow cytometry. (A) After the adequate cell population was selected by R1 (left panel), both dead cells incorporating TO-PRO-1 and the Mac-1+ cells were eliminated by R2 (middle panel), and the cells expressing DiI were observed (right panel). DiI+ cells selected by R3 were analyzed (B-E). (B) Isotype control. (C-E) Expression of CD45 (C), CD31 (PECAM) (D), and CD34 (E) by DiI+ cells.
Flow cytometry analysis on DiI+ isolated cells of YS after 1 hour of culture. The tissues of the inoculated embryos after 1 hour of culture were incubated in a dispased solution and examined by flow cytometry. (A) After the adequate cell population was selected by R1 (left panel), both dead cells incorporating TO-PRO-1 and the Mac-1+ cells were eliminated by R2 (middle panel), and the cells expressing DiI were observed (right panel). DiI+ cells selected by R3 were analyzed (B-E). (B) Isotype control. (C-E) Expression of CD45 (C), CD31 (PECAM) (D), and CD34 (E) by DiI+ cells.
Erythroid cells are generated from cells incorporating Ac-LDL-DiI
Since nonspecific uptake of Ac-LDL-DiI or DiI by blood cells could not be excluded, peripheral blood obtained from embryos at 10 dpc was cultured in the continuous presence of Ac-LDL-DiI (10 μg/mL) for 12 hours. No DiI+ cells, except macrophages expressing Mac-1, were detected at the end of the culture period, indicating that erythroid cells, white blood cells (except macrophages), and progenitor cells in circulation did not uptake Ac-LDL-DiI.
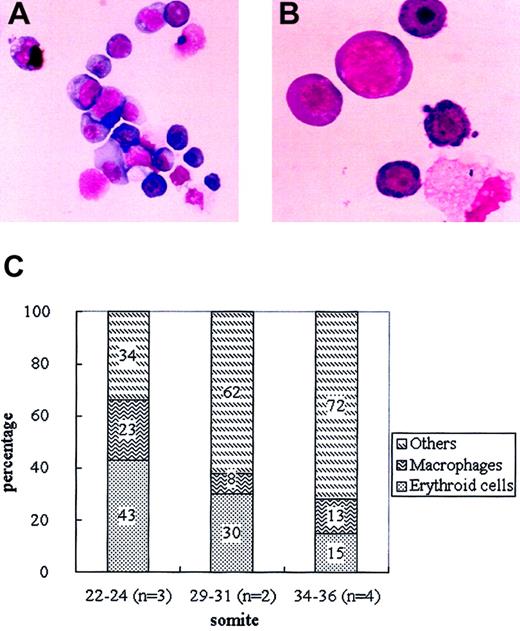
To investigate whether cells that had incorporated Ac-LDL-DiI were released into the circulation, peripheral blood samples obtained from inoculated embryos developed for 12 hours were analyzed. Circulating blood cells (1.4% ± 0.4%; n = 5) were positive for DiI by flow cytometry. DiI+ cells were first detected in the circulation after 6 hours of culture, and their number gradually increased to reach a peak at 12 hours. To find out which type of circulating cells displayed DiI, DiI+ cells were sorted from the blood samples and analyzed after May-Grünwald-Giemsa staining. Half of the DiI+ cells belonged to the definitive erythroid lineage at various stages of maturation as evidenced by the morphological aspect. In addition, macrophages and other myeloid cells were also detected (Figure 4A-B).8-11 Globin types were analyzed by RT-PCR on the DiI+ cell fraction using oligonucleotides specific for embryonic βH1 globin and adult-type β-major globin gene transcripts. As shown in Figure 5B, DiI+ cells expressed the β-major, but not the βH1 globin, gene, demonstrating that DiI+ erythroid cells belonged to the definitive lineage.
DiI+ cells in the circulation of inoculated embryos after 12 hours of culture. (A) Appearance of DiI+ cells sorted from blood (May-Grünwald-Giemsa staining). Original magnification, × 100. (B) Detail of sorted DiI+ cells. Original magnification, × 400. (C) Proportion of erythroid cells, macrophages, and other myeloid cells in the sorted DiI+ population at various somite stages. Numbers in each column indicate the means obtained from several independent experiments.
DiI+ cells in the circulation of inoculated embryos after 12 hours of culture. (A) Appearance of DiI+ cells sorted from blood (May-Grünwald-Giemsa staining). Original magnification, × 100. (B) Detail of sorted DiI+ cells. Original magnification, × 400. (C) Proportion of erythroid cells, macrophages, and other myeloid cells in the sorted DiI+ population at various somite stages. Numbers in each column indicate the means obtained from several independent experiments.
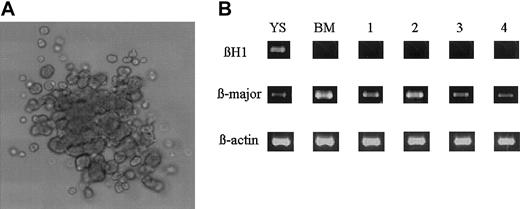
Erythroid colony formation from the DiI+ cells. (A) Appearance of an erythroid burst formed from the sorted DiI+ cells. Original magnification, × 100. (B) RT-PCR analysis for the expression of βH1 and β-major globin gene by the sorted DiI+ cells (lane 1) and the erythroid bursts formed from the DiI+ cells (lanes 2 to 4). YS indicates 8.5-dpc YS cells; BM, adult bone marrow cells.
Erythroid colony formation from the DiI+ cells. (A) Appearance of an erythroid burst formed from the sorted DiI+ cells. Original magnification, × 100. (B) RT-PCR analysis for the expression of βH1 and β-major globin gene by the sorted DiI+ cells (lane 1) and the erythroid bursts formed from the DiI+ cells (lanes 2 to 4). YS indicates 8.5-dpc YS cells; BM, adult bone marrow cells.
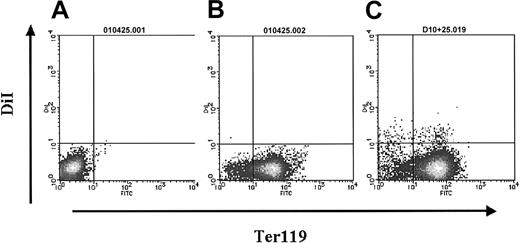
Figure 4C shows the proportion of erythroid cells, macrophages, and other myeloid cells in the circulating DiI+ fraction according to the embryonic stage. Prior to the 22 to 24 somite stage, 43% of the DiI+ cells were from the erythroid lineage, whereas from the 29 somite stage onward, the number of DiI+ erythroid cells decreased. At the 34 to 36 somite stage, the proportion was one third that found at the 22 to 24 somite stage. The expression of Ter119, a marker for erythroid cells, was analyzed by flow cytometry in the DiI+ cells on peripheral blood from the 22 to 24 somite stage embryos. Half (51% ± 7%; n = 4) of the DiI+ cells were positive for Ter119 (Figure 6C), consistent with the proportion deduced from morphological observation.
Ter119 expression on DiI+ cells analyzed by flow cytometry. (A) Isotype control. (B-C) Profiles of the samples from noninoculated (B) and inoculated embryos (C) with Ac LDL-DiI after 12 hours of culture.
Ter119 expression on DiI+ cells analyzed by flow cytometry. (A) Isotype control. (B-C) Profiles of the samples from noninoculated (B) and inoculated embryos (C) with Ac LDL-DiI after 12 hours of culture.
The number of circulating erythroid progenitors was next determined in methylcellulose clonal cultures of the DiI+ cells in erythroid conditions. When 1 × 104 DiI+ cells were cultured in the presence of SCF, IL-3, and Epo, 5.7 ± 4.0 erythroid colonies (n = 3) and 47.7 ± 8.5 erythroid bursts (n = 3) were formed (Figure 5A). In addition to erythroid progenitors, a few macrophage and megakaryocytic colonies were detected. Hemoglobin types were analyzed by RT-PCR on 10 individually picked erythroid bursts. All the colonies examined expressed the β-major but not the βH1 gene (lanes 2 to 4 in Figure 5B), confirming the definitive erythroid origin.
To investigate whether DiI+ cells also contained multipotent progenitors, the culture conditions were changed to a combination of growth facters and cytokines (SCF, IL-3, IL-6, G-CSF, EPO, and TPO), so that mixed colonies could be formed. When 1 × 104 DiI+ cells were cultured (n = 3), 18.7 ± 6.1 mixed colonies were formed.
Discussion
The experimental design did not impair hematopoietic cell development
Using a combination of in vivo intracardiac inoculation followed by a whole mouse embryo culture system, we provide direct evidence for the generation of HCs by vessel-associated ECs during embryogenesis. Intracardiac inoculations were performed at 10 dpc, a time when definitive hematopoiesis is detected both in the YS and the embryo proper.1-6,17-19,21 Despite efforts, attempts to perform inoculation earlier than 10 dpc failed, owing to hemorrhages. Inoculated embryos were cultured up to 10.5 dpc. All cultured embryos exhibiting abnormal development were discarded. Blood samples obtained after inoculation and culture displayed an identical hematopoietic composition to that of control embryos, indicating that the experimental design did not impair hematopoiesis.
Cells incorporating Ac-LDL-DiI displayed an endothelial phenotype
Ac-LDL is considered to be a specific marker for macrophages and ECs which is specifically receptor-mediated endocytosed.30-33 The first DiI+ illumination was observed as early as 30 minutes after inoculation. One hour later, the whole vascular tree in the YS and the embryo proper was labeled. On section, DiI staining was restricted to flat cells lining the blood vessels that were definitely identified as ECs. These DiI+ cells expressed CD31 and CD34 but not CD45. Cells displaying DiI uptake together with such an antigenic make-up are likely to belong to the endothelial lineage.17,19,20 These antigenic criteria, combined or not with the DiI uptake, have been considered by many authors as characteristic of the endothelial lineage. Using similar antigenic criteria, Oberlin and colleagues recently reported the in vitro derivation of HCs by YS and aortic ECs.20 This is also in agreement with experiments reporting that VE-cadherin+CD45- Ter119- ECs contributed to definitive hematopoiesis in vitro.19 Recently, it was shown that CD41 was expressed upstream of CD45 and could thus be used as an earlier marker for HCs commitment.34 Even though these data will have to be taken into account in the future, contamination by committed HCs is unlikely in our experiments, since (1) on section the DiI signal was restricted to flat cells, thus displaying an endothelial phenotype, and (2) 10 dpc blood samples (ie, at the time of intracardiac inoculation) incubated in Ac-LDL-DiI–containing medium were never stained by Ac-LDL-DiI at 10 μg/mL, indicating that committed HCs are unable to uptake Ac-LDL. It was reported that even 10 × concentrations of Ac-LDL (100 μg/mL) administered in vivo did not reveal additional Ac-LDL-DiI+ cell types, suggesting that uptake is not dependent on concentration.34
Definitive erythropoiesis emerged through an EC intermediate
The design of the experiments did not enable us to identify which compartment, among the YS and the embryo proper, generated the definitive, DiI+, erythroid cells. Definitive erythropoiesis was shown to occur at the same time in the YS and the embryo at the onset of circulation.5 It is well known that the first (primitive) wave of erythropoiesis occurs at the level of the YS blood islands, which consist of groups of undifferentiated mesodermal cells, the so-called hemangioblasts, that further differentiate into ECs for external-most cells and HCs for internal-most cells. Alternatively, intraembryonic hematopoietic cell production is thought to occur through a completely different process involving the production of HCs by a mature, already differentiated endothelium. In vivo production of HCs by ECs has been demonstrated at the level of the aorta in the avian model22,23 and is strongly assumed for the mouse AGM.35 Consistent with data from gene inactivation in mice, several genes have been identified that affect one lineage but not the other. Transcriptional factors c-Myb and AML-1 are essential for definitive hematopoiesis, but not for primitive hematopoiesis.36,37 Whether definitive erythropoiesis generated at the level of the yolk sac occurs from an EC-derived process remains to be determined. Our results are consistent with EC-derived production.
Generation of definitive erythroid cells from tagged endothelial cells
EC-derived erythropoiesis belonged to the definitive type. No βH1 globin mRNA was detected by RT-PCR in sorted DiI+ cells, suggesting that the sorted cells did not contain YS-derived primitive erythroid cells. Moreover, DiI+ cells were not stained by antibodies recognizing primitive erythrocytes (data not shown).38 The absence of DiI+ primitive erythrocytes is consistent with the demonstrated time course for erythroid cell release: a first one occurring early at E7.5 and involving blood island–derived HCs and a second one slightly later, involving both YS and embryonic compartments. It is likely that, at the time of staining, the first wave had undergone full differentiation and was thus incapable of Ac-LDL uptake. Although ECs were tagged at the same time, the DiI+ cells consisted of erythroid cells in various steps of maturation. Both erythroid colonies and erythroid bursts were detected in methylcellulose clonal cultures. According to the time they were released, erythroid cells may display different potential, or alternatively, progenitors may undergo maturation in the circulation. In addition to erythropoiesis, DiI+ multipotential hematopoietic progenitors were also present, as revealed by a mixed-colony formation, suggesting additional differentiation properties of ECs. Moreover, our preliminary data indicate that the DiI+ fraction also contained HSCs (D.S. et al, manuscript in preparation).
The ratio of the DiI+ erythroid cells in the circulation was strongly dependent on the developmental stage. Beyond the 29 somite stage, the number of DiI+ circulating erythroid cells gradually decreased. At the same time, definitive erythroid progenitors appear in the fetal liver,9,39-42 suggesting that these erythroid progenitors may colonize this organ. The derivation of erythroid cells from ECs is also strengthened by the presence of EPO/EPO-R at 10.0 dpc in a restricted number of vessels.43 According to the authors, ECs expressing EpoR could be responsive to Epo and generate erythropoiesis, both in YS and in the embryo proper.
This EC-derived erythropoiesis seems likely to occur only in the embryo. Although we injected AC-LDL-DiI into adult mice, we never detected DiI+ cells in hematopoietic cells isolated from bone marrow after 12 and 24 hours of inoculation (data not shown).
These EC-derived erythroid cells and progenitors can rapidly enter the circulation at preliver stage, colonize the fetal liver, and give rise to the mature definitive erythrocytes before HSCs enter the fetal liver. Whether or not the differential emergence of erythroid cells and HSCs relies on qualitative differences of the vessel endothelium is not known. Such a question will be of special interest in the near future.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-09-2799.
Supported by the Program for Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan, the Mitsubishi Pharma Research Foundation, and the Association pour la Recherche contre le Cancer (grants 5131 and French MRT ACI 22-2002-296).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr T. Atsumi for antimurine embryonic hemoglobin sera; Drs T. Nakahata and S.-I. Nishikawa for helpful discussion; Drs K. Downs, N. Osumi, T. Inoue, H. Yagasaki, S. Nishikawa, Y. Soda, and H. Yoshida for technical advice; Dr S. Iwai, Mr A. Takeshita, Miss M. Satoh, and Miss M. Itoh for technical support; and Drs F. Stuart and F. Dieterlen-Lièvre for critical reading of this paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal