Abstract
Mast cells play a critical role in host defense against a number of pathogens. Increased mast cell infiltration has been described in allergic asthma, in rheumatoid arthritis, and during helminthes infection. Despite the importance of mast cells in allergic disease and defense against infection, little is known about the mechanisms by which mast cells migrate to various tissues under steady state conditions or during infection or inflammation. Here, we show that activation of c-Kit by its ligand, stem cell factor (SCF), cooperates with α4 integrin in inducing directed migration of mast cells on fibronectin. A reduction in migration and activation of a small G protein, Rac, was observed in mast cells derived from class IA phosphoinositide-3 kinase (PI-3kinase)–deficient mice in response to SCF stimulation and in mast cells expressing the dominant-negative Rac (RacN17), as well as in mast cells deficient in the hematopoietic-specific small G protein, Rac2. In addition, a PI-3kinase inhibitor inhibited α4- as well as SCF-induced migration in a dose-dependent fashion. In contrast, a mitogen-activated protein kinase (MAPK) inhibitor had little effect. Consistent with the pharmacologic results, abrogating the binding of the p85α subunit of class IA PI-3kinase to c-Kit also resulted in inhibition of SCF-induced migration on fibronectin. These genetic and biochemical data demonstrate that both c-Kit and α4 integrin signaling are linked to class IA PI-3kinase and Rac pathways and regulate integrin-directed (haptotactic) migration in mast cells.
Introduction
Migration of hematopoietic stem and progenitors cells during embryogenesis, retention of these cells in the adult bone marrow microenvironment, and the distribution of committed progenitors to various adult tissues under steady state conditions or during inflammation are regulated in part by adhesion receptors, including integrins.1-3 However, our current understanding of integrin-directed (haptotactic) migration/homing of hematopoietic cells and the potential impact of concurrent stimulation by growth factors on haptotactic migration is limited. Further, the biochemical pathways that regulate haptotaxis in primitive or mature hematopoietic cells are largely unknown.
Integrins are a family of heterodimeric transmembrane glyco-proteins that can function as cell–extracellular matrix (ECM) or cell-cell adhesion receptors.3 The α4 integrins are particularly important owing to their involvement in various developmental and physiologic processes.4,5 The α4 chain can associate with either of the 2 β chains, β1 and β7. The α4β1 mediates adhesion to vascular cell adhesion molecule–1 (VCAM-1) and to the alternatively spliced CS-1 region of fibronectin. In contrast, α4β7 predominantly mediates adhesion to mucosal addressin cell adhesion molecule–1 (MAdCAM-1).6,7
Mast cells are bone marrow (BM)–derived cells that play an essential role in normal host defense and various allergic diseases.8-10 Mast cells are recruited into tissues by the release of their precursors from the bone marrow into the peripheral blood, followed by the migration of these precursors into various tissues, including mucosal and connective tissues.8-12 Mast cells express β1 and β7 integrins, and ligation of these integrin receptors modulates mast cell functions.13 In vivo, functional blockade of α4 integrin inhibits mast cell activation in a rat model of airway hyperresponsiveness, owing in part to impaired migration of these cells to the sites of airway inflammation.14 Further, mast cells isolated following an intestinal nematode infection express high levels of α4β7 integrin, while mast cells isolated from peritoneal cavity express mainly α4β1.15 Thus, integrins may be differentially expressed by mast cells in response to environmental cues. Alternatively, selective expression of integrins might occur during the development of different mast cell subtypes, which could dictate the migration of committed mast cell precursors to appropriate tissues.16
Recently, Gurish et al,13 using β7 integrin–deficient mice, demonstrated a critical role for α4β7 in the migration of mast cells to the small intestine, but not to other tissues. Along with a specific role for α4β7, a role for the c-Kit receptor in migration of mast cell progenitors to the small intestine was suggested.13 Interestingly, similar to the mast cell deficiency seen in β7 integrin–deficient mice, mutations in the c-Kit receptor or its ligand, stem cell factor (SCF), are also associated with reduced intestinal mast cell progenitors, despite normal numbers of mast cell progenitors in the BM.13 Thus, cooperation between c-Kit and α4 integrin may play an essential role in the migration of mast cells to the small intestine.
Evidence for functional cooperation between c-Kit and integrins has been demonstrated previously.17,18 Exposure of mast cells to SCF increases adherence of these cells to fibronectin (FN) by “inside-out signaling.”19-23 Mast cells from c-Kit–mutant mice exhibit diminished basal adhesion to stromal cells, and SCF-induced adhesion to FN requires c-Kit receptor tyrosine kinase activity. More recently, with the use of a coculture system involving mast cells and human umbilical vein endothelial cells (HUVECs), mast cell adhesion and proliferation were shown to be dependent on c-Kit and α4β1 interaction with SCF and VCAM-1, respectively, as neutralizing antibodies directed against c-Kit and α4β1 inhibited both adhesion and proliferation of mast cell progenitors.24 Further, our laboratory has recently shown that α4β1 and α5β1 can differentially regulate survival and proliferation of erythroid progenitors in response to c-Kit activation.25 However, in spite of these previous studies, the role of c-Kit– and integrin-derived signals in regulating haptotactic migration of BM-derived mast cell progenitors in vitro or in vivo has not been directly examined.
In fibroblasts, small G proteins of the Rho family are regulators of actin cytoskeleton.26 Microinjection of RhoA, Rac1, or Cdc42 in fibroblasts triggers the formation of stress fibers, lamellipodia, or filopodia, respectively,27,28 and these cellular processes contain a high concentration of integrin complexes. Studies have shown that dominant-negative Rac blocks lamellipodia formation induced by hepatocyte growth factor in Madin Darby canine kidney (MDCK) cells,29 and constitutively active Rac and Cdc42 stimulate the migration of mammary carcinoma cells.30 Further, activation of class IA phosphoinositide-3 kinase (PI-3kinase) by integrins stimulates Rac-dependent migration of colon carcinoma cells.31 Studies using dominant-negative or constitutively active mutants of Rho-like small G proteins have suggested a role of integrins in the regulation of Rho, Rac, and Cdc42.32-36 However, the role of these signaling molecules in directed migration of primary mast cells is not known.
In mast cells, various receptor signaling pathways, including those mediated by c-Kit, can activate class IA PI-3kinase.37 The role of class IA PI-3kinase on Rac activation and consequently on integrin-directed migration is poorly understood. Given the importance of c-Kit– and α4 integrin–generated signals in hematopoietic cell development and function, the essential requirement for α4 and c-Kit in the migration of mast cells in the small intestine, and the known interactions between receptor tyrosine kinases (RTKs) and integrins in directed migration of nonhematopoietic cells, we hypothesized that coordinate activation of α4 integrin and c-Kit controls the directed migration of mast cells. In the present study, we define a novel role for c-Kit receptor tyrosine kinase in regulating integrin-directed migration on fibronectin via the class IA PI-3kinase and Rac pathway in primary mast cells.
Materials and methods
Mice
The p85α+/- (129/SV × C57BL/6) and Rac2+/- (C57BL/6 N12) mice have been previously described.38,39 Rac2-/- mice were derived by mating Rac2+/- mice. The p85α-/- embryos were derived by mating of p85α+/- mice. Fetal livers from 15.5-day-old wild-type and p85α-/- embryos were dispersed mechanically. The genotypes of Rac2-/- mice and p85α-/- embryos were determined by polymerase chain reaction analysis as previously described.38,39 These studies were conducted with a protocol approved by the Indiana University Laboratory Animal Research Center (Indianapolis).
Generation of wild-type, p85α–/–, and Rac2–/– mast cells
Bone marrow (BM)–derived wild-type and Rac2-/- mast cells were generated by culturing BM cells in Iscove Modified Dulbecco Medium (IMDM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and recombinant murine interleukin-3 (IL-3) (Pepro Tech, Rocky Hill, NJ) for 4 to 5 weeks. Fetal liver–derived wild-type and p85α-/- mast cells were generated by culturing 15.5-day-old fetal liver cells in the presence of IL-3.
Antibodies and flow cytometric analysis
Phycoerythrin (PE)–conjugated monoclonal antibodies (MoAbs) were directed against c-Kit and α5β1. Fluorescence isothyocyanate (FITC)–conjugated antibodies were directed against α4β1. All the PE- and FITC-conjugated MoAbs, including the isotype control antibodies, were purchased from Pharmingen (San Diego, CA). Mast cells (1 × 106) were incubated at 4°C for 30 minutes with 1 μg the primary MoAb. Cells were washed 3 times with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma, St Louis, MO), and analyzed by fluorescence-activated cell sorter (FACS) (Becton Dickinson, San Jose, CA).
Cloning and expression of dominant-negative Rac (RacN17) in mast cells
Hemagglutinin (HA)-tagged RacN17 cDNA in pcDNA3 (a kind gift of Dr X. D. Ren, Stony Brook, NY) was digested with HindIII and NotI, ligated into StuI and NotI sites of a bicistronic retroviral vector MIEG340 by blunting the HindIII site in the RacN17 cDNA. The cloned product was verified by sequencing. To produce MIEG3-RacN17 viral supernatants for infection of mast cells, phoenix ecotropic cells (obtained from American Type Culture Collection, Manassas, VA) were transiently transfected with MIEG3-RacN17 retroviral construct by means of the Lipofectamine Plus reagent (Invitrogen). Supernatants were collected 48 hours after transfection, filtered through 0.45 μM membranes, and used. Cells were infected with 2 mL virus supernatant in the presence of 8 μg/mL polybrene. Virus-infected cells were harvested 48 hours later, sorted by fluorescence-activated cell sorter (FACS), and expanded in culture.
Construction of chimeric wild-type and 719-mutant c-Kit receptor
A chimeric receptor (CHR) encoding amino acids (aa's) 1 through 513 of the human macrophage–colony stimulating factor (M-CSF) receptor (containing the extracellular domain) and aa's 528 through 977 of the murine c-Kit receptor (containing the membrane-spanning and cytoplasmic tail) joined at an EcoRI site was constructed. A plasmid containing the human full-length M-CSF receptor cDNA (a kind gift of Dr Sherr, St Judes, Memphis, TN) was used. Forward (NotI-containing) and reverse (EcoRI-containing) primers corresponding to the start site, and transmembrane region of the M-CSF receptor were used to clone, by polymerase chain reaction (PCR), the extracellular domain of the M-CSF receptor. Forward (EcoRI-containing) and reverse (XhoI-containing) primers corresponding to the transmembrane (TM) and the stop site were used for a PCR of the TM and the cytoplasmic domain (CD) of the murine c-Kit receptor. The PCR product was digested and ligated into the NotI and XhoI sites of MIEG3 bicistronic retroviral expression vector.40 The sequence of the CHR was verified. To generate a mutant c-Kit CHR defective in the binding and activation of p85α subunit of class IA PI-3kinase, the NotI-XhoI wild-type CHR DNA fragment (2.9 kilobase [kb]) spanning the sites to be mutated was subcloned into Bluescript. Quikchange site-directed mutagenesis kit (Stratagene, Menasha, WI) and primers containing the appropriate mutations were used to mutate tyrosine 719 (the p85α-binding site) to phenylalanine (Phe). The NotI-XhoI fragment containing mutation at 719 in murine c-Kit receptor was verified by sequencing released from Bluescript and religated into the NotI-XhoI site of MIEG3 retroviral vector.
Integrin-directed (haptotaxis) migration assays
Integrin-directed migration (haptotaxis) assays were performed as previously described.41 Briefly, transwell plates (Costar, Corning, NY) were coated on the underside with 20 μg/mL FN peptides (Takara, Shiga, Japan) for 2 hours at 37°C, rinsed once with 2% PBS, and then placed into the lower chamber containing 500 μL complete medium with or without SCF. Mast cells were resuspended at 2 × 105 cells in 100 μL IMDM medium, added to the top of each chamber, and allowed to migrate toward the underside of the top chamber. Nonmigratory cells on the upper membrane surface were removed with a cotton swab, and migrated cells attached to the bottom surface of the membrane were stained with 0.1% crystal violet in 0.1 M borate, pH 9.0, and 2% ethanol for 15 minutes at room temperature as previously described.41 The number of migrated cells per membrane were counted with an inverted microscope with a 20 × objective lens. As a control, cell migration on BSA was also determined and was less than 0.001% of the total cell population.
Immunoprecipitation
Immunoprecipitations (IPs) were performed as previously described.42 Briefly, factor-starved mast cells expressing either the wild-type or the 719-mutant CHR were stimulated with M-CSF for 10 minutes. Thereafter, cells were lysed in lysis buffer (10 mM K2HPO4, 1 mM EDTA [ethylenediaminetetraacetic acid], 50 mM EGTA [ethylene glycol tetraacetic acid], 10 mM MgCl2, 1 mM Na2VO4, 50 mM β-glycerol phosphate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 μg/mL pepstatin A [pH 7.2]). Lysates were clarified by centrifugation at 10 000g, 4°C, for 30 minutes. IPs were performed by incubating equivalent amounts of cell lysates with an anti–M-CSF receptor antibody (Peprotech) overnight at 4°C. Protein A– or protein G–sepharose beads (Amersham Biosciences, Piscataway, NJ) were used to collect the antigen-antibody complexes. IPs were separated by sodium dodecyl sulfate–polyacylamide gel electrophoresis (SDS-PAGE), and proteins were electrophoretically transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). After blocking residual binding sites on the transfer membrane by incubating the membrane with 5% milk overnight, Western blot (WB) analysis using an anti-p85α antibody was performed (Santa Cruz Biotechnology, CA). Supersignal west dura extended-duration detection system (Pierce, Rockford, IL) was used according to the manufacturer's instructions.
Rac activation
The expression of HA-RacN17 was determined by WB analysis. Equal amounts of protein from mast cells expressing either the empty vector (MIEG3 only) or MIEG3-RacN17 were fractionated on 10% SDS-PAGE gel and transferred to nitrocellulose membrane. Expression of HA-RacN17 was determined by using an anti-HA antibody (Santa Cruz Biotechnology). Rac activation was performed by depriving mast cells of serum and growth factor for 20 to 24 hours, followed by stimulation for various lengths of time with 100 ng/mL SCF (Amgen, Thousand Oaks, CA). Rac activation was determined by means of a Rac activation assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's protocol and as described previously.43
Enumeration of mast cells in vivo
Six-week-old female mice were used for determination of mast cell numbers in the dermis (ear) and the peritoneal lavage of wild-type and Rac2-/- mice. Tissues were fixed and stained with toluidine blue. Peritoneal mast cells were obtained from wild-type and Rac2-/- mice by injecting 10 mL PBS into the peritoneal cavity of mice. The peritoneal cavity was washed for 1 minute with constant agitation, and 5 mL peritoneal lavage cells were harvested, centrifuged, and resuspended in 1 mL PBS. Total cells were counted by means of a hemocytometer, and differential cell counts were performed by cytospin cell preparations stained with Diff-Quik Stain Set (Dade Behring, Düdingen, Switzerland). Peritoneal mast cells were identified morphologically according to the criteria described previously.44
Results
Activation of c-Kit induces α4 integrin–directed (haptotactic) migration in mast cells
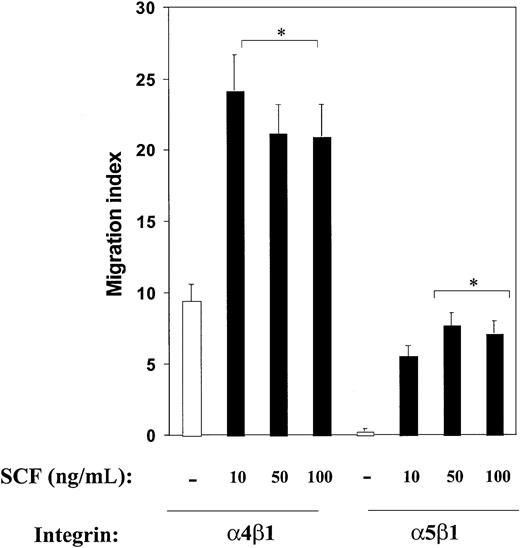
Previous work, including the analysis of mice deficient in the expression of c-Kit and α4 integrin, has suggested an essential role for these 2 molecules in normal hematopoietic development and function. However, the role of these 2 molecules in directed migration of hematopoietic cells, including mast cells, is poorly understood, and it is not known whether c-Kit and α4 integrin can collaborate in regulating directed migration of hematopoietic cells on fibronectin. To test whether c-Kit and α4 integrins cooperate in regulating directed cell migration, we used FN peptides that encode the binding site for integrin α4β1 (H296), α5β1 (CH271), or both α4β1 and α5β1 (CH296). In previous studies, we have shown specificity of these peptides with blocking antibodies to different integrins.45 As shown in Figure 1, bone marrow–derived mast cell progenitors demonstrated significantly higher directed migration on fibronectin via integrin α4β1 (H296) compared with α5β1 (CH271) in the absence of SCF. Importantly, α4β1-induced migration was profoundly enhanced in the presence of SCF (Figure 1). Addition of SCF to mast cells did not alter the cell surface expression of α4β1 on mast cells (data not shown). Interestingly, while migration on FN via α5β1 was minimal in the absence of SCF, there was enhanced migration in the presence of SCF in these cells (Figure 1). A similar increase in the migration of mast cells was observed in transwells coated with a fibronectin peptide that contains the binding sites for α4β1 as well as α5β1 (CH296) or the whole fibronectin molecule (data not shown). These results demonstrate that in the presence of SCF, α4- as well as α5-directed migration in mast cells is significantly enhanced, although directed migration via α4β1 appears to be higher under these culture conditions.
Effect of c-Kit activation on integrin-directed migration in mast cells. Activation of c-Kit modulates integrin-directed (haptotactic) migration in mast cells. Migration assays were performed with the use of mast cells and FN (H296, which contains binding site for α4β1; or CH271, which contains binding site for α5β1) in the presence or absence of increasing SCF concentrations as described in “Materials and methods.” Cell migration is expressed as a migration index and calculated as the fold increase over the negative control (BSA-coated wells). Cell migration on BSA was less than 0.001% of the total cell population. Shown are the means ± standard errors of the means (SEMs) of 40 different fields from 2 independent experiments performed in duplicate. *P < .05 for α4β1 or α5β1 in the presence of SCF (all concentrations) versus α4β1 or α5β1 in the absence of SCF (all concentrations).
Effect of c-Kit activation on integrin-directed migration in mast cells. Activation of c-Kit modulates integrin-directed (haptotactic) migration in mast cells. Migration assays were performed with the use of mast cells and FN (H296, which contains binding site for α4β1; or CH271, which contains binding site for α5β1) in the presence or absence of increasing SCF concentrations as described in “Materials and methods.” Cell migration is expressed as a migration index and calculated as the fold increase over the negative control (BSA-coated wells). Cell migration on BSA was less than 0.001% of the total cell population. Shown are the means ± standard errors of the means (SEMs) of 40 different fields from 2 independent experiments performed in duplicate. *P < .05 for α4β1 or α5β1 in the presence of SCF (all concentrations) versus α4β1 or α5β1 in the absence of SCF (all concentrations).
Antibodies to α4β1 and c-Kit inhibit directed (haptotactic) migration in mast cells
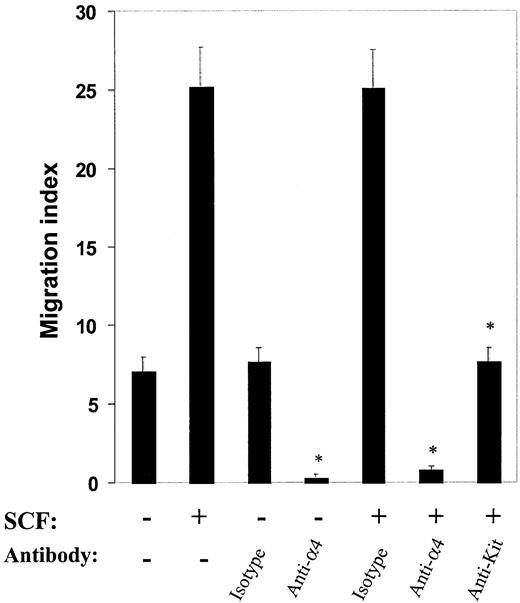
To further examine the effect of α4 and c-Kit in directed migration of mast cells, we repeated migration assays after pretreating cells with blocking antibodies against α4 and c-Kit. As shown in Figure 2, compared with isotype control antibody, anti-α4 antibody treatment significantly inhibited haptotactic migration, both in the absence as well as in the presence of SCF. Further, SCF-induced migration via integrin α4 was also inhibited by pretreatment with an anti–c-Kit antibody. Collectively, these results suggest that both α4 integrin and c-Kit play roles in regulating directed migration of mast cells on fibronectin.
Effect of antibodies to α4β1 and c-Kit on directed migration in mast cells. Antibodies to α4β1 and c-Kit inhibit directed (haptotactic) migration in mast cells. Migration assays were performed with the use of mast cells in the presence or absence of SCF after preincubating the cells with 20 μg/mL anti-α4, anti–c-Kit, or appropriate isotype control antibodies. Cell migration is expressed as in Figure 1. Shown are the means ± SEMs of 40 different fields from 2 independent experiments performed in duplicate.*P < .05 for isotype control versus anti-α4 or anti–c-Kit antibody.
Effect of antibodies to α4β1 and c-Kit on directed migration in mast cells. Antibodies to α4β1 and c-Kit inhibit directed (haptotactic) migration in mast cells. Migration assays were performed with the use of mast cells in the presence or absence of SCF after preincubating the cells with 20 μg/mL anti-α4, anti–c-Kit, or appropriate isotype control antibodies. Cell migration is expressed as in Figure 1. Shown are the means ± SEMs of 40 different fields from 2 independent experiments performed in duplicate.*P < .05 for isotype control versus anti-α4 or anti–c-Kit antibody.
α4β1- and c-Kit–directed migration is dependent on the PI-3kinase pathway
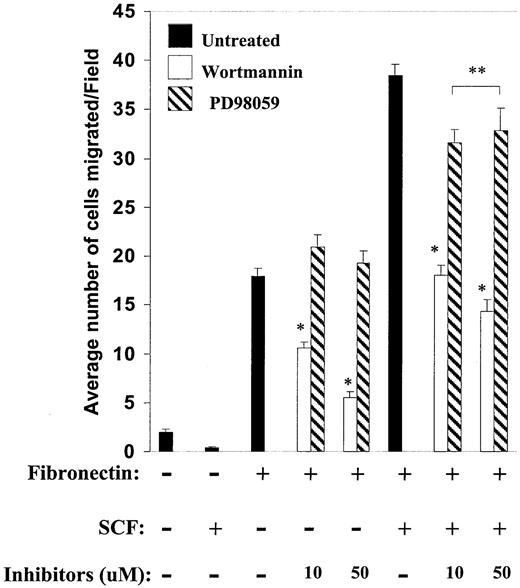
Previous studies have implicated the activation of PI-3kinase and the extracellular signal-regulated kinase–mitogen-activated protein kinase (ERK-MAP kinase) pathway in directed migration of nonhematopoietic cells.41,46 To determine which of these pathways plays a predominant role in directed migration of mast cells, we used pharmacologic inhibitors of PI-3kinase and MAP-kinase signaling pathways. Mast cells were preincubated with different concentrations of the PI-3K inhibitor wortmannin or the MAPK inhibitor PD98059 for 1 hour at 37°C, before migration assays were performed. As shown in Figure 3, pretreatment of mast cells with wortmannin resulted in a dose-dependent decrease in migration in the absence or in the presence of SCF. In contrast, treatment of these same cells with PD98059 had no effect in the absence of SCF and a minimal, although statistically significant, effect on decrease in migration (Figure 3). These results suggest that the PI-3kinase activation plays a dominant role in regulating c-Kit– and α4 integrin–directed migration of mast cells.
Integrin-directed migration and the PI-3kinase pathway. integrin-directed migration is dependent on the PI-3kinase pathway. Migration assays were performed with mast cells in the presence or absence of SCF and indicated concentrations of wortmannin and PD98059 inhibitors. Cells were first preincubated with indicated concentrations of inhibitors for 1 hour at 37°C. Thereafter, cells were washed and migration was performed as described in “Materials and methods.” Data are the means ± SEMs of 40 different fields from 2 independent experiments performed in duplicate and are expressed as the average number of migrated cells per field. *P < .05 for untreated versus wortmannin.**P < .05 for untreated versus PD98059.
Integrin-directed migration and the PI-3kinase pathway. integrin-directed migration is dependent on the PI-3kinase pathway. Migration assays were performed with mast cells in the presence or absence of SCF and indicated concentrations of wortmannin and PD98059 inhibitors. Cells were first preincubated with indicated concentrations of inhibitors for 1 hour at 37°C. Thereafter, cells were washed and migration was performed as described in “Materials and methods.” Data are the means ± SEMs of 40 different fields from 2 independent experiments performed in duplicate and are expressed as the average number of migrated cells per field. *P < .05 for untreated versus wortmannin.**P < .05 for untreated versus PD98059.
Although informative, the interpretation of results using pharmacologic inhibitors is limited because there are 4 classes of PI-3kinases and wortmannin can inactivate all 4 classes. Thus, while the use of wortmannin suggests PI-3kinase is critical in migration, it does not distinguish which class is physiologically relevant. To address this issue, we used a biochemical and a genetic approach to specifically examine the role of class IA PI-3kinase in c-Kit–induced haptotaxis on fibronectin. A mutant c-Kit receptor was generated in which tyrosine 719 was mutated to Phe, thus ablating the binding site for the p85α subunit of class IA PI-3kinase. To bypass c-Kit receptors in wild-type mast cells, a chimeric c-Kit receptor (CHR) was constructed. The M-CSF receptor and c-Kit belong to the same subfamily but have different ligand-binding specificities.47 Mast cells do not express endogenous M-CSF receptor and show no biologic response to M-CSF.48 Therefore, we constructed a gene encoding a chimeric receptor protein that contains the extracellular ligand-binding domain of the human M-CSF receptor, and the transmembrane and cytoplasmic domains of murine c-Kit. Both wild-type (M-CSF/c-Kit) and the class IA PI-3kinase–mutant CHRs (M-CSF/c-Kit719) were cloned into a bicistronic retroviral vector MIEG3, in which the expression of the enhanced green fluorescent protein (EGFP) gene is under the control of the Moloney leukemia stem cell virus (MSCV) long terminal repeat (LTR) and uses an internal ribosome entry site (IRES) element.40,49 Cells were transduced with these retroviruses, and EGFP+ cells were sorted, expanded, and used to perform biochemical and functional studies. To confirm that the tyrosine 719 to phenylalanine–mutant receptor is defective in the binding of p85α subunit of class IA PI-3kinase, we performed Western blots on immunoprecipitated M-CSF/c-Kit719 and M-CSF/c-Kit after stimulating the cells with M-CSF. Equal amounts of lysates were subjected to IP with the use of an anti–M-CSF receptor antibody, followed by Western blot analysis using an anti-p85α antibody. As expected, immunoprecipitates of cells expressing the M-CSF/c-Kit demonstrated coimmunoprecipitated p85α subunit of class IA PI-3kinase after M-CSF stimulation (Figure 4A). In contrast, and as previously demonstrated,23 IP of cells expressing the M-CSF/c-Kit719 did not result in coimmunoprecipitation of p85α in spite of similar cell surface expression (Figure 4B), suggesting defective binding to this mutant (Figure 4A). Consistent with a role for PI-3kinase in directed migration of mast cells suggested by treatment with wortmannin, abrogating the binding of the p85α subunit of class IA PI-3kinase to c-Kit was associated with a markedly reduced migration of these cells in the presence of M-CSF (Figure 4C).
Effect of Tyr719Phe substitution in c-Kit on PI-3kinase and haptotactic migration in mast cells. Tyrosine-to-phenylalanine substitution at position 719 in c-Kit abrogates the binding of the p85α subunit of PI-3kinase and impairs c-Kit–induced haptotactic migration in mast cells. (A) Lack of association of the p85α subunit of PI-3kinase with the 719 chimeric c-Kit receptor. Cells expressing either the M-CSF/c-Kit or the M-CSF/c-Kit719 receptor were starved and stimulated with M-CSF for indicated times. Equal amounts of protein were subjected to IP with the use of an anti–M-CSF receptor antibody, and Western blot analysis was performed with an anti-p85α antibody. Shown is the position of p85α. (B) Flow cytometric analysis of mast cells expressing M-CSF/c-Kit (left panel) and M-CSF/c-Kit719 (right panel). Solid histogram demonstrates staining using an isotype control antibody. Open histogram indicates the level of expression of the M-CSF receptor. (C) Mast cells expressing either M-CSF/c-Kit or M-CSF/c-Kit719 were subjected to migration in the presence or absence of M-CSF as described in “Materials and methods.” Shown are the mean ± standard deviation (SD) of 3 independent experiments. *P < .05 for M-CSF/c-Kit versus M-CSF/c-Kit719.
Effect of Tyr719Phe substitution in c-Kit on PI-3kinase and haptotactic migration in mast cells. Tyrosine-to-phenylalanine substitution at position 719 in c-Kit abrogates the binding of the p85α subunit of PI-3kinase and impairs c-Kit–induced haptotactic migration in mast cells. (A) Lack of association of the p85α subunit of PI-3kinase with the 719 chimeric c-Kit receptor. Cells expressing either the M-CSF/c-Kit or the M-CSF/c-Kit719 receptor were starved and stimulated with M-CSF for indicated times. Equal amounts of protein were subjected to IP with the use of an anti–M-CSF receptor antibody, and Western blot analysis was performed with an anti-p85α antibody. Shown is the position of p85α. (B) Flow cytometric analysis of mast cells expressing M-CSF/c-Kit (left panel) and M-CSF/c-Kit719 (right panel). Solid histogram demonstrates staining using an isotype control antibody. Open histogram indicates the level of expression of the M-CSF receptor. (C) Mast cells expressing either M-CSF/c-Kit or M-CSF/c-Kit719 were subjected to migration in the presence or absence of M-CSF as described in “Materials and methods.” Shown are the mean ± standard deviation (SD) of 3 independent experiments. *P < .05 for M-CSF/c-Kit versus M-CSF/c-Kit719.
p85α–/– mast cells show reduced c-Kit–induced migration and Rac activation
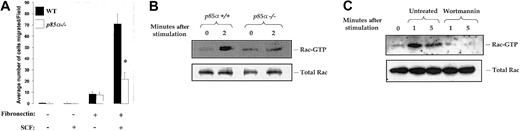
To further confirm the role of p85α in c-Kit–induced migration of mast cells, we examined mast cells from mice deficient in the expression of the p85α subunit of class IA PI-3kinase.38 Consistent with our previous observations (Figures 3 and 4C), p85α-/- mast cells demonstrated significantly reduced haptotaxis on fibronectin after activation of c-Kit with SCF (Figure 5A).
Reduced c-Kit–induced migration and reduced Rac activation in p85α–/–mast cells. (A) Mast cells from wild-type and p85α-/-mice were generated and subjected to migration as described in Figure 1. Data are the mean ± SEM of 20 different fields from 1 of 3 independent experiments and are expressed as the average number of migrated cells per field. *P < .05 for WT versus p85α-/-. (B) Wild-type (p85α+/+) and p85α-/-mast cells were starved for 18 to 24 hours and stimulated with SCF for indicated times. Equal amounts of cell lysates were subjected to a P21-activated kinase (PAK)–binding pull-down assay, which measures active, GTP-bound Rac as described in “Materials and methods.” Shown is the position of activated Rac (Rac-GTP). (C) Wild-type mast cells were starved for 18 to 24 hours and left untreated or treated with wortmannin for 1 hour at 37°C. Subsequently, cells were washed and stimulated with SCF for indicated times. Equal amounts of cells lysates were subjected to a Rac-GTP pull-down assay as described in “Materials and methods.” Top panel: the position of activated Rac (Rac-GTP). Bottom panel: total Rac protein in each lane.
Reduced c-Kit–induced migration and reduced Rac activation in p85α–/–mast cells. (A) Mast cells from wild-type and p85α-/-mice were generated and subjected to migration as described in Figure 1. Data are the mean ± SEM of 20 different fields from 1 of 3 independent experiments and are expressed as the average number of migrated cells per field. *P < .05 for WT versus p85α-/-. (B) Wild-type (p85α+/+) and p85α-/-mast cells were starved for 18 to 24 hours and stimulated with SCF for indicated times. Equal amounts of cell lysates were subjected to a P21-activated kinase (PAK)–binding pull-down assay, which measures active, GTP-bound Rac as described in “Materials and methods.” Shown is the position of activated Rac (Rac-GTP). (C) Wild-type mast cells were starved for 18 to 24 hours and left untreated or treated with wortmannin for 1 hour at 37°C. Subsequently, cells were washed and stimulated with SCF for indicated times. Equal amounts of cells lysates were subjected to a Rac-GTP pull-down assay as described in “Materials and methods.” Top panel: the position of activated Rac (Rac-GTP). Bottom panel: total Rac protein in each lane.
Previous studies (conducted primarily in fibroblasts) implicated class IA PI-3kinase in activation of the Rho GTPase, Rac, and subsequent Rac-regulated cytoskeletal-mediated functions, including chemotaxis.50-52 To determine if lack of the p85α subunit of class IA PI-3kinase impairs Rac activation, which may in part be responsible for the impaired haptotactic migration in response to SCF, we analyzed the activation of Rac by GTP binding in p85α-deficient mast cells in response to SCF stimulation. As shown in Figure 5B, GTP-bound Rac was reduced in p85α-/- mast cells in response to SCF stimulation. Consistent with these observations, treatment of wild-type mast cells with wortmannin also inhibited Rac activation in response to SCF stimulation (Figure 5C).
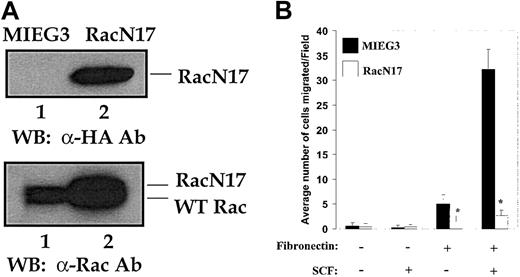
Expression of dominant-negative Rac (RacN17) in mast cells impairs α4- and c-Kit–induced haptotaxis
To directly assess the role of Rac in c-Kit– and α4 integrin–directed migration in mast cells, we expressed a dominant-negative Rac (RacN17) in wild-type mast cells. After infection of mast cells with either the empty vector (MIEG3) expressing EGFP or RacN17-EGFP, EGFP+ cells were sorted and used to perform functional and biochemical studies. Figure 6A (top panel) demonstrates the expression of RacN17 by Western blot analysis using an anti-HA antibody (lane 2), while a comparison of expression of endogenous Rac–with exogenous HA–tagged RacN17, as determined by Western blotting using an anti-Rac antibody, is shown in Figure 6A (bottom panel). Expression of RacN17 was associated with significantly reduced haptotactic migration of mast cells in the absence as well as in the presence of SCF compared with cells expressing the empty vector (Figure 6B). These results strongly implicate Rac-GTP in the haptotactic migration of mast cells. However, since RacN17 will inhibit all isoforms of Rac (Rac1, Rac2, and Rac3), these studies do not determine which specific Rac is critical for this migration.
Effect of expression of dominant-negative Rac (RacN17) in mast cells. Expression of dominant-negative Rac (RacN17) in mast cells impairs integrin- and c-Kit–induced haptotaxis. Mast cells from wild-type mice were generated as described in “Materials and methods.” These cells were transduced with either the empty MIEG3-EGFP or the MIEG3-RacN17-EGFP retrovirus and sorted to homogeneity. (A) Demonstrated are the expression of HA-RacN17 (lane 2) as determined by Western blotting using an anti-HA antibody (top panel), and the expression of endogenous Rac protein as well as of HA-tagged RacN17 detected with an anti-Rac antibody (bottom panel). The positions of endogenous Rac (lanes 1-2) and the slow-migrating HA-RacN17 (lane 2) are indicated. (B) Migration assay was performed with the use of mast cells transduced with either the empty vector (MIEG3) or HA-RacN17 in the presence or absence of SCF. Data are the means ± SEMs of 3 independent experiments. *P < .05 for MIEG3 versus RacN17.
Effect of expression of dominant-negative Rac (RacN17) in mast cells. Expression of dominant-negative Rac (RacN17) in mast cells impairs integrin- and c-Kit–induced haptotaxis. Mast cells from wild-type mice were generated as described in “Materials and methods.” These cells were transduced with either the empty MIEG3-EGFP or the MIEG3-RacN17-EGFP retrovirus and sorted to homogeneity. (A) Demonstrated are the expression of HA-RacN17 (lane 2) as determined by Western blotting using an anti-HA antibody (top panel), and the expression of endogenous Rac protein as well as of HA-tagged RacN17 detected with an anti-Rac antibody (bottom panel). The positions of endogenous Rac (lanes 1-2) and the slow-migrating HA-RacN17 (lane 2) are indicated. (B) Migration assay was performed with the use of mast cells transduced with either the empty vector (MIEG3) or HA-RacN17 in the presence or absence of SCF. Data are the means ± SEMs of 3 independent experiments. *P < .05 for MIEG3 versus RacN17.
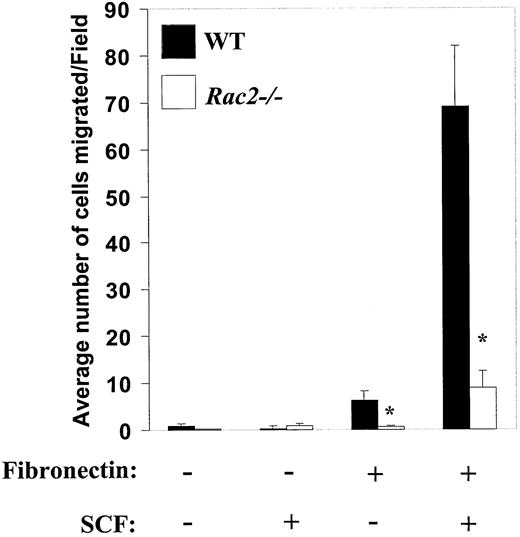
Rac2–/– mast cells demonstrate impaired integrin- and c-Kit–induced haptotactic migration
Directed migration involves multiple steps, including cytoskeleton reorganization, polarization, cell adhesion, and detachment. In nonhematopoietic cells, a significant part of this process is regulated by Rho family GTPases, including Rac. Since we have previously demonstrated an essential role for the hematopoietic-specific Rho GTPase, Rac2, in SCF-induced chemotaxis in mast cells,40 we next determined whether integrin-directed migration was affected by the deficiency of Rac2. Rac2-/- mast cells were generated and analyzed for migration on FN (CH296) in the absence or presence of SCF. As shown in Figure 7, loss of Rac2 resulted in approximately 90% inhibition in integrin-directed migration in spite of the presence of Rac1 in these cells.40 While deficiency of Rac2 also reduced adhesion to FN (data not shown),40 the effect on migration was significantly greater than the effect on adhesion. Consistent with the reduced haptotactic migration in Rac2-/- mice cells in vitro, Rac2 deficiency in vivo was also associated with a slight, but significant, decrease in the number of dermal (ear) and peritoneal mast cells (data not shown). Collectively, these results identify a biochemical pathway downstream of c-Kit and integrin involving class IA PI-3kinase and Rac in regulating c-Kit–induced migration on FN in primary mast cells.
Impaired α4 integrin– and c-Kit–induced haptotactic migration in Rac2–/–mast cells. Mast cells from WT and Rac2-/- mice were subjected to migration assay as described in “Materials and methods.” Data are mean ± SEM from 1 of 3 representative experiments, and are expressed as the average number of migrated cells per field. At least 10 different fields were scored. *P < .05 for WT versus Rac2-/-.
Impaired α4 integrin– and c-Kit–induced haptotactic migration in Rac2–/–mast cells. Mast cells from WT and Rac2-/- mice were subjected to migration assay as described in “Materials and methods.” Data are mean ± SEM from 1 of 3 representative experiments, and are expressed as the average number of migrated cells per field. At least 10 different fields were scored. *P < .05 for WT versus Rac2-/-.
Discussion
Integrin-stimulated (haptotactic) cell migration is a complex and dynamic process. Migrating cells must adhere to and retract from the substratum on which they move.53 Haptotaxis differs from chemotactic-induced cell migration in that it requires adhesion molecules, such as integrins, to generate motility-signaling events.54 Activation of integrins by cytokines, such as SCF, can result in enhanced binding of integrins to their cognate ligands, and these changes contribute to an integrated signal for coordinated cellular events such as directed cell migration. Since SCF is expressed by endothelial cells,55,56 it is likely to cooperate with integrins in modulating biologic responses in circulating mast cells. This was recently illustrated by means of a coculture system in which proliferation of mast cells was shown to be dependent on the cooperation between c-Kit and α4β1 integrins on mast cells and VCAM-1 and SCF on HUVEC cells.24 In addition to modulating proliferation, SCF can act as a chemoattractant, and together with adhesion to ECM, activation of Kit may facilitate the migration of mast cells and hence influence tissue localization. Thus, SCF may participate in the tissue distribution of mast cells under physiologic conditions.
Previous studies in fibroblast have demonstrated that integrin-derived signals synergize with growth factor signals to produce the structural changes necessary for directed cell migration. In fibroblasts, directed cell migration is triggered by a gradient of chemotactic factors, such as platelet-derived growth factor (PDGF).3,57,58 Among the pathways triggered by PDGF receptor (PDGFR) stimulation, class IA PI-3kinases and the small G proteins of the Rho family, including Rac1, have been implicated in actin cytoskeletal reorganization and cell migration.50-52 Using dominant-negative mutants of PI-3kinase and Rac, studies have shown a role for PI-3kinase–induced Rac activation in regulating cytoskeletal changes, such as PDGF-induced lamellipodia formation, and accompanying directed migration.59 In the present study, using mast cells deficient in the expression of class IA PI-3K, Rac2, and by expressing a dominant-negative form of Rac (RacN17), we also demonstrate an essential role for the PI-3K/Rac pathway in regulating haptotactic migration in primary mast cells downstream from c-Kit and α4 integrin.
Our results demonstrating impaired in vitro haptotaxis in mast cells deficient in the expression of the p85α subunit of class IA PI-3kinase are consistent with significant reductions in the number of mast cells in the ear dermis, back dermis, and peritoneum and in the gastrointestine of PI-3kinase-/- mice in vivo.60 Of note, the severity of deficiency of mast cells in the small intestine is greater than in other tissues in these mice, although c-Kit is required for mast cell development in tissues and mutants of c-Kit are severely deficient in mast cells in nearly all tissues.8 These results suggest that PI-3kinase may play a more significant role in the migration of gastrointestinal mast cells, and that other pathways downstream from c-Kit may be involved in the migration/homing of mast cells in other tissues.
In vitro, we demonstrate that loss of PI-3kinase activity and reduced haptotactic migration in PI-3kinase-/- mice cells correlates with a significant reduction in GTP-bound (active) Rac in response to SCF stimulation. A similar decrease in Rac activation was recently reported in mast cells expressing a mutant c-Kit, impaired in the binding and activation of p85α subunit of PI-3kinase.37 However, these studies did not examine the role of this biochemical phenomenon in integrin-directed migration of mast cells. Consistent with a role for Rac in regulating haptotactic migration in fibroblasts, the expression of dominant-negative Rac in mast cells and the deficiency of the hematopoietic-specific Rho GTPase, Rac2, in mast cells also results in impaired haptotactic migration. Interestingly, in contrast to a profound reduction in the number of tissue-specific mast cells reported in PI-3kinase-/- mice, loss of Rac2 is associated with only a modest (but significant) reduction in the number of mast cells in vivo, including dermal (ear) and peritoneal mast cells (data not shown). Thus, in vivo, PI-3kinase may regulate other Rho family members, such as Rac1, and Cdc42, which may play a role in the migration of mast cells in other tissues. This is consistent with the suggested role of Cdc42 in orientation and migration of macrophages.61 Alternatively, PI-3kinase may regulate the activity of other signaling proteins implicated in directed migration, including phospholipase C–γ (PLC-γ).62 Finally, since c-Kit also regulates proliferation in primary mast cells, and since PI-3kinase-/- mice cells show proliferative defects in response to SCF, the reduced number of tissue mast cells in PI-3kinase-/- mice could be attributed to impaired c-Kit–induced proliferation in vivo.60 Regardless of the mechanism, our in vitro results clearly demonstrate a role for PI-3kinase in regulating Rac activity and, consequently, haptotactic migration in mast cells. Further, the requirement for Rac in mediating haptotaxis was demonstrated by introducing a dominant-negative form of Rac in primary mast cells and also by examining mast cells from mice deficient in the expression of a hematopoietic-specific Rho GTPase, Rac2.
One of the important questions that will require further investigation is how integrin- and c-Kit–derived signals are integrated and modulate the activation of PI-3kinase and Rac in mast cells. One mechanism might involve the physical interaction (clustering) of key components of both signaling pathways.63,64 Coclustering of integrins and growth factor receptors appears to require association with the cytoskeleton and recruitment of downstream signaling molecules. Aggregation of these molecules has been thought to bring both adhesion- and growth factor–mediated signaling closer to manifest activity.64 Recent reports have documented the interaction of αvβ3 with the insulin and PDGF receptors in fibroblasts.65 However, we did not observe a physical association between c-Kit and α4 integrin in mast cells. More recently, focal adhesion kinase (FAK) was demonstrated to be an important proximal link between PDGF and EGF receptors and β1 integrins during migration in fibroblasts.66 We have not been able to demonstrate a role of FAK in c-Kit– and α4 integrin–mediated haptotaxis in mast cells (data not shown). Thus, alternate mechanisms must exist via which c-Kit and integrin modulate haptotaxis in mast cells. Regardless of the mechanism, our studies clearly demonstrate a role for c-Kit and α4 integrin in regulating haptotaxis in primary mast cells via the PI-3kinase/Rac pathway.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-08-2521.
Partially supported by National Institutes of Health (NIH) grant 2 R01 DK48605 (to D.A.W.). D.I. is a recipient of a Basil O'Connor Award from the March of Dimes (5-FY02-254), and is partially supported by NIH grant 1KO8 CA096579-01.
R.K. is a recipient of an American Society of Hematology Junior Faculty Scholar Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Takara Bio (Otsu, Japan) for providing fibronectin peptides and Dr Lewis Cantley for providing p85α-deficient mice. We thank Drs Mervin Yoder, Wade Clapp, and Ed Srour for critically reviewing the manuscript and members of our laboratories for useful discussions. We also thank Marsha Hippensteel for assistance in preparation of this manuscript and expert administrative assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal