Abstract
The human endothelial cell protein C receptor (hEPCR) is normally expressed by the endothelium of large blood vessels, but the molecular basis for its in vivo specificity is uncertain. In this study, DNaseI hypersensitive site mapping demonstrated the presence of a hypersensitive site in the 5′ flanking region of the hEPCR gene in endothelial cells and certain transformed cells (HeLa and U937) known to express hEPCR in vitro. Conversely, this site was only weakly hypersensitive in HepG2 cells, cells which do not express hEPCR mRNA. Functional analysis of this 5′ flanking region by in vivo dimethylsulfate footprinting in cultured endothelial cells identified multiple regions, containing high and low homology consensus Sp1 binding sequences, that were protected from methylation in endothelial cells. These sequences were not protected in HepG2 cells. Reporter gene analysis of this region in endothelial cells demonstrated the presence of promoter activity conferred by the proximal 572 bp but failed to identify a functional TATA-box. This promoter was inactive in HepG2 cells. Electrophoresis mobility shift assays using endothelial cell nuclear extracts identified Sp1 family proteins binding to sites that were protected during footprinting. Sp1 sites were identified in regions at –368, –232, –226, –201, –146, and –102 bp relative to the translation start site. With the exception of the site at –102 bp, each identified Sp1 binding site made a positive contribution to reporter gene expression, although no individual site was critically important. We conclude that transcription factor binding to Sp1 binding sites in the 5′ flanking region is critical for normal hEPCR gene expression in endothelial cells.
Introduction
One of the key regulatory mechanisms controlling physiologic thrombin generation in the vasculature is the protein C anticoagulant pathway. This pathway becomes activated when thrombin binds to the endothelial cell receptor, thrombomodulin. In binding thrombomodulin the substrate specificity of thrombin is altered which results in the activation of protein C. Activated protein C can then inactivate the clotting cofactors Va and VIIIa and so inhibit further thrombin generation (reviewed in Esmon1 and Dahlback2 ). Consequently, defects in this pathway invariably result in an increased risk of thrombosis, illustrating its essential regulatory role.3 Activation of protein C is enhanced by an additional cell surface receptor, the endothelial cell protein C receptor (EPCR). The EPCR binds both protein C and activated protein C with high affinity (Kd approximately 30 nM)4,5 and functions by reducing the Km for protein C activation by the thrombin:thrombomodulin complex. The physiologic importance of the EPCR has been demonstrated in a baboon model in which activated protein C generation was reduced by approximately 90% following thrombin infusion when EPCR function was blocked by an inhibitory monoclonal antibody.6
The human EPCR (hEPCR) gene consists of 4 exons and spans approximately 6 kb of genomic DNA.7,8 The initial report of the hEPCR gene placed the transcription initiation site at a single point at –79 bp relative to the translation initiation point.7 A later report by Hayashi et al8 placed the major transcription initiation site at –82 bp and a minor transcription start site at –162 bp. The murine EPCR (mEPCR) gene has also been sequenced and exhibits a conserved structure when compared with the hEPCR gene.9 However, unlike the hEPCR gene, transcription of the mEPCR gene occurs from multiple sites, with 6 major initiation loci located between –100 bp and –109 bp. Furthermore, it appears that most of the murine promoter activity is mediated by single AP-4 and Sp1 binding sites located between –220 and –180 bp upstream of exon 1.10
The EPCR is one of a number of proteins with diverse functions whose normal expression in vivo is restricted almost exclusively to the vascular endothelium.11 We currently have an incomplete understanding of the mechanisms that restrict gene expression to this cell type. Endothelial cell-specific gene expression has, at least in part, been attributed to widely distributed transcription factors, such as Sp1, GATA, and Ets family members, as opposed to a set of truly endothelial cell-specific factors.12, 13, 14, 15, 16, 17, 18 Intronic sequences, extracellular forces, and other extrinsic factors (such as tissue microenvironment) have also been shown to influence the expression of some endothelial cell-specific genes.19, 20, 21 Despite its reported selective expression and distribution in vivo, EPCR gene expression has also been detected in a variety of cell lines in culture.22 In this report, we mapped DNaseI hypersensitive sites around the hEPCR gene and performed a functional characterization of the region immediately 5′ to the hEPCR gene to gain first insight into the molecular basis of hEPCR gene expression.
Materials and methods
Mapping of DNaseI hypersensitive sites
DNaseI hypersensitive sites were assayed in a variety of cells in culture as described previously.23,24 Only selected, representative, hypersensitive site data on human umbilical vein endothelial cell (HUVEC), HeLa, U937, and HepG2 cells are presented. In brief, nuclei were isolated from cultured cells and digested for 3 minutes at 22°C with a range of 1 to 20 μg/mL DNaseI. Optimally, digested DNA samples were digested with BamHI or EcoRI, electrophoresed on agarose gels, transferred to nylon membranes, and probed with 32P-labeled probes. DNaseI hypersensitive sites downstream of a BamHI site located 4.7 kb upstream of the EPCR gene were probed with a mixture of 2 nonrepetitive sequences located downstream of this BamHI site. These probes were generated by polymerase chain reaction (PCR) using the primer sets R1 (AGCTTTATTTTATTTATTTTTTG and AAAAGCTATAATTTGAAAGTT) and R2 (GTGATGCTAGTGAAACCCAT and AGATGGAGGCCTTTCTAATC).
Multiplex RT-PCR analysis of hEPCR gene expression
DNA-free total RNA was isolated from HUVECs, human dermal microvascular endothelial cells (DMVECs), EA.hy926 cells, and HepG2 cells using the RNeasy mini kit (Qiagen, Valencia, CA). Reverse transcription (RT)–PCR was performed using the OneStep RT-PCR kit (Qiagen) according to the manufacturer's instructions. Primers were included to coamplify hEPCR cDNA and the cDNA of a housekeeping gene, TPI1. Each reaction contained 10 pg RNA, 30 pmol EPCR.RT-PCR1 (AGTCCTACCTGCTCCAGTTCCA) and EPCR.RT-PCR2 (AGTCCTACCTGCTCCAGTTCCA), together with 15 pmol TPIF1 (GCGCAGACACTGACCTTCAG) and 29449 (CACCATCAGAAGCATATGC). Expected amplification products were 125 bp and 884 bp for the hEPCR and TPI1, respectively. RT-PCR reaction conditions involved 50°C for 30 minutes (for reverse transcription), 95°C for 15 minutes followed by 30 cycles of 94°C for 30 seconds, 54°C for 30 seconds, 72°C for 1 minute, and a final extension at 72°C for 10 minutes. Amplification products were visualized on 2% agarose gels.
In vivo footprinting
Dimethylsulfate (DMS) treatment of cells and naked genomic DNA followed by ligation mediated-PCR (LM-PCR) were performed as previously described.25 PCR amplification for LM-PCR was carried out using Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). PCR products were labeled by primer extension using 32P-5′-labeled primers. Presented autoradiographs are representative of experiments performed in triplicate. Gels were imaged using a BioRad Molecular Imager FX Phosphorimager. Bands were quantified using QuantityOne software (Bio-Rad, Hercules, CA). The primers used were designed to examine the region immediately 5′ to the transcription start site. The lower strand primers were GACTTCTCACCAAGCCCTTCTC, TTCCGCTCCCTGTTCCTGGT, and CGCTCCCTGTTCCTGGTTCCTA; the upper strand primers were GCTGGCTGCAGTCTAGGGAA, CTAGGGAAAAGAGAAGTGAGGACCGT, and CTAGGGAAAAGAGAAGTGAGGACCGTCTGGG. As footprinting data observed using EA.hy926 cells and HUVECs gave identical results, only EA.hy926 footprints are presented.
Sequencing and plasmid generation
The sequence of the hEPCR gene 5′ region was determined from the PAC clone 212-C5 using the primer walking method as previously described.7 The sequence spanning –2336 to –9 bp was amplified by PCR and cloned into vector pSP72 (Promega, Madison, WI). The 5′ deletions of this “full-length” hEPCR promoter fragment were then generated by either PCR or restriction enzyme digestion and subsequently subcloned into the reporter vector pSEAP-basic (encoding the gene for secreted alkaline phosphatase [SEAP]; Clontech, Palo Alto, CA) via XhoI and EcoRI sites to ensure the correct orientation. Restriction enzymes and primers used for cloning are indicated in Table 1.
Restriction enzymes and oligonucleotide primers used to clone 5′ deletion constructs
Construct* . | Deletion-specific enzymes/oligonucleotide primers† . |
|---|---|
| SEAP.-2336 | TCTCATGGTACCAAATGAAATATTTCAGGCTGTGC |
| SEAP.-1194 | BsmI |
| SEAP.-691 | BstEII |
| SEAP.-572 | TCTCATGGATCCGGATTATGTAATTGTTAT |
| SEAP.-518 | BstXI |
| SEAP.-265 | TCTCATGGATCCGGCAGGTCTGGGCAGGAG |
| SEAP.-227 | TCTCATGGATCCAGAGGGAGGGCAGGAGGGAG |
| SEAP.-137 | PfIMI |
| Downstream primer | TCTCATGGTACCTGAGGCTCCGGACCTGGGT |
Construct* . | Deletion-specific enzymes/oligonucleotide primers† . |
|---|---|
| SEAP.-2336 | TCTCATGGTACCAAATGAAATATTTCAGGCTGTGC |
| SEAP.-1194 | BsmI |
| SEAP.-691 | BstEII |
| SEAP.-572 | TCTCATGGATCCGGATTATGTAATTGTTAT |
| SEAP.-518 | BstXI |
| SEAP.-265 | TCTCATGGATCCGGCAGGTCTGGGCAGGAG |
| SEAP.-227 | TCTCATGGATCCAGAGGGAGGGCAGGAGGGAG |
| SEAP.-137 | PfIMI |
| Downstream primer | TCTCATGGTACCTGAGGCTCCGGACCTGGGT |
Constructs were initially prepared in vector pSP72 and subcloned into pSEAP. basic. The number indicates the 5′ most hEPCR nucleotide contained within the construct.
For constructs generated using restriction enzymes, vector pSP72.-2336 was digested with BamHI and the enzyme listed. The fragment containing vector and hEPCR sequence was blunt ended, and the vector was recircularized. Constructs generated by PCR used the primers listed and the same downstream primer.
Site-directed mutagenesis was performed using the Quikchange mutagenesis kit (Stratagene), according to the manufacturer's instructions. The sequences of the oligonucleotide primers used for mutagenesis are available on request. The nature of each mutation is indicated in Table 2. Large-scale preparations of all reporter constructs were prepared using Endofree Plasmid Maxiprep kits (Qiagen). The integrity of all DNA sequences synthesized in vitro was confirmed by sequencing.
Cell culture, transfection, and reporter assays
Single donor HUVECs and DMVECs (Clonetics, Walkersville, MD) were maintained in complete endothelial cell growth medium (EGM-2; Clonetics), according to the supplier's instructions. All experiments were performed on cells at passage 4 or lower. The endothelial cell line EA.hy92626 (kindly provided by Dr Cora-Jean Edgell, University of North Carolina, Chapel Hill) was maintained in Dulbecco HAT (hypoxanthine, aminopterin, and thymidine) media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Sigma, St Louis, MO). HepG2 cells were obtained from the European Collection of Cell Cultures (Salisbury, United Kingdom) and maintained in minimal essential media (Sigma) supplemented with 1 × nonessential amino acids (Sigma), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 10% FCS. All cells were maintained at 37°C, 5% CO2 in a humidified incubator.
Both HUVECs and EA.hy926 cells were transiently transfected with the SEAP reporter constructs using TransIT-LT1 transfection reagent (Mirus, Madison, WI) according to the manufacturer's instructions. This method reproducibly achieved a transfection efficiency of approximately 10% for both EA.hy926 cells and HUVECs. This efficiency was determined by staining for β-galactosidase following transfection with a vector encoding the LacZ gene and was performed in duplicate for each set of transfections (data not shown). To allow for normalization of results with respect to variations in transfection efficiency, SEAP constructs were cotransfected with the vector pGL3-control (Promega), encoding firefly luciferase under control of the SV40 promoter and enhancer. HepG2 cells were transfected using the calcium phosphate method previously described.27 Briefly, cells were seeded in 6-well plates at 1 × 106 cells per well, 18 hours prior to transfection. Cells were then transfected with 10 μg SEAP reporter vectors for 6 hours.
Conditioned media (containing the product of the SEAP gene) and cell lysates (containing the luciferase gene product) were collected 48 hours after transfection by standard techniques. SEAP levels were determined by chemiluminescent assay of 15 μL conditioned media according to the manufacturer's instructions (Clontech). Luciferase levels in cell lysates were established by chemiluminescent assay (Promega).
Electrophoresis mobility shift assay (EMSA)
Nuclear proteins were prepared as previously described28 and stored at –70°C. Double-stranded probes for EMSA corresponding to specific sequences of the hEPCR promoter were prepared by annealing 100 pmol complementary oligonucleotides. The sequence of each sense strand oligonucleotide is shown in Table 2. Complementary oligonucleotides were designed to leave a 5′ overhang to facilitate labeling with 32P using Klenow fragment of DNA polymerase I (NEB, Beverly, MA). Each 20 μL EMSA binding reaction contained 25 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.5), 50 mM KCl, 3 mM MgCl2, 1 mM DTT (dithiothreitol), 50 μg/mL poly (dI-dC) (Amersham Pharmacia Biotech), 250 μg/mL bovine serum albumin (BSA), 0.01% IGEPAL-630, 10 μg nuclear protein, and 30 to 300 fmol labeled duplex oligonucleotide, depending on specific activity. Preliminary competition experiments, using increasing amounts of unlabeled specific competitor or nonspecific/mutated control oligonucleotide, were used to determine the minimum and maximum amounts of oligonucleotide that could be used for specific competition (data not shown). Presented competition experiments have been simplified by showing 100 pmol unlabeled double-stranded oligonucleotide, a great excess that still resulted in specific competition. Supershift experiments included 100 μg/mL of the appropriate polyclonal antibody (Santa Cruz Biotech, Santa Cruz, CA) added to the binding reaction 10 minutes after addition of the labeled oligonucleotide. In all cases, reactions were incubated on ice for 1 hour and electrophoresed on a 5% TBE (Tris Borate EDTA (ethylenedia-minetetraacetic acid)) polyacrylamide gel (Bio-Rad). Thereafter, gels were dried, and labeled oligonucleotides were visualized by autoradiography. As bandshift patterns using EA.hy926 or HUVEC nuclear extracts were identical, only EA.hy926 nuclear protein data are presented.
Results
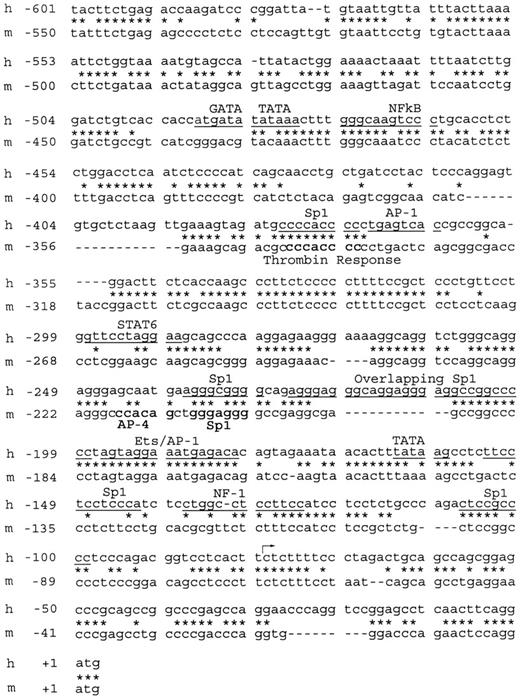
The previously published sequence of the hEPCR gene included 495 bp of 5′ flanking sequence,7 and we now present a further 1762 bp upstream of this point (GenBank Accession no. AF106202). All numbering in this report is relative to the ATG start codon (+1). Alignment of the hEPCR gene with that of the mEPCR sequence showed that approximately 600 bp upstream of exon 1 is approximately 60% conserved between the 2 species (Figure 1). The Sp1 binding site and the thrombin response element identified in the murine promoter10 are also conserved between the 2 species. This 600-bp region contains elements with numerous matches to consensus transcription factor binding sites29 (Figure 1). Only 2 potential TATA-motifs were identified in this region; however, these are located far (82 bp and 470 bp) from the major transcription start site. Furthermore, disruption of the TATA-motif at 82 bp upstream of the major transcription start site resulted in unaffected transcriptional activity in reporter gene analysis, suggesting that this element does not constitute a functional TATA-box (data not shown).
Alignment of the immediate upstream promoter sequences of the human and murineEPCRgenes. The nucleotide sequences spanning –601 to +3 bp of the hEPCR gene and spanning –550 to +1 bp of the mEPCR gene were aligned to identify regions of sequence similarity. All numbering is relative to the A of the ATG initiation codons. Stars indicate aligned nucleotides; dashes indicate gaps in the alignment. Predicted transcription factor binding sites are underlined in the human (h) sequence. Transcription factor binding sites identified in the murine (m) promoter10 are highlighted in bold. The previously identified transcription start site of the human gene7 is indicated by the arrow.
Alignment of the immediate upstream promoter sequences of the human and murineEPCRgenes. The nucleotide sequences spanning –601 to +3 bp of the hEPCR gene and spanning –550 to +1 bp of the mEPCR gene were aligned to identify regions of sequence similarity. All numbering is relative to the A of the ATG initiation codons. Stars indicate aligned nucleotides; dashes indicate gaps in the alignment. Predicted transcription factor binding sites are underlined in the human (h) sequence. Transcription factor binding sites identified in the murine (m) promoter10 are highlighted in bold. The previously identified transcription start site of the human gene7 is indicated by the arrow.
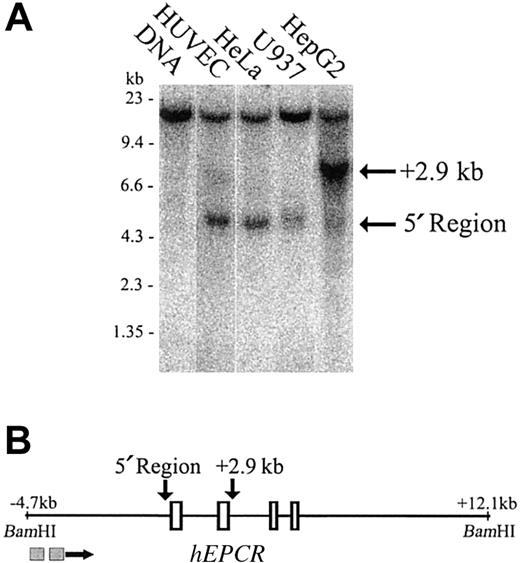
The EPCR promoter forms a DNaseI hypersensitive site
To identify potential regulatory regions, we first mapped DNaseI hypersensitive sites over 16.8 kb of genomic sequence that encompassed all of the introns and exons of the hEPCR gene. This analysis extended between BamHI sites located at –4.7 kb and +12.1 kb relative to the translation start site. A prominent DNaseI hypersensitive site existed at the region immediately 5′ to the hEPCR gene in HUVECs and also in HeLa and U937 cells (cells that express hEPCR in vitro22 and unpublished observations, September 2002) (Figure 2A, lanes 2-4). However, it was only weakly hypersensitive in HepG2 cells (Figure 2A, lane 5). A second DNaseI hypersensitive site of presently uncertain functional significance was located 2.9 kb downstream of the translation start site. This site was weakly hypersensitive in HUVECs (Figure 2A, lane 2) but strongly hypersensitive in HepG2 cells (Figure 2A, lane 5). The existence, intensity, and location of the 2 DNaseI hypersensitive sites in the hEPCR gene was confirmed by probing the same samples from an EcoRI site located 4.3 kb downstream of the translation start site (data not shown).
Mapping of DNaseI hypersensitive sites around thehEPCRgene. (A) DNaseI hypersensitivity sites were mapped in HUVEC, HeLa, U937, and HepG2 cells (lanes 2-5). Lane 1 indicates DNA undigested with DNaseI. The approximate band size in kilobase (kb) is indicated on the left of the figure. The location of sites in relation to the hEPCR gene is indicated on the right. (B) Map of the BamHI fragment encompassing the hEPCR gene. Exons 1 to 4 of the hEPCR gene are indicated as rectangles, and the identified hypersensitive sites are indicated (above the figure) by the arrows. The locations of the probes used in this analysis are indicated as gray boxes below the figure.
Mapping of DNaseI hypersensitive sites around thehEPCRgene. (A) DNaseI hypersensitivity sites were mapped in HUVEC, HeLa, U937, and HepG2 cells (lanes 2-5). Lane 1 indicates DNA undigested with DNaseI. The approximate band size in kilobase (kb) is indicated on the left of the figure. The location of sites in relation to the hEPCR gene is indicated on the right. (B) Map of the BamHI fragment encompassing the hEPCR gene. Exons 1 to 4 of the hEPCR gene are indicated as rectangles, and the identified hypersensitive sites are indicated (above the figure) by the arrows. The locations of the probes used in this analysis are indicated as gray boxes below the figure.
hEPCR mRNA transcripts are present in endothelial cells but not in HepG2 cells
Multiplex RT-PCR was performed on total RNA extracts from the endothelial cell line EA.hy926, HUVECs, DMVECs, and HepG2 cells to assess the presence of hEPCR mRNA transcripts (Figure 3, lanes 3, 5, 6, and 4, respectively). The integrity of the mRNA preparations was confirmed by RT-PCR of a housekeeping gene, TPI1 (Figure 3, lanes 3-6). A hEPCR RT-PCR amplification product was detected in the EA.hy926 cells, HUVECs, and DMVECs (Figure 3, lanes 3, 5, and 6). HepG2 cells were devoid of detectable EPCR transcripts (Figure 3, lane 4).
Multiplex RT-PCR analysis of EA.hy926, HUVEC, and HepG2 mRNA extracts. Multiplex RT-PCR was performed on RNA prepared from EA.hy926 cells, HepG2 cells, HUVECs, and DMVECs (lanes 3-6). Primers were designed to span intron 3 of the hEPCR gene, and the size of the expected hEPCR transcript-dependent PCR amplification product was 125 bp. To confirm the integrity of RNA, RT-PCR was performed in multiplex with a housekeeping gene (TPI1). The expected size of amplification product was 884 bp. The amplified products were separated on a 2% agarose gel and compared with a 123 bp oligonucleotide ladder (lanes 1 and 8) and a no-RNA template negative control (lanes 2 and 7).
Multiplex RT-PCR analysis of EA.hy926, HUVEC, and HepG2 mRNA extracts. Multiplex RT-PCR was performed on RNA prepared from EA.hy926 cells, HepG2 cells, HUVECs, and DMVECs (lanes 3-6). Primers were designed to span intron 3 of the hEPCR gene, and the size of the expected hEPCR transcript-dependent PCR amplification product was 125 bp. To confirm the integrity of RNA, RT-PCR was performed in multiplex with a housekeeping gene (TPI1). The expected size of amplification product was 884 bp. The amplified products were separated on a 2% agarose gel and compared with a 123 bp oligonucleotide ladder (lanes 1 and 8) and a no-RNA template negative control (lanes 2 and 7).
In vivo DMS footprinting of the hEPCR promoter and immediate upstream sequences
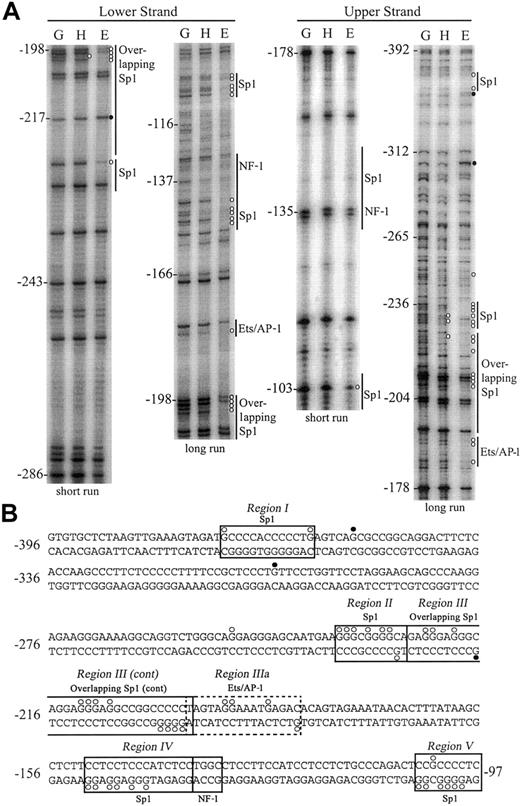
By in vivo DNA footprinting, we compared the DMS reactivity of both upper and lower DNA strands of the putative hEPCR promoter in cells in which the EPCR gene is active (EA.hy926 and HUVEC) or inactive (HepG2). Identical results were observed for footprints of EA.hy926 cells or HUVECs. Experiments were performed in triplicate with representative long and short runs shown of both upper and lower DNA strands from –392 to –97 bp relative to the ATG start codon (Figure 4A). Following standardization for lane intensity, individual mean band intensities for endothelial or HepG2 cells were quantified relative to genomic DNA (complete quantification data not shown). The positions of specific guanidine residues that displayed significant (> 2-fold) enhancement or reduction in DMS reactivity in endothelial cells or HepG2 cells, relative to naked DNA, are indicated in Figure 4.
In vivo DMS footprinting of both DNA strands of the hEPCR promoter. (A) Analysis of lower strand (left panel) and upper strand (right panel). DMS methylation of naked genomic DNA (G) was compared with in vivo methylation of HepG2 (H) or EA.hy926 (E) DNA. A representative experiment with long and short gel runs from each primer set is presented. Potential binding sites for transcription factors are indicated at the right of each gel image. Positions relative to the ATG site are indicated at the left of each gel. Open circles (○) represent 3-fold or greater protection, and closed circles (•) represent 2-fold or greater enhancements of DMS reactivity of E and H relative to G. (B) DNA sequence of the hEPCR promoter from –396 bp to –97 bp with transcription factor binding sites and DMS protections/enhancements indicated as described in panel A. Boxed regions (I-V) indicate binding sites corroborated by EMSA. The dashed box indicates a putative binding site not identified by EMSA.
In vivo DMS footprinting of both DNA strands of the hEPCR promoter. (A) Analysis of lower strand (left panel) and upper strand (right panel). DMS methylation of naked genomic DNA (G) was compared with in vivo methylation of HepG2 (H) or EA.hy926 (E) DNA. A representative experiment with long and short gel runs from each primer set is presented. Potential binding sites for transcription factors are indicated at the right of each gel image. Positions relative to the ATG site are indicated at the left of each gel. Open circles (○) represent 3-fold or greater protection, and closed circles (•) represent 2-fold or greater enhancements of DMS reactivity of E and H relative to G. (B) DNA sequence of the hEPCR promoter from –396 bp to –97 bp with transcription factor binding sites and DMS protections/enhancements indicated as described in panel A. Boxed regions (I-V) indicate binding sites corroborated by EMSA. The dashed box indicates a putative binding site not identified by EMSA.
We identified 5 regions in the hEPCR proximal promoter that exhibited differential DMS reactivity between endothelial and HepG2 cells. The first region (region I) spanned –376 to –336 bp. Guanidine residues were protected from DMS methylation either side of a Sp1 consensus sequence. In addition, a strong enhancement of guanidine –354 bp was observed in endothelial cells. We did not footprint the lower strand of this region. Extensive protection, in excess of 30-fold relative to genomic DNA, was found within region II, located between –239 and –228 bp. These protected guanidine residues correspond to a Sp1 site located at –232 bp. Region III, spanning –227 to –188 bp, contains 3 overlapping Sp1 sites with low homology to the consensus sequence. The lower DNA strand also showed extensive protection over these same consensus sequences in the endothelial cells. The quantification of bands within region II and region III indicated that every guanidine residue on the upper strand was at least 3-fold protected from methylation in endothelial cells when compared with naked genomic DNA. An additional footprint was observed 3′ to region III (region IIIa), which contained an overlapping consensus Ets/AP-1 binding site located at –191 bp.
Region IV, located at –150 to –133 bp, corresponds to 2 overlapping, low homology Sp1 consensus binding sites. Guanidine residues here were strongly protected on the lower strand in endothelial cells. No alterations in DMS reactivity were observed over a consensus nuclear factor 1 (NF-1) binding site located at –135 bp. Region V, spanning –105 to –97 bp, contains a consensus Sp1 binding site and displayed significant protection of all guanidine residues on both DNA strands in endothelial cells.
Transcriptional activity of hEPCR promoter 5′ deletion constructs
To further delineate the location of functional elements that govern expression of the hEPCR gene, 8 constructs were generated, containing progressive deletions of the 5′ region fused to a reporter gene (SEAP). These constructs were transfected into EA.hy926 cells and HUVECs, and their relative transcriptional activity was assessed (Figure 5). After normalization, constructs SEAP.–1194, SEAP.–691, and SEAP.–572 each exhibited similar, maximal, reporter gene activity in both EA.hy926 cells and HUVECs. The activity of SEAP.–2336 was approximately 50% that of SEAP.–1194 in EA.hy926 cells and approximately 75% in HUVECs. These expression levels corresponded to approximately 10% that of the SEAP-control vector (containing the SV40 promoter and enhancer, data not shown). All results fell within the linear range of the assays used (data not shown). Progressive deletion of bases between –572 and –228 bp resulted in a stepwise reduction in reporter gene activity to approximately 50% in EA.hy926 cells and approximately 15% in the HUVECs. In HUVECs, the largest decrease in activity within this region was observed on deletion of sequences located between –518 and –265 bp. A final deletion, which removed the DNA sequence spanning –227 to –138 bp, reduced reporter gene activity to near baseline levels in HUVECs, and to approximately 10% of the maximal value in EA.hy926 cells.
Deletion analysis of sequences 5′ to the hEPCR gene in endothelial cells. Constructs containing serial deletions of the 5′ sequence of the hEPCR gene fused to the gene for SEAP (represented schematically on the left of the figure) were transiently transfected into EA.hy926 cells (▪) and HUVECs (▦) as described in “Materials and methods.” The relative normalized reporter activity is shown on the right of the figure, ± standard error.
Deletion analysis of sequences 5′ to the hEPCR gene in endothelial cells. Constructs containing serial deletions of the 5′ sequence of the hEPCR gene fused to the gene for SEAP (represented schematically on the left of the figure) were transiently transfected into EA.hy926 cells (▪) and HUVECs (▦) as described in “Materials and methods.” The relative normalized reporter activity is shown on the right of the figure, ± standard error.
The activity of each 5′ deletion construct was also determined in HepG2 cells. All cotransfected cells expressed the control luciferase reporter to high levels and SEAP-control vector values were 50to 100-fold higher than that detected for the endothelial cells. In stark contrast to the endothelial cells, none of the tested 5′ deletion constructs were capable of inducing normalized reporter activity above baseline levels in the HepG2 cells (data not shown).
Nuclear protein binding to hEPCR 5′ flanking sequences
The identities of the nuclear proteins binding to hEPCR 5′ flanking sequences were investigated using EMSA to determine specific residues involved in nuclear protein interactions. Supershift assays were performed to identify the specific proteins mediating the shifted bands. All oligonucleotide sequences used (sense strand) are presented in Table 2.
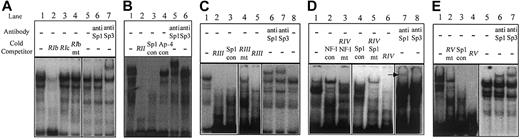
Region I. Oligonucleotide RI (Table 2), corresponding to –401 to –347 bp, encompassed protected region I that was identified in Figure 3. This oligonucleotide could bind nuclear protein specifically and reproducibly, resulting in a distinctive pattern of 2 major shifted bands (Figure 6A, lane 1). This sequence contained consensus transcription factor binding sites for Sp1 and AP-1 (Table 2). Oligonucleotide RIb (corresponding to –374 to –355 bp) contained a consensus Sp1 site and could compete for nuclear protein binding (Figure 6A, lane 2), whereas oligonucleotide RIc, corresponding to –361 to –347 bp (and containing only the consensus AP-1 site), did not (Figure 6A, lane 3). Introduction of a 4-base mutation within the core of the Sp1 consensus sequence (CACC to ACTT) eliminated the ability of oligonucleotide RIb to compete for nuclear protein binding (Figure 6A, lane 4). Supershift analysis identified Sp3 as the nuclear protein whose binding resulted in the formation of the faster migrating complex (Figure 6A, lane 7). We were unable to demonstrate Sp1 binding in region I by supershift analysis (Figure 6A, lane 6).
Mapping of transcription factor binding sites in thehEPCRgene promoter by EMSA. Double-stranded oligonucleotides, corresponding to active regions identified in 5′ deletion analysis, were labeled with α-32P, incubated with EA.hy926 cell nuclear extracts, and separated by EMSA using native-polyacrylamide gel electrophoresis (PAGE; see “Materials and methods”). The probes used were as follows: (panel A) RI; (panel B) RII; (panel C) RIII; (panel D) RIV; and (panel E) RV (Table 2). Where indicated, an excess of unlabeled oligonucleotides, containing mutated bases within putative transcription factor binding sites, was included during incubation (Table 2). For supershift analysis, 100 μg/mL polyclonal antibodies to Sp1 or Sp3 was included as indicated. The arrow in panel D indicates the presence of a supershifted band (con = consensus, mt = mutant).
Mapping of transcription factor binding sites in thehEPCRgene promoter by EMSA. Double-stranded oligonucleotides, corresponding to active regions identified in 5′ deletion analysis, were labeled with α-32P, incubated with EA.hy926 cell nuclear extracts, and separated by EMSA using native-polyacrylamide gel electrophoresis (PAGE; see “Materials and methods”). The probes used were as follows: (panel A) RI; (panel B) RII; (panel C) RIII; (panel D) RIV; and (panel E) RV (Table 2). Where indicated, an excess of unlabeled oligonucleotides, containing mutated bases within putative transcription factor binding sites, was included during incubation (Table 2). For supershift analysis, 100 μg/mL polyclonal antibodies to Sp1 or Sp3 was included as indicated. The arrow in panel D indicates the presence of a supershifted band (con = consensus, mt = mutant).
Region II. Protected region II comprises a sequence that is homologous to the Sp1 site that has previously been demonstrated to confer most of the activity to the murine EPCR promoter.10 In contrast to the mouse promoter, deletion of this region in the human sequence resulted in only a limited decrease in reporter activity in both HUVECs and EA.hy926 cells (Figure 5, compare SEAP.–265 and SEAP.–227). In the murine sequence, there is also a functionally important AP-4 consensus sequence located immediately 5′ to the Sp1 site. This AP-4 site is not conserved in the human sequence, and this region is not protected from DMS methylation in endothelial cells (Figures 1 and 4). However, reproducible and specific nuclear protein binding to an oligonucleotide (RII, Table 2) that spanned this region was observed (Figure 6B, lane 1). Furthermore, nuclear protein binding resulting in the lower mobility species was effectively competed for by a consensus Sp1 oligonucleotide and supershifted by an anti-Sp1 antibody (Figure 6B, lanes 3 and 5). We were unable to demonstrate Sp3 binding (Figure 6B, lane 6). Addition of the AP-4 consensus oligonucleotide had no effect on the DNA protein interactions (Figure 6B, lane 4).
Region III. Protected region III includes the 3 overlapping consensus Sp1 binding sites that were protected from in vivo DMS methylation in endothelial cells (Figure 4). We used RIII as the probe in EMSA and demonstrated that protein binding to this sequence was competed by unlabeled RIII, indicating specificity (Figure 6C, lanes 1-2). Formation of the DNA/protein complexes corresponding to the upper prominent band and the upper band of the higher mobility doublet could also be prevented with a Sp1 consensus oligonucleotide (Figure 6C, lane 3). Point mutations introduced into all 3 potential Sp1 sites, followed by competition, resulted in restoration of the original pattern of shifted bands (Figure 6C, lane 4). Supershift analysis, using antibodies to Sp1 family members, demonstrated binding of both Sp1 and Sp3 to this region (Figure 6C, lanes 6-7).
Region IIIa. Region IIIa contains an overlapping consensus Ets/AP-1 binding site that was protected from in vivo DMS methylation in endothelial cells. We were unable to demonstrate nuclear protein binding to an oligonucleotide that spanned this sequence. Furthermore, an oligonucleotide representing this Ets/AP-1 binding site was unable to compete for nuclear protein binding to DNA sequence encompassing the overlapping Sp1 sites (within region III) and the Ets/AP-1 binding site (data not shown).
Region IV. Protected region IV contained 2 overlapping, low homology, consensus Sp1 binding sites that were protected from in vivo DMS methylation (Table 2 and Figure 4). The oligonucleotide used, corresponding to –155 to –117 bp (which overlaps these Sp1 binding sites, denoted RIV), also contained a high homology consensus NF-1 binding site that was not protected during in vivo footprinting (Figure 4). Nuclear protein binding to oligonucleotide RIV resulted in a complex band shift pattern (Figure 6D, lane 1). Competition with a consensus NF-1 oligonucleotide resulted in the central, high intensity, band being partially eliminated (Figure 6D, lane 2). The Sp1 consensus oligonucleotide competed for protein binding mediating the upper of the bands (Figure 6D, lane 4). Mutation of the consensus NF-1 binding site within oligonucleotide RIV reduced its ability to compete for NF-1–like factor binding (Figure 6D, lane 3 compared with lane 6). Conversely, when point mutations were introduced into both the Sp1 sites within oligonucleotide RIV, this resulted in a similar bandshift pattern to when the consensus NF-1 oligonucleotide was used (Figure 6D, compare lanes 5 and 2). This result indicated that the Sp1 and NF-1 sites within wild-type RIV were mediating the binding of the 2 major shifted species. Supershift assays demonstrated Sp1 binding to the wild-type sequence, but no clear evidence of Sp3 binding (Figure 6D, lanes 7-8). The protein binding through the NF-1 consensus sequence was not formally identified.
Region V. Protected region V was examined using an oligonucleotide spanning –131 to –92 bp (denoted RV, Table 2) (Figure 6E). This sequence contained a sequence with limited homology to the Sp1 consensus (Figure 1) but was protected from in vivo DMS methylation (Figure 4). Reproducible-specific nuclear protein binding to this sequence was demonstrated (Figure 6E, lanes 1 and 4), and the binding mediating the 3 lowest mobility bands could be competed for by excess consensus Sp1 oligonucleotide (Figure 6E, lane 3). Mutation of the Sp1 consensus within RV resulted in less efficient competition than with the wild-type oligonucleotide, indicating that protein bound through this Sp1 site (Figure 6E, lanes 2 and 4). Supershift experiments identified Sp1 and Sp3 as 2 of the proteins involved in binding RV (Figure 6E, lanes 6-7).
Functional analysis of transcription factor binding sites
To attribute functional significance to those DNA sequences exhibiting nuclear protein binding, we introduced numerous point mutations into construct SEAP.–518 that were shown to abolish nuclear protein binding in the EMSA competition experiments. In the case in which a mutant oligonucleotide was not used in EMSA (the Sp1 site at –232 bp [RII, Figure 6B]), the specific mutation introduced is indicated in Table 2. To aid clarity, each mutant construct has been named according to the corresponding panel in Figure 6 in which the EMSA was shown. The results of transient transfection of EA.hy926 cells and HUVECs are shown in Figure 7, compared with wild-type construct SEAP.–518. The data for both cell types was comparable for most of the mutants studied. Therefore, the results are only discussed for individual cell types when there is an apparent functional difference between them.
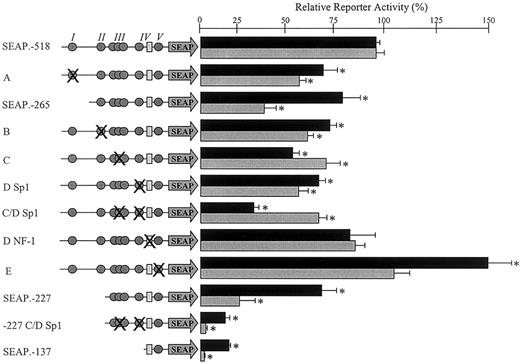
Functional significance of mapped transcription factor binding sites. The function of each mapped transcription factor binding site was investigated by introducing an appropriate mutation into the construct SEAP.–518 or SEAP.–227. These mutations are represented schematically on the left side of the figure. All of the identified binding sites within the wild-type constructs SEAP.–518, SEAP.–265, SEAP.–227, and SEAP.–137 are shown. The Sp1 family protein binding sites are represented by gray circles, and the NF-1 binding site is represented by a pale gray rectangle. The overlapping circles represent the cluster of 3 overlapping Sp1 sites, all of which were mutated for this investigation. Symbol(s) that are crossed represent those site(s) mutated in each construct. The precise mutation(s) introduced were those indicated in Table 2. Mutant constructs (named according to the corresponding EMSA in Figure 6) were transiently transfected into EA.hy926 cells (▪) or HUVECs (▦) as described in “Materials and methods.” Normalized reporter activity is shown relative to the wild-type SEAP.–518 construct ± standard error. The log activity of each mutant was compared with this using a paired t test; those with significantly different activities (P < .05) are indicated with an asterisk.
Functional significance of mapped transcription factor binding sites. The function of each mapped transcription factor binding site was investigated by introducing an appropriate mutation into the construct SEAP.–518 or SEAP.–227. These mutations are represented schematically on the left side of the figure. All of the identified binding sites within the wild-type constructs SEAP.–518, SEAP.–265, SEAP.–227, and SEAP.–137 are shown. The Sp1 family protein binding sites are represented by gray circles, and the NF-1 binding site is represented by a pale gray rectangle. The overlapping circles represent the cluster of 3 overlapping Sp1 sites, all of which were mutated for this investigation. Symbol(s) that are crossed represent those site(s) mutated in each construct. The precise mutation(s) introduced were those indicated in Table 2. Mutant constructs (named according to the corresponding EMSA in Figure 6) were transiently transfected into EA.hy926 cells (▪) or HUVECs (▦) as described in “Materials and methods.” Normalized reporter activity is shown relative to the wild-type SEAP.–518 construct ± standard error. The log activity of each mutant was compared with this using a paired t test; those with significantly different activities (P < .05) are indicated with an asterisk.
Mutation of the Sp1/Sp3 binding site at –368 bp within region I reduced reporter activity by approximately 35% (Figure 7A). This mutation mimicked the effect seen in EA.hy926 cells following deletion of this entire region (Figure 7, black bars, compare SEAP.–518 with SEAP.–265 and mutant A). In the HUVECs, mutation of this site accounted for at least half of the loss of activity observed on deletion of the whole region (Figure 7, gray bars, compare SEAP.–518 with SEAP.–265 and mutant A). Mutation of the Sp1 binding site within region II that is conserved between the hEPCR and mEPCR promoters reduced reporter activity by approximately 30% when compared with the wild-type SEAP.–518 (Figure 7B). As discussed previously, deletion of this entire region resulted in only a limited decrease in reporter activity (Figures 5 and 7, compare SEAP.–265 and SEAP.–227).
When point mutations were introduced into all of the potential Sp1 binding sites located in the cluster (spanning –226 to –201 bp), reporter activity was reduced by approximately 40% compared with wild-type construct SEAP.–518 (Figure 7C). A similar reduction in reporter activity was observed on introduction of a point mutation within the Sp1 sites around –146 bp in region IV (Figure 7D, Sp1). When both of these Sp1 binding regions were altered by point mutations, reporter activity was reduced by approximately 70% from the wild type in the EA.hy926 cells and approximately 30% in the HUVECs (Figure 7C-D, Sp1). This finding suggests an additive effect in the cell line that was not apparent in the primary cells. When this double mutation was introduced into reporter construct SEAP.–227, activity was reduced dramatically and approximated to that of construct SEAP.–137 (Figure 7, –227 C/D Sp1). This indicated that the difference in activity between constructs SEAP.–227 and SEAP.–137 could be mediated by these Sp1 binding sites (Figure 7, compare constructs SEAP.–227, SEAP.–137, and SEAP.–227 C-D Sp1).
The remaining Sp1 binding site identified by in vivo DMS footprinting and EMSA was located at –102 bp (Figures 3 and 6E). A point mutation within this Sp1 binding site resulted in a statistically insignificant increase of approximately 10% in the HUVECs and an increase of approximately 50% in the EA.hy926 cells (Figure 7E). We also investigated the protein binding site identified by EMSA that corresponded to a NF-1 consensus sequence (located at –135 bp, Figure 6D) that was not protected from in vivo DMS methylation in endothelial cells (Figure 4). Point mutation within the NF-1 consensus sequence resulted in no significant alteration in reporter activity from the wild type (Figure 7D, NF-1).
Discussion
In this study we characterize the transcriptional activity of the 5′ flanking region of the hEPCR gene in endothelial cells. Promoter activity is located within the initial 572 bp upstream of exon 1 of the hEPCR gene, and this probably constitutes a TATA-less promoter. Mapping of DNaseI hypersensitive sites around the hEPCR gene indicated that this region contained a hypersensitive site in several cell types, and alignments indicated that this region was significantly conserved between the human and murine form of the gene. Furthermore, analysis of this sequence by in vivo footprinting identified multiple Sp1 consensus sequences and a composite consensus Ets/AP-1 binding site within this region that were protected from in vivo DMS methylation. EMSAs were used to map transcription factor binding to this region and demonstrated directly that the protected Sp1 consensus sequences could bind Sp1 family proteins (Figure 6). No protein binding was detected to the putative Ets/AP-1 binding site.
Functional reporter gene studies revealed that no single Sp1 binding site was essential for activity of the hEPCR promoter. Mutation of each individual site (or cluster of sites), with the exception of that at –102 bp, resulted in a small yet significant decrease in reporter gene activity (Figure 7). The cluster of Sp1 binding sites at –226 to –201 bp and those at –146 bp appeared to confer the most transcriptional activity and could be responsible for most of the reporter activity of the 5′ deletion construct SEAP.–227. This finding suggests that the potential Ets/AP1 binding site at –191 bp is likely to be of minor importance in basal hEPCR gene expression. The potential NF-1 site identified by EMSA was unoccupied in endothelial cells in vivo and did not confer activity to reporter gene constructs. It is, therefore, unlikely to contribute to basal hEPCR gene expression under the conditions in which this study was set.
The findings of multiple overlapping low homology Sp1 sites and the modest reductions in reporter activity observed when these were mutated suggest a certain degree of redundancy within the promoter. In this situation, should the favored Sp1 binding site be disrupted, alternative Sp1 binding sites may participate in nuclear protein binding resulting in unaltered gene expression. This implies that only mutation of all potential Sp1 binding sites could completely abrogate the activity of the promoter. Such redundancy may constitute a mechanism by which expression of an essential receptor30 is guaranteed. However, it is possible that high-level expression of hEPCR in vivo requires the cooperative action of all transcription factor binding sites as has been described elsewhere.31
The transcription factors Sp1 and Sp3 are ubiquitously expressed zinc finger proteins and bind their recognition sequence with similar affinity.32 Sp1 family members are thought to be responsible for the transactivation of numerous genes in endothelial cells,12, 13, 14 although their specific role in the expression of genes restricted to endothelial cells is not fully understood. No significant differences in bandshift patterns were observed when EMSA of the Sp1 binding regions identified in this study were performed using nonendothelial cell nuclear extracts (data not shown). This suggests that tissue-specific activity of the Sp1 family proteins alone is unlikely to be exclusively responsible for cell-restricted hEPCR gene expression. One alternative mechanism for cell-restricted hEPCR gene expression is selective access of these factors to their cis-acting DNA sequences. Indeed, our in vivo footprinting data indicate that transcription factors can gain access to and bind Sp1 consensus binding sites in the hEPCR promoter in endothelial cells, but not in hepatic cells that do not express hEPCR mRNA. Similar data have been reported for the selective binding of transcription factors to the endothelial cell specific KDR/flk-1 promoter in endothelial cells rather than fibroblasts or HeLa cells.33
In contrast to normal in vivo observations, in which EPCR expression is restricted to endothelial cells,11 it is known that a number of transformed cells, including U937, express hEPCR.22,34 Moreover, we have observed low levels of hEPCR transcripts in most commonly cultured fibroblasts, including HeLa cells (data not shown). Mirroring this pattern of expression we show here that both U937 and HeLa cells contain the immediate upstream DNaseI hypersensitive site (Figure 2). In addition, we have observed that in HeLa cells, this site is partly occupied in vivo (by footprinting, data not shown). These observations suggest that this hypersensitive site is directly related to transcription of hEPCR. Whether this is the cause of the recently described expression of EPCR in normal monocytes35 remains to be clarified. However, it seems likely that additional cell-specific mechanisms must be invoked in vivo for high-level endothelial cell expression. One such mechanism may involve elements distinct from those analyzed in this study, such as far upstream or downstream enhancer elements that may cooperate with the promoter and regulate/enhance gene expression in a cell-restricted manner. In transgenic studies of several endothelial cell-specific genes, elements in addition to the 5′ region are required to replicate endogenous gene expression patterns.36,37
An additional complexity with EPCR is provided by its differential expression in the endothelium of differently sized vessels in the vasculature. Immunohistochemistry has indicated that EPCR expression is absent in most capillary endothelial cells, is increased in arterioles and postcapillary venules, and is maximal in the larger vessels.11 We attempted to address this issue by examining hEPCR expression in primary DMVECs. However, RT-PCR analysis demonstrated the presence of abundant hEPCR mRNA in the DMVECs (Figure 3). In addition, FACS analysis has indicated that both DMVECs and a microvascular cell line (MVEC-138 ) express cell surface EPCR to an equivalent level to the EA.hy926 cells (data not shown). These observations suggest that it may be difficult to analyze differential endothelial cell hEPCR expression in cell culture unless a method for culturing a pure population of capillary endothelial cells can be developed. More likely, the removal of cells from their in vivo microenvironment for in vitro culture may alter their phenotype and gene expression. This question is currently being addressed by use of a transgenic mouse model. This approach will also help establish the efficacy of our promoter constructs within an intact chromosomal environment.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-05-1570.
Supported by grants from the British Heart Foundation (FS/99002) and the Special Trustees of the Charing Cross Hospital. Research in the laboratories of C.B. and P.N.C. is funded by grants from the Wellcome Trust, the Leukaemia Research Fund, Yorkshire Cancer Research, and the BBSRC. G.A.F. is a MRC Clinical Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Gamble and M. Vadas for the HUVECs used for DNaseI hypersensitive site analyses.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal